An Integrated Numerical Study of CO2 Storage in Carbonate Gas Reservoirs with Chemical Interaction between CO2, Brine, and Carbonate Rock Matrix
Abstract
1. Introduction
2. Simulation
2.1. Scenarios
2.2. Simulation of CO2 Injection
2.3. CO2 Dissolution in Residual Brine
2.4. CO2–Brine–Carbonate Interaction
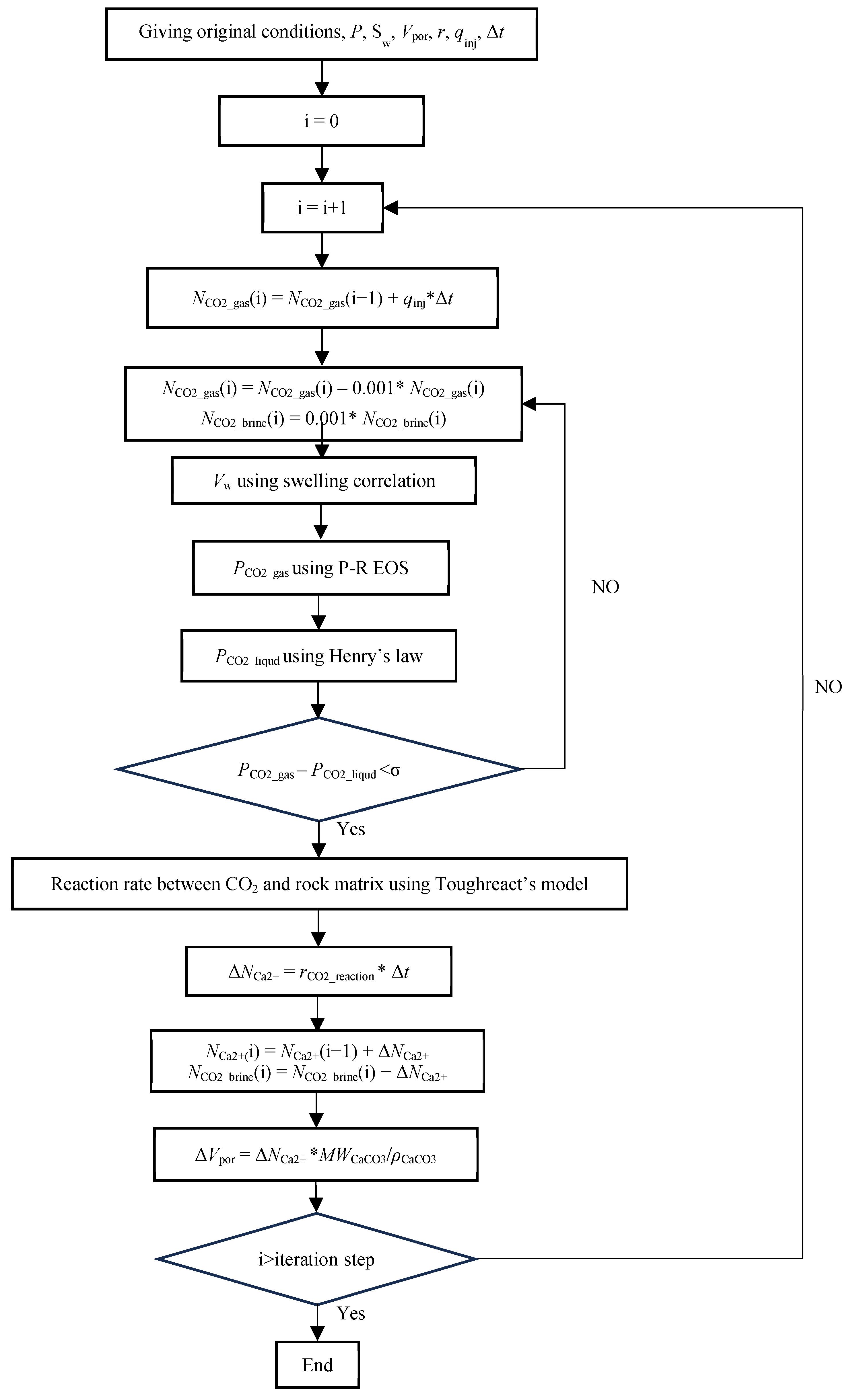
2.5. Simulation Workflow
3. Results and Discussion
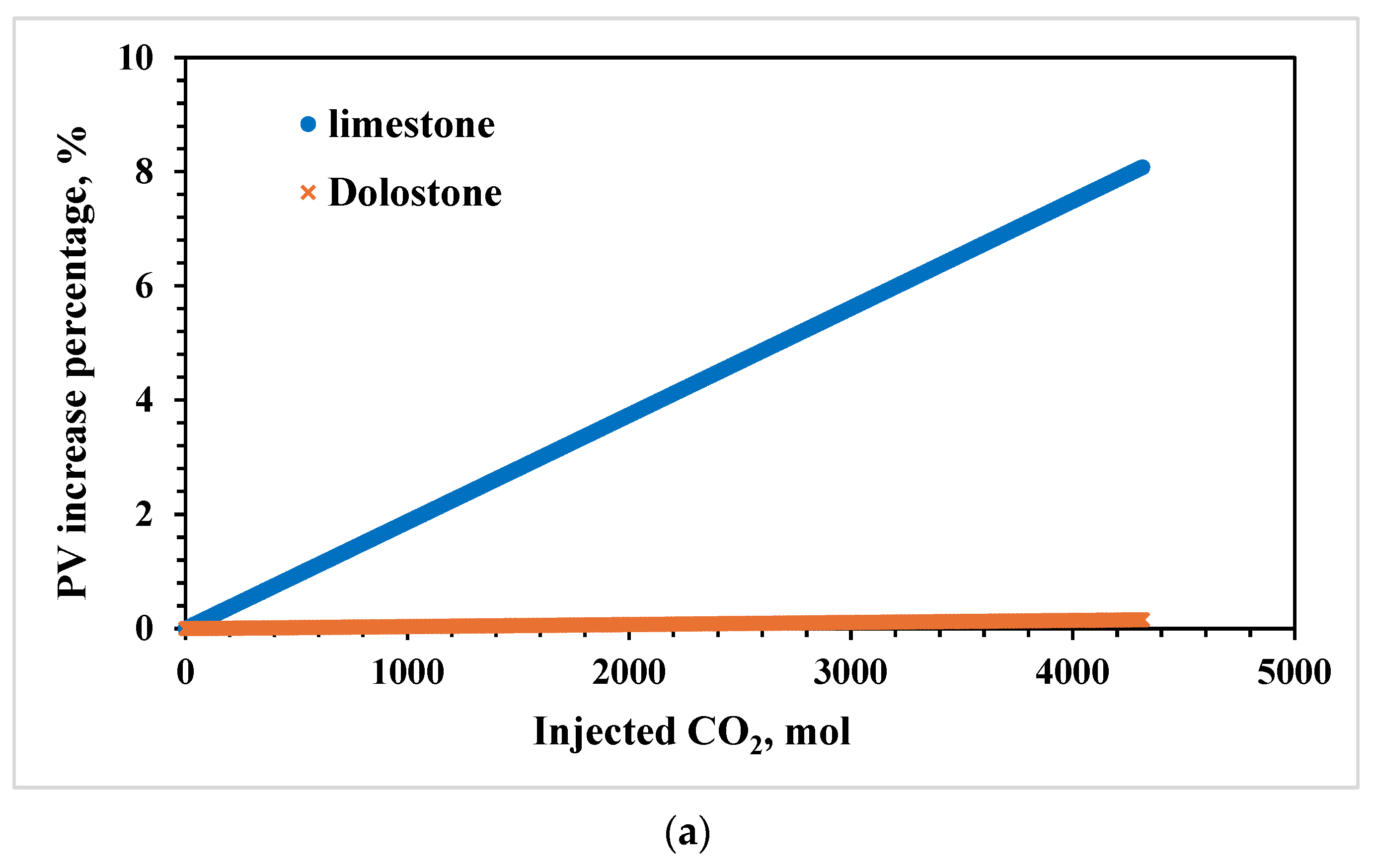
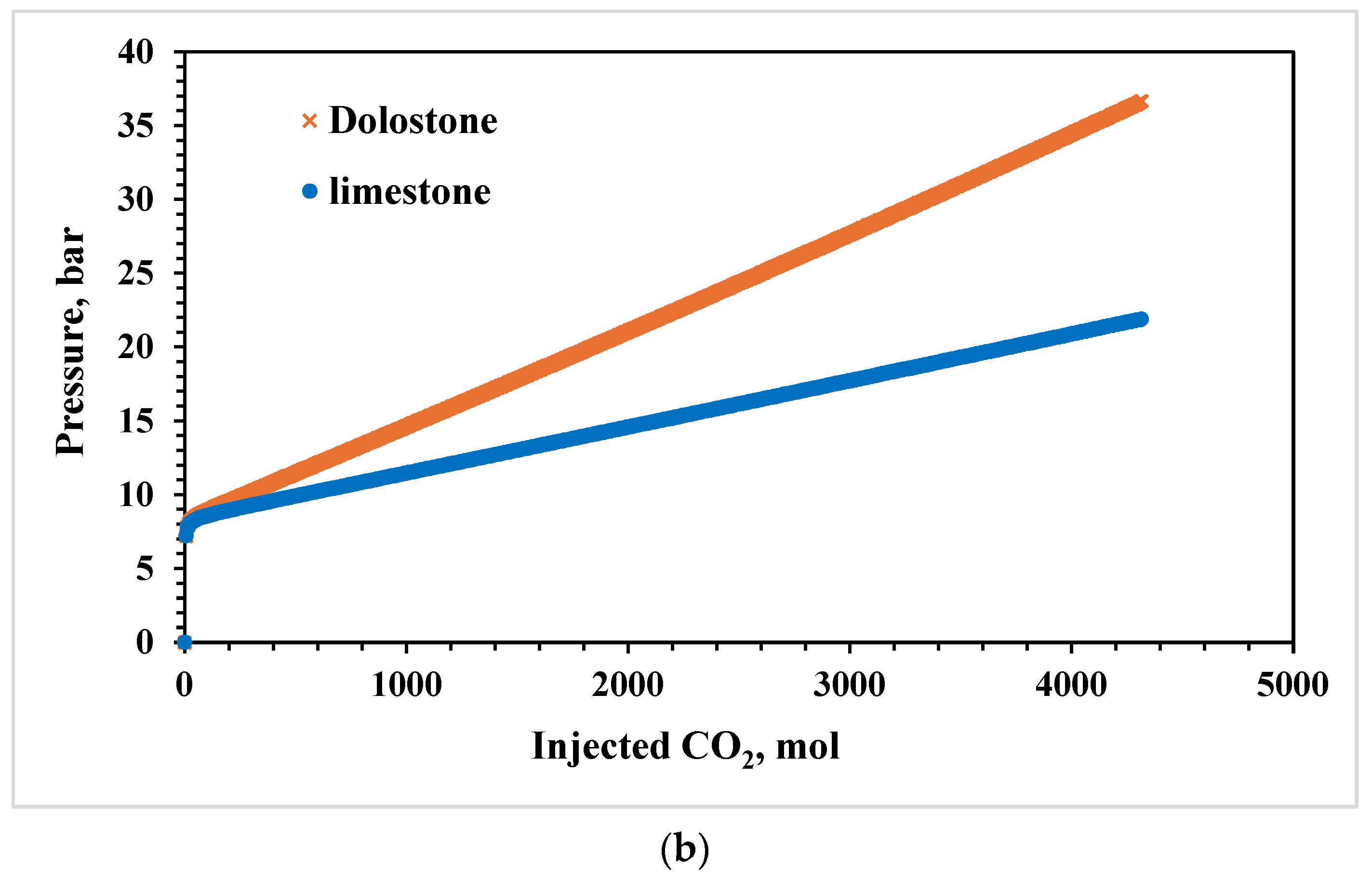
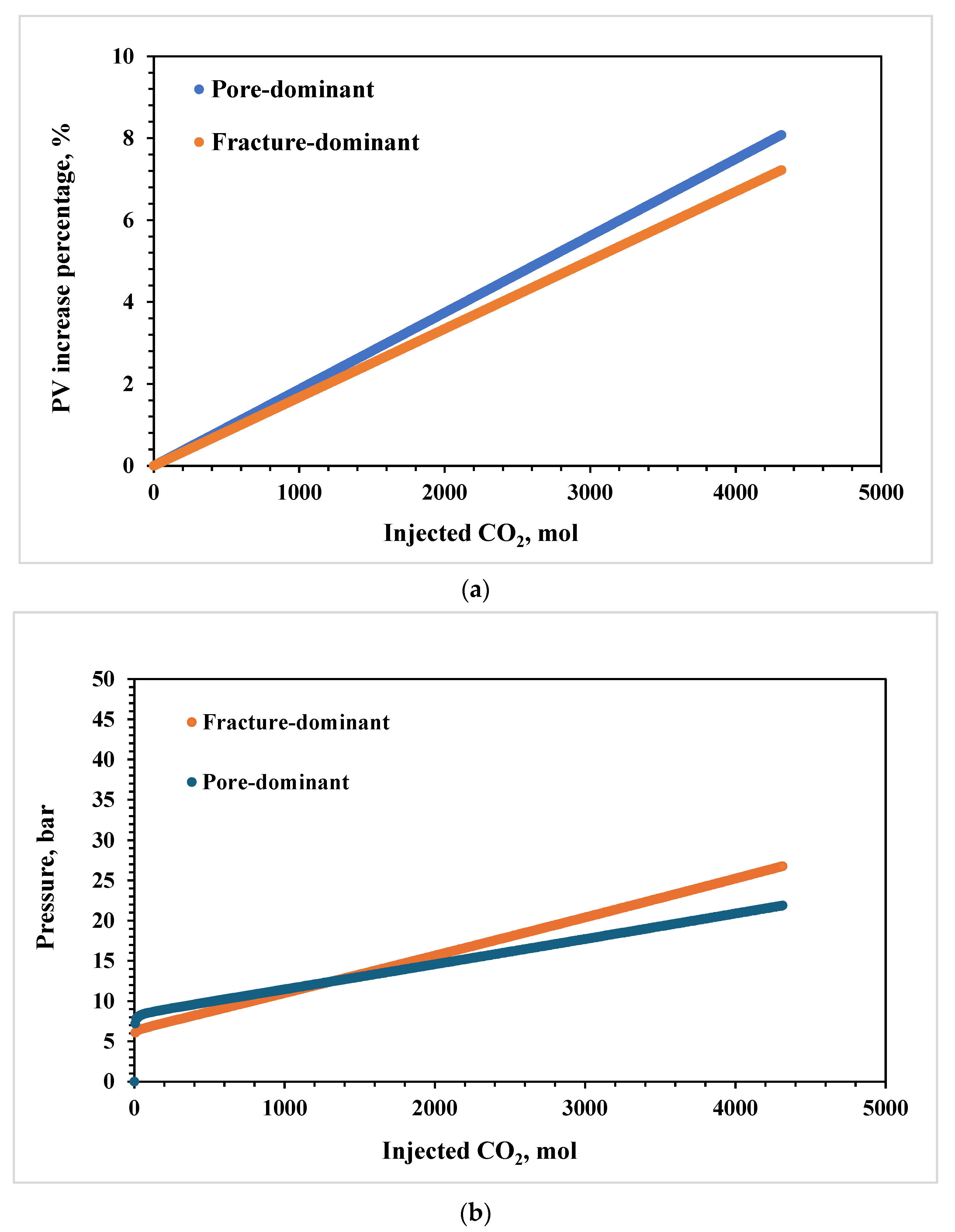
3.1. Effects of Lithology
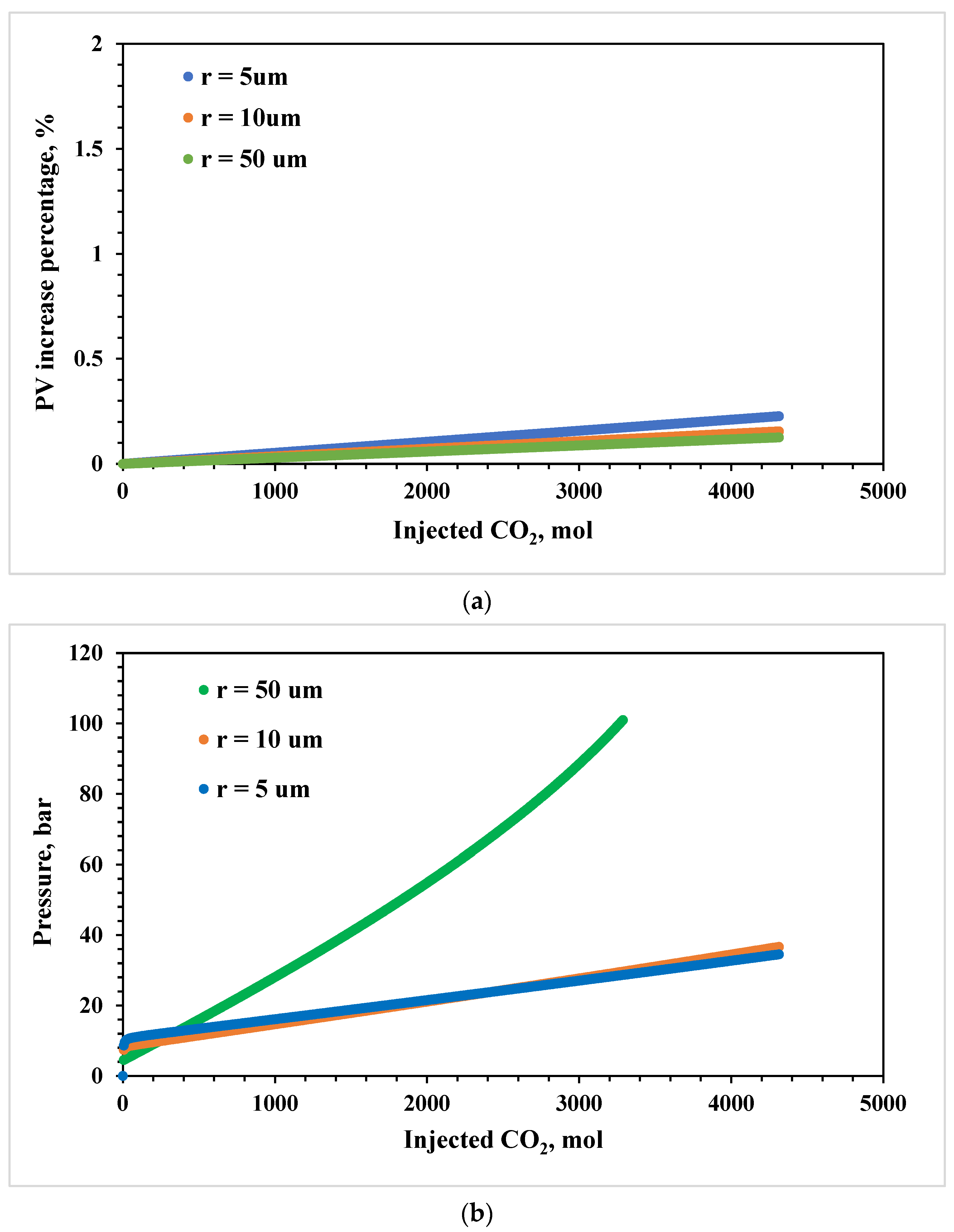
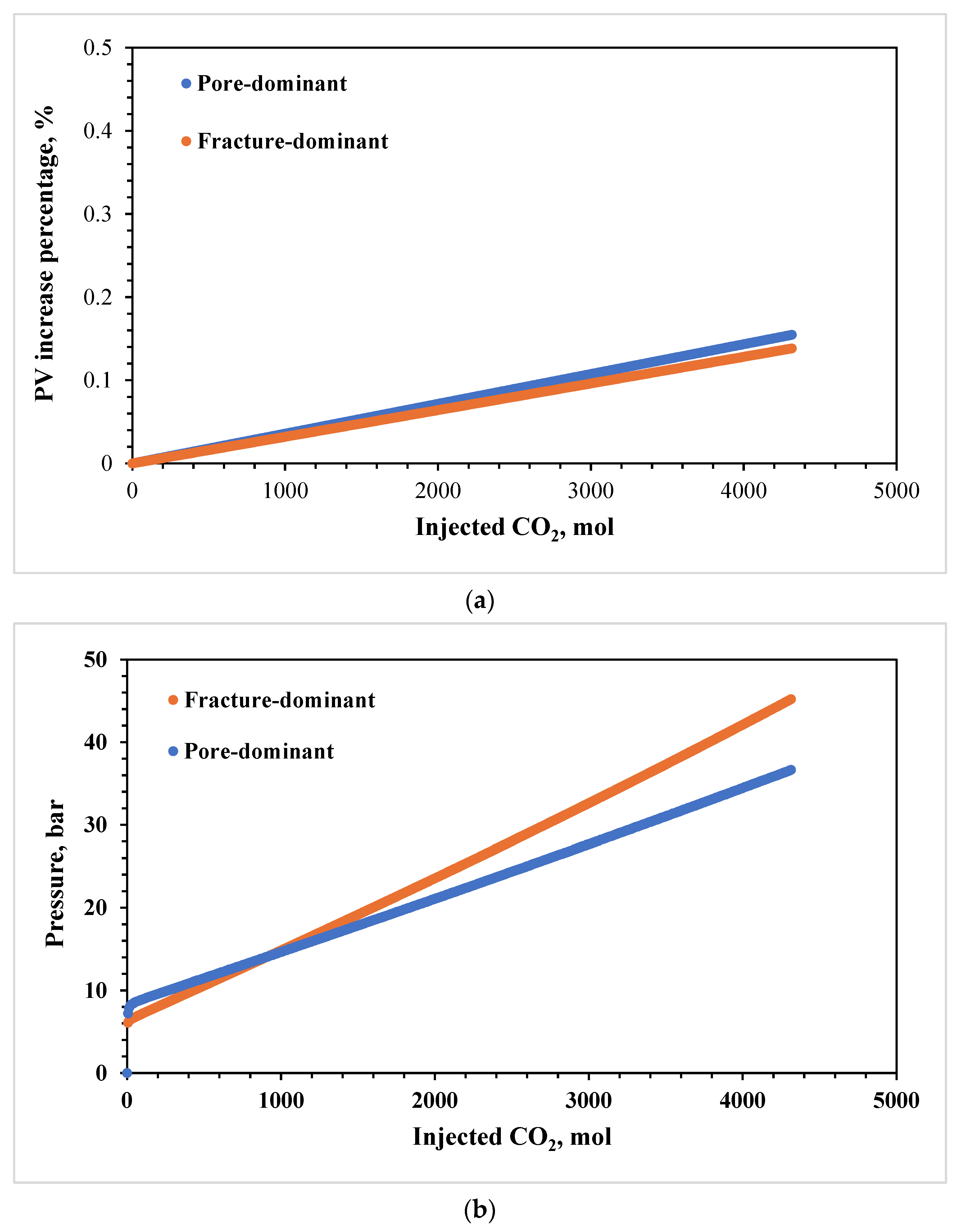
3.2. Effects of Pore Size
3.3. Effects of Pore–Throat Structures
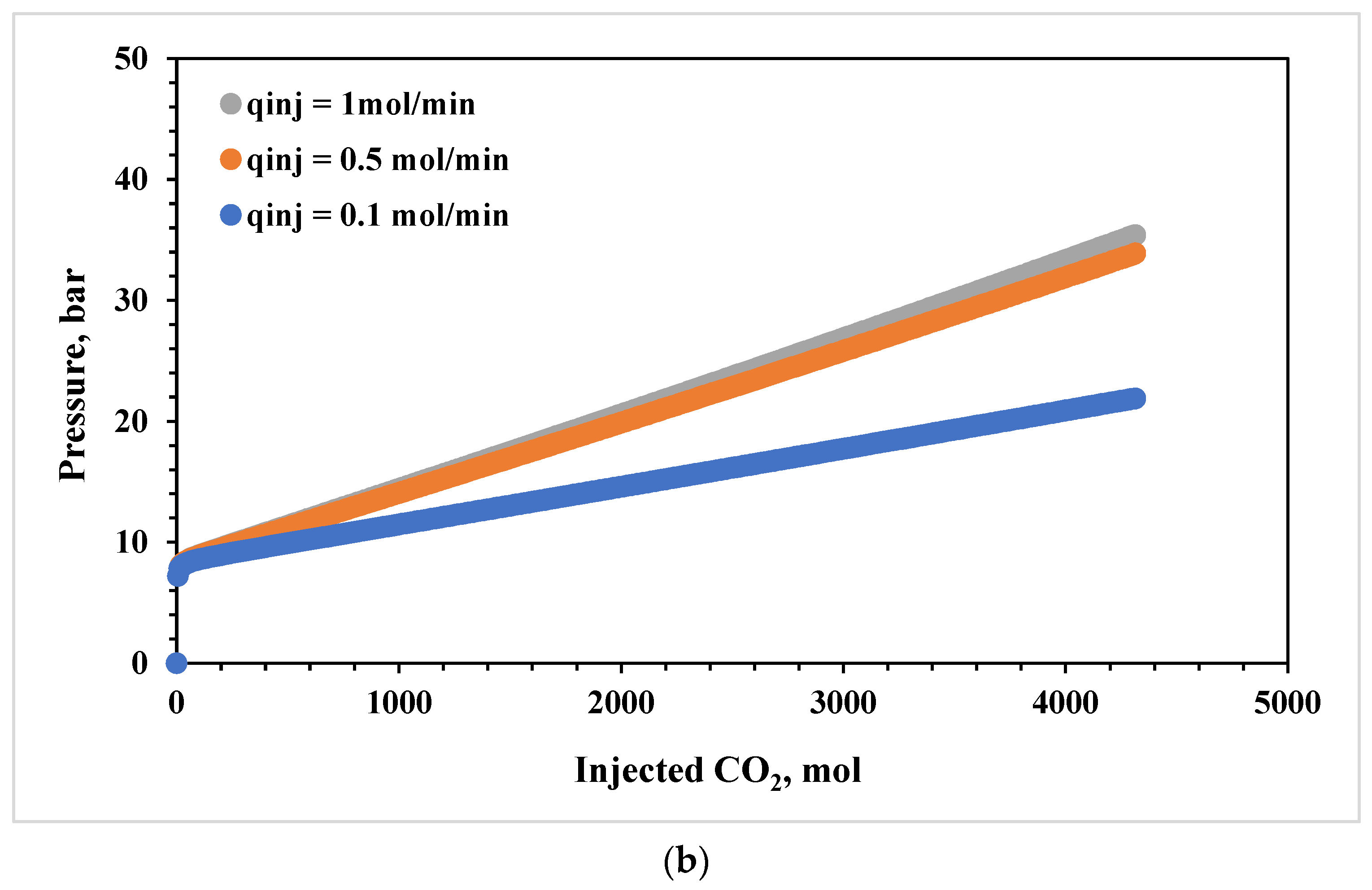
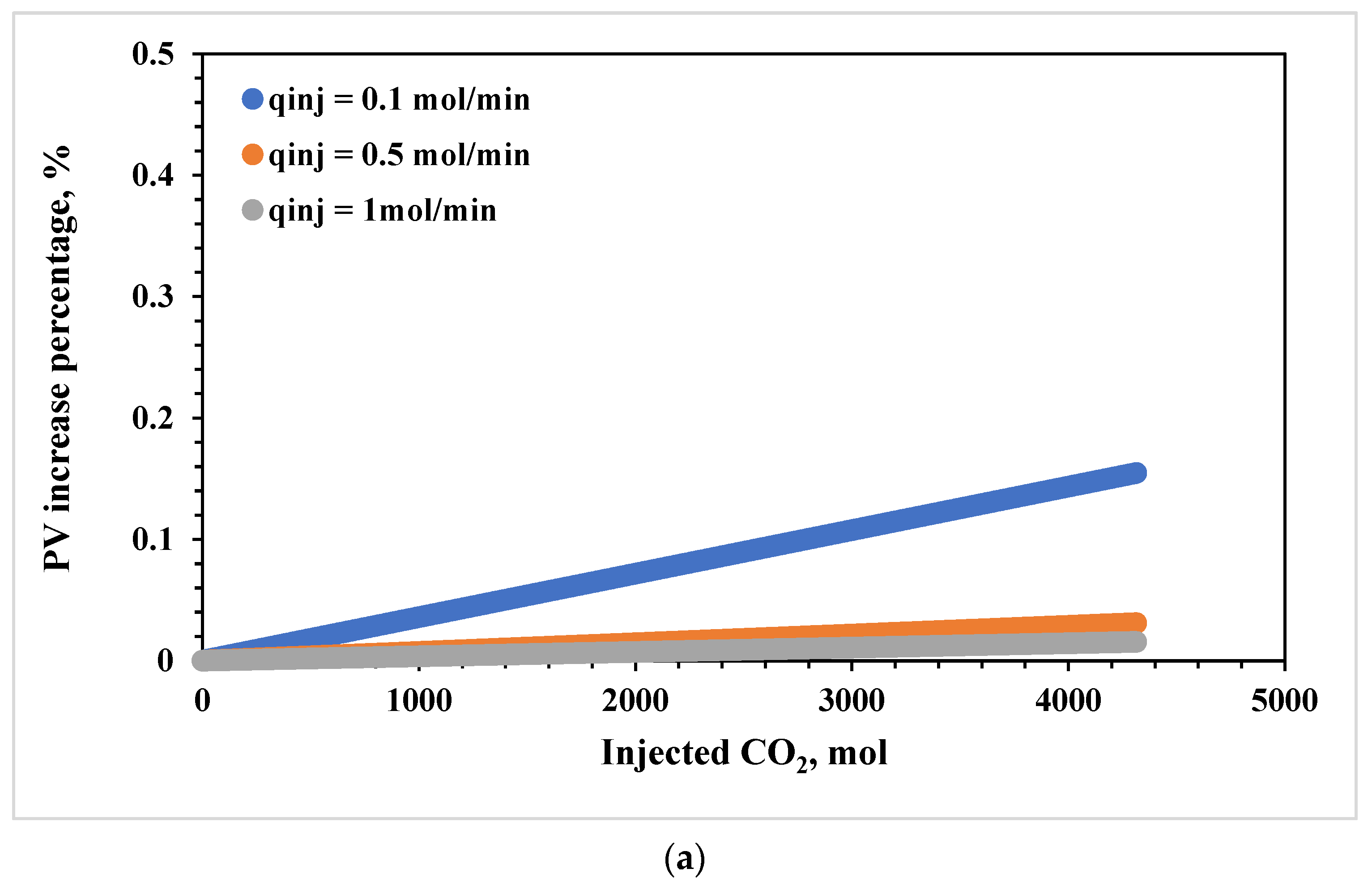
3.4. Effects of CO2 Injection Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baban, A.; Ali, M.; Arif, M.; Keshavarz, A.; Amin, R.; Iglauer, S. Effect of Organic Acid on CO2 Trapping in Carbonate Geological Formations: Pore-Scale Observations Using NMR. Energy Fuels 2023, 37, 2983–2992. [Google Scholar] [CrossRef]
- Yao, J.; Yuan, W.; Peng, X.; Chen, Z.; Gu, Y. A Novel Multi-Phase Strategy for Optimizing CO2 Utilization and Storage in an Oil Reservoir. Energies 2023, 16, 5289. [Google Scholar] [CrossRef]
- Kolawole, O.; Millikan, C.; Kumar, M.; Ispas, I.; Schwartz, B.; Weber, J.; Badurina, L.; Segvic, B. Impact of Microbial-Rock- CO2 interactions on Containment and Storage Security of Supercritical CO2 in Carbonates. Int. J. Greenh. Gas. Con. 2022, 120, 103755. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E.C. Chapter 8—Naturally Fractured Reservoirs. In Petrophysics, 4th ed.; Tiab, D., Donaldson, E.C., Eds.; Gulf Professional Publishing: Oxford, UK, 2016; pp. 415–481. [Google Scholar] [CrossRef]
- Postma, T.J.W.; Bandilla, K.W.; Peters, C.A.; Celia, M.A. Field-Scale Modeling of CO2 Mineral Trapping in Reactive Rocks: A Vertical Integrated Approach. Water Resour. Res. 2022, 58, e2021WR030626. [Google Scholar] [CrossRef]
- Agada, S.; Geiger, S.; Doster, F. Wettability, Hysteresis and Fracture–Matrix Interaction during CO2 EOR and Storage in Fractured Carbonate Reservoirs. Int. J. Greenh. Gas. Con. 2016, 16, 57–75. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Wang, J.; Sung, W. Relationship between Oil Production and CO2 Storage during Low-Salinity Carbonate Water Injection in Acid Carbonate Reservoirs. J. Ind. Eng. Chem. 2020, 88, 215–223. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, Y.; Zhang, D.; Yan, D.; Shen, Y. Seismic Fragility and Resilience Assessment of Shallowly Buried large-Section Underground Civil Defense Structure in Soft Soils: Framework and Application. Tunn. Undergr. Space Technol. 2024, 146, 145640. [Google Scholar] [CrossRef]
- Kumar, R.; Campbell, S.; Sonnenthal, E.; Cunningham, J. Effect of Brine Salinity on the Geological Sequestration of CO2 in a Deep Saline Carbonate Formation. Greenh. Gases 2019, 10, 296–312. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Preliminary Assessments of CO2 Storage in Carbonate Formations: A Case Study from Malaysia. J. Geophy. Eng. 2017, 14, 533–554. [Google Scholar] [CrossRef]
- Smith, M.M.; Hao, Y.; Carroll, S.A. Development and Calibration of a Reactive Transport Model for Carbonate Reservoir Porosity and Permeability Changes Based on CO2 Core-Flood Experiments. Int. J. Greenh. Gas. Con. 2017, 57, 73–88. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yao, J.; Iglauer, S.; Luqout, L.; Zhang, K.; Sun, H.; Zhang, L.; Song, W.; Wang, Z. Dynamic Pore-Scale Dissolution by CO2-Saturated Brine in Carbonates: Impact of Homogeneous Versus Fractured Versus Vuggy Pore Structure. Water Resour. Res. 2020, 56, e2019WR026112. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson, G.W.; Brink, F.; Southam, G.; Paterson, D.; Hall, N.; Heath, R.; Greer, D.; Kirste, D.; Golding, S.D. Impure CO2 Storage Reactions of Sandstone, Mudstone and Carbonate Cemented Cores: Experimental CO2, SO2, NOxO2 Reaction Metal Mobilisation and Fate. Int. J. Coal Geol. 2023, 277, 104352. [Google Scholar] [CrossRef]
- Hao, Y.; Smith, M.M.; Carroll, S.A. Multiscale Modeling of CO2-Induced Carbonate Dissolution: From Core to Meter Scale. Int. J. Greenh. Gas. Con. 2019, 88, 272–289. [Google Scholar] [CrossRef]
- Mitiku, A.B.; Li, D.; Bauer, S.; Beyer, C. Geochemical Modelling of CO2-Water-Rock Interactions in a Potential Storage Formation of the North German Sedimentary Basin. Appl. Geochem. 2013, 36, 168–186. [Google Scholar] [CrossRef]
- Menke, H.P.; Reynolds, C.A.; Andrew, M.G.; Pereira, J.P.; Bijeljic, B.; Blunt, M.J. 4D Multi-Scale Imaging of Reactive Flow in Carbonates: Assessing the Impact of Heterogeneity on Dissolution Regimes Using Streamlines at Multiple Length Scales. Chem. Geol. 2016, 481, 27–37. [Google Scholar] [CrossRef]
- Salem, A.M.; Shedid, S.A. Variation of Petrophysical Properties due to Carbon Dioxide (CO2) Storage in Carbonate Reservoirs. 2013. J. Pet. Gas. Eng. 2013, 4, 91–102. [Google Scholar]
- Hosseini, M.; Ali, M.; Fahimpour, J.; Keshavarz, A.; Iglauer, S. Calcite−Fluid Interfacial Tension: H2 and CO2 Geological Storage in Carbonates. Energy Fuels 2023, 37, 5986–5994. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2–water–rock interactions and their effects. J. CO2 Uti. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Seyyedi, M.; Mahmud, H.K.B.; Verrall, M.; Giwelli, A.; Esteban, L.; Ghasemiziarani, M.; Clennell, B. Pore Structure Changes Occur during CO2 injection into Carbonate Reservoirs. Sci. Rep. 2020, 10, 3624. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Liu, S.; Zhao, C.; Dong, S.; Song, Y. CO2 Transport and Carbonate Precipitation in the Coupled Diffusion-Reaction Process during CO2 Storage. Fuel 2023, 334, 126805. [Google Scholar] [CrossRef]
- Valle, L.M.; Grima, C.; Rodriguez, R.; Llopis, C. Effect of sc CO2-Brine Mixture on Injectivity and Storage Capacity in Rock Samples of Naturally Fractured Carbonate Formations. J. Nat. Gas Sci. Eng. 2020, 81, 103452. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Arif, M.; Lebedev, M.; Andreas, B.; Sarmadivaleh, M.; Iglauer, S. Carbonate Rock Mechanical Response to CO2 Flooding Evaluated by a Combined X-ray Computed Tomography—DEM Method. J. Nat. Gas Sci. Eng. 2020, 24, 103675. [Google Scholar] [CrossRef]
- Shokri, J.; Ruf, M.; Lee, D.; Mohammadrezaei, S.; Steeb, H.; Niasar, V. Exploring Carbonate Rock Dissolution Dynamics and the Influence of Rock Mineralogy in CO2 Injection. Environ. Sci. Technol. 2024, 58, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, Q.; Xu, L.; Onyekwena, C.C.; Yu, T.; Xu, L. Effects of SiO2 Nanoparticles on Wettability and CO2-Brine-Rock interactions in Carbonate Reservoirs: Implications for CO2 Storage. Energy Fuels 2024, 38, 1258–1270. [Google Scholar] [CrossRef]
- Siqueira, T.A.; Iglesia, R.S.; Ketzer, J.M. Carbon Dioxide Injection in Carbonate Reservoirs—A Review of CO2-Water-Rock Interaction Studies. Greenh. Gases 2017, 7, 802–816. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Xu, T.; Kharaka, Y.K.; Doughty, C.; Freifeld, B.M.; Daley, T.M. Reactive Transport Modeling to Study changes in Water Chemistry Induced by CO2 Injection at the Frio-I Brine Pilot. Chem. Geol. 2010, 271, 153–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Chen, X. Experimental Investigation on Effect of Water Flim Thickness in Unsaturated Sandstone Cores on CO2 Transport during Geologic Storage. J. Hydrol. 2021, 601, 126595. [Google Scholar] [CrossRef]
- Andre, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical Modeling of Fluid–Rock Chemical Interactions at the Supercritical CO2–Liquid Interface during CO2 Injection into a Carbonate Reservoir, the Dogger Aquifer (Paris Basin, France). Energy Conv. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Schmoker, J.W.; Krystinik, K.B.; Halley, R.B. Selected Characteristics of Limestone and Dolomite Reservoirs in the United States. AAPG Bull. 1985, 69, 733–741. [Google Scholar]
- Marchado, M.V.B.; Khanal, A.; Delshad, M. Unveiling the Essential Parameters Driving Mineral Reactions during CO2 storage in Carbonate Aquifers through Proxy Models. Appl. Sci. 2024, 14, 1465. [Google Scholar] [CrossRef]



| Scenario No. | Lithology | Pore Space Type | Pore Size (µm) | Injection Rate (mol/min) |
|---|---|---|---|---|
| 1 | Limestone | Pore-dominant | 5 | 1.0 |
| 2 | Limestone | Pore-dominant | 5 | 0.5 |
| 3 | Limestone | Pore-dominant | 5 | 0.1 |
| 4 | Limestone | Pore-dominant | 10 | 0.1 |
| 5 | Limestone | Pore-dominant | 50 | 0.1 |
| 6 | Limestone | Fracture-dominant | 5 | 1.0 |
| 7 | Limestone | Fracture-dominant | 5 | 0.5 |
| 8 | Limestone | Fracture-dominant | 5 | 0.1 |
| 9 | Limestone | Fracture-dominant | 10 | 0.1 |
| 10 | Limestone | Fracture-dominant | 50 | 0.1 |
| 11 | Dolostone | Pore-dominant | 5 | 1.0 |
| 12 | Dolostone | Pore-dominant | 5 | 0.5 |
| 13 | Dolostone | Pore-dominant | 5 | 0.1 |
| 14 | Dolostone | Pore-dominant | 10 | 0.1 |
| 15 | Dolostone | Pore-dominant | 50 | 0.1 |
| 16 | Dolostone | Fracture-dominant | 5 | 1.0 |
| 17 | Dolostone | Fracture-dominant | 5 | 0.5 |
| 18 | Dolostone | Fracture-dominant | 5 | 0.1 |
| 19 | Dolostone | Fracture-dominant | 10 | 0.1 |
| 20 | Dolostone | Fracture-dominant | 50 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Chen, Z.; Jiang, C.; Peng, X. An Integrated Numerical Study of CO2 Storage in Carbonate Gas Reservoirs with Chemical Interaction between CO2, Brine, and Carbonate Rock Matrix. Appl. Sci. 2024, 14, 6002. https://doi.org/10.3390/app14146002
Yao J, Chen Z, Jiang C, Peng X. An Integrated Numerical Study of CO2 Storage in Carbonate Gas Reservoirs with Chemical Interaction between CO2, Brine, and Carbonate Rock Matrix. Applied Sciences. 2024; 14(14):6002. https://doi.org/10.3390/app14146002
Chicago/Turabian StyleYao, Jiangyuan, Zhuoheng Chen, Chunqing Jiang, and Xiaolong Peng. 2024. "An Integrated Numerical Study of CO2 Storage in Carbonate Gas Reservoirs with Chemical Interaction between CO2, Brine, and Carbonate Rock Matrix" Applied Sciences 14, no. 14: 6002. https://doi.org/10.3390/app14146002
APA StyleYao, J., Chen, Z., Jiang, C., & Peng, X. (2024). An Integrated Numerical Study of CO2 Storage in Carbonate Gas Reservoirs with Chemical Interaction between CO2, Brine, and Carbonate Rock Matrix. Applied Sciences, 14(14), 6002. https://doi.org/10.3390/app14146002






