Sustainable Energy Solutions: Utilising UGS for Hydrogen Production by Electrolysis
Abstract
:1. Introduction
2. Methodology and Concept Description
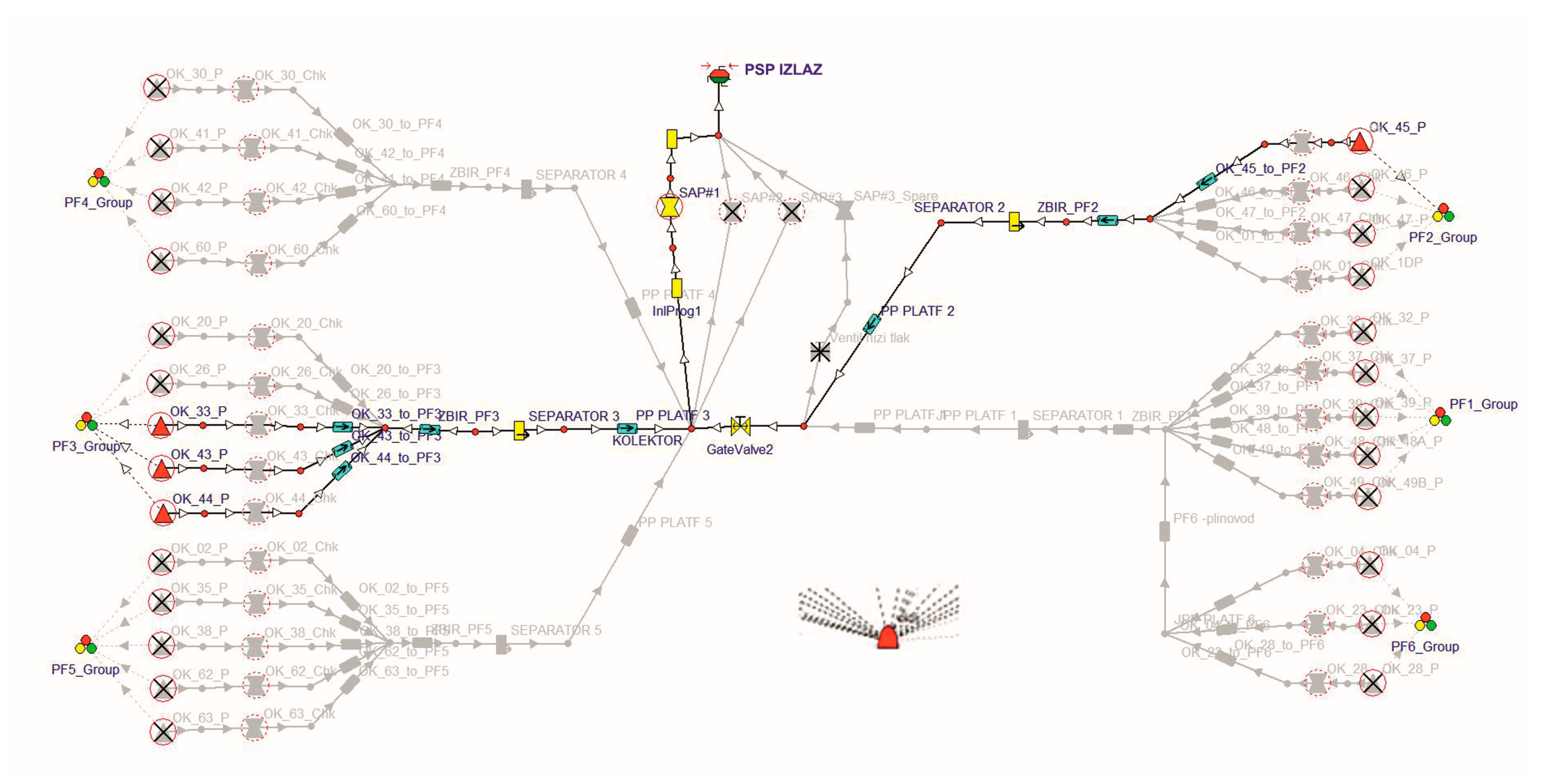
2.1. UGS Facility Description
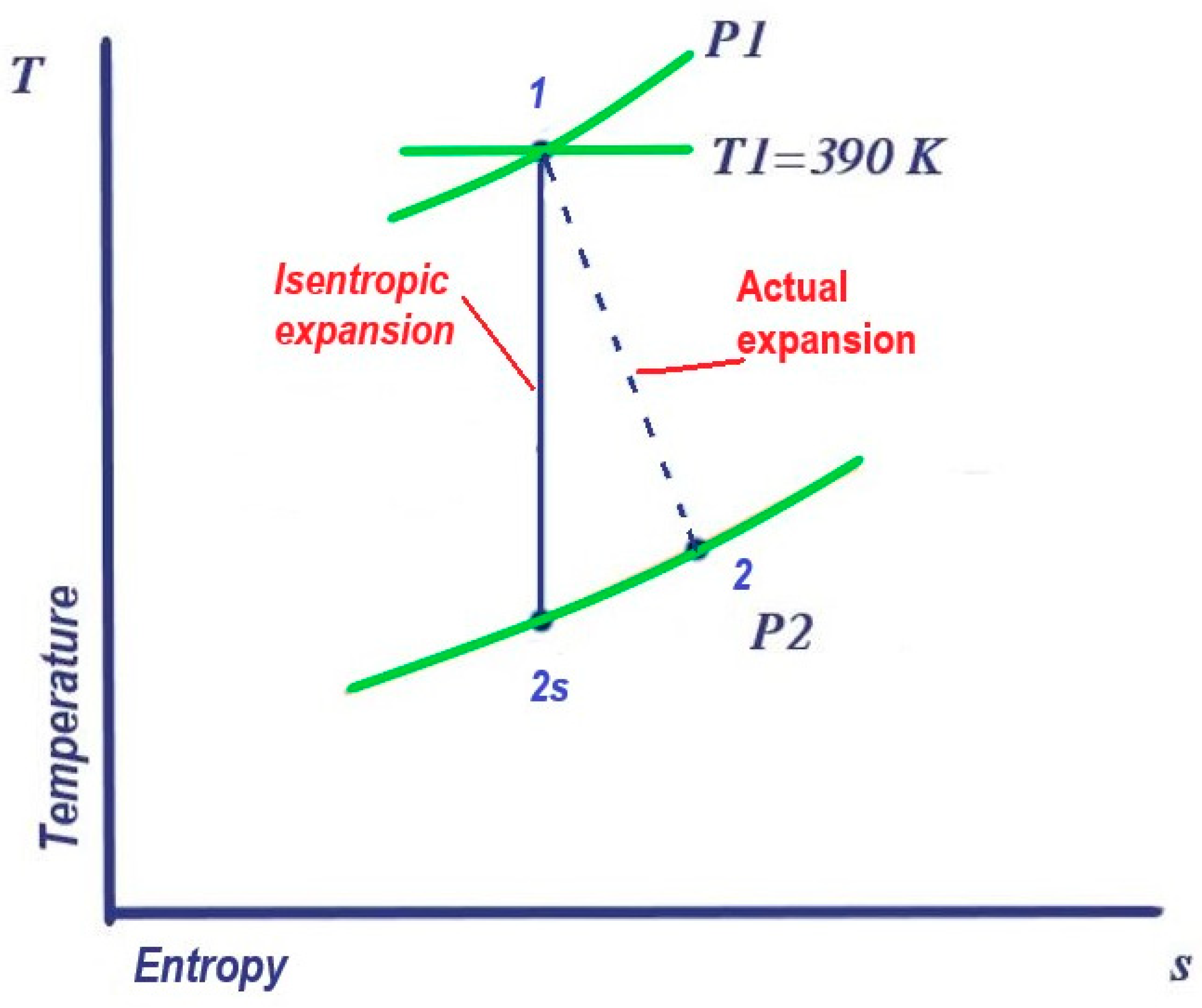
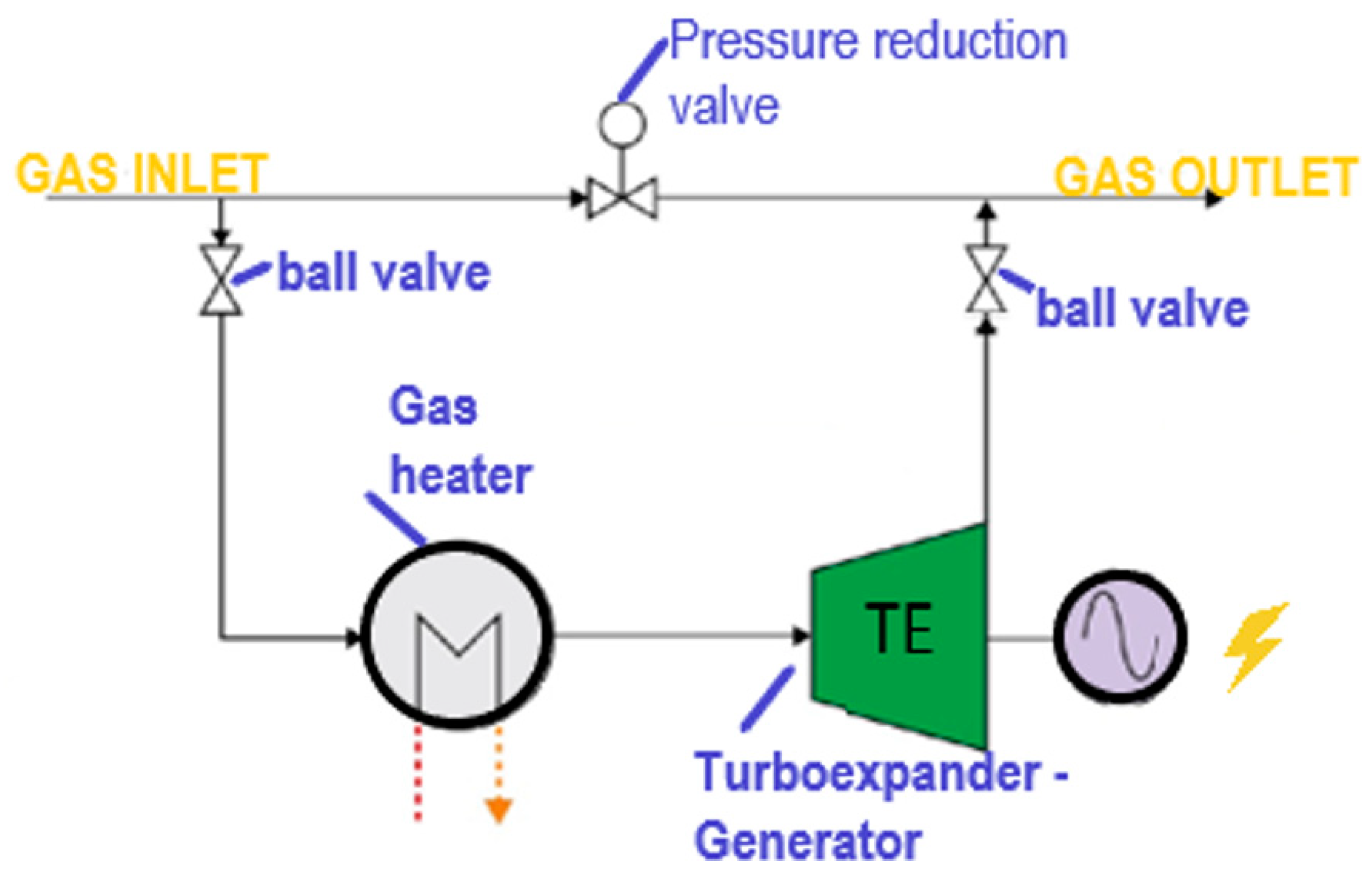
2.2. Exergy Process Description in Pressure Reduction Station at UGS Facility
3. Mathematical Model Description
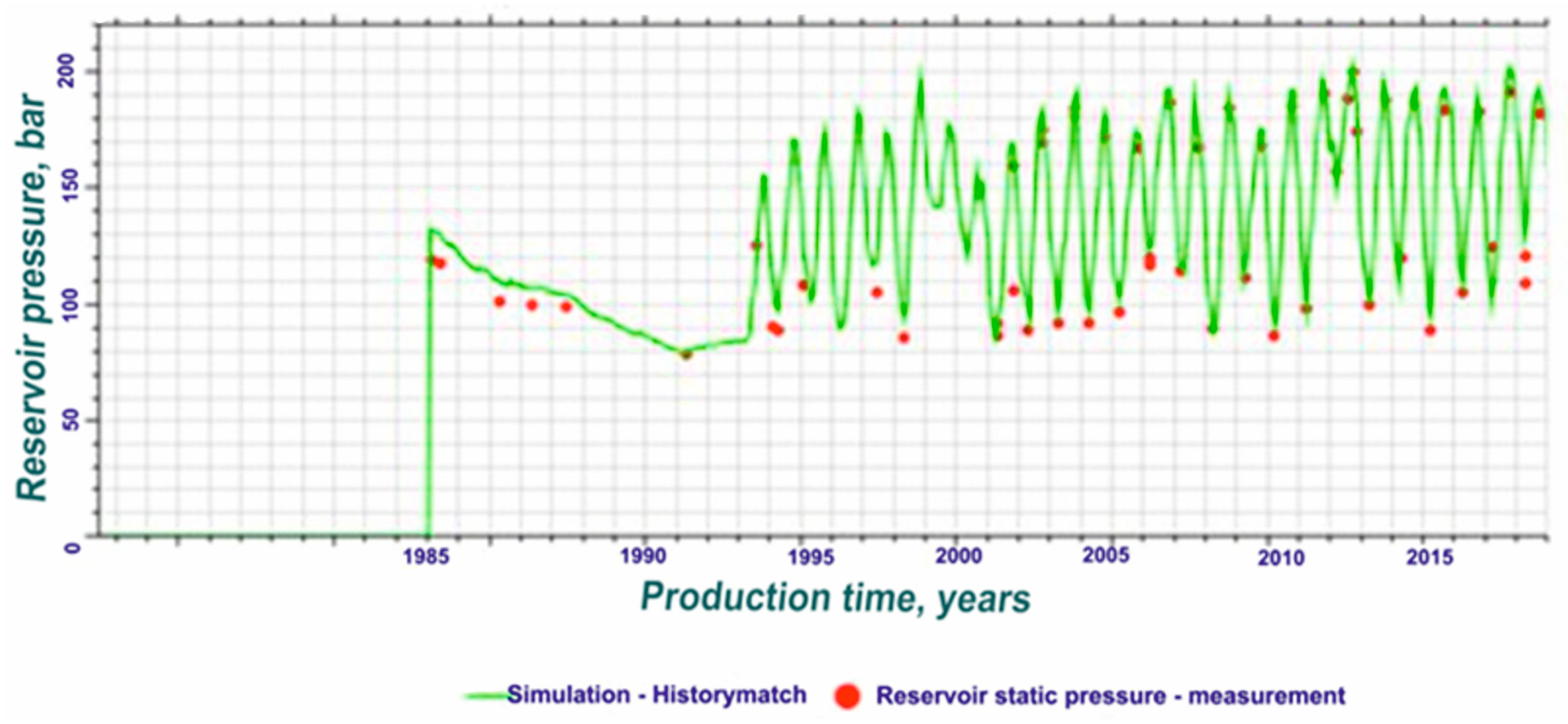
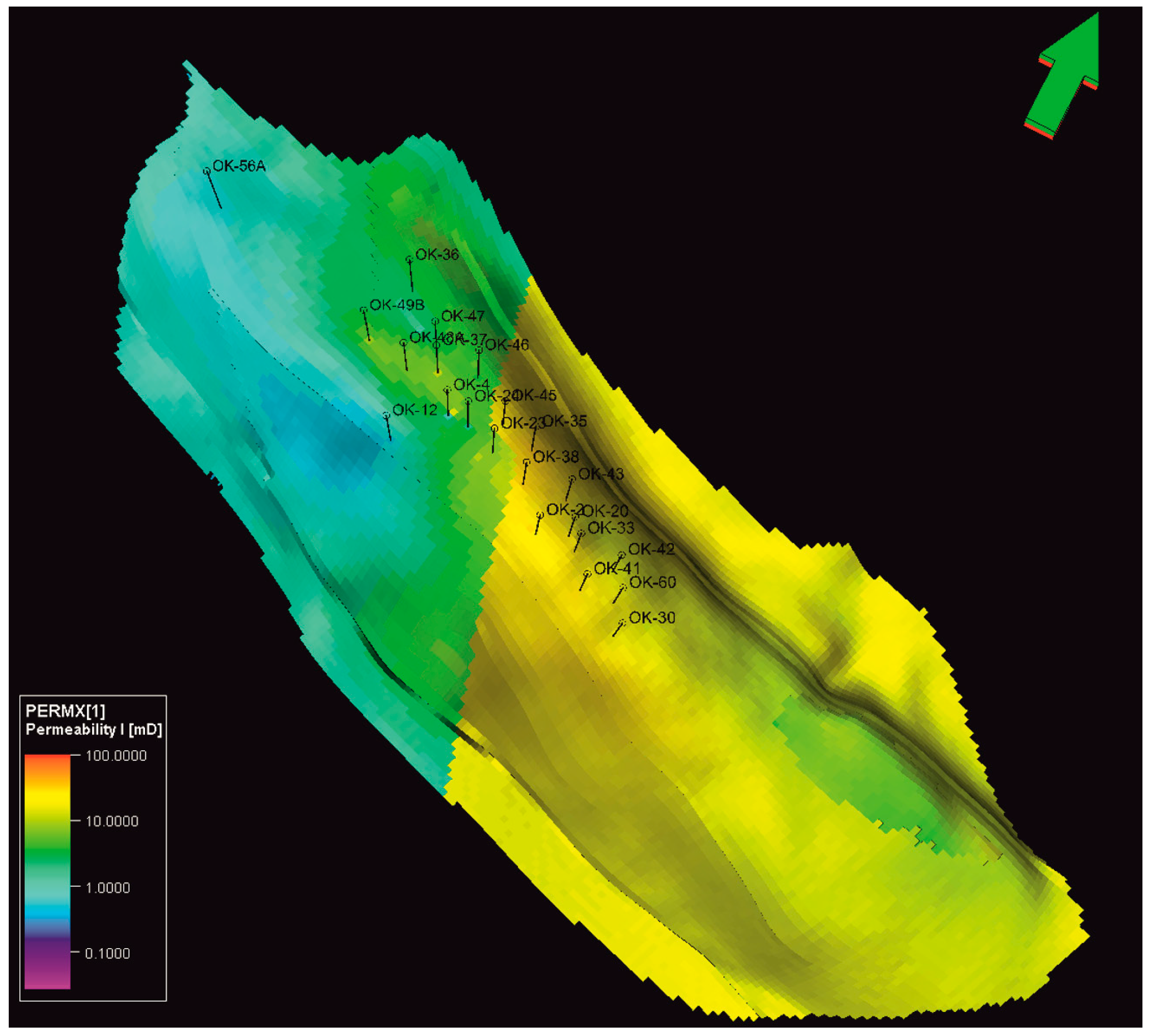
3.1. Reservoir Model Definition
3.2. Pipeline Surface Model and Wells Model
- Pressure gradient kinetic component was negligible;
- Horizontal steady-state flow;
- Pressure and temperature differentials across pipelines were calculated based on mass balances and energy incremental;
- The same pipe material was assumed for all pipes, and heat transfer to the ground was assumed to be at steady state.
3.3. Gas Composition and PVT Model of Reservoir Fluid
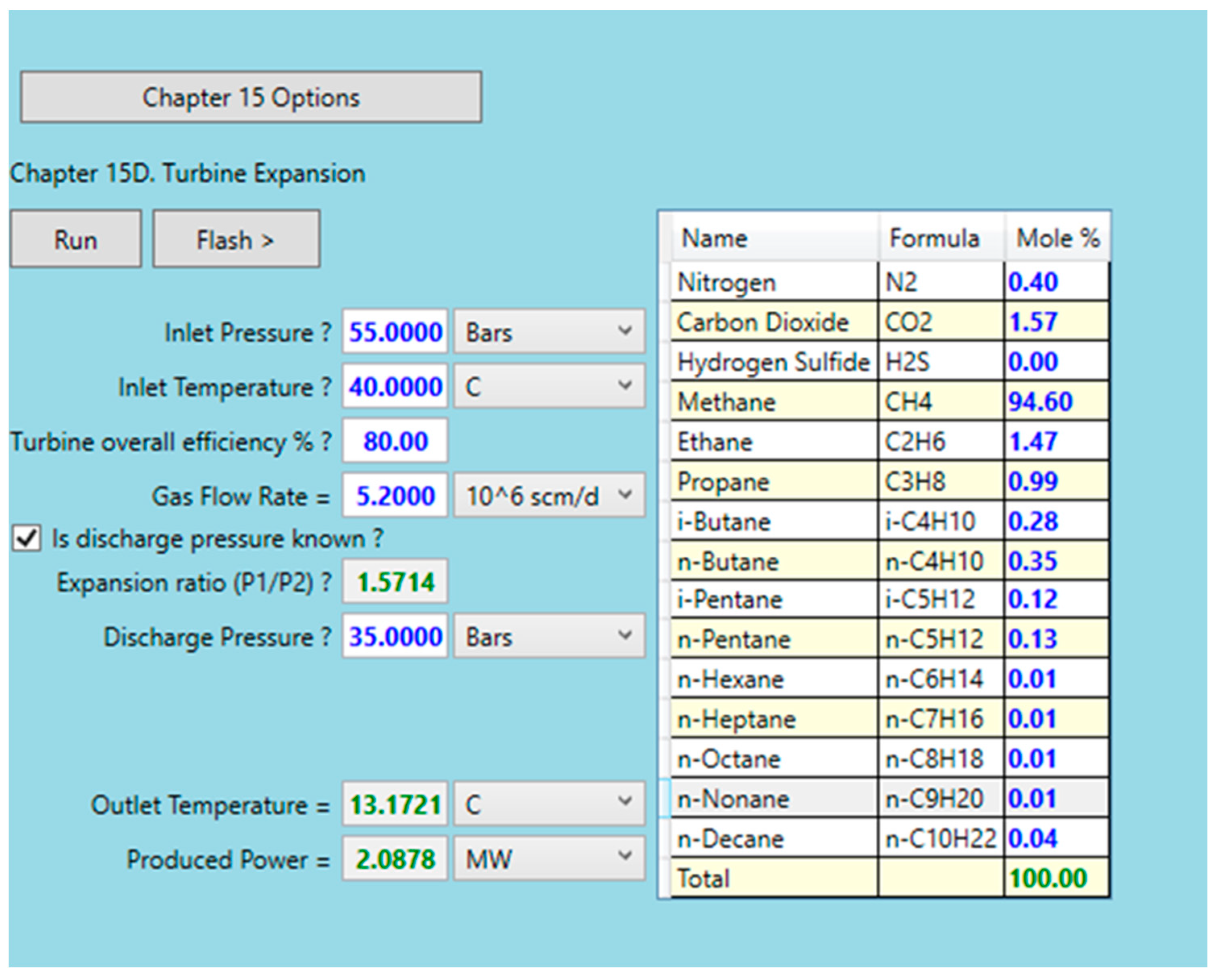
3.4. Integration of Turboexpander in Mathematical Model, and Case Scenario Definition
- Gas inlet pressure, MPa;
- Gas inlet temperature, K;
- Mole fraction of individual gas component, –;
- Gas flow rate, kg/h.
- Last system node pressure (gas separator pressure) = 3 MPa;
- Number of active withdrawal wells = 22;
- Maximum drawdown available at each well = 3 MPa;
- Maximum allowable gas production = 220,000 Sm3/h;
- Fixed pressure upstream of the turboexpander (IGPE) = 5.5 MPa;
- Maximum permitted gas rate for injection = 160,000 m3/h;
- Limit value for withdrawal gas rate = 240,000 m3/h;
- Minimum average reservoir pressure = 8 MPa;
- Maximum average reservoir pressure = 19.6 MPa;
- Minimum permissible pressure at the wellhead = 5.5 MPa;
- Maximum permissible downhole pressure value = 21 MPa;
- The withdrawal cycle began on the first of October and ended at the beginning of April. The injection cycle began on the first of April and ended at the beginning of October.
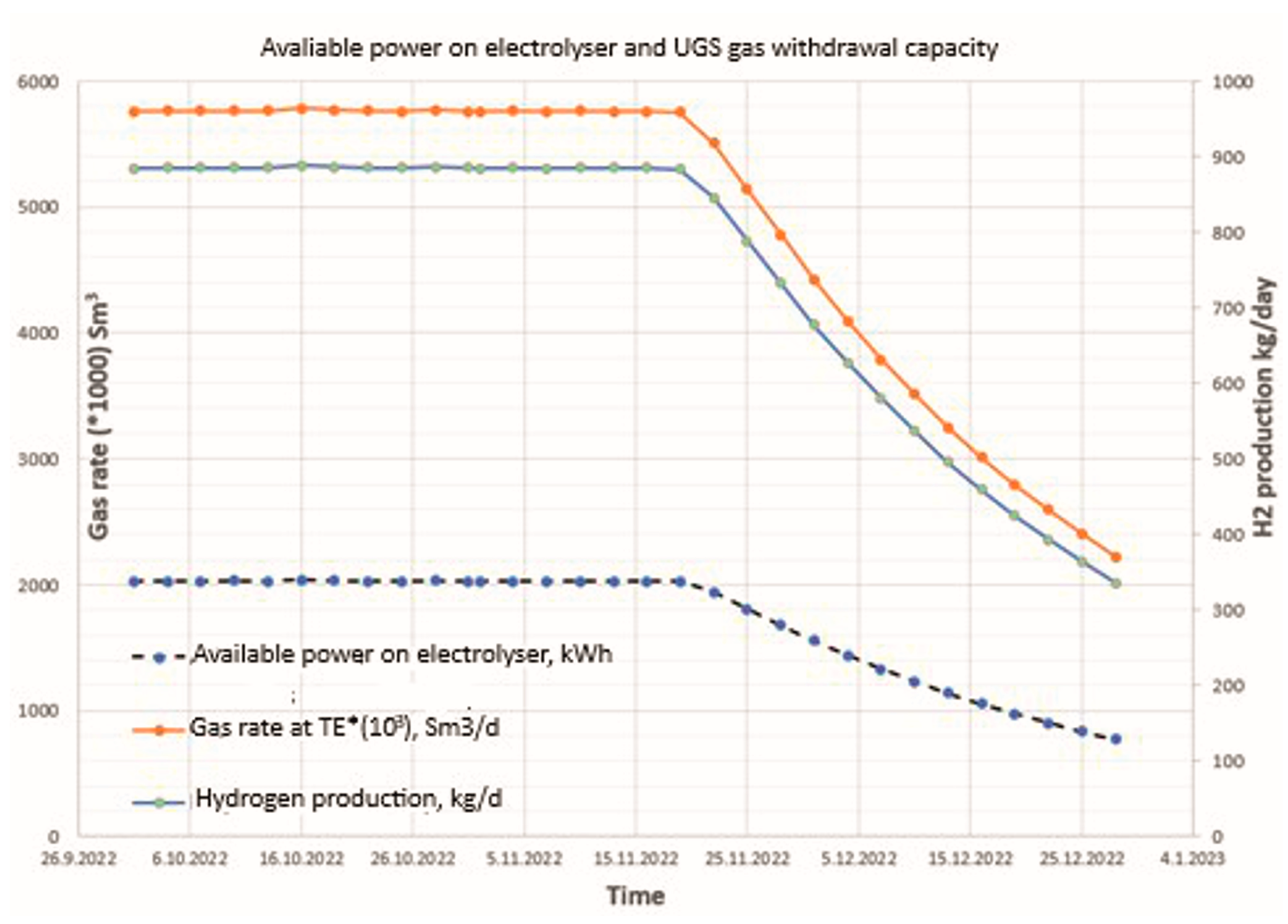
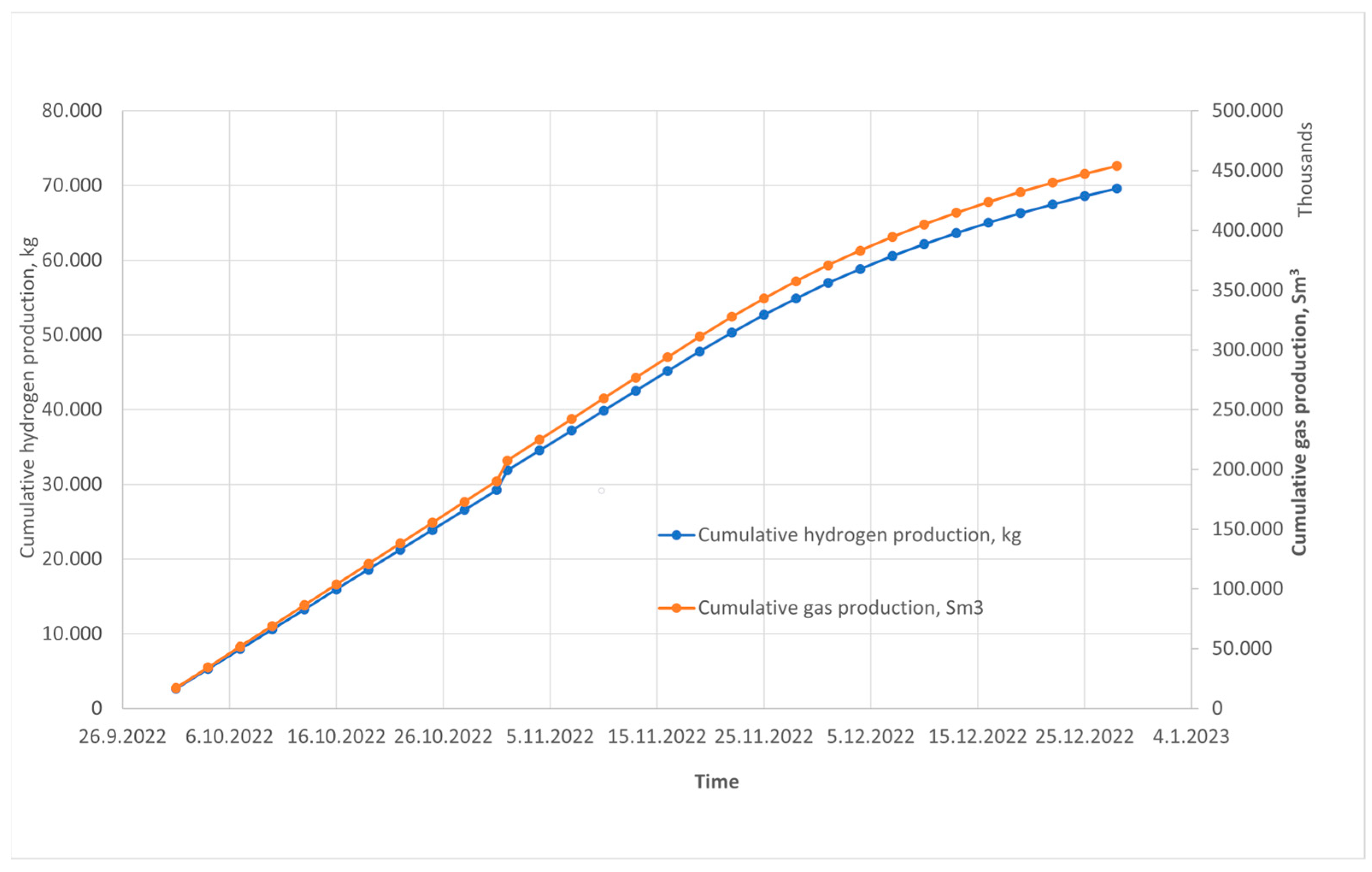
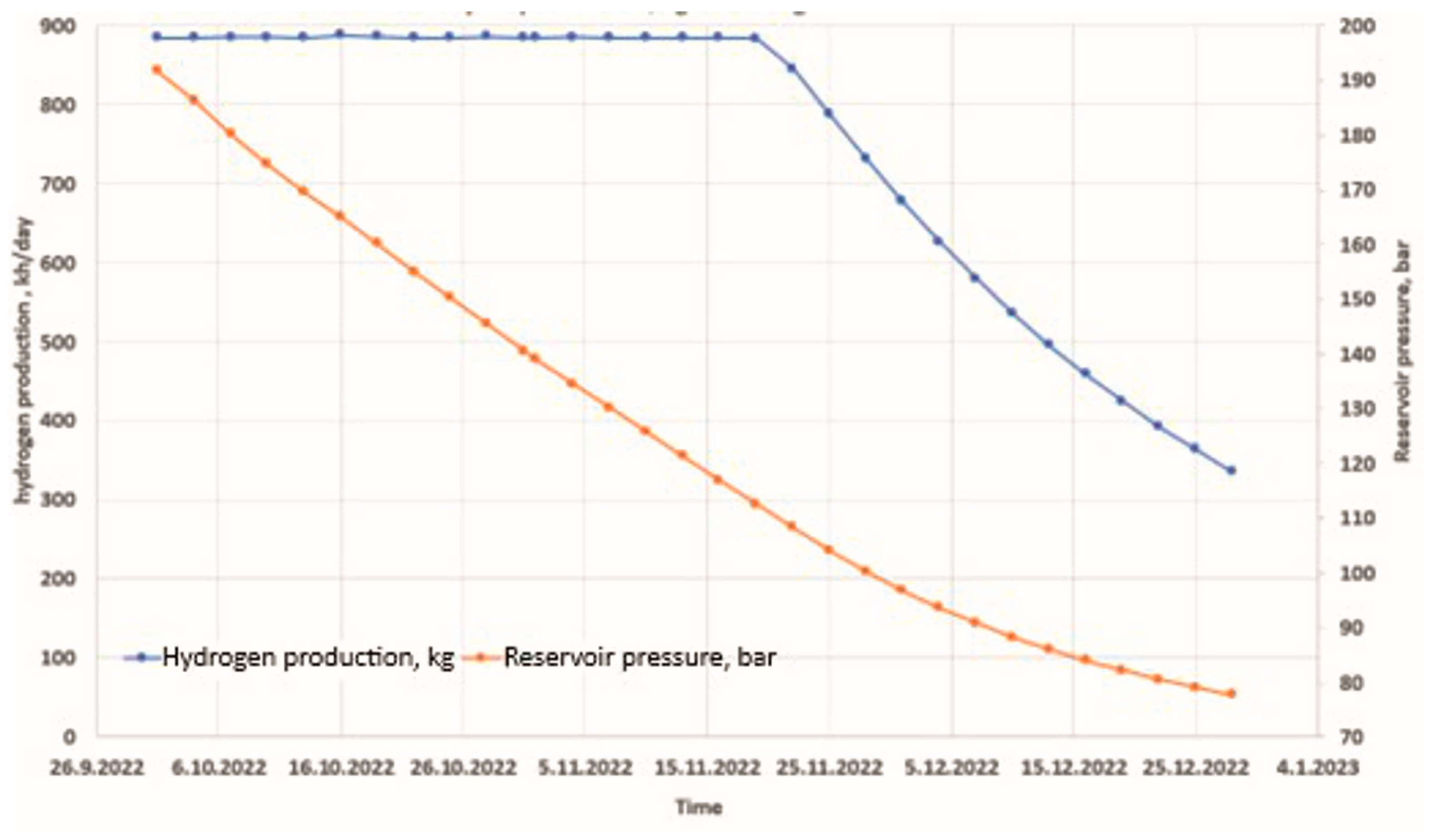
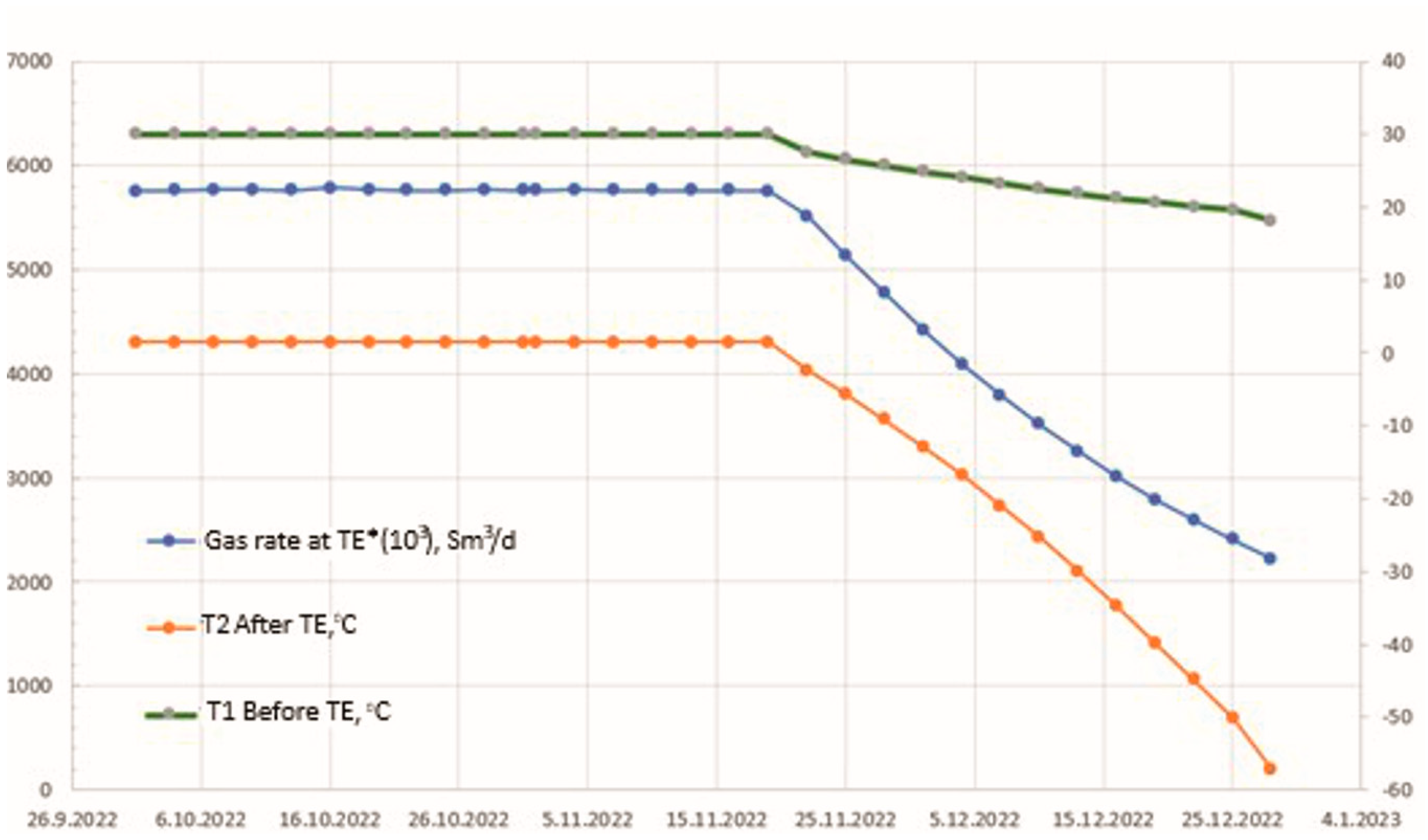
4. Simulation Model Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. The Role of Waste-to-Energy in the Circular Economy; European Commission (EU COM): Brussels, Belgium, 2017. [Google Scholar]
- European Commission (EC). Communication from the Commission. The European Green Deal; European Commission (EU COM): Brussels, Belgium, 2019. [Google Scholar]
- Caferra, R.; D’Adamo, I.; Morone, P. Wasting energy or energizing waste? The public acceptance of waste-to-energy technology. Energy 2023, 263, 126123. [Google Scholar] [CrossRef]
- Moustakas, K.; Loizidou, M.; Klemes, J.; Hao, J.L.; Varbanov, P. New developments in sustainable waste-to-energy systems. Energy 2023, 284, 129270. [Google Scholar] [CrossRef]
- UN General Assembly (UN). Transforming Our World: The 2030 Agenda for Sustainable Development, A/RES/70/1; UN General Assembly GAOR, 70th sess., Suppl. no. 49; 2015. Available online: https://sdgs.un.org/ (accessed on 26 June 2024).
- European Commission (EC); International Renewable Energy Agency (IRENA). Renewable Energy Prospects for the European Union; European Union: Maastricht, The Netherlands; IRENA: Abu Dhabi, United Arab Emirates, 2018; pp. 1–120. [Google Scholar]
- Brown, S.; Jones, D.; Fulghum, N.; Bruce-Lockhart, C.; Candlin, A.; Ewen, M.; Czyżak, P.; Rangelova, K.; Heberer, L.; Rosslowe, C.; et al. European Electricity Review. EMBER 2024, 1–95. Available online: https://ember-climate.org/app/uploads/2024/02/European-Electricity-Review-2024.pdf (accessed on 26 June 2024).
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Mazza, A.; Bompard, E.; Chicco, G. Applications of power to gas technologies in emerging electrical systems. Renew. Sustain. Energy Rev. 2018, 92, 794–806. [Google Scholar] [CrossRef]
- Glenk, G.; Holler, P.; Reichelstein, S. Advances in power-to-gas technologies: Cost and conversion efficiency. Energy Environ. Sci. 2023, 16, 6058–6070. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; Technical Report NREL/TP-5600-51995; National Renewable Energy Laboratory: Golden, CO, USA, 2013; pp. 1–131.
- Zelenika, I.; Pavlović, D.; Rajič, P.; Kovačić, T.; Srpak, M. Hydrogen Underground Storage as a Critical Factor in the Energy Transition Period. Tech. Gaz. 2021, 28, 1480–1487. [Google Scholar]
- Sorgulu, F.; Dincer, I. Analysis and techno-economic assessment of renewable hydrogen production and blending into natural gas for better sustainability. Int. J. Hydrogen Energy 2022, 47, 19977–19988. [Google Scholar] [CrossRef]
- Erdener, B.C.; Sergi, B.; Guerra, O.J.; Chueca, A.L.; Pambour, K.; Brancucci, C.; Hodge, B.M. A review of technical and regulatory limits for hydrogen blending in natural gas pipelines. Int. J. Hydrogen Energy 2023, 48, 5595–5617. [Google Scholar] [CrossRef]
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Powering a Climate-Neutral Economy: An EU Strategy for Energy System Integration; European Commission (EU COM): Brussels, Belgium, 2020. [Google Scholar]
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Hydrogen Strategy for a Climate-Neutral Europe; European Commission (EU COM): Brussels, Belgium, 2020. [Google Scholar]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Voitic, G.; Pichler, B.; Basile, A.; Iulianelli, A.; Malli, K.; Bock, S.; Hacker, V. Hydrogen production. In Fuel Cells and Hydrogen, 1st ed.; Hacker, V., Mitsushima, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–241. [Google Scholar]
- Idriss, H. Hydrogen production from water: Past and present. Curr. Opin. Chem. Eng. 2020, 29, 74–82. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of renewable-energy-based green hydrogen into the energy future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). The Future of Hydrogen: Seizing Today’s Opportunities; OECD: Paris, France, 2019. [Google Scholar]
- IRENA and Bluerisk. Water for Hydrogen Production; International Renewable Energy Agency, Bluerisk: Abu Dhabi, United Arab Emirates, 2023. [Google Scholar]
- Alamiery, A. Advancements in materials for hydrogen production: A review of cutting-edge technologies. ChemPhysMater 2024, 3, 64–73. [Google Scholar] [CrossRef]
- Folgado, H.J.; Isaías González, I.; Antonio José Calderón, A.J. PEM Electrolyzer Digital Replica based on internal resistance determination applied to hydrogen energy storage. J. Energy Storage 2024, 75, 109694. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Loomba, S.; Khan, M.W.; Haris, M.; Mousavi, S.M.; Zavabeti, A.; Xu, K.; Tadich, A.; Thomsen, L.; McConville, C.F.; Li, Y.; et al. Nitrogen-doped porous nickel molybdenum phosphide sheets for efficient seawater splitting. Small 2023, 19, 2207310. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Miao, J.; Zhang, L.; Gao, J.; Wang, H.; Tao, H.; Hung, S.F.; Vasileff, A.; Qiao, S.Z.; Liu, B. An earth-abundant catalyst-based seawater photoelectrolysis system with 17.9% solar-to-hydrogen efficiency. Adv. Mater. 2018, 30, 1707261. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [PubMed]
- Al-Baghdadi, M.A.S.; Abdolhamid, H.R.; Mkhatresh, O.A. Using of produced water associated with oil and gas production as a source of hydrogen: Solar electrolysis cell application. Ovidius Univ. Ann. Chem. J. 2009, 20, 44–47. [Google Scholar]
- Poživil, J. Use of expansion turbines in natural gas pressure reduction stations. Acta Montan. Slovaca 2004, 3, 258–260. [Google Scholar]
- Kostowski, W. The possibility of energy generation within the conventional natural gas transport system. Strojarstvo 2010, 52, 429–440. [Google Scholar]
- Kaneko, K.I.; Ohtani, K.; Tsujikawa, Y.; Fuji, S. Utilization of the cryogenic exergy of LNG by a mirror gas-turbine. Appl. Energy 2004, 79, 355–369. [Google Scholar] [CrossRef]
- Szargut, J. Exergy Method: Technical and Ecological Applications, 1st ed.; WIT Press: Southampton, UK, 2005; pp. 1–161. [Google Scholar]
- Szargut, J.; Szczygiel, I. Utilization of the cryogenic exergy of liquid natural gas (LNG) for the production of electricity. Energy 2009, 34, 827–837. [Google Scholar] [CrossRef]
- Danieli, P.; Carraro, G.; Lazzaretto, A. Thermodynamic and Economic Feasibility of Energy Recovery from Pressure Reduction Stations in Natural Gas Distribution Networks. Energies 2020, 13, 4453. [Google Scholar] [CrossRef]
- Borelli, D.; Devia, F.; Cascio, E.L.; Schenone, C. Energy recovery from natural gas pressure reduction stations: Integration with low temperature heat sources. Energy Convers. Manag. 2018, 159, 274–283. [Google Scholar] [CrossRef]
- Saied, O.; Abdellatif, A.; Shaaban, S.; Elsafty, A.F. Efficient Energy Recovery Scenarios from Pressure-Reducing Stations Intended for New Al-Alamein City in Egypt. Energies 2022, 15, 9077. [Google Scholar] [CrossRef]
- Bielka, P.; Kuczyński, S. Energy Recovery from Natural Gas Pressure Reduction Stations with the Use of Turboexpanders: Static and Dynamic Simulations. Energies 2022, 15, 8890. [Google Scholar] [CrossRef]
- GCAP [Software]. Available online: https://www.jmcampbell.com/gcap-software.php (accessed on 15 July 2024).
- Petroleum Experts Ltd. Integrated Production Modelling Toolkit (IPM) [Software]. Available online: http://www.petex.com/products/ipm-suite/ (accessed on 15 July 2024).
- Zelenika, I. The Underground Gas Storage Volume Modelling with Respect to Reservoir Parameters and Production Equipment. Ph.D. Thesis, University of Zagreb, Faculty of Mining, Geology and Petroleum Engineering, Zagreb, Croatia, 2017. [Google Scholar]
- Campbell, J.M.; Hubbard, R.A. Gas Conditioning and Processing: The Equipment Modules, 8th ed.; John, M., Ed.; Campbell and Company: Norman, OK, USA, 2004; Volume 2. [Google Scholar]
- Brkić, V.; Zelenika, I.; Mijić, P.; Medved, I. Underground gas storage process optimisation with respect to reservoir parameters and production equipment. Energies 2021, 14, 4324. [Google Scholar] [CrossRef]
- Singh, K.; Fevang, Ø.; Whitson, C.H. Whitson Consistent Black-Oil PVT Table Modification. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Available online: https://assets.new.siemens.com/ (accessed on 15 July 2024).



| Water Properties | |
|---|---|
| Salinity (NaCl) | 7352 mg/dm3 |
| Electric resistivity | 0.862 Ohm·m |
| pH | 6.31 |
| Density | 1005 kg/dm3 |
| TDS (Total dissolved solids) | 8720 mg/dm3 |
| Cations (Na+, Ca2+, Mg2+, Fe3+) | 3260 mg/dm3 |
| Anions (Cl−, HCO3−, SO42−) | 5550 mg/dm3 |
| Fe (ICP) | 284 mg/dm3 |
| Month/Year | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 |
|---|---|---|---|---|---|---|---|---|
| November | 2.4 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0.53 |
| December | 78.2 | 0 | 53.19 | 0 | 8.2 | 0 | 0.92 | 30.03 |
| January | 53.7 | 2.7 | 78.7 | 42.2 | 43.8 | 14.33 | 14.35 | 86.28 |
| February | 150.3 | 27 | 150.5 | 135.7 | 124.7 | 52.07 | 123.46 | 159.29 |
| March | 370 | 107.5 | 280.76 | 214.8 | 114.8 | 177.03 | 181.18 | 152.63 |
| April | 103.1 | 160.3 | 212.5 | 100.6 | 200.7 | 174.18 | 285.58 | 5.95 |
| May | 36.4 | 74.4 | 24 | 33.7 | 4.8 | 8.23 | 236.71 | 0 |
| Σ/year, m3 | 794.1 | 371.9 | 800.25 | 527 | 497 | 425.84 | 842.2 | 434.71 |
| Trap Type | Anticline |
|---|---|
| HC contact | 70 m |
| Formation | Kloštar Ivanić Formation |
| Average net thickness (a1 + a2 + a3) | 16 m + 19 m + 7 m |
| Porosity | 5.5–23% |
| Permeability | 0.1–170 mD |
| Water saturation | 29% |
| Rock compressibility | 5.476 × 10−6 |
| Reservoir temperature | 185 °C |
| Initial pressure | 196.2 bar |
| Storage working pressure range | 80–196 bar |
| Gas–water contact | 1800 m |
| Gas Component | Mol (%) |
|---|---|
| N2 | 0.400 |
| CO2 | 1.570 |
| C1 | 94.604 |
| C2 | 1.470 |
| C3 | 0.990 |
| iC4 | 0.280 |
| nC4 | 0.351 |
| iC5 | 0.122 |
| nC5 | 0.132 |
| C6 | 0.005 |
| C7 | 0.010 |
| C8 | 0.014 |
| C9 | 0.010 |
| C11 | 0.042 |
| Pressure (bar) | Bg (m3/m3) | µg (cP) | Pressure (bar) | Bg (m3/m3) | µg (cP) |
|---|---|---|---|---|---|
| 1.00 | 1.32568 | 0.01357 | 181.52 | 0.00671 | 0.01794 |
| 13.03 | 0.10046 | 0.01367 | 193.55 | 0.00631 | 0.01845 |
| 25.07 | 0.05163 | 0.01379 | 205.59 | 0.00596 | 0.01898 |
| 37.10 | 0.03451 | 0.01394 | 217.62 | 0.00565 | 0.01952 |
| 49.14 | 0.02581 | 0.01411 | 229.66 | 0.00538 | 0.02007 |
| 61.17 | 0.02055 | 0.01432 | 241.69 | 0.00513 | 0.02064 |
| 73.21 | 0.01704 | 0.01455 | 253.72 | 0.00492 | 0.02121 |
| 85.24 | 0.01453 | 0.01481 | 265.76 | 0.00472 | 0.02180 |
| 97.28 | 0.01266 | 0.01510 | 277.79 | 0.00455 | 0.02238 |
| 109.31 | 0.01122 | 0.01542 | 289.83 | 0.00439 | 0.02297 |
| 121.35 | 0.01007 | 0.01577 | 301.86 | 0.00424 | 0.02356 |
| 133.38 | 0.00914 | 0.01615 | 313.90 | 0.00411 | 0.02415 |
| 145.41 | 0.00837 | 0.01656 | 325.93 | 0.00399 | 0.02474 |
| 157.45 | 0.00772 | 0.01700 | 337.97 | 0.00388 | 0.02533 |
| 169.48 | 0.00718 | 0.01746 | 350.00 | 0.00378 | 0.02592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenika, I.; Novak Mavar, K.; Medved, I.; Pavlović, D. Sustainable Energy Solutions: Utilising UGS for Hydrogen Production by Electrolysis. Appl. Sci. 2024, 14, 6434. https://doi.org/10.3390/app14156434
Zelenika I, Novak Mavar K, Medved I, Pavlović D. Sustainable Energy Solutions: Utilising UGS for Hydrogen Production by Electrolysis. Applied Sciences. 2024; 14(15):6434. https://doi.org/10.3390/app14156434
Chicago/Turabian StyleZelenika, Ivan, Karolina Novak Mavar, Igor Medved, and Darko Pavlović. 2024. "Sustainable Energy Solutions: Utilising UGS for Hydrogen Production by Electrolysis" Applied Sciences 14, no. 15: 6434. https://doi.org/10.3390/app14156434





