Abstract
The seeds of Boraginaceae species constitute a source of γ-linolenic acid (GLA, 18:3n-6)-rich oils, mainly those of Borago officinalis. However, there are many wild unexplored Boraginaceae taxa that are potentially GLA producers. This work scrutinizes the seeds of several Greek Boraginaceae species for fatty acids (FAs), phenolic compounds, antioxidant activity, and in vitro antiproliferative activity against HT-29 colorectal cancer cells. The FA profiles were checked by GC-FID, and GLA (FA% of total FA) highlights in Symphytum bulbosum (26.2), S. creticum (23.2), and Lithodora zahnii (17.4). The total FA content ranged from 9.2 in Alkanna methanaea to 27.3 g/100 g of seeds in Alkanna corcyrensis. The antioxidant activity of the water/methanol extracts, evaluated by the ABTS and DPPH methods, was in line with other GLA producer seeds. Phenolic acids were analyzed by HPLC-DAD and LC-MS, and rosmarinic acid ranged from 160.2 in S. bulbosum to 479.7 mg/100 g in L. zahnii. The MTT assay showed dose- and time-dependent inhibitory effects of the water/methanol extracts against HT-29 cancer cells, especially those of C. major and L. zahnii (GI50 of 600 and 375 μg/mL for 72 h-exposed cells). This work constitutes the first approach to evaluate the seeds of Boraginaceae taxa from Greece as functional oil providers.
1. Introduction
Preserving natural resources that are still unknown in terms of their biochemical composition is crucial for scientific advancements and the development of new applications in various fields. Many undiscovered organisms and environments may possess unique biochemical compounds with significant potential for medicine, biotechnology, and industry []. Preserving these resources will ensure the preservation of untapped reservoirs of novel molecules, enzymes, and genetic material that could lead to the discovery of new drugs, bioactive compounds, and sustainable materials [,].
Bioactive fatty acid (FA)-containing oils constitute an invaluable nutritional resource. For instance, the discovery of new oils containing γ-linolenic acid (GLA, 18:3n-6) and stearidonic acid (SDA, 18:4n-3) is significant in the realm of nutrition and health. GLA and SDA are “conditionally essential” omega-6 (n-6) and omega-3 (n-3) polyunsaturated FA (PUFA) types, respectively, and offer distinct health benefits []. GLA has been linked to anti-inflammatory properties (e.g., []), skin health [], cancer prevention and treatment [,], and hormonal balance [], and has potential therapeutic applications for rheumatoid arthritis and atopic dermatitis []. On the other hand, SDA is a precursor to nutritionally valuable eicosapentaenoic acid (EPA, 20:5n-3); then, increasing dietary intake of SDA-rich oils can enhance the body’s ability to convert SDA into EPA, which is associated with cardiovascular health, anti-inflammatory effects, and cognitive function [,]. Therefore, exploring and identifying new oil sources containing such FAs can improve nutritional profiles and provide potential avenues for therapeutic interventions [,,].
Both GLA and SDA sources are scarce in nature. Besides some microbiological sources, GLA is primarily obtained from three plants: borage (Borago officinalis L., ~21–23% GLA of total FAs); evening primrose (Oenothera biennis L., ~9–12% GLA of total FAs); and blackcurrant (Ribes nigrum L., ~15–20% GLA of total FAs) []. Concerning SDA, the oils from Buglossoides arvensis (Ahiflower® oil, ~17% SDA) and E. plantagineum and E. vulgare seeds (~14% SDA) are the richest commercial known sources of SDA. Moreover, SDA is also found in some other marketed seed oils, such are those of blackcurrant (R. nigrum, ~2–4% SDA) []. Consequently, the FA composition of Boraginaceae seeds, in which both GLA and SDA occur, highlights their potential as valuable sources of these conditionally essential FAs and their potential applications in promoting human health [].
Phenolic compounds present antioxidant activity, helping to neutralize harmful free radicals and protecting cells from oxidative stress. The presence of phenolic compounds in Boraginaceae seed oils suggests their potential as natural antioxidants, which can be beneficial for human health and the prevention of chronic diseases []. These contribute to the stability and nutritional quality of the oils while offering potential health benefits. Boraginaceae seeds, such as those of borage, contain phenolic compounds, including phenolic acids, flavonoids, and tannins [,,].
Antioxidant-rich oils, as those having high amounts of phenolic compounds, have been associated with various health benefits, including reducing the risk of chronic diseases such as cardiovascular disease, cancer, and neurodegenerative disorders. For instance, extra virgin olive oil, which is abundant in phenolic compounds, exhibits potent antioxidant properties that contribute to its protective effects on heart health. These antioxidants contribute to the stability and shelf life of oils while offering potential health benefits when consumed. Therefore, the antioxidant activity of oils is of paramount importance for maintaining overall well-being and reducing the risk of oxidative-stress-related ailments [,].
The main phenolic compounds identified in Boraginaceae seeds include phenolic acids, flavonoids, and tannins. Phenolic acids, such as rosmarinic, caffeic, chlorogenic, and vanillic acids, are commonly found in these seeds and exhibit antioxidant properties. Flavonoids, including quercetin, kaempferol, and rutin, are another class of phenolic compounds occurring in Boraginaceae seeds, which exercise antioxidant, anticancer, cardioprotective, and anti-inflammatory effects [,,,].
Boraginaceae oil plays a crucial role in neutralizing free radicals and reducing oxidative stress, which are implicated in the development and progression of colorectal cancer. For instance, the phenolic compounds found in Boraginaceae oils can help to protect against DNA damage, inhibit cancer cell proliferation, and induce apoptosis in colorectal cancer cells. The potential chemopreventive and therapeutic effects of Boraginaceae oils against colorectal cancer are related to their antioxidant activity [].
Greece is renowned for its rich biodiversity, and the family Boraginaceae contributes significantly to the country’s plant diversity, especially of the islands. Boraginaceae is well represented in various regions of Greece, with a diverse array of species inhabiting different ecosystems. The family includes both native and endemic plants, adding to the unique biodiversity of the region. Species from the genera Alkanna, Lithodora, Symphytum, and Onosma are among the notable members of Boraginaceae found in Greece. These plants exhibit a range of morphological characteristics, growth habits, and ecological adaptations, reflecting the diverse habitats they occupy, including mountains, plains, coastal areas, and islands. Therefore, the biodiversity of Boraginaceae in Greece is of great value for ecological balance, conservation efforts, and the potential discovery of new medicinal plants and bioactive compounds []. Previously, some Greek Boraginaceae species have been analyzed. For instance, the chemical compositions of hexane extracts of the lipid fraction of the roots of some Boraginaceae species from Greece and their phytochemical relevance were evaluated []. Furthermore, the phytochemical composition of aerial parts of Rindera graeca was analyzed, and seven caffeic acid derivatives and two flavonol glucosides were found [].
The increasing interest in SDA- and GLA-rich oils from the pharmaceutical and food industries highlights the need for research to assess the biological activity of dietary SDA- and GLA-rich oils as healthy components of functional foods. On the other hand, elucidating the chemical composition and biological activity of Boraginaceae seeds from Greece will help in understanding the potential of these natural resources and will provide arguments to develop programs aimed at preserving natural habitats. Accordingly, this work was focused on disentangling the FA and phenolic compound profiles, the antioxidant activity, and the antitumor activity against the HT-29 human colorectal cancer cell line of selected Greek Boraginaceae seeds.
2. Materials and Methods
2.1. Reagents and Chemicals
All the chemicals used, including the solvents, were of analytical grade. Water was purified using a Milli-Q system (Millipore, Burlington, MA, USA). Aluminum chloride (99% purity), doxorubicin (98.0–102%, D1515), and sodium carbonate (99.5% purity) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (98% purity), acetic acid (≥99.8%), amphotericin (suitable for cell culture), dimethylsulfoxide (DMSO, ≥99.7%, suitable for cell culture), F-C reagent, L-glutamine (suitable for cell culture), methanol (LC-grade), hydrochloric acid (ACS reagent, 37%), penicillin-streptomycin (suitable for cell culture), petroleum ether (puriss. p.a., ACS reagent), and sodium pyruvate (suitable for cell culture, ≥99%) were purchased from Merck (Madrid, Spain). Phenolic standards—gallic acid (97.5%, 91,215), protocatechuic acid (≥97%, 37,580), 4-hydroxybenzoic acid (99%, H20059), DL-p-hydroxyphenyllactic acid (≥97%, H3253), 3,4-dihydroxyhydrocinnamic acid (98%, 102,601), chlorogenic acid (≥95%, C3878), caffeic acid (≥98%, C0625), vanillic acid (≥97%, 94,770), vanillin (99%, V1104), syringic acid (≥95%, S6881), sinapic acid (≥98%, D7927), salicylic acid (≥99%, 247,588), trans-p-coumaric acid (≥98%, 55,823), salicylic acid (≥99%, 247,588), ferulic acid (99%, 128,708), naringenin (≥95%, N5893), rutin (≥94%, R5143), rosmarinic acid (≥98%, R4033), 2-hydroxy-4-methoxybenzoic acid (99%, 173,479), quercetin (≥95%, Q4951), luteolin (≥95%, GP5376), kaempferol (≥90%, K0133), apigenin (≥97%, PC15106), lithospermic acid (≥98%, BP0878), quercetin-3-β-D-glucoside (≥95%, GC9169), and apigenin-7-glucoside (≥99%, T4S0295)—were purchased at high purity grade from Merck (Spain).
2.2. Samples
Data on the seeds collected for the current study are shown in Table 1. Seeds from each wild species were collected from three well-differentiated subpopulations in each location. Upon arrival to the laboratory, moisture was determined using 2 g of each sample, and this procedure was carried out in a forced-air oven at 105 °C until constant weight. The remaining seeds were labeled and placed in a glass desiccator until analysis. Just prior to analysis, the seeds were ground to powder with a mortar. Moisture for all seeds was 8.5 ± 0.9%, and all results are reported on a dry weight (dw) basis.

Table 1.
Data on Boraginaceae seeds collected in Greece.
2.3. Fatty Acids Analysis
This methodology is fully detailed in Supplementary File S1. Direct derivatization of seed oils to FA methyl esters (FAMEs) was performed. FAMEs were analyzed in a Focus GC equipped with an FID and an OmegawaxTM 250 Fused Silica Capillary as previously described [].
2.4. Extraction of Phenolic Compounds
This methodology is fully detailed in Supplementary File S1. Extraction and analysis of phenolic compounds from seeds were carried out according to Lyashenko et al. [].
2.5. Determination of Total Phenolic and Flavonoid Contents
TPC was measured using the F-C assay, as reported by Singleton et al. [], with minor modifications. This methodology is fully detailed in Supplementary File S1. The results were expressed as mg of gallic acid equivalent (GAE) per 100 g fw using a standard curve of GA. TFC of the phenolic extract of seeds was determined according to Zou et al. [], with minor modifications, and this methodology is fully detailed in Supplementary File S1. The results were expressed as mg of Quercetin Equivalents (QE)/100 g fw using a standard curve of quercetin (10–500 μg/mL).
2.6. Characterization of Phenolic Compounds by HPLC-DAD
This methodology is detailed in Supplementary File S1. High-Performance Liquid Chromatography (HPLC) analyses of phenolic compounds were conducted utilizing a Finnigan Surveyor chromatograph equipped with a diode array detector (DAD) and a reverse-phase C18 column (Luna® Omega, 250 × 4.6 mm i.d., 3 µm particle size, from Phenomenex, Torrance, CA, USA). Compounds were separated employing a gradient elution using acidified water (1% acetic acid) (A) and acetonitrile (B) as the mobile phase at 25 °C. The total running time was 105 min. The flow rate was kept at 0.4 mL/min and the injection volume was 10 μL. Peaks were monitored at 254, 280, and 320 nm and identified by retention times in comparison with pure standards. A HPLC-DAD chromatogram of the L. zahnii seed extract is shown in Figure 1. Quantification of the compounds was made using external calibration curves obtained from pure standards (Sigma-Aldrich, St. Louis, MO, USA) in the HPLC-DAD system (Supplementary Materials Table S1). Another HPLC-DAD chromatogram of S. creticum seed extract is shown Supplementary Figure S1.
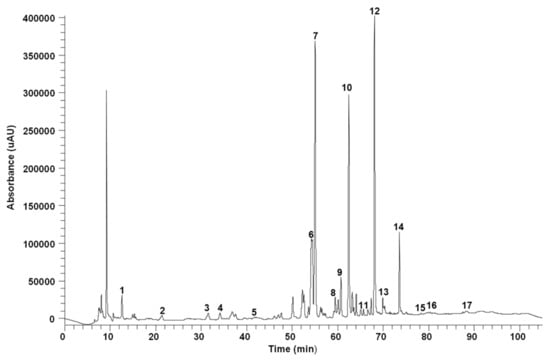
Figure 1.
A 254 nm HPLC chromatogram of the phenolic-containing methanol/water extract of L. zahnii (M9). 1. Gallic acid. 2. Protocatechuic acid. 3. Chlorogenic acid. 4. 4-Hydroxybenzoic acid. 5. Vanillic acid. 6. Vanillin. 7. Trans-p-Coumaric acid. 8. Rutin. 9. Sinapic acid. 10. Quercetin 3-O-glucoside. 11. Apigenin-7-O-glucoside. 12. Rosmarinic acid. 13. Salicylic acid. 14. Salvianolic acid. 15. Luteolin. 16. Naringenin. 17. Kaempferol.
2.7. Characterization of Phenolic Compounds by LC-MS
This methodology is fully detailed in Supplementary File S1. The chromatographic separations were performed on a Vanquish Flex Quaternary LC equipped with a reverse-phase C18 column (Hypersil Gold, 100 mm × 2.1 mm, 1.9 μm) at a flow rate of 0.2 mL/min. The total running time was 39 min. The LC system was coupled to a hybrid mass spectrometer, Q-Orbitrap, Thermo Fisher Scientific, using electrospray ionization (ESI) in positive- and negative-ion mode. The basis for the identification of phenolic compounds by LC-MS in the hydroalcoholic extracts (MeOH:H2O, 60:40 v/v) of Boraginaceae seeds from Greece is detailed in Supplementary Table S2.
2.8. Antioxidant Activity
The extraction was carried out using methanol/water (60:40, v/v) according to the methodology described by Forbes-Hernández et al. [], with some modifications. This methodology is fully explained in Supplementary File S1. The antioxidant activity using the ABTS method was determined using a solution of ABTS•+ radical (2.2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) in ethanol (2.45 mM). The DPPH method was carried out according to Skenderidis et al. [], which is fully described in Supplementary File S1. The absorbance of the solution was measured at 517 nm. The values of ABTS•+ and DPPH were expressed as mmol of Trolox Equivalent/100 g dry weight (mmol TE/100 g dw).
2.9. Antitumor Assays
This methodology is fully detailed in Supplementary File S1. The antiproliferative activity of the hydroalcoholic extracts (methanol/water, 60:40, v/v) from seeds was used to check antiproliferative activities against the HT-29 colon cancer cell line and the CCD-18 colonic human myofibroblasts cell line. Cell cultures were supplied by the Technical Instrumentation Service of the University of Granada (Granada, Spain) [].
2.10. Statistical Analysis
Data on seeds correspond to the analyses performed on seeds collected from three different species populations, each of which was analyzed in triplicate. The statistical significance was calculated using Student’s t-tests and a one-way analysis of variance (ANOVA) and expressed as the average ± SD. Differences among mean values were tested by Duncan’s test at p < 0.05 and for antiproliferative activity at p < 0.05 and p < 0.01. Statistical analyses were performed using Statgraphics Centurion XVIII version. 18.1.12 (Warrenton, VA, USA).
3. Results
3.1. Fatty Acids Content
Table 2 details the total FAs and FA profiles of the targeted seeds. The total FAs in the seeds analyzed here ranged from 9.2 in Alkanna methanaea to 27.3 g/100 g of seeds in Alkanna corcyrensis. The saturated FA (SFA) fraction ranged from 11.0 (Alkanna sartoriana) to 31.5% (A. corcyrensis) of total FAs, and the main SFA in all scrutinized species was palmitic acid (PA, 16:0), which is the most frequent SFA found in animals and plants, followed by stearic acid (SA, 18:0). PA ranged from 7.8 in A. sartoriana to 24.5% of total FAs in A. corcyrensis, and SA was between 1.7% in Symphytum bulbosum SB2 and 7.0 in A. corcyrensis. The monounsaturated FA (MUFA) group was from 5.0 (A. corcyrensis) to 25.5% (Onosma frutescens), and oleic acid (OA, 18:1n-9), which was the main MUFA, ranged from 6.8 (A. corcyrensis) to 25.6% (O. frutescens) of total FA. The n-6 PUFA fraction ranged from 18.5 in Echium italicum subsp. biebersteinii to 62.2% of total FAs in Symphytum creticum. Linoleic acid (LA, 18:2n-6 cis) was the most prominent contributor to this PUFA group, showing percentages within 12.9 (E. italicum biebersteinii) to 39.0% (S. creticum). Within this group, GLA percentages were taxa-dependent. For instance, the two analyzed Onosma species showed ~4% of total FAs; Echium species displayed 5–8%; in Alkanna, it ranged between 8.1 (A. methanaea) and 13.0% (A. tinctoria); in Lithodora zahnii it reached 17.4%; and in both Symphytum species it was ~23–26%.

Table 2.
Total FA and FA profiles of Boraginaceae seeds collected in Greece A,B,C.
Both ALA and SDA make up the n-3 PUFA group, and these were found in all species. Such a group ranged from 2.0 (S. creticum) to 50.7% of total FAs (E. italicum biebersteinii); ALA reached the highest percentages, ranging between 1.3 in S. creticum and 41.2% of total FAs in E. italicum biebersteinii; and SDA was found in low percentages: it was between 0.7 in S. creticum and 9.6% of total FAs in E. italicum biebersteinii. On the other hand, the n-6/n-3 PUFA ratio was from 0.4 (E. italicum biebersteinii) to 31.2 (S. creticum).
3.2. Phenolic Compounds
Spectrophotometrically determined total phenolics content (TPC) and total flavonoids content (TFC) are detailed in Table 3. TPC ranged from 267.1 (O. frutescens) to 844.0 mg GAE/100 g (A. methanaea), and TFC ranged between 163.5 (O. frutescens) and 674.1 mg of QE/100 g (A. pindicola). The total amounts of both phenolic acids and flavonoids determined by HPLC-DAD as the sum of quantified compounds are given in Table 3. A partial agreement between the amounts of phenolics quantified using both methodologies was noted, which is discussed below.

Table 3.
Antioxidant activity and phenolic compounds of Boraginaceae seeds collected in Greece A,B,C.
Table 4 details the phenolic compound composition of the methanol/water extracts of the selected Boraginaceae seeds collected in Greece. Concerning phenolic acids, among the hydroxybenzoic ones, the following compounds were detected: gallic acid (GA, a trihydroxy benzoic acid) was found in some samples, highlighting A. methanaea (19.1 mg/100 g); protocatechuic acid (a dihydroxybenzoic acid) was found at very low amounts, and S. bulbosum SB2 contains the highest amounts (5.0 mg/100 g); 4-hydroxy-benzoic acid, which ranged from 0.9 (Cerinthe major) to 12.2 mg/100 g (A. methanaea); vanillic acid (a dihydroxybenzoic acid derivative), which was present in some species at low quantities, highlighting A. methanaea (8.2 mg/100 g); and syringic acid (3,5-dimethoxy-4-hydroxybenzoic acid), occurring only in Onosma graeca (8 mg/100 g). Among the hydroxycinnamic acids, the following were found: chlorogenic acid (a cinnamate ester of caffeic acid and (-)-quinic acid), detected at disparate quantities in the various samples and standing out in S. creticum (228.5 mg/100 g); 3,4-dihydroxy-hydrocinnamic acid, which was quantified in few species, and A. sartoriana had the highest amount (1.9 mg/100 g); caffeic acid (3,4-dihydroxy-cinnamic acid), which was present only in three species, and S. creticum had the highest amount (5.0 mg/100 g); trans-p-coumaric acid (a hydroxy derivative of cinnamic acid), which showed disparate values in the various samples, and A. methanaea had the highest amount (160.7 mg/100 g); sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid), which was found in few species, and L. zahnii reached the highest value (16.4 mg/100 g); and rosmarinic acid (an ester of cinnamic acid and 3,4-dihydroxyphenyl lactic acid), which was present at very high amounts in most species, ranging from 33.9 (O. frutescens) to 762.7 mg/100 g in A. methanaea. As for phenolic aldehydes, vanillin was the only compound detected in three samples, and L. zahnii had the highest value (8.3 mg/100 g). Regarding polyphenols, lithospermic acid (a polycyclic phenolic carboxylic acid) was present in all samples, although it reached low amounts in most cases, but in highlights in S. bulbosum SB1(211.8 mg/100 g). The flavonoid glycosides that were detected in all species were rutin (quercetin-3-O-rutinoside), from 3.8 (O. frutescens) to 41.3 mg/100 g (A. graeca subsp. graeca); isoquercetin (quercetin 3-O-glucoside), from 15.0 (A. tinctoria) to 112.9 mg/100 g (S. creticum); and apigetrin (apigenin-7-O-glucoside), from 11.3 (C. major) to 137.7 mg/100 g (A. methanaea). The aglycones of flavonoids had a more random distribution among the different taxa, and were absent in many of them; for instance, luteolin was present in six taxa, standing out in C. major (35.9 mg/100 g); quercetin was detected in three taxa, reaching 13.8 mg/100 g in A. sartoriana; naringenin occurred in seven taxa, particularly in A. methanaea (52.5 mg/100 g); and kaempferol was also found in seven taxa, with the highest value found in S. bulbosum SB1 (16.9 mg/100 g). The total identified phenolics as the sum of the phenolics individually quantified by HPLC-DAD ranged from 102.8 (O. frutescens) to 1524.7 mg/100 g (A. methanaea). These last species were also the ones that contained the lowest and highest amount of phenolics, respectively, according to the Folin–Ciocalteu (F-C) method. The basis for the identification of phenolic compounds by LC-MS is detailed in Supplementary Table S2. Providing consistency to the analyses, all compounds detected by HPLC-DAD were confirmed by LC-MS, as detailed in Supplementary Table S3.

Table 4.
Phenolic compounds (mg/100 g seeds) of methanol/water extracts of selected Boraginaceae seeds collected in Greece A,B.
3.3. Antioxidant Activity
The antioxidant activities of the phenolic-containing seed extracts of all checked taxa are detailed in Table 3. By the DPPH assay, values ranged between 0.92 (C. major) and 4.72 mmol TE/100 g dw (A. methanaea). The values obtained through the ABTS•+ method were typically between one half and one third of those obtained through the DPPH assay and ranged from 0.61 (E. vulgare subsp. pustulatum) to 2.49 mmol TE/100 g (A. methanaea). Overall, the highest antioxidant activities determined by the DPPH and ABTS•+ methods were recorded in this last species. The antioxidant activity was also assayed using pure molecules for comparison purposes, and through the DPPH methodology it was between 16.52 (ascorbic acid) and 23.44 mmol of TE/100 g (α-tocopherol), while in the ABTS•+ assay the values ranged between 8.67 (α-tocopherol) and 10.42 mmol TE/100 g (caffeic acid).
3.4. Antitumor Activity
The methanol/water (60:40, v/v) seed extracts of all samples were assessed for inhibitory effects on HT-29 human colorectal cancer cells measured through the MTT assay. The more active extracts against HT-29 cancer cells after 48 and 72 h of treatment are depicted in Figure 2A,B. Cell growth inhibition was notably exercised by A. graeca, C. major, O. frutescens, L. zahnii, and S. creticum, which at 500 µg/mL and after 72 h of cell exposure induced 81.5, 69.1, 90.6, 30.5, and 75.0% of cancer cell viability in comparison with controls without extract addition. The GI50 values of the latter species and those of selected phenolic compounds occurring in the samples are shown in Figure 3. After a 72 h incubation period, the GI50 values for A. graeca, C. major, O. frutescens, L. zahnii, S. creticum, chlorogenic acid, caffeic acid, rutin, rosmarinic acid, quercetin, and doxorubicin were 990, 600, 900, 375, 880, 680, 500 680, 35, 75, and 3 µg/mL, respectively.
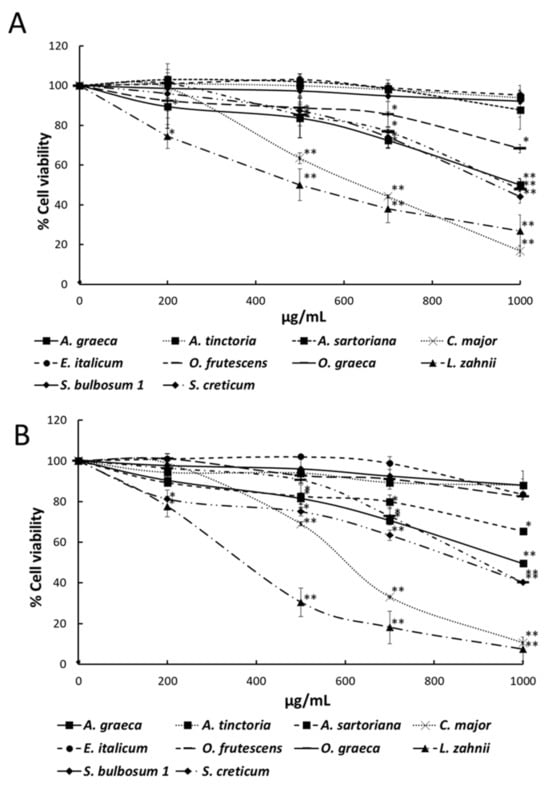
Figure 2.
MTT assay. Concentration–response plot for HT-29 cells after exposure to seed extracts for 48 (A) and 72 h (B). Data represent the mean of three complete independent experiments ± SD (error bars) with statistical significance equal to * p < 0.05 and ** p < 0.001.
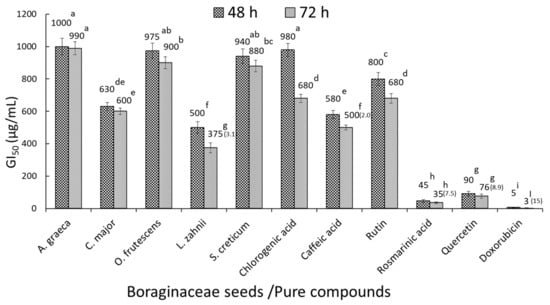
Figure 3.
MTT assay. GI50 for HT-29 cells after treatment with selected seeds extracts, pure phenolics, and doxorubicin for 48 and 72 h. The GI50 value is detailed over columns, and the selectivity index (SI) for 72 h-exposed cells to seeds extracts/pure compounds is shown in parentheses. Data represent the mean of three complete independent experiments ± SD (error bars). In a bar, means followed by different letters are significantly different at p < 0.05.
The selectivity index (SI) of HT-29 versus normal CCD-18 cells was calculated for extracts and pure compounds having GI50 ≤ 500 µg/mL. The SIs at 72 h were 3.1 (L. zahnii), 2.0 (caffeic acid), 7.5 (rosmarinic acid), 8.9 (quercetin), and 15 (doxorubicin); thus, these extracts and pure compounds have a high selectivity against HT-29 human colorectal cancer cells.
4. Discussion
4.1. Fatty Acids Content
This study shows that some Boraginaceae Greek species, i.e., both Symphytum species and L. zahnii, stand out for their high GLA content. Lithodora comprises three range-restricted species, and this genus has a Mediterranean distribution with its diversity center in the western Mediterranean []. This is the first report on the FA composition of L. zahnii, which is a vulnerable endemic species of Greece found only in certain rocky places of the south Peloponnese peninsula (see Table 1). Previously, the GLA% of total FAs of Lithodora fruticosa was set at 13.3% []; thus, L. zahnii constitutes an advantageous GLA producer within this genus. Glandora is a Lithospermeae genus closely related to Lithodora, which is native to the western and central Mediterranean region, that was split from the former in 2008 []. Previously, the GLA% of other Glandora species has been reported: G. nitida (19.2%, []; G. maroccana (20.2%) []; and G. diffusa, G. oleifolia, and G. rosmarinifolia (12.8, 12.5, and 12.4%, respectively) []. Therefore, there are two types of members belonging to both genera, that is, high- and medium-GLA-producer species. Given that the GLA% in Boraginaceae seed oil depends on several genes (FA desaturase2 (BaFAD2), FA desaturase3 (BaFAD3), and Delta-6-desaturase (BaD6D-1 and BaD6D-2)) [], the GLA% of both Glandora and Lithodora species should be considered as a possible taxonomical marker, while the molecular characterization of the microsomal FA desaturase pathway gene of these species could shed light on the phylogenetic relationships between both taxa.
So far, all surveyed Symphytum spp. have high GLA% of total FAs in their seed oil, for instance, S. grandiflorum (21.1), S. ibiricum (18.9), and S. tuberosum subsp. tuberosum (27.0%) []. In this work, the two checked species showed high GLA percentages. Previously, S. bulbosum collected in Reggio Calabria (Italy) showed GLA at 27.6% of total FAs [], which is in good agreement with the percentages found in this work for the last species. Small variations in GLA% could be related to environmental factors (temperature, light, and humidity), which influence the expression of genes involved in GLA biosynthesis, although the existence of chemotypes within this species cannot be ruled out.
Concerning S. creticum, the native range of this species is S. Greece to E. Aegean Islands. As for Aegean Symphytum, S. icaricum and S. naxicola are included in the Graeca section, while S. circinale, S. insulare, and S. creticum belong to the Procopiania section. These sections correspond to clear clades obtained through a phylogenetic analysis using ITS total and trnL-F []. Taking into account the habitats in which these species develop, they could become advantageous crops adapted to highly arid conditions, which could produce not only a GLA-rich seed oil, but a large number of useful products that are typically obtained from Symphyptum species []; thus, expanding knowledge on the chemical composition, food applications, and phytopharmacology of Aegean Symphytum taxa constitutes a promising research area.
The remaining species showed low GLA percentages, thus lacking interest for the obtainment of GLA oils, although they could be used for the recovery of other compounds contained in the unsaponifiable fraction, as discussed below. Concerning SDA, another PUFA of interest typically obtained from Boraginaceae seed oil, none of the surveyed species could be considered a good source of it.
According to both the total FAs detected in the seeds of S. bulbosum SB1 and L. zahnii (19.8 and 26.4 g/100 g) and their GLA levels (26.2 and 17.4% of total FA), the whole seeds of these species contain 5.2 and 4.6 g GLA/100 g.
Considering healthy n-3 PUFA, ALA was found in all samples at highly variable amounts. It is an essential FA (EFA) for humans [], acting as substrate for the biosynthesis of the conditionally EFAs EPA and docosahexaenoic acid (DHA, 22:6n-3), although such bioconversion is relatively limited and depends on several factors, e.g., dosage, gender, age, and body condition [,]. ALA improves anti-metabolic syndrome while exercising anticancer, anti-inflammatory, antioxidant, anti-obesity, neuroprotection, and intestinal flora regulation properties [,]. Therefore, finding new sources of this PUFA is desirable. Interestingly, Boraginae species are poor sources of ALA, and in this work the maximum percentage of it was found in S. bulbosum SB2 (11.4% of total FAs). Conversely, all Lithospermeae taxa exceed this ceiling, highlighting E. italicum biebersteinii and A. sartoriana, with 41.2 and 37.3% of total FAs. However, considering absolute ALA amounts, C. major (15.9) and E. italicum biebersteinii (13.2 g/100 g of seeds) reached the highest values. Other FAs found in the seeds under study are commonly found in most vegetable oils, thus lacking significance for giving added value to these oils.
The FA profiles detected in this work could vary somewhat for seeds from different annual cycles or collected in different places. For instance, small variations in GLA% have been found in the seed oil of B. officinalis collected in two successive years []. This situation is expected, since, as discussed above, the FA profiles in the seed oil of Boraginaceae depend on the activity of specific enzymes, which present characteristic isoforms in the various taxa [].
4.2. Phenolic Compound Content
TPC values (Table 2) showed significant differences among species when assessed through the F-C method. By using this methodology, most seeds showed significant amounts of phenolic compounds, although all figures were similar to other ones previously reported for Boraginaceae taxa, e.g., B. officinalis, which was reported to contain phenolic acids at 245-1090 mg GAE/100 g dw []; however, Borowy and Kapłan [] indicated for B. officinalis 1490-1690 mg of phenolic acids as caffeic acid equivalents by 100 g dw); for E. vulgare and B. officinalis, 122.6 and 130.4 mg GAE/100 g dw were reported []; and for Echium pycnanthum, 2731 mg GAE/100 g dw were found []. Concerning other GLA-rich species, evening primrose (O. biennis L.) contains higher amounts than most Boraginaceae taxa, for instance 736.47 mg/100 g dw [], a figure in line with the results of this work; for R. nigrum L. cultivars, it ranged from 160.4 to 230.7 mg/100 g []. As for flavonoids, the figures for the species analyzed in this work were higher than those reported for B. officinalis by Borowy and Kapłan [], 10–14 mg QE/100 g dw, but lower than the ones obtained for E. pycnanthum by Chaouche et al. [], 1626 mg of catechin equivalents by 100 g dw.
Assessed as the sum of individual phenolics through HPLC-DAD (Table 3), phenolic compounds ranged from 247.8 in S. bulbosum SB2 to 1524.7 mg/100 g seeds in A. methanaea. Using the same methodology, within Boraginaceae taxa, in Buglossoides species such content ranged from 62.1 to 645.5 mg/100 g dw []; in Borago species, it was between 55.36 and 255.86 mg/100 g seeds []. Concerning other GLA-rich taxa, total phenolic compounds assessed by HPLC-DAD typically reach ~2/3 of the amount evaluated by the F-C method, as reported by Bakowska-Barczak et al. []. This disagreement can be explained considering the following: (i) the F-C method provides the total amount of phenolic compounds, while the HPLC reports only amounts for identified compounds, and (ii) the unspecificity of this methodology toward phenolics, which leads to an overestimation of the actual phenolic content [].
Overall, the samples checked here contain higher amounts of phenolic compounds than other previously reported Boraginaceae taxa. This fact can be due to the fact that warm summer Mediterranean climates, like the one in which the analyzed species occur, lead to stressful conditions for plants, which can activate a multi-gene response that induces the synthesis of secondary metabolites, for instance phenolic compounds [].
Need to be considered that refined oils usually contain low amounts of polyphenols, which are mainly removed during the refining process due to their hydrophilic nature. Accordingly, in the event that any of the oils from the analyzed seeds are commercialized, it is recommended that to benefit from the bioactive compounds contained in the seeds, such as phenolics, the target oils are extracted by cold pressing.
4.3. Phenolic Compound Profiles
The identified compounds had high molar extinction coefficients at 280, 300, and 320 nm, which allowed the chromatographic separations to be monitored (Supplementary Table S3). Compounds were identified by comparing the absorption spectra of each peak identified in Boraginaceae seed extracts with those of pure standards and retention time (Rt). The precision/injection repeatability test showed good precision in peak area (standard deviation < 1%) and peak retention time (±2%). The results of the regression equation, LOD, LOQ, and recoveries for each compound are detailed in Supplementary Table S3.
Rutin and rosmarinic and chlorogenic acids were the main phenolic compounds quantified in the evaluated seeds. Rosmarinic acid occurs in all species within the Boraginaceae family [,,], while rutin and chlorogenic acid have also been identified in several Boraginaceae species []. Rosmarinic acid is widely cited in all organs of Boraginaceae plants, and it is useful as a chemotaxonomic tool. This phenolic is highly bioactive, although is considered safe, and it has several properties, i.e., antimutagenic, anti-inflammatory, antiviral, and antibacterial []. For instance, for the treatment of Herpes simplex, rosmarinic acid is a common component of pharmaceutical formulae. The cosmetic industry also includes this phenolic in creams for skincare, given its antioxidant and UVB sun radiation-protective properties. Moreover, studies are being developed on its antitumor activity [,]. Consistently, given the high concentrations that rosmarinic acid reached in A. methanaea, C. major, S. creticum, A. pindicola, A. corcyrensis, and A. graeca graeca (>400 mg/100 g), these constitute promising candidates for the obtainment of this important phenolic compound. Interestingly, previous levels of rosmarinic acid found in the seeds of other Boraginaceae species are notably lower than those found in this work. For example, in Mertensia spp., a range of 110 to 180 mg/100 g was found []; in B. officinalis, the reported figures were 165 mg/100 g [] and 12–103 mg/100 g []; in wild-endemic Borago spp., it was 14–105 mg/100 []; and in Lithospermum officinale, the range of values was 305–323 mg/100 g []. Therefore, the Greek seeds mentioned above could be used for agricultural production to obtain this acid.
The flavonoid class represents a low fraction of the quantified phenolics; it accounts for ~1/4 of total phenolics for most species and ranged from 14.8 (C. major) to 48.7% (O. frutescens) of total phenolics. Most of the flavonoids were glycoside derivatives, and A. methanaea reached the highest values (218.8 mg/100 g). Alkanna species had the highest amounts of apigetrin (apigenin-7-O-glucoside), especially A. methanaea (137.7 mg/100 g), while in the remaining taxa isoquercetin (quercetin 3-O-glucoside) reached the highest values, except A. graeca graeca, in which rutin (quercetin-3-O-rutinoside) was higher than isoquercetin. Although the TFC fraction is lower than the phenolic acids one, such compounds can apport important benefits to the health status. Flavonoids display diverse biological activities, i.e., antioxidant, immunomodulatory, antimicrobial, and estrogenic ones. Furthermore, glycosylation modifies their biological activities, changing the in vivo absorption, bioavailability, and interaction with ligands []. In any case, in all tested species the rosmarinic acid content was higher than that of any flavonoid.
4.4. Antioxidant Activity
For assessing this property, two different methodologies were accomplished, and a good correlation in the values obtained from both methods was noted. There are reports that some compounds that react with peroxyl radicals do not react well with the DPPH radical due to steric hindrance between the compounds, because DPPH· is a highly stable nitrogen radical; conversely, ABTS•+ is a product of a reaction with potassium persulfate, and thus more unstable []. This fact leads to differences in the antioxidant activity obtained using both methods. Table 3 details the results obtained by both methodologies. Notice that the species showing the highest antioxidant activity measured by both procedures were Alkanna taxa. The remaining species in most cases had values slightly higher than 1 (DPPH method) or somewhat lower than 1 mmol TE/100 g (ABTS method). To the best of our knowledge, there are no values in the literature on the antioxidant capacity of Boraginaceae seeds evaluated by the same methodology used here. However, other GLA-rich seeds of various R. nigrum cultivars were evaluated, and the ABTS method had values in the 1.4–1.7 range, while the DPPH one gave values between 1.1 and 1.3 mmol TE/100 g dw []; therefore, these values are in line with those found in the Boraginaceae seeds evaluated here.
The antioxidant activity of pure compounds (ascorbic acid, α-tocopherol, and caffeic acid) was also checked, and the results obtained by the ABTS•+ procedure method were approximately half of those obtained by the DPPH method. Interestingly, the values obtained by each method for all checked compounds were quite similar (Table 3). However, as expected, the figures obtained for the antioxidant activity of all seed extracts were much lower than those obtained for such pure molecules.
4.5. Antiproliferative Activity of the Water/Methanol Seed Extracts on HT-29 Cancer Cells
A colon cancer human cell line was selected for evaluating the antiproliferative activity of the phenolic-containing extracts because colorectal cancer is one of the most common cancers worldwide and the fourth leading cause of death, with mortality close to 50% in Western countries. Also, several studies suggested that it is a disease strongly influenced by diet, which can modulate inflammatory processes and affect gene expression in the body [].
The effects of the seed extracts of selected taxa on HT-29 cell viability are shown in Figure 2. After 48 and 72 h of treatment, the MTT assay revealed concentration- and time-dependent inhibitory effects on HT-29 cells mediated by most seed extracts. The extracts of C. major and L. zahnii induced the greatest decrease in cell viability at 72 h at the maximum concentration tested (1000 μg/mL), which was ~5–30% lower than that obtained at 48 h. The doses of extracts that inhibited cell growth by 50% (GI50), as well as those of some pure phenolic compounds occurring in the checked extracts, are depicted in Figure 3. GI50 values after a 72-h treatment period for L. zahnii, C. major, S. creticum, O. frutescens, and A. graeca were 375, 600, 880, 900, and 990 μg/mL. These results are in good agreement with those obtained for the phenolic-containing seed extracts from several Borago species [] and Buglossoides taxa []. Finding inhibitory effects against HT-29 cells exercised by the phenolic extract of any Boraginaceae seed is noteworthy, given that such cells tend to be unresponsive to phenolics. For instance, the phenolics extracts from another GLA species, Oenothera paradoxa, induced apoptosis in Caco-2 cells, while HT-29 cells were slightly affected [].
The antiproliferative activity against HT-29 cells of pure phenols occurring in the studied extracts was also checked: rosmarinic acid, quercetin, cafeic acid, rutin, and chlorogenic acid. These showed GI50 of 35, 76, 500, 680, and 680 µg/mL. Interestingly, the species that displayed higher antitumor activity, i.e., C. major, contained high amounts of rosmarinic acid, which is the more active phenolic against HT-29 cells; thus, this activity was probably influenced partially by this phenolic.
Finally, the SI of HT-29 vs. normal cells (CCD-18) was calculated for the more active extract, i.e., L. zahnii (see Materials and Methods–Section 2). Extracts having SI > 2 are classified as highly selective against cancer cells, while the ones with SI < 2 exhibited toxicity to normal cells []. For research on biological extracts and/or isolated compounds, this figure is critical for deciding further research on the subject []. Although data were favorable, this research should be continued with appropriate experiments before considering this extract as an anticancer drug candidate. This is mandatory, because although in vitro drug/extract dose–response experiments constitute the cornerstone of the preclinical evaluation of anticancer drugs, the transition from in vitro anticancer activity to clinical application needs careful analysis and validation of results.
It is timely to indicate that in the case of trying to obtain oils exhibiting antitumor bioactivity from the analyzed Greek Boraginaceae taxa, it would be necessary to process the seeds by cold pressing. This is so because oils extracted through organic solvent use, e.g., n-hexane, lack phenolic compounds, given the hydrophilic nature of the latter.
4.6. Correlation among Parameters
Figure 4 shows a heat map of the correlations among the different variables evaluated here in the Greek Boraginaceae seeds samples, i.e., ALA, GLA, LA, total FAs, SFAs, MUFAs, n-3 PUFAs, n-6 PUFAs, n-6/n-3 ratio, TPC, TFC, DPPH, ABTS, and prominent phenolics (chlorogenic, trans-p-coumaric, rosmarinic, salvianolic, and lithospermic acids; rutin, isoquercetin, and apigetrin). The GI (%) exercised by 1 mg/mL of seed extract added to cell cultures measured with the MTT test (GImg/mL) was also used for checking correlations. Note that the antioxidant activity measured by both the DPPH and ABTS•+ methods, in addition to the fact that both methods were highly correlated, has a significant and positive correlation with both TFC and TPC. This correlation indicated that the compounds that have significant reducing ability contributed largely to the antioxidant activity found in the various seed extracts. A positive correlation was found between TPC and TFC, suggesting that the metabolic pathways leading to phenols are activated for related enzyme systems to yield both flavonoids and phenolic compounds. As expected, these last two parameters have positive correlation with most of the quantified phenolics: trans-p-coumaric, rosmarinic, and salvianolic acids, rutin, and apigetrin. Moreover, both the DPPH and ABTS•+ methods had positive correlations with the latter compounds, which suggests that such compounds exert the measured antioxidant activity. Interestingly, the last phenolic compounds are the ones that present positive correlations with the methods that quantified them spectrophotometrically, i.e., TPC and TFC. On the other hand, GImg/mL lacks positive correlations with any of the tested parameters. This suggests that the antitumor activity was exerted by a synergy of the compounds/bioactivities of the seed extracts, but any of them could have predominantly developed this activity. Finally, other correlations between the various FA and FA groups occur as expected; for example, the positive correlation between n-6 PUFA with LA and GLA.
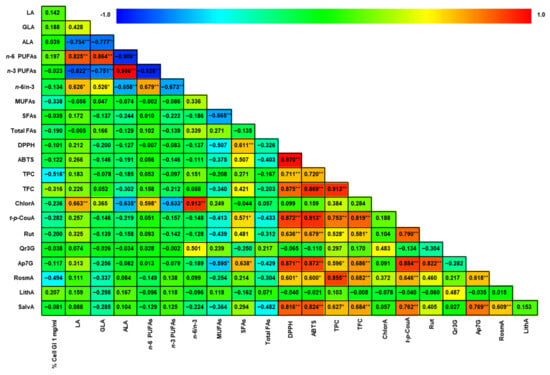
Figure 4.
Pearson Product–Moment Correlations. Heat map of the correlation between the different variables. The redder colors indicate a stronger positive correlation, while the bluer colors indicate a stronger negative correlation. Correlations were evaluated with a significance of p < 0.05 (*) and p < 0.01 (**). Abbreviations: LA—linoleic acid; GLA—gamma-linolenic acid; ALA—alpha-linolenic acid; MUFAs—monounstaturated FAs; SFAs—saturated FAs; Total FAs—total fatty acids; TPC—total phenolics content; TFC—total flavonoids content; ChlorA—chlorogenic acid; t-p-CouA—trans-p-coumaric acid; Isoquer—isoquercetin; Apiget—apigetrin, LithA—lithospermic acid; SalvA—salvianolic acid; GImg/mL—percentage of cell growth inhibition exercised by adding 1 mg/mL of seed extract to cell cultures, measured with the MTT test.
5. Conclusions
Among the Greek Boraginaceae taxa analyzed in this work, three species showed high amounts of GLA in their seed oils, i.e., both Symphytum species and L. zahnii, and most of them showed high amounts of rosmarinic acid and rutin, highlighting C. major, Alkanna spp., S. creticum, and L. zahnii, while their antioxidant activity was in line with other GLA-rich seeds. The phenolic extract of several extracts, especially those of C. major and L. zahnii, showed dose- and time-dependent activity against the human colorectal cancer cell line HT-29. This activity is noteworthy, given that HT-29 cells tend to be unresponsive to phenolics. The antiproliferative bioactivity effect of phenolic extracts was probably due to a synergy among compounds having high antiproliferative activity. The GLA-rich species analyzed in this work have optimal FA and phenolics profiles, as well as good antioxidant and antitumor activities; thus, new crops could be developed from these taxa to supply GLA-rich oils to the food, cosmetic, and pharmaceutical industries. It is advisable to perform cold-press extraction of these oils, since when extracting oils with organic solvents during the refining process bioactive compounds are removed, thus they lose most of their functional properties. In this work, most of the seeds have been collected in a single location; therefore, some variation in the concentration of bioactive compounds and/or bioactivities could be expected from seeds collected in other locations, as well as from seeds collected in different years. This variation would be marginal in the case of FAs, since their percentages are a consequence of genetically regulated enzymatic activities. However, as discussed above, phenolic concentrations are more variable within the same taxon. Therefore, it would be interesting to investigate to what extent the richness in rosmarinic acid shown by some species is environmentally dependent.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app14146026/s1: Supplementary File S1: Material and Methods; Supplementary Figure S1: A 254 nm-HPLC chromatogram of the phenolic-containing methanol:water extract of S. creticum (M7); Supplementary Table S1: HPLC-DAD parameters used for the analysis of phenolic-enriched extracts of Boraginaceae seeds from Greece; Supplementary Table S2: Phenolic compounds identified by LC-MS in the hydroalcoholic extracts (MeOH:H2O, 60:40 v/v) of Boraginaceae seeds from Greece and identification basis; Supplementary Table S3: Phenolic compounds occurrence detected in Boraginaceae seeds from Greece by LC-MS.
Author Contributions
Conceptualization, M.E., T.C.-C. and J.L.G.-G.; Methodology, M.E., T.C.-C., R.L.-R. and J.L.G.-G.; Software, T.C.-C., H.B., R.L.-R., R.L.-R. and J.L.G.-G.; Validation, T.C.-C., H.B., R.L.-R. and J.L.G.-G.; Formal Analysis, M.E., T.C.-C., R.L.-R. and J.L.G.-G.; Investigation, M.E., T.C.-C., M.Á.R.-C., F.G.-M. and J.L.G.-G.; Resources, R.L.-R. and J.L.G.-G.; Data Curation, T.C.-C., H.B., R.L.-R. and J.L.G.-G.; Writing—Original Draft Preparation, M.E. and J.L.G.-G.; Writing—Review and Editing, M.E. and J.L.G.-G.; Visualization, F.G.-M., R.L.-R. and J.L.G.-G.; Supervision, J.L.G.-G., Project Administration, R.L.-R. and J.L.G.-G.; Funding Acquisition, R.L.-R. and J.L.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from Junta de Andalucía (Grant P20_00806), University of Almería (Grant P_LANZ_2023/003), Campus de Excelencia Internacional Agroalimentario (ceiA3) (Grant 001), Centro de Investigación en Agrosistemas Intensivos Mediterráneos y biotecnología Agroalimentaria (CIAMBITAL) (Grant 002), and from MICIU/AEI/10.13039/501100011033 (Grant PID2022-143070NB-I00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials, and further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Segelbacher, G.; Bosse, M.; Burger, P.; Galbusera, P.; Godoy, J.A.; Helsen, P.; Hvilsom, C.; Iacolina, L.; Kahric, A.; Manfrin, C.; et al. New developments in the field of genomic technologies and their relevance to conservation management. Conserv. Genet. 2022, 23, 217–242. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2020, 75, 311–315. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L. Stearidonic acid (18: 4n-3): Metabolism, nutritional importance, medical uses, and natural sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1226–1236. [Google Scholar] [CrossRef]
- Baker, E.J.; Valenzuela, C.A.; van Dooremalen, W.T.; Martínez-Fernández, L.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Gamma-Linolenic and Pinolenic Acids Exert Anti-Inflammatory Effects in Cultured Human Endothelial Cells Through Their Elongation Products. Mol. Nutr. Food Res. 2020, 64, 2000382. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.J.; Ortea, I.; Guil-Guerrero, J.L. α-Linolenic and γ-linolenic acids exercise differential antitumor effects on HT-29 human colorectal cancer cells. Toxicol. Res. 2020, 9, 474–483. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Gong, L. Gamma linolenic acid suppresses hypoxia-induced proliferation and invasion of non-small cell lung cancer cells by inhibition of HIF1α. Genes Genom. 2020, 42, 927–935. [Google Scholar] [CrossRef]
- da Costa, L.D.F.C.; Lopes, C.M.C.; Roa, C.L.; Zuchelo, L.T.S.; Baracat, E.C.; de Andrade, J.; Soares, J.M., Jr. Is there a beneficial effect of gamma-linolenic acid supplementation on body fat in postmenopausal hypertensive women? A prospective randomized double-blind placebo-controlled trial. Menopause 2021, 28, 699–705. [Google Scholar] [CrossRef]
- Greupner, T.; Koch, E.; Kutzner, L.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Single-dose SDA-rich Echium oil increases plasma EPA, DPAn3, and DHA concentrations. Nutrients 2019, 11, 2346. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- D’Helft, J.; Caccialanza, R.; Derbyshire, E.; Maes, M. Relevance of ω-6 GLA Added to ω-3 PUFAs Supplements for ADHD: A Narrative Review. Nutrients 2022, 14, 3273. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Rincón-Cervera, M.Á. Sardinian Boraginaceae are new potential sources of gamma-linolenic acid. Food Chem. 2017, 218, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Chelh, T.C.; Lyashenko, S.; Lahlou, A.; Belarbi, E.H.; Rincón-Cervera, M.Á.; Rodríguez-García, I.; Urrestarazu-Gavilán, M.; López-Ruiz, R.; Guil-Guerrero, J.L. Buglossoides spp. seeds, a land source of health-promoting n-3 PUFA and phenolic compounds. Food Res. Int. 2022, 157, 111421. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.; Shahidi, F.; Amarowicz, R.; Abou-Zaid, M.M. Phenolic acids in defatted seeds of borage (Borago officinalis L.). Food Chem. 2001, 75, 49–56. [Google Scholar] [CrossRef]
- Mhamdi, B.; Wannes, W.A.; Bourgou, S.; Marzouk, B. Biochemical characterization of borage (Borago officinalis L.) seeds. J. Food Biochem. 2009, 33, 331–341. [Google Scholar] [CrossRef]
- Fabrikov, D.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Rodríguez-García, I.; Gómez-Mercado, F.; Urrestarazu, M. Borage oil: Tocopherols, sterols and squalene in farmed and endemic-wild Borago species. J. Food Compos. Anal. 2019, 83, 103299. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Dietary fatty acids in the secondary prevention of coronary heart disease: A systematic review, meta-analysis and meta-regression. BMJ Open 2021, 11, e041120. [Google Scholar] [CrossRef] [PubMed]
- Borowy, A.; Kapłan, M. Chemical composition and antioxidant activity of borage (Borago officinalis L.) seeds. Acta Sci. Polonorum. Hortorum Cultus 2020, 19, 79–90. [Google Scholar] [CrossRef]
- Lyashenko, S.; Fabrikov, D.; González-Fernández, M.J.; Gómez-Mercado, F.; Ruiz, R.L.; Fedorov, A.; de Bélair, G.; Urrestarazu, M.; Rodríguez-García, I.; Álvarez-Corral, M.; et al. Phenolic composition and in vitro antiproliferative activity of Borago spp. seed extracts on HT-29 cancer cells. Food Biosci. 2021, 42, 101043. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Fabrikov, D.; Rincón-Cervera, M.Á.; Urrestarazu, M.; Gómez-Mercado, F. γ-Linolenic and Stearidonic Acids from Boraginaceae of Diverse Mediterranean Origin. Chem. Biodivers. 2020, 17, e2000627. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N. Lipids of the hexane extract from the roots of medicinal Boraginaceous species. Phytochem. Anal. 2003, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ganos, C.; Aligiannis, N.; Chinou, I.; Naziris, N.; Chountoulesi, M.; Mroczek, T.; Graikou, K. Rindera graeca (Boraginaceae) phytochemical profile and biological activities. Molecules 2020, 25, 3625. [Google Scholar] [CrossRef] [PubMed]
- Tzanoudakis, D.; Panitsa, M. The flora of the Greek islands. Ecol. Mediterr. 1995, 21, 195–212. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of flavonoid-rich extracts of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Weigend, M.; Hilger, H.H. Phylogeny and systematics of Lithodora (Boraginaceae-Lithospermeae) and its affinities to the monotypic genera Mairetis, Halacsya and Paramoltkia based on ITS1 and trnLUAA-sequence data and morphology. Toxicol. Rep. 2008, 57, 79–97. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; López-Martínez, J.C.; Gómez-Mercado, F.; Campra-Madrid, P. Gamma-linolenic and stearidonic acids from Moroccan Boraginaceae. Eur. J. Lipid Sci. Technol. 2006, 108, 43–47. [Google Scholar] [CrossRef]
- Ferrero, V.; Arroyo, J.; Castro, S.; Navarro, L. Unusual heterostyly: Style dimorphism and self-incompatibility are not tightly associated in Lithodora and Glandora (Boraginaceae). Ann. Bot. 2012, 109, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; Rincón-Cervera, M.Á.; Venegas-Venegas, E. Restricted-range Boraginaceae species constitute potential sources of valuable fatty acids. J. Am. Oil Chem. Soc. 2014, 91, 301–308. [Google Scholar] [CrossRef]
- Prasad, P.; Sreedhar, R.V. Identification and functional characterization of Buglossoides arvensis microsomal fatty acid desaturation pathway genes involved in polyunsaturated fatty acid synthesis in seeds. J. Biotech. 2020, 308, 130–140. [Google Scholar] [CrossRef]
- Hacıoğlu, B.T.; Erik, S. Phylogeny of Symphytum L. (Boraginaceae) with special emphasis on Turkish species. Afr. J. Biotechnol. 2011, 10, 15483–15493. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Boyunegmez Tumer, T.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, B.; Wannes, W.A.; Sriti, J.; Jellali, I.; Ksouri, R.; Marzouk, B. Effect of harvesting time on phenolic compounds and antiradical scavenging activity of Borago officinalis seed extracts. Ind. Crops Prod. 2010, 31, e1–e4. [Google Scholar] [CrossRef]
- Nogala-Kalucka, M.; Rudzinska, M.; Zadernowski, R.; Siger, A.; Krzyzostaniak, I. Phytochemical content and antioxidant properties of seeds of unconventional oil plants. J. Am. Oil Chem. Soc. 2010, 87, 1481–1487. [Google Scholar] [CrossRef]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A.M.; Schieber, A.; Kolodziejczyk, P. Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues. J. Agric. Food Chem. 2009, 57, 11528–11536. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotech. 2012, 32, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Lyashenko, S.; González-Fernández, M.J.; Borisova, S.; Belarbi, E.H.; Guil-Guerrero, J.L. Mertensia (Boraginaceae) seeds are new sources of γ-linolenic acid and minor functional compounds. Food Chem. 2021, 350, 128635. [Google Scholar] [CrossRef]
- Lyashenko, S.; Yunusova, S.; López-Ruiz, R.; Vasfilova, E.; Kiseleva, O.; Chimitov, D.; Bahanova, M.; Bojko, N.; Guil-Guerrero, J.L. Lipid fractions, fatty acid profiles, and bioactive compounds of Lithospermum officinale L. seeds. J. Am. Oil Chem. Soc. 2021, 98, 425–437. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Gorlach, S.; Wagner, W.; Podsedek, A.; Sosnowska, D.; Dastych, J.; Koziołkiewicz, M. Polyphenols from evening primrose (Oenothera paradoxa) defatted seeds induce apoptosis in human colon cancer Caco-2 cells. J. Agric. Food Chem. 2011, 59, 6985–6997. [Google Scholar] [CrossRef] [PubMed]
- Vichitsakul, K.; Laowichuwakonnukul, K.; Soontornworajit, B.; Poomipark, N.; Itharat, A.; Rotkrua, P. Anti-proliferation and induction of mitochondria-mediated apoptosis by Garcinia hanburyi resin in colorectal cancer cells. Heliyon 2023, 22, e16411. [Google Scholar] [CrossRef] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).