Concrete Gas Permeability: Implications for Hydrogen Storage Applications
Abstract
:1. Introduction
Concrete Permeability
- The porosity of the material and its structure: the volume, size, and distribution of the pores regulate the rate at which it absorbs liquids, mainly water containing certain aggressors and gases.
- The viscosity of the considered fluid, which is affected by its temperature.
- The pressure to which the fluid is subjected.
- Calcium silicate hydrate (C-S-H): It is the main hydration product of Portland cement and forms through the reaction of cement with water. This gel is amorphous and extends throughout the concrete matrix, filling the pores and voids of the material.
- Calcium hydroxide (CH): This is another common hydration product formed through the reaction of cement with water. CH is crystalline and is deposited in the pores and voids of concrete, where it can react with other compounds to form additional products. It gives the concrete a highly alkaline character, ranging from pH 12 to 13 (protective for reinforcement).
- Ettringite: This is a compound of calcium sulfate hydrate and aluminum that is formed when sulfate-containing cement is used. This product can be deposited in the pores and voids of concrete, contributing to the formation of the material’s crystalline structure.
- Hydrated silica: This is a compound that forms through the reaction of cement with silica. This hydration product is deposited in the pores and voids of concrete, contributing to its strength and durability.
2. Experimental Development
2.1. Materials
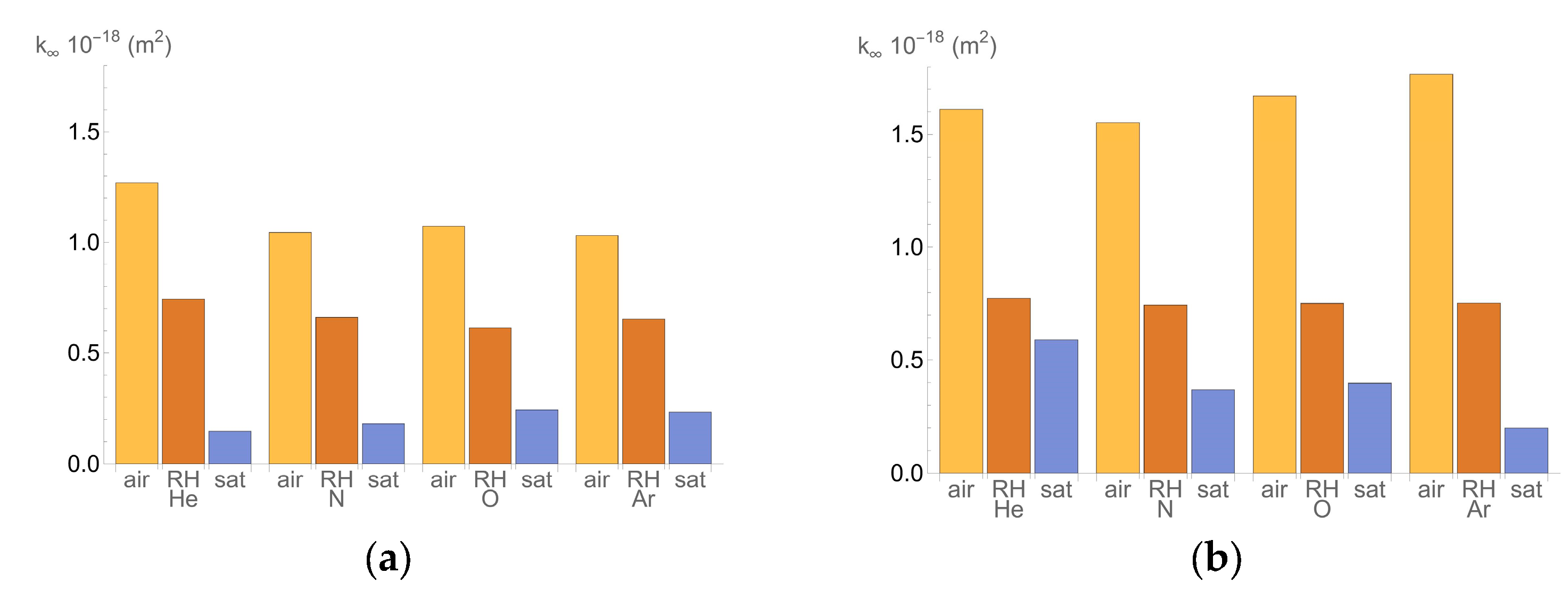
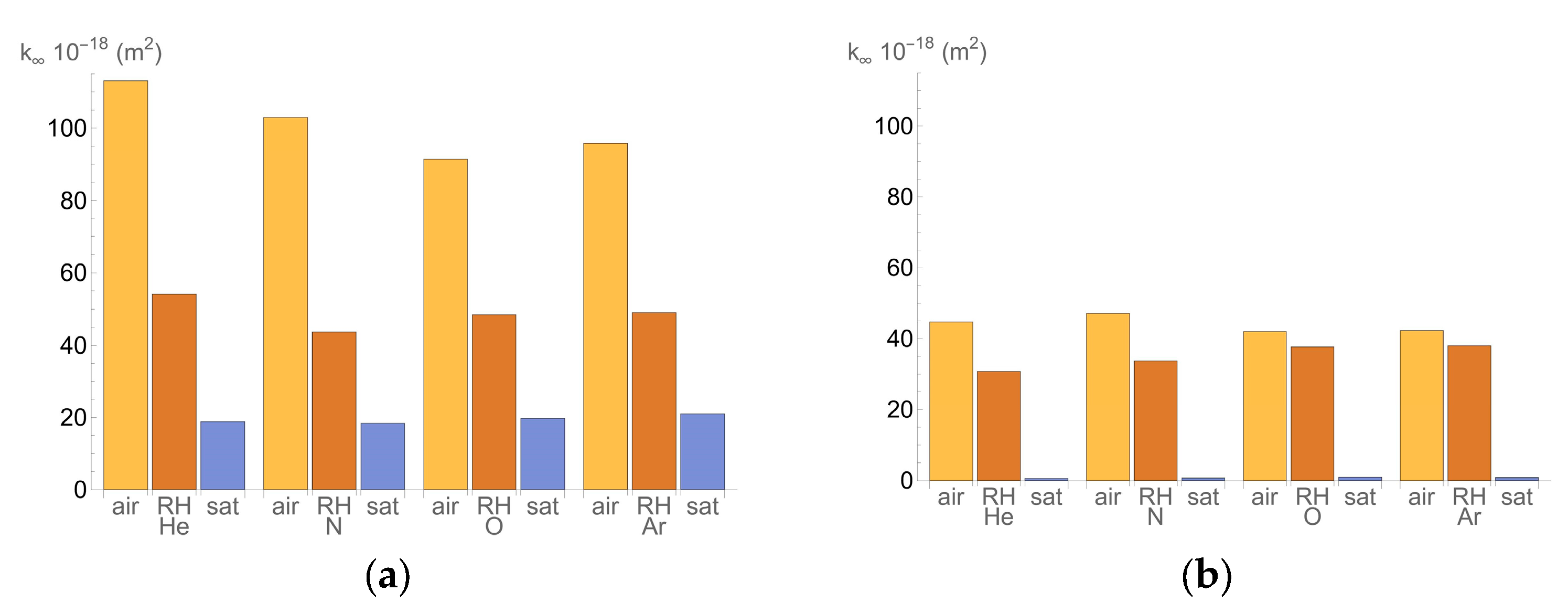
- Air: Specimens were placed in the oven at 50 °C, as described in the UNE 83966 procedure [32], and weighed daily until there was no further mass variation. They were then left under laboratory conditions (20 ± 2 °C and relative humidity above 45%) for two to four days before testing.
- Controlled relative humidity (RH): specimens conditioned to 65% RH.
- Saturated: specimens were submerged in water and weighed daily until there was no mass variation (100% RH).
2.2. Techniques and Normative
- Permeability Measurement: The permeability of hardened concrete to oxygen has been determined following the procedure specified in the UNE 83981 standard. Additionally, three other gases (helium, nitrogen, and argon) have been tested to observe any significant differences between them.
- Preparation and Curing of Specimens: the preparation and curing of concrete specimens for resistance tests have been carried out according to the UNE-EN 12390-2:2020 standard [33].
- Conditioning of Concrete Specimens: the UNE 83966 standard has been used to condition the concrete specimens for gas and capillarity permeability tests.
- Resistivity Measurement: the resistivity of the concrete specimens has been measured directly, following the UNE-EN 12390-19:2023 [34].
- Ultrasonic Testing: ultrasonic testing has been conducted as per the UNE-EN 12504-4:2022 standard [35].
2.3. Procedures
| Gas | Viscosity (10−5 Pa·s) | Atomic Mass (g/mol) | Density (g/mL) |
|---|---|---|---|
| Hydrogen | 0.8400 | 1.00797 | 0.071 |
| Helium | 1.9460 | 4.0026 | 0.126 |
| Nitrogen | 1.7660 | 14.0067 | 0.810 |
| Oxygen | 2.0260 | 15.9994 | 1.429 |
| Argon | 2.2150 | 39.9480 | 1.400 |
- K is the permeability coefficient of oxygen (m2);
- Q is the gas flow through the specimen (m3/s);
- p0 is the pressure at which Q (m3/s) is determined (considered equal to pa) (N/m2);
- L is the thickness of the specimen (m);
- ⴄ is the viscosity of the gas (N·s/m2);
- A is the cross-sectional area of the specimen (m2);
- p is the pressure applied during the test (N/m2);
- pa is the atmospheric pressure (N/m2).
- is the distance between each of the four equally spaced electrodes (m);
- is the geometry factor for semi-infinite concrete elements, expressed as a non-dimensional parameter;
- is the electrical resistance (Ω);
- is the cross-section area of the sample (m2);
- is the height of the specimen or the distance between electrodes (m).
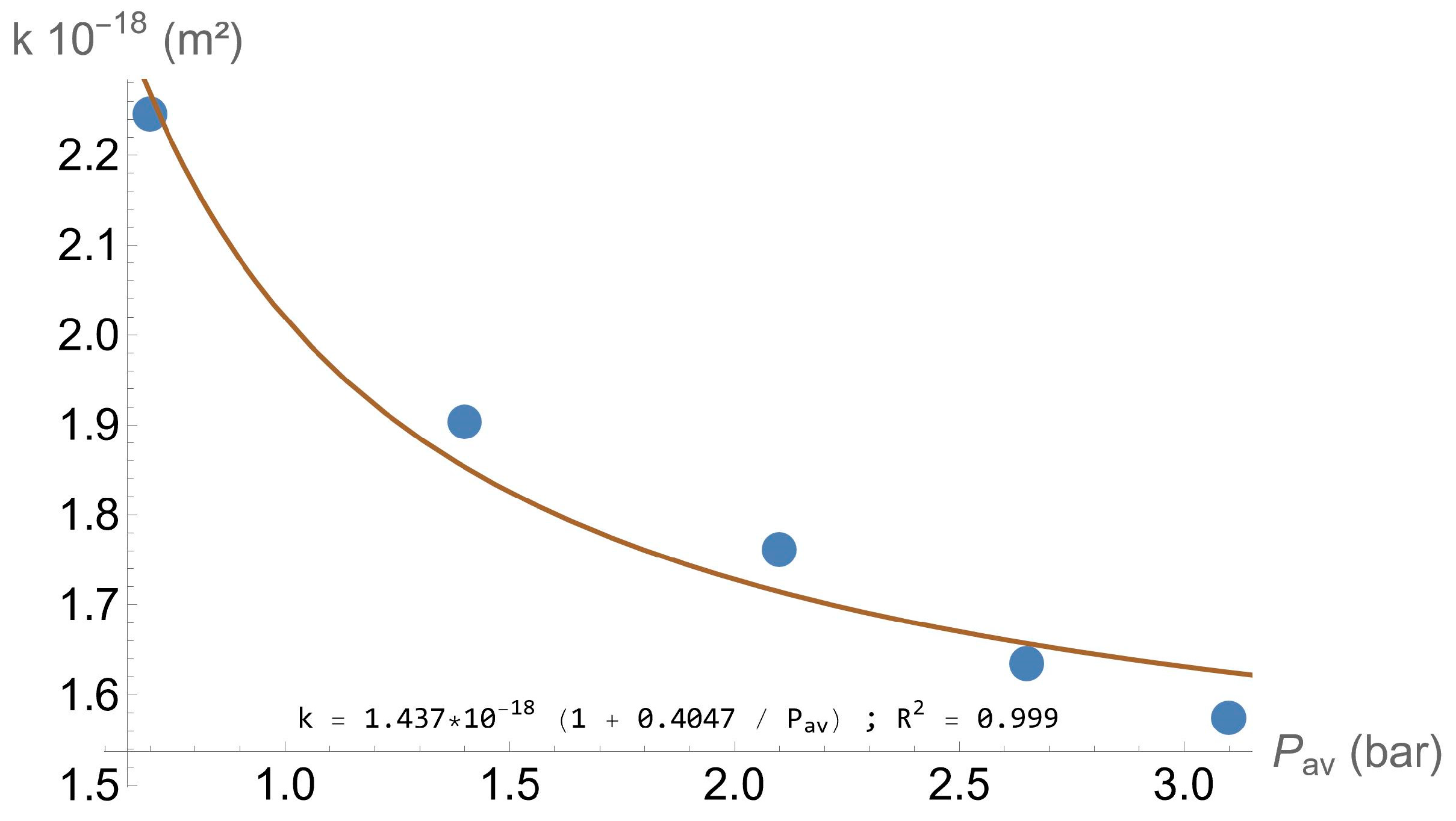
2.4. Permeability Calculation
- k∞ is the permeability when the pressure is sufficiently high (m2);
- b is an adjustment parameter, expressed as a non-dimensional parameter;
- p is the pressure applied during the test (N/m2);
- pa is the atmospheric pressure (N/m2);
- Pav is the average pressure (N/m2).
- εp is the porosity of the concrete, expressed as a non-dimensional parameter;
- ρ is the gas density (kg/m3);
- k is the permeability, experimentally obtained (m2);
- u is the flow velocity of the gas (m/s);
- µ is the gas viscosity (N·s/m2).
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajda, D.; Lutyński, M. Hydrogen Permeability of Epoxy Composites as Liners in Lined Rock Caverns—Experimental Study. Appl. Sci. 2021, 11, 3885. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Gajda, D.; Lutyński, M. Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage. Energies 2022, 15, 2663. [Google Scholar] [CrossRef]
- Amirthan, T.; Perera, M. Underground hydrogen storage in Australia: A review on the feasibility of geological sites. Int. J. Hydrogen Energy 2023, 48, 4300–4328. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Peng, T.; Wan, J.; Liu, W.; Li, J.; Xia, Y.; Yuan, G.; Jurado, M.J.; Fu, P.; He, Y.; Liu, H. Choice of hydrogen energy storage in salt caverns and horizontal cavern construction technology. J. Energy Storage 2023, 60, 106489. [Google Scholar] [CrossRef]
- Harris, A.; Atkinson, A.; Claisse, P. Transport of gases in concrete barriers. Waste Manag. 1992, 12, 155–178. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Jung, H.; Ha, J.-C.; Kim, E.-H. Gas threshold pressure and gas permeability of silo concrete specimens for a low- and intermediate-level waste disposal facility in Korea. Ann. Nucl. Energy 2013, 55, 1–8. [Google Scholar] [CrossRef]
- Scott, D.S.; Dullien, F.A.L. Diffusion of ideal gases in capillaries and porous solids. AIChE J. 1962, 8, 113–117. [Google Scholar] [CrossRef]
- Hamami, A.; Turcry, P.; Aït-Mokhtar, A. Influence of mix proportions on microstructure and gas permeability of cement pastes and mortars. Cem. Concr. Res. 2012, 42, 490–498. [Google Scholar] [CrossRef]
- Heede, P.V.D.; Gruyaert, E.; De Belie, N. Transport properties of high-volume fly ash concrete: Capillary water sorption, water sorption under vacuum and gas permeability. Cem. Concr. Compos. 2010, 32, 749–756. [Google Scholar] [CrossRef]
- Zhang, D.; Li, K. Concrete gas permeability from different methods: Correlation analysis. Cem. Concr. Compos. 2019, 104, 103379. [Google Scholar] [CrossRef]
- Issaadi, N.; Hamami, A.; Belarbi, R.; Aït-Mokhtar, A. Experimental assessment of the variability of concrete air permeability: Repeatability, reproducibility and spatial variability. Energy Procedia 2017, 139, 537–543. [Google Scholar] [CrossRef]
- Boumaaza, M.; Huet, B.; Pham, G.; Turcry, P.; Aït-Mokhtar, A.; Gehlen, C. A new test method to determine the gaseous oxygen diffusion coefficient of cement pastes as a function of hydration duration, microstructure, and relative humidity. Mater. Struct. Mater. Constr. 2018, 51, 51. [Google Scholar] [CrossRef]
- Lamouchi, T.; Levasseur, S.; Potier, L.; Dubois, T.; Skoczylas, F. About Gas Permeability and Diffusion through Concrete. Mater. Proc. 2023, 13, 42. [Google Scholar] [CrossRef]
- Chao, Z.; Gong, B.; Yue, W.; Xu, X.; Shi, D.; Yang, C.; Hu, T. Experimental study on stress-dependent gas permeability and porosity of artificially cracked cement mortar. Constr. Build. Mater. 2022, 359, 129290. [Google Scholar] [CrossRef]
- Qian, R.; Liu, C.; Liu, G.; Liu, Z.; Pang, B.; She, W.; Zhang, Y. Effects of various inlet-gas mediums on apparent permeability of concrete under steady-state flow: Comparison between carbon-dioxide and oxygen. Cem. Concr. Compos. 2021, 119, 103995. [Google Scholar] [CrossRef]
- Rosti, M.E.; Cortelezzi, L.; Quadrio, M. Direct numerical simulation of turbulent channel flow over porous walls. J. Fluid Mech. 2014, 784, 396–442. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Abdelhady, A.; Yang, J.; Wang, H. Effects of specimen shape and size on the permeability and mechanical properties of porous concrete. Constr. Build. Mater. 2021, 266, 121074. [Google Scholar] [CrossRef]
- Abbas, A.; Carcasses, M.; Ollivier, A.-P. Gas permeability of concrete in relation to its degree of saturation. Mater. Struct./Mater. Constr. 1999, 32, 3–8. [Google Scholar] [CrossRef]
- Jooss, M.; Reinhardt, H.W. Permeability and diffusivity of concrete as function of temperature. Cem. Concr. Res. 2002, 32, 1497–1504. [Google Scholar] [CrossRef]
- Andrade, C.; Bettencourt-Ribeiro, A.; Buenfeld, N.R.; Carcasses, M.; Carino, N.J.; Ehrenberg, F.; Ewertson, C.; Garbocczi, E.; Geiker, M.; Gjorv, O.E.; et al. Permeability of Concrete as a Criterion of its Durability. Mater. Struct. 1999, 32, 174–179. [Google Scholar]
- Yiğiter, H.; Yazıcı, H.; Aydın, S. Effects of cement type, water/cement ratio and cement content on sea water resistance of concrete. Build. Environ. 2007, 42, 1770–1776. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S. Influence of water-to-cement ratio and curing period on pore structure of cement mortar. Constr. Build. Mater. 2013, 38, 804–812. [Google Scholar] [CrossRef]
- Illangakoon, G.B.; Asamoto, S.; Nanayakkara, A.; Trong, L.N. Concrete cold joint formation in hot weather conditions. Constr. Build. Mater. 2019, 209, 406–415. [Google Scholar] [CrossRef]
- Yoo, S.-W.; Kwon, S.-J. Effects of cold joint and loading conditions on chloride diffusion in concrete containing GGBFS. Constr. Build. Mater. 2016, 115, 247–255. [Google Scholar] [CrossRef]
- Kishen, J.C.; Rao, P.S. Fracture of cold jointed concrete interfaces. Eng. Fract. Mech. 2007, 74, 122–131. [Google Scholar] [CrossRef]
- UNE 83981; Concrete Durability. Test Methods. Determination to Gas Permeability of Hardened Concrete. AENOR: Madrid, Spain, 2008.
- Jung, J.K.; Lee, J.H.; Jang, J.S.; Chung, N.K.; Park, C.Y.; Baek, U.B.; Nahm, S.H. Characterization technique of gases permeation properties in polymers: H2, He, N2 and Ar gas. Sci. Rep. 2022, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Care, S.; Derkx, F. Determination of relevant parameters influencing gas permeability of mortars. Constr. Build. Mater. 2011, 25, 1248–1256. [Google Scholar] [CrossRef]
- Dutzer, V.; Dridi, W.; Poyet, S.; Le Bescop, P.; Bourbon, X. The link between gas diffusion and carbonation in hardened cement pastes. Cem. Concr. Res. 2019, 123, 105795. [Google Scholar] [CrossRef]
- UNE 83966; Concrete Durability. Test Methods. Conditioning of Concrete Test Pieces for the Purpose of Gas Permeability and Capilar Suction Tests. AENOR: Madrid, Spain, 2008.
- UNE-EN 12390-2; Testing Hardened Concrete—Part 2: Making and Curing Specimens for Strength Tests. AENOR: Madrid, Spain, 2020.
- UNE-EN 12390-19; Testing of Hardened Concrete—Part 19: Determination of Electrical Resistivity. AENOR: Madrid, Spain, 2023.
- UNE-EN 12504-4:2022; Testing Concrete in Structures—Part 4: Determination of Ultrasonic Pulse Velocity. AENOR: Madrid, Spain, 2022.
- Franco-Luján, V.A.; Maldonado-García, M.A.; Jiménez-Quero, V.G.; Montes-García, P. Reliability of electrical resistivity on the long-term monitoring of concrete. Results Eng. 2023, 18, 101154. [Google Scholar] [CrossRef]
- Aragoncillo, A.M.M.; Cleary, D.B.; Lomboy, G.R. Estimating the permeability of porous aggregate concretes using electrical resistivity based tests. Constr. Build. Mater. 2023, 364, 129909. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Electrical Resistivity of Concrete for Durability Evaluation: A Review. Adv. Mater. Sci. Eng. 2017, 2017, 8453095. [Google Scholar] [CrossRef]
- Cheytani, M.; Chan, S. The applicability of the Wenner method for resistivity measurement of concrete in atmospheric conditions. Case Stud. Constr. Mater. 2021, 15, e00663. [Google Scholar] [CrossRef]
- Fitzka, M.; Karr, U.; Granzner, M.; Melichar, T.; Rödhammer, M.; Strauss, A.; Mayer, H. Ultrasonic fatigue testing of concrete. Ultrasonics 2021, 116, 106521. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.A.D.N.; Moreno, A.L.; Ferreira, G.C.d.S. Ultrasonic testing on evaluation of concrete residual compressive strength: A review. Constr. Build. Mater. 2023, 373, 130887. [Google Scholar] [CrossRef]
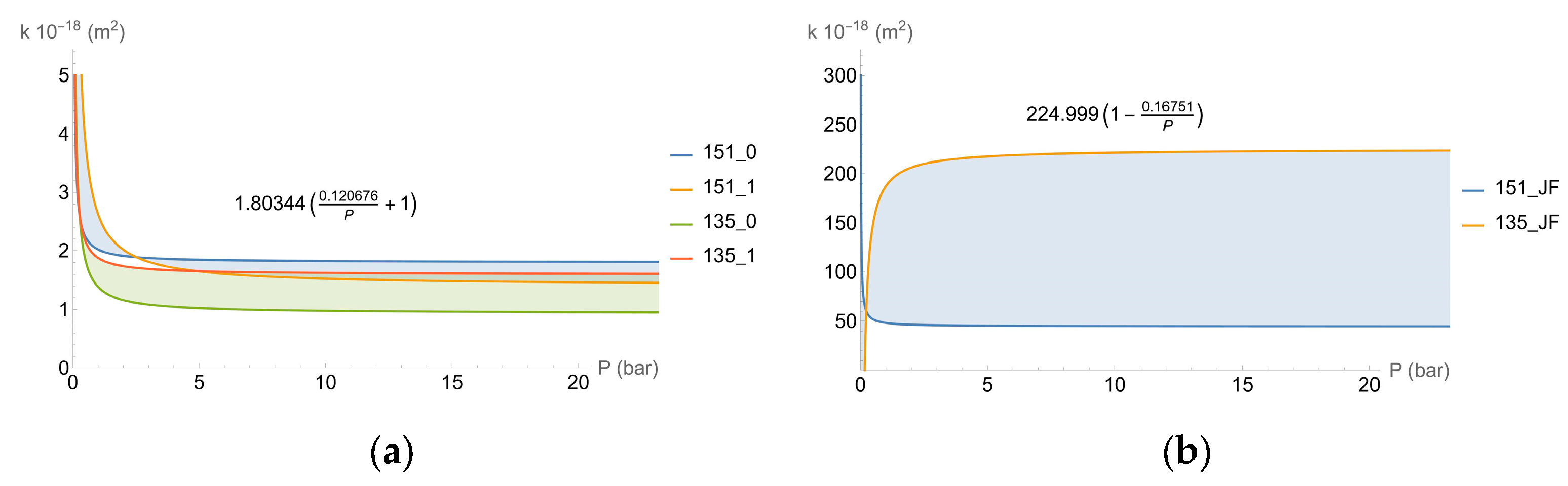

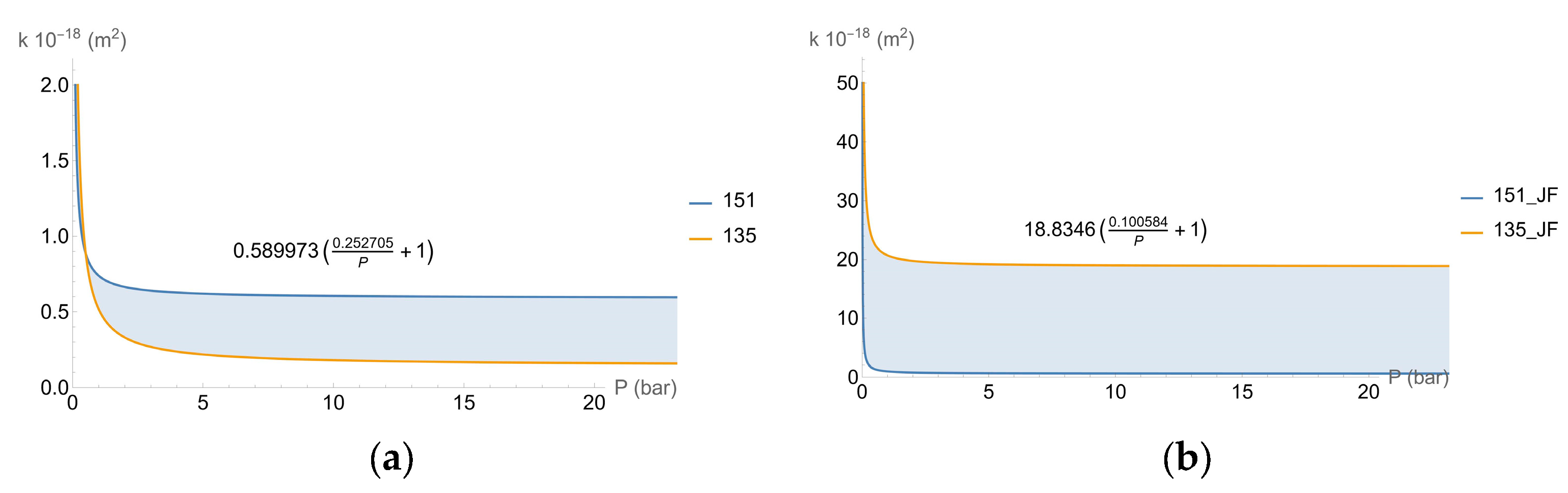
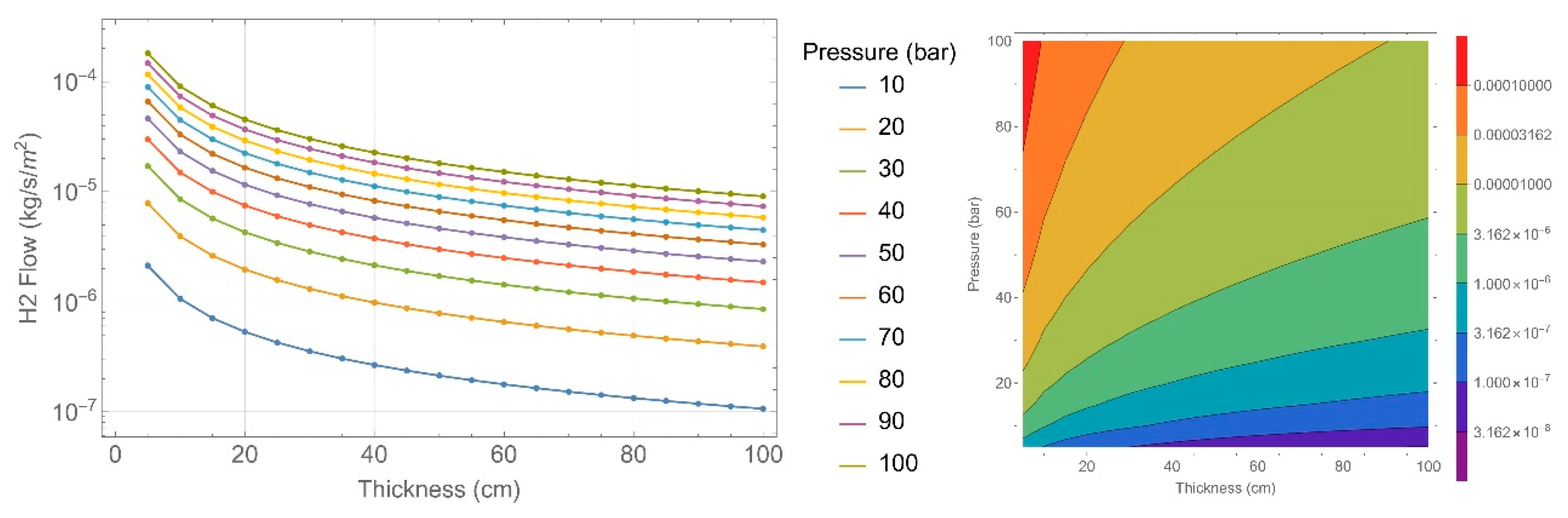

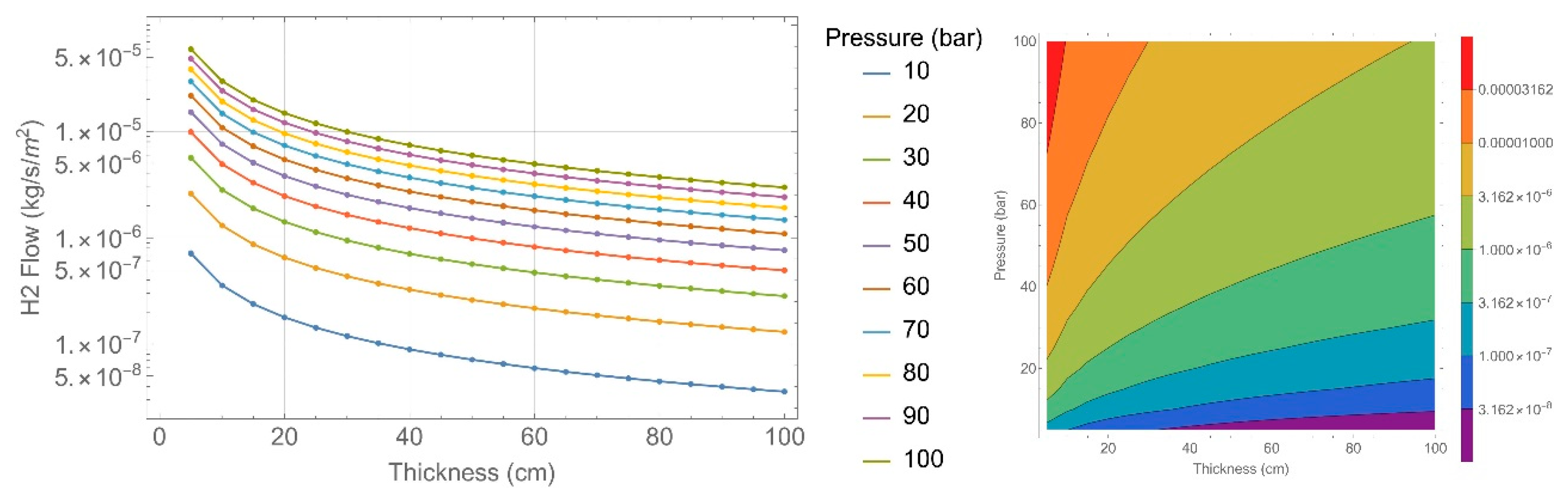
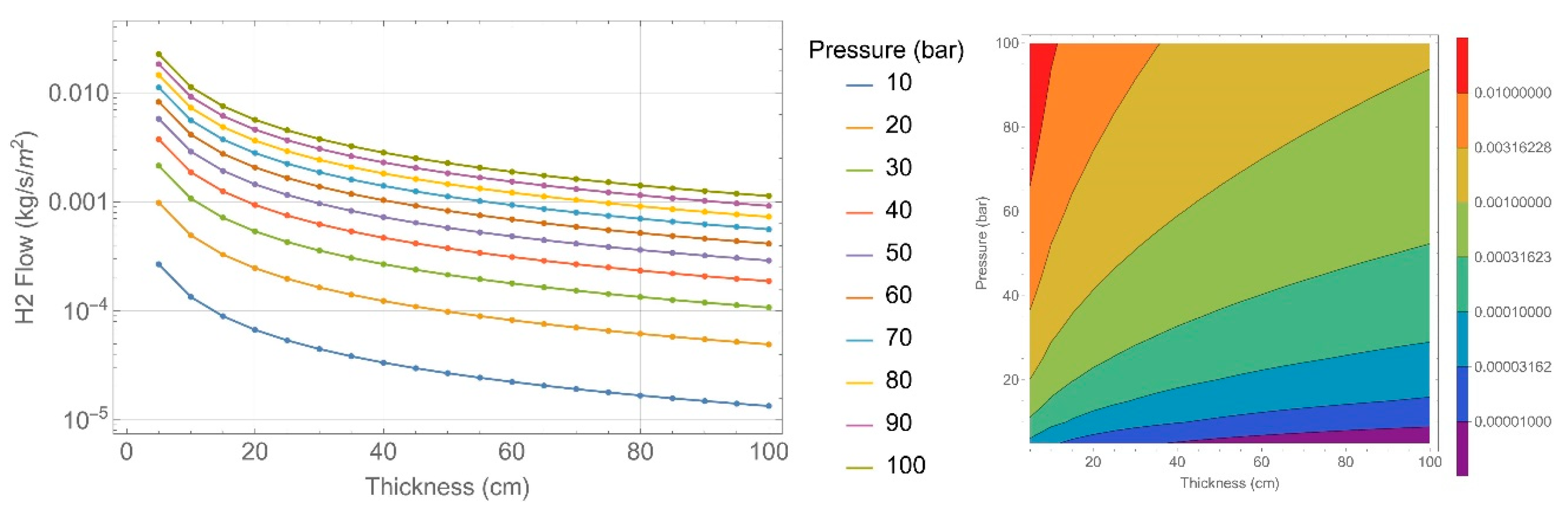
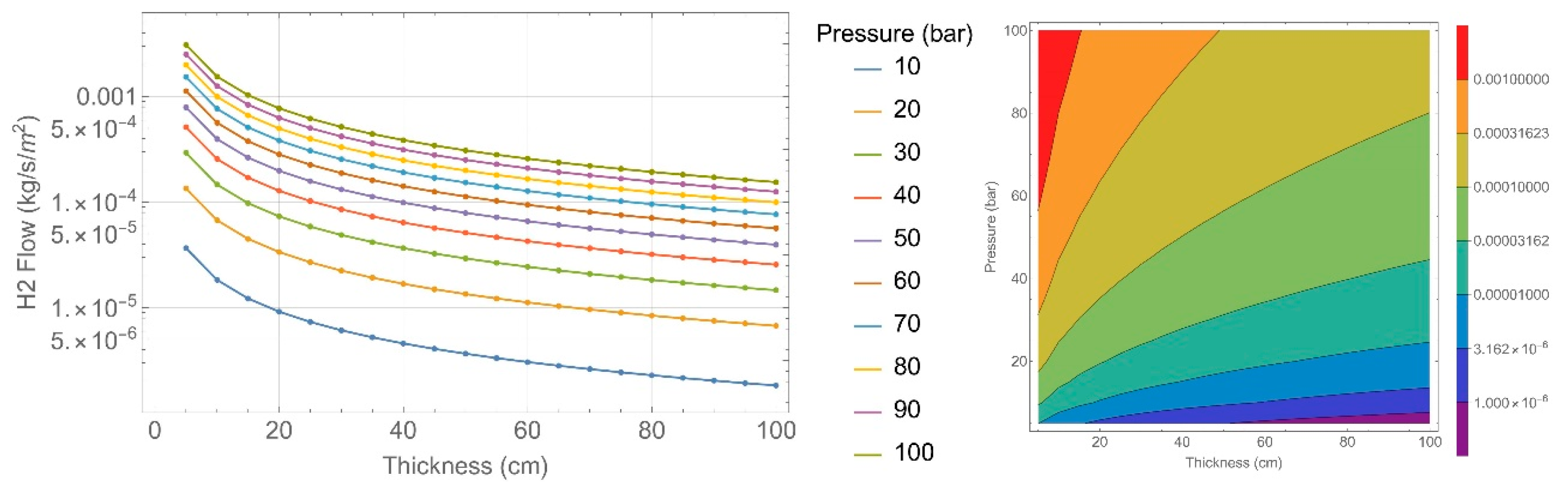
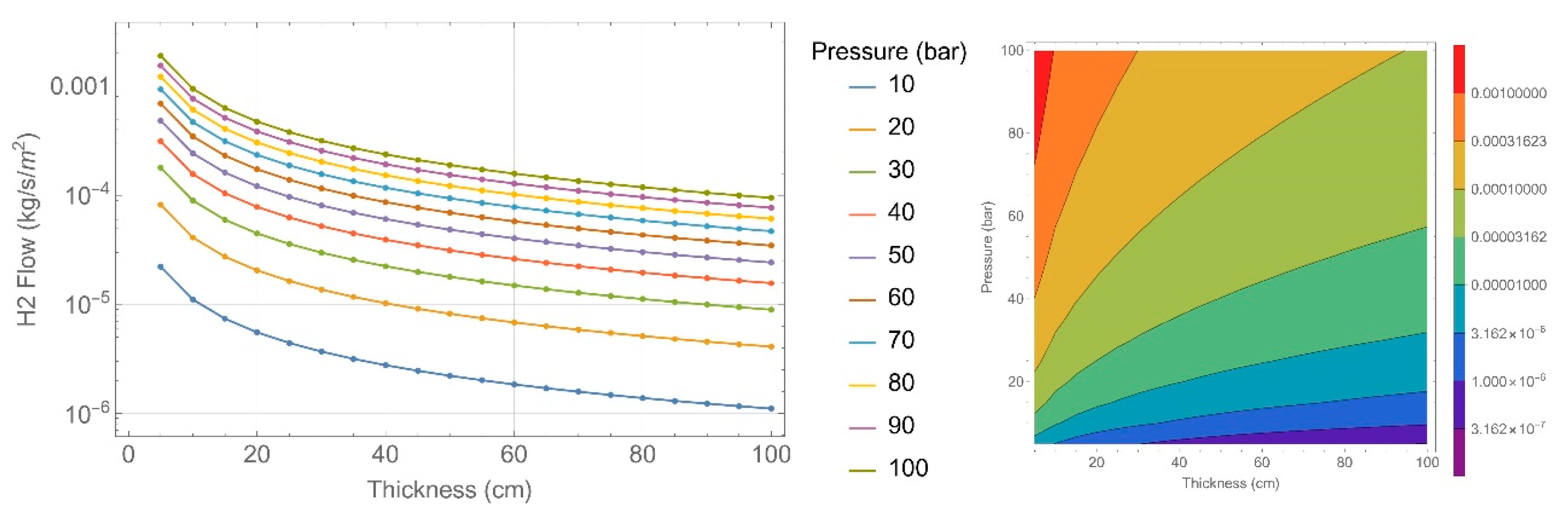
| Concrete Characteristics | 135 Specimens | 151 Specimens |
|---|---|---|
| Type | Concrete for precast elements with high early-age strengths | Slip-form concrete |
| Cement | CEM III/A 42.5 N/SRC | CEM II/A-S 42.5 R/SRC |
| Slump test | 18 cm | 22 cm |
| Cement | 425 kg/m3 | 385 kg/m3 |
| W/C ratio | 0.35 | 0.40 |
| Density | 2383 kg/m3 | 2323 kg/m3 |
| 28-day compressive strength | 62 MPa | 47 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu Araujo, L.; Rebolledo Ramos, N.; Torres Martín, J.E.; Chinchón-Payá, S.; Sánchez Montero, J.; Lample Carreras, R.M.; Vera-Agullo, J.; Jimenez-Vicaria, J.D. Concrete Gas Permeability: Implications for Hydrogen Storage Applications. Appl. Sci. 2024, 14, 6408. https://doi.org/10.3390/app14156408
Abreu Araujo L, Rebolledo Ramos N, Torres Martín JE, Chinchón-Payá S, Sánchez Montero J, Lample Carreras RM, Vera-Agullo J, Jimenez-Vicaria JD. Concrete Gas Permeability: Implications for Hydrogen Storage Applications. Applied Sciences. 2024; 14(15):6408. https://doi.org/10.3390/app14156408
Chicago/Turabian StyleAbreu Araujo, Luana, Nuria Rebolledo Ramos, Julio Emilio Torres Martín, Servando Chinchón-Payá, Javier Sánchez Montero, Rosa Maria Lample Carreras, Jose Vera-Agullo, and Jose David Jimenez-Vicaria. 2024. "Concrete Gas Permeability: Implications for Hydrogen Storage Applications" Applied Sciences 14, no. 15: 6408. https://doi.org/10.3390/app14156408






