Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
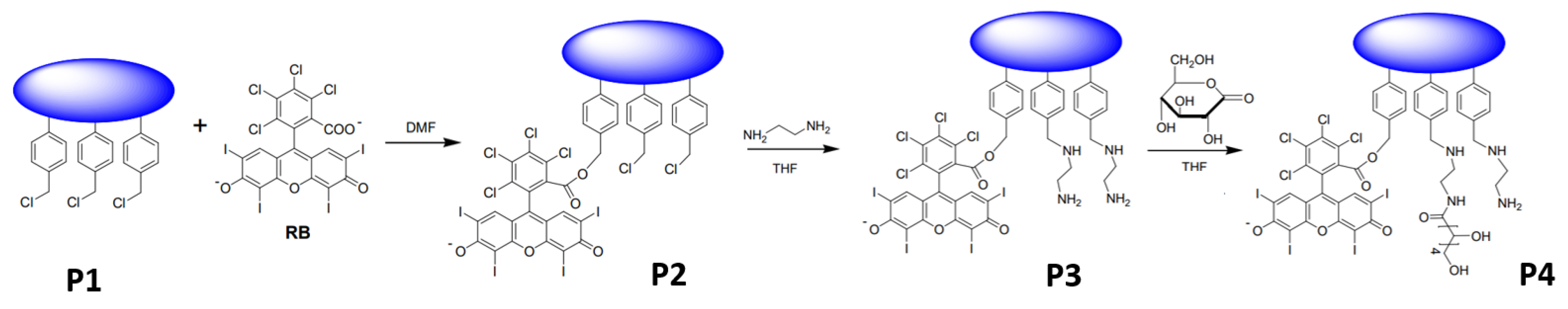
2.1. Synthesis and Characterization of Photoactive Polymers
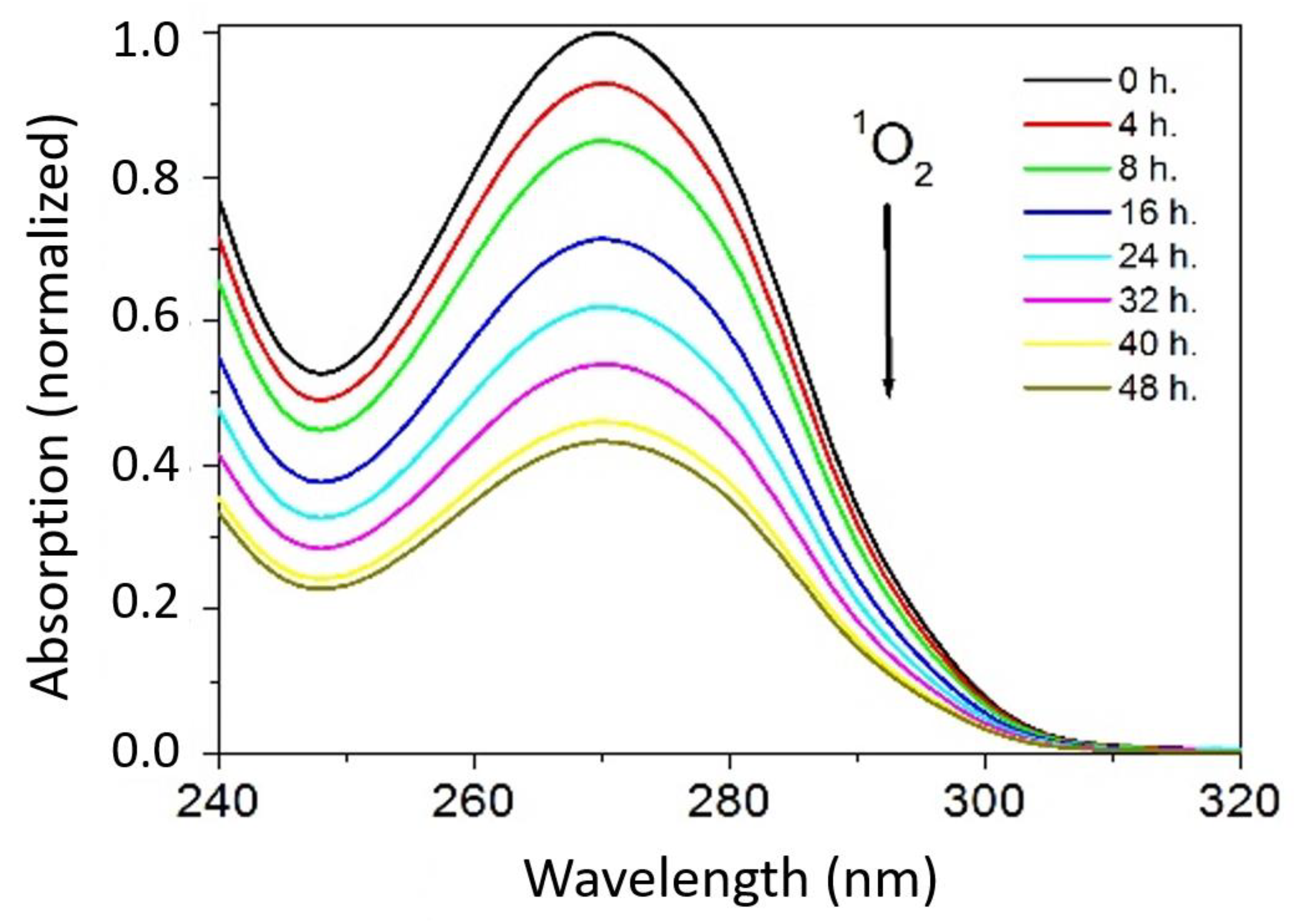
2.2. Photo-Oxidation Kinetics of AHMPD with Polymers P2, P3, and P4 as Photosensitizers
2.3. AHMPD Degradation Studies with P3 and P4 Polymers as Photosensitizers in Industrial WWTP Samples
3. Results and Discussion
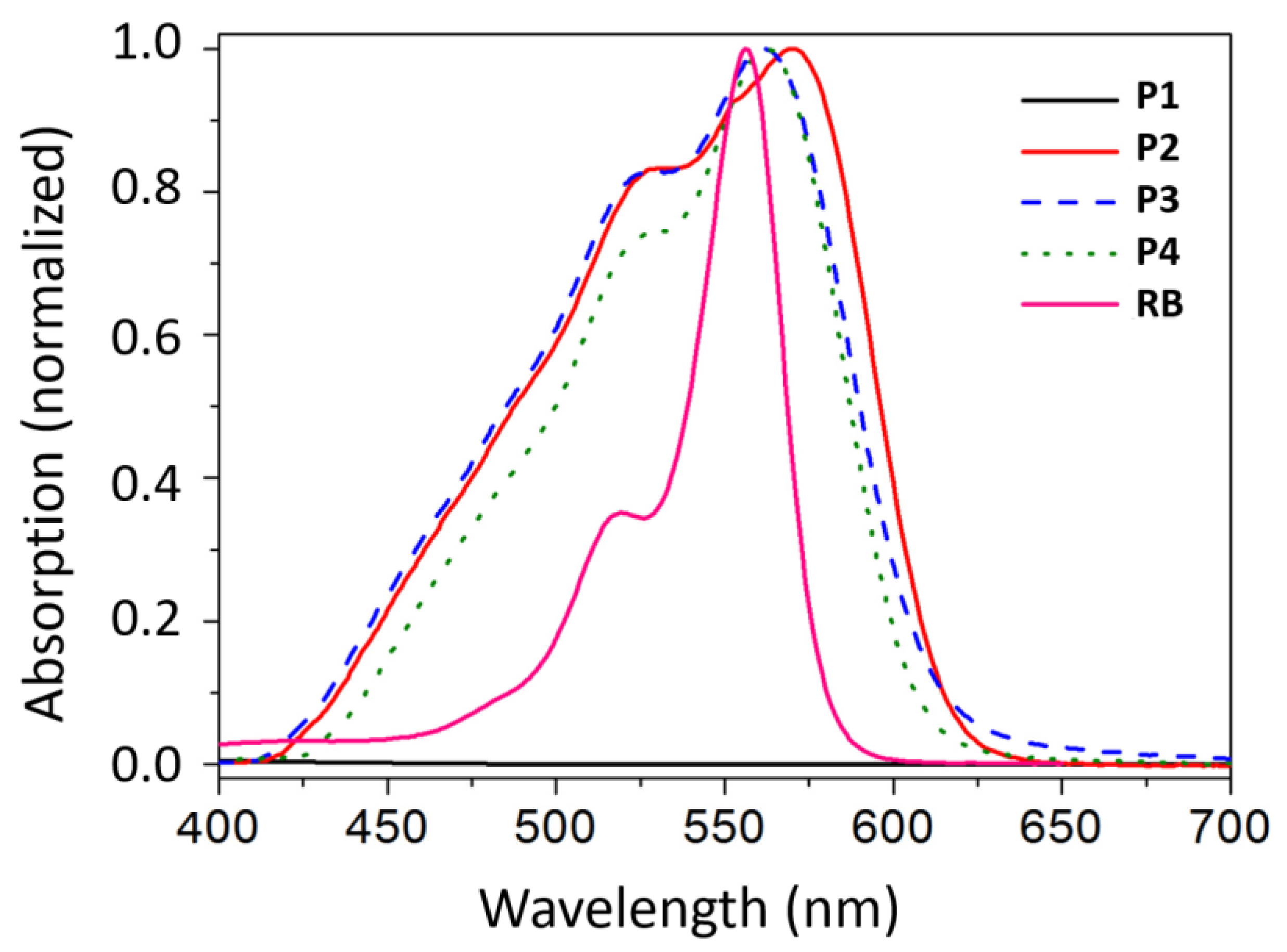
3.1. Synthesis and Characterization of Photoactive Polymers
3.2. Study of the Photo-Oxidation Kinetics of AHMPD with Polymers P2, P3, and P4 as Photosensitizers
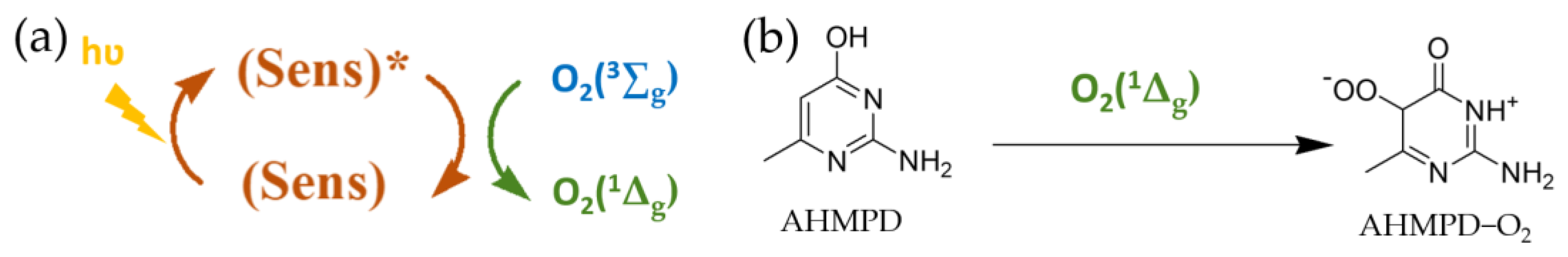
3.3. Interpretation of the Photocatalytic Mechanism for the Photo-Oxidation of AHMPD Using Rose of Bengal
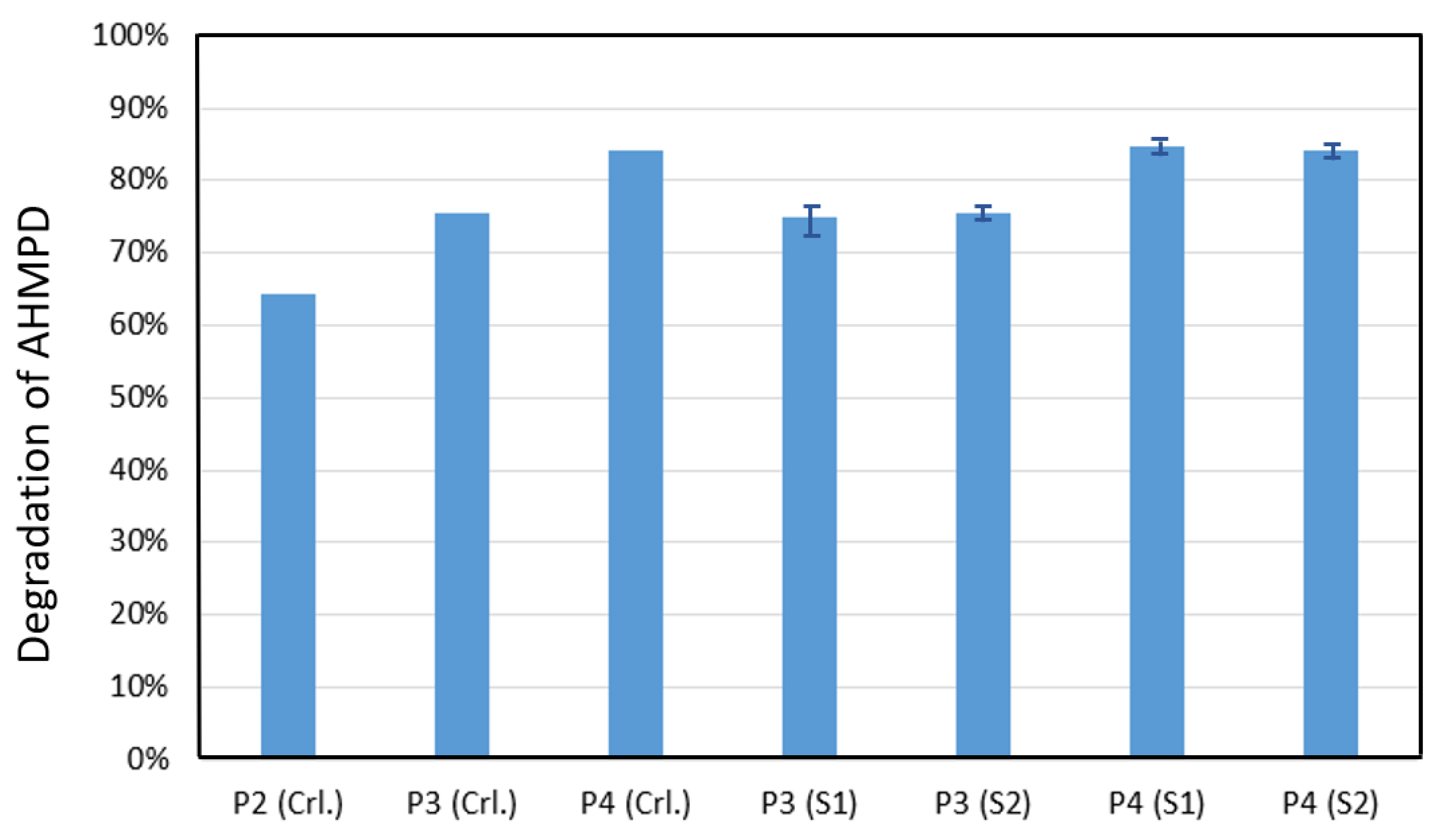
3.4. AHMPD Degradation Studies with P3 and P4 Polymers as Photosensitizers in Industrial WWTP Samples
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A review on emerging pollutants in the water environment: Existences, health effects and treatment processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Patel, N.A.V.E.E.N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation-a review. Pollution 2020, 6, 99–113. [Google Scholar]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Crini, G. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water–A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Madureira, J.; Melo, R.; Margaça, F.M.; Verde, S.C. Ionizing radiation for treatment of pharmaceutical compounds: A review. J. Water Process Eng. 2022, 49, 103179. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent developments in photocatalysis of industrial effluents: A review and example of phenolic compounds degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Barros, L.; Cabo Verde, S.; Margaça, F.M.; Santos-Buelga, C.; Ferreira, I.C. Ionizing radiation technologies to increase the extraction of bioactive compounds from agro-industrial residues: A review. J. Agric. Food Chem. 2020, 68, 11054–11067. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Margaça, F.M.; Santos-Buelga, C.; Ferreira, I.C.; Verde, S.C.; Barros, L. Applications of bioactive compounds extracted from olive industry wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Life Clean Up. Available online: https://www.lifecleanup.eu/ (accessed on 1 July 2024).
- Rizzi, V.; Gubitosa, J.; Fini, P.; Petrella, A.; Romita, R.; Agostiano, A.; Cosma, P. A “classic” material for capture and detoxification of emergent contaminants for water purification: The case of tetracycline. Environ. Technol. Innov. 2020, 19, 100812. [Google Scholar] [CrossRef]
- Rizzi, V.; Romita, R.; Gómez, V.M.; Gubitosa, J.; Gabaldón, J.A.; Fortea, M.I.; Gómez, T.; Cosma, P.; Fini, P. The synergistic action of cyclodextrin-based adsorbent and advanced oxidation processes for sulfamethoxazole removal from water. Int. J. Environ. Sci. Technol. 2022, 19, 10663–10676. [Google Scholar] [CrossRef]
- Gubitosa, J.; Mongiovi, C.; Romita, R.; Cosma, P.; Nuzzo, S.; Rizzi, V.; Fini, P. Removal of emerging contaminants from water using cyclodextrin-based polymers and advanced oxidation processes: The case of carbamazepine. Processes 2022, 10, 1703. [Google Scholar] [CrossRef]
- Fabregat, V.; Pagán, J.M. Technical–Economic Feasibility of a New Method of Adsorbent Materials and Advanced Oxidation Techniques to Remove Emerging Pollutants in Treated Wastewater. Water 2024, 16, 814. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Galindo, F. Singlet oxygen generation by photoactive polymeric microparticles with enhanced aqueous compatibility. Environ. Sci. Pollut. Res. 2014, 21, 11884–11892. [Google Scholar] [CrossRef]
- Fabregat, V.; Burguete, M.I.; Luis, S.V.; Galindo, F. Improving photocatalytic oxygenation mediated by polymer supported photosensitizers using semiconductor quantum dots as ‘light antennas’. RSC Adv. 2017, 7, 35154–35158. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Schnorr, C.; Oviedo, L.R.; Knani, S.; Silva, L.F.; Silva, W.L.; Bohn Rhoden, C.R. Adsorption and photocatalytic degradation of pesticides into nanocomposites: A review. Molecules 2022, 27, 6261. [Google Scholar] [CrossRef]
- Saien, J.; Khezrianjoo, S. Degradation of the fungicide carbendazim in aqueous solutions with UV/TiO2 process: Optimization, kinetics and toxicity studies. J. Hazard. Mater. 2008, 157, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Andiappan, M. Structure–property–performance relationships of cuprous oxide nanostructures for dielectric Mie resonance-enhanced photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Ashar, A.; Qayyum, A.; Bhatti, I.A.; Aziz, H.; Bhutta, Z.A.; Abdel-Maksoud, M.A.; Eletmany, M.R. Photo-Induced Super-Hydrophilicity of Nano-Calcite@ Polyester Fabric: Enhanced Solar Photocatalytic Activity against Imidacloprid. ACS Omega 2023, 8, 35722–35737. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, S.; Tirumala, R.T.A.; Andiappan, M.; Bristow, A.D. Size-and shape-dependent charge-carrier dynamics in sub-micron cuprous oxide nanoparticles. In Frontiers in Optics; Optica Publishing Group: Washington, DC, USA, 2022; p. JTu4A-86. [Google Scholar]
- Mohammadparast, F.; Tirumala, R.T.A.; Ramakrishnan, S.B.; Dadgar, A.P.; Andiappan, M. Operando UV–Vis spectroscopy as potential in-line PAT system for size determination of functioning metal nanocatalysts. Chem. Eng. Sci. 2020, 225, 115821. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T.; Khatri, N.; Ramakrishnan, S.B.; Mohammadparast, F.; Khan, M.T.; Tan, S.; Andiappan, M. Tuning Catalytic Activity and Selectivity in Photocatalysis on Mie-Resonant Cuprous Oxide Particles: Distinguishing Electromagnetic Field Enhancement Effect from the Heating Effect. ACS Sustain. Chem. Eng. 2023, 11, 15931–15940. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Cabezuelo, O.; Martinez-Haya, R.; Schmidt, L.C.; Bosca, F.; Marin, M.L. Organic photoredox catalysts: Tuning the operating mechanisms in the degradation of pollutants. Pure Appl. Chem. 2023, 95, 899–912. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Moya, P.; Marin, M.L.; Bosca, F. Synthesis of novel heterogeneous photocatalysts based on Rose Bengal for effective wastewater disinfection and decontamination. Catal. Today 2023, 413, 113948. [Google Scholar] [CrossRef]
- Flores, J.; Moya, P.; Bosca, F.; Marin, M.L. Photoreactivity of new rose bengal-SiO2 heterogeneous photocatalysts with and without a magnetite core for drug degradation and disinfection. Catal. Today 2023, 413, 113994. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Bosio, A.; Gamba, S.; Bosca, F.; Marin, M.L. Covalent or ionic bonding of Eosin Y to silica: New visible-light photocatalysts for redox wastewater remediation. J. Environ. Chem. Eng. 2023, 11, 111024. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]
- Lambert, C.R.; Kochevar, I.E. Does rose bengal triplet generate superoxide anion? J. Am. Chem. Soc. 1996, 118, 3297–3298. [Google Scholar] [CrossRef]
- Neckers, D.C. Rose bengal. J. Photochem. Photobiol. A Chem. 1989, 47, 1–29. [Google Scholar] [CrossRef]
- Marsh, R.W. (Ed.) Systemic Fungicides; CABI Digital Library: London, UK, 1972. [Google Scholar]
- Pajares, A.; Gianotti, J.; Stettler, G.; Haggi, E.; Miskoski, S.; Criado, S.; García, N.A. Kinetics of the dye sensitized photooxidation of 2-amino-4-hydroxy-6-methylpyrimidine, a model compound for some fungicides. J. Photochem. Photobiol. A Chem. 2000, 135, 207–212. [Google Scholar] [CrossRef]
- Haggi, E.; Bertolotti, S.; Miskoski, S.; Amat-Guerri, F.; García, N.A. Environmental photodegradation of pyrimidine fungicides Kinetics of the visible-light-promoted interactions between riboflavin and 2-amino-4-hydroxy-6-methylpyrimidine. Can. J. Chem. 2002, 80, 62–67. [Google Scholar] [CrossRef]
- Afroz, Z.; Faizan, M.; Alam, M.J.; Rodrigues, V.H.N.; Ahmad, S.; Ahmad, A. Synthesis, structural, hirshfeld surface, spectroscopic studies and quantum chemical calculation of the proton transfer complex between 2-amino-4-hydroxy-6-methylpyrimidine and salicylic acid. J. Mol. Struct. 2018, 1171, 438–448. [Google Scholar] [CrossRef]
- Tamagaki, S.; Liesner, C.E.; Neckers, D.C. Polymer-based sensitizers for photochemical reactions. Silica gel as a support. J. Org. Chem. 1980, 45, 1573–1576. [Google Scholar] [CrossRef]
- Wang, Q.C.; Svec, F.; Frechet, J.M. Hydrophilization of porous polystyrene-based continuous rod column. Anal. Chem. 1995, 67, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, B.; Paczkowski, J.; Neckers, D.C. Heterogeneous and semiheterogeneous photosensitization: Photochemical processes using derivatives of rose bengal. Macromolecules 1986, 19, 863–870. [Google Scholar] [CrossRef]
- Lindig, B.A.; Rodgers, M.A.; Schaap, A.P. Determination of the lifetime of singlet oxygen in water-d2 using 9, 10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]
- Dixon, S.R.; Wells, C.H. Dye-sensitised photo-oxidation of 2-dimethylamino-5, 6-dimethylpyrimidin-4-01 in aqueous solution. Pestic. Sci. 1983, 14, 444–448. [Google Scholar] [CrossRef]
- García, N.A. New trends in photobiology: Singlet-molecular-oxygen-mediated photodegradation of aquatic phenolic pollutants. A kinetic and mechanistic overview. J. Photochem. Photobiol. B Biol. 1994, 22, 185–196. [Google Scholar] [CrossRef]
- Schaap, A.P.; Thayer, A.L.; Blossey, E.C.; Neckers, D.C. Polymer-Based Sensitizers for Photooxidations. II. J. Am. Chem. Soc. 1975, 97, 3741–3745. [Google Scholar] [CrossRef]
- Blossey, E.C.; Neckers, D.C.; Thayer, A.L.; Schaap, A.P. Polymer-based sensitizers for photooxidations. J. Am. Chem. Soc. 1973, 95, 5820–5822. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T.; Ramakrishnan, S.B.; Mohammadparast, F.; Khatri, N.; Arumugam, S.M.; Tan, S.; Andiappan, M. Structure–property–performance relationships of dielectric Cu2O nanoparticles for Mie resonance-enhanced dye sensitization. ACS Appl. Nano Mater. 2022, 5, 6699–6707. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Loh, H.; Andiappan, M.; Bristow, A.D. Photocarrier Recombination Dynamics in Highly Scattering Cu2O Nanocatalyst Clusters. J. Phys. Chem. C 2024, 128, 2003–2011. [Google Scholar] [CrossRef]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of charge transfer from plasmonic nanostructures to chemically attached materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef]

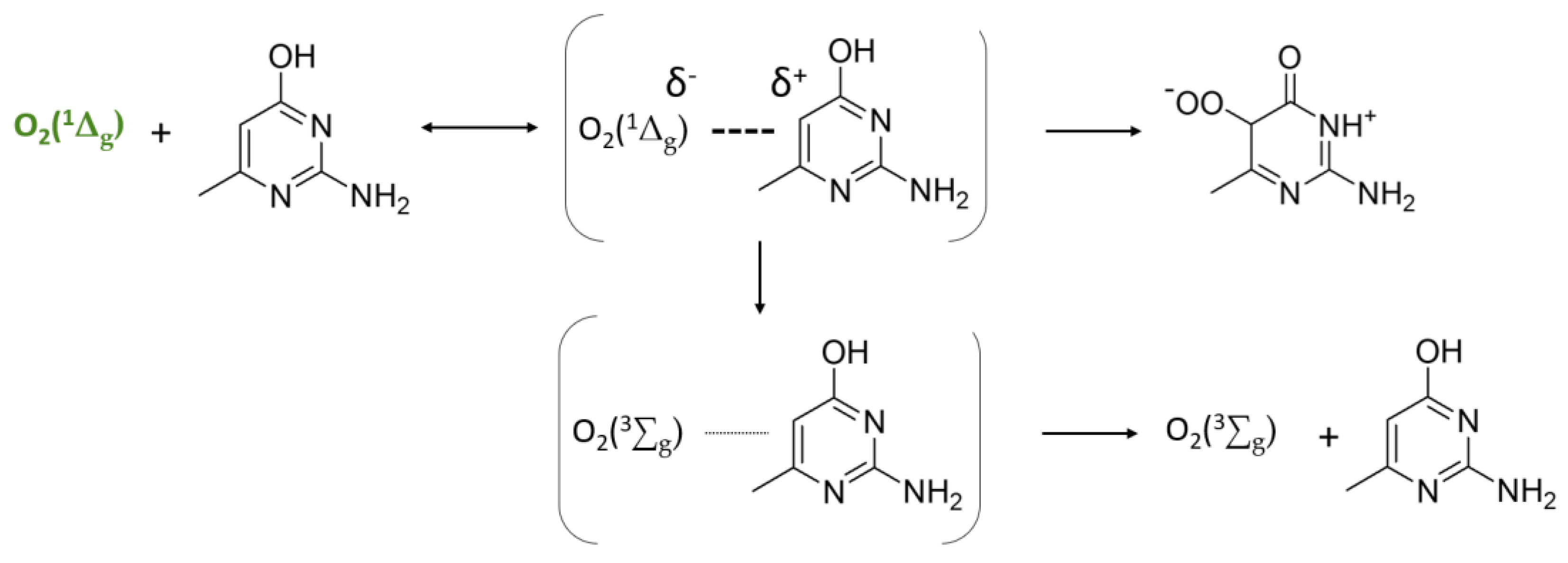
| Photosensitizer | Water (pH = 7) | Water (pH = 11) | |
|---|---|---|---|
| P2 | Conversion (%) | 3 | 64 |
| kobs (10−4 h−1) | 7.4 | 214 | |
| P3 | Conversion (%) | 12 | 76 |
| kobs (10−4 h−1) | 27.5 | 294 | |
| P4 | Conversion (%) | 13 | 84 |
| kobs (10−4 h−1) | 30.1 | 385 | |
| Polymer | Photo-Oxidation Reaction of AHMPD at pH = 11 | Photo-Oxidation Reaction of AHMPD at pH = 7 | Quantum Yield in Singlet Oxygen Generation Measured with ADPA Reaction | ||||
|---|---|---|---|---|---|---|---|
| Maximum Conversion | Normalized Relative Effectiveness | Maximum Conversion | Normalized Relative Effectiveness | Maximum Conversion | Normalized Relative Effectiveness | kobs (10−4 min−1) | |
| Darkness | 0% | - | 0% | - | 0% | - | - |
| P1 | 0% | - | 0% | - | 0% | - | - |
| P2 | 64% | 0.75 | 3% | 0.23 | 85% | 0.85 | 432 |
| P3 | 76% | 0.89 | 12% | 0.92 | >99% | 1.00 | 635 |
| P4 | 84% | 1.00 | 13% | 1.00 | >99% | 1.00 | 640 |
| Photosensitizer | Sample 1 | Sample 2 | |
|---|---|---|---|
| Darkness | Concentration of AHMPD at t = 0 h | 0.027 ppm | 0.016 ppm |
| Degradation at t = 48 h | 0% | 0% | |
| P3 | kobs (10−4 h−1) | 289 ± 12 | 292 ± 7 |
| Degradation at t = 48 | 75 ± 2% | 75 ± 1% | |
| P4 | kobs (10−4 h−1) | 391 ± 10 | 383 ± 8 |
| Degradation at t = 48 | 85 ± 1% | 84 ± 1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabregat, V. Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents. Appl. Sci. 2024, 14, 6308. https://doi.org/10.3390/app14146308
Fabregat V. Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents. Applied Sciences. 2024; 14(14):6308. https://doi.org/10.3390/app14146308
Chicago/Turabian StyleFabregat, Víctor. 2024. "Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents" Applied Sciences 14, no. 14: 6308. https://doi.org/10.3390/app14146308
APA StyleFabregat, V. (2024). Enhancing Emerging Pollutant Removal in Industrial Wastewater: Validation of a Photocatalysis Technology in Agri-Food Industry Effluents. Applied Sciences, 14(14), 6308. https://doi.org/10.3390/app14146308







