Comparison of Three Gas Chromatographic Methods—Identification of Terpenes and Terpenoids in Cannabis sativa L.
Abstract
1. Introduction
2. Experimental
2.1. Methods
2.2. Experimental Conditions for HS
2.3. Experimental Conditions for SPME
2.4. Experimental Condition for All Three Analyses
2.5. Identification
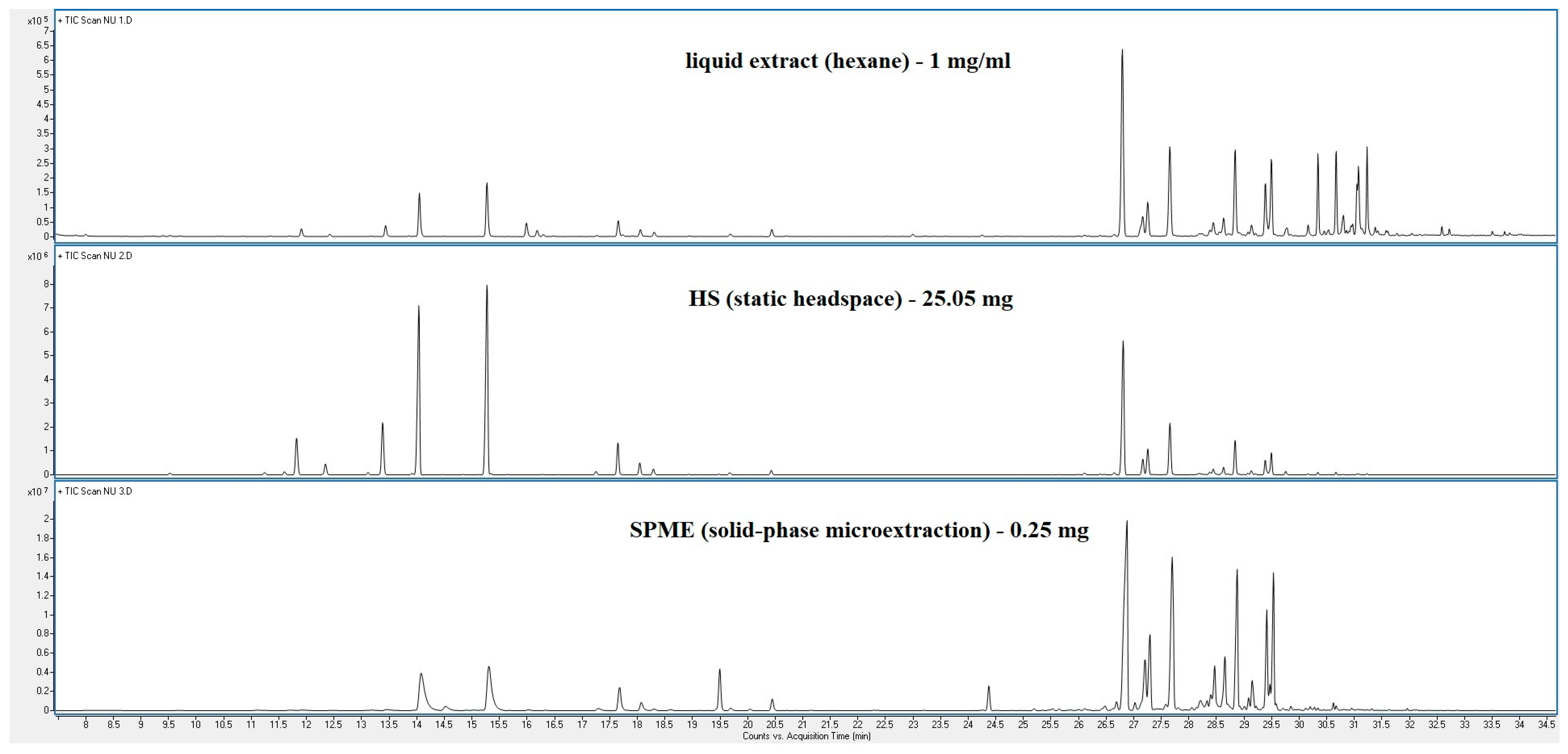
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef] [PubMed]
- Booth, J. Terpene and Isoprenoid Biosynthesis in Cannabis sativa. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2020; 223p. [Google Scholar]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.; Xiao, Y.; Liu, H.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Ann. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The plants’ 1-deoxy-D-xylulose-5-phosphate pathway for biosynthesis of isoprenoids. Fett/Lipid 1998, 100, 128–138. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. The isolation and structure of cannabinolic, cannabidiolic and cannabigerolic acids. Tetrahedron 1965, 21, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Borah, R.; Sharma, B.; Pandhi, S.; Tripathi, V.; Yadav, H.S.; Devi, S.; Patil, U.; et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: An overview. Phytother. Res. 2021, 35, 6010–6029. [Google Scholar] [CrossRef] [PubMed]
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.M.; Largent-Milnes, T.M. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 1269–1297. [Google Scholar] [CrossRef]
- Laws, J.S., III; Shrestha, S.; Smid, S.D. Cannabis terpenes display variable protective and anti-aggregatory actions against neurotoxic β amyloid in vitro: Highlighting the protective bioactivity of α-bisabolol in motorneuronal-like NSC-34 cells. Neurotoxicology 2022, 90, 81–87. [Google Scholar] [CrossRef]
- Rodriguez, C.E.B.; Ouyang, L.; Kandasamy, R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav. Pharmacol. 2022, 33, 130–157. [Google Scholar] [CrossRef]
- Di Sotto, A.; Gullì, M.; Acquaviva, A.; Tacchini, M.; Di Simone, S.C.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; et al. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022, 183, 114980. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; Garcia-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Worth, T. Unpicking the entourage effect. Nature 2019, 572, S12–S13. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of entourage: Terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021, 11, 8232. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, F.E.; Smith Donn, L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Dewey, W.L.; Harris, L.S.; Howes, J.F.; Nuite, J.A. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J. Pharmacol. Exp. Ther. 1970, 175, 435–442. [Google Scholar]
- Pertwee, R.G. The ring test: A quantitative method for assessing the ‘cataleptic’ effect of cannabis in mice. Br. J. Pharmacol. 1972, 46, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Little, P.J.; Compton, D.R.; Johnson, M.R.; Melvin, L.S.; Martin, B.R. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 1988, 247, 1046–1051. [Google Scholar]
- Tang, X.; Cancelada, L.; Rapp, V.H.; Russell, M.L.; Maddalena, R.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Heated Terpenoids Present in Vaporizable Cannabis Concentrates. Environ. Sci. Technol. 2021, 55, 6160–6170. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, R.A.; Marr, A.G. The oxidation of terpenes. I. Mechanism and reaction products of D-limonene autoxidation. Food Res. 1960, 25, 517–530. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Magnusson, K.; Nilsson, U. Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Contact Dermat. 1992, 26, 332–340. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Shao, L.P.; Nilsson, U.; Gäfvert, E.; Nilsson, J.L.G. Hydroperoxides in oxidized d-limonene identified as potent contact allergens. Arch. Dermatol. Res. 1994, 286, 97–103. [Google Scholar] [CrossRef]
- Nilsson, U.; Bergh, M.; Shao, L.P.; Karlberg, A.T. Analysis of contact allergenic compounds in oxidized d-limonene. Chromatographia 1996, 42, 199–205. [Google Scholar] [CrossRef]
- Calogirou, A.; Larsen, B.R.; Kotzias, D. Gas-phase terpene oxidation products: A review. Atmos. Environ. 1999, 33, 1423–1439. [Google Scholar] [CrossRef]
- Christensson, J.B. Clinical and Experimental Studies on Oxidized Fragrance Terpenes as Contact Allergens. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2009; 55p. [Google Scholar]
- Christensson, J.B.; Karlberg, A.T.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez, A.A.; Goh, C.L.; Nixon, R.; White, I.R. Oxidized limonene and oxidized linalool—Concomitant contact allergy to common fragrance terpenes. Contact Dermat. 2016, 74, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bennike, N.H.; Palangi, L.; Christensson, J.B.; Nilsson, U.; Zachariae, C.; Johansen, J.D.; Hagvall, L. Allergic contact dermatitis caused by hydroperoxides of limonene and dose-response relationship-A repeated open application test (ROAT) study. Contact Dermat. 2019, 80, 208–216. [Google Scholar] [CrossRef]
- Sköld, M.; Börje, A.; Matura, M.; Karlberg, A.T. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermat. 2002, 46, 267–272. [Google Scholar] [CrossRef]
- Sköld, M.; Börje, A.; Harambasic, E.; Karlberg, A.T. Contact Allergens Formed on Air Exposure of Linalool. Identification and Quantification of Primary and Secondary Oxidation Products and the Effect on Skin Sensitization. Chem. Res. Toxicol. 2004, 17, 1697–1705. [Google Scholar] [CrossRef]
- Christensson, J.B.; Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A.T. Linalool—A significant contact sensitizer after air exposure. Contact Dermat. 2010, 62, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Christensson, J.B.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Arnau, A.G.; Goh, C.L.; Nixon, R.; White, I.R. Air-oxidized linalool—A frequent cause of fragrance contact allergy. Contact Dermat. 2012, 67, 247–259. [Google Scholar] [CrossRef]
- Grosjean, D.; Williams, E.L.; Seinfeld, J.H. Atmospheric Oxidation of Selected Terpenes and Related Carbonyls: Gas-Phase Carbonyl Products. Environ. Sci. Technol. 1992, 26, 1526–1533. [Google Scholar] [CrossRef]
- Matura, M.; Sköld, M.; Boerje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermat. 2005, 52, 320–328. [Google Scholar] [CrossRef]
- Sköld, M.; Karlberg, A.T.; Matura, M.; Börje, A. The fragrance chemical β-caryophyllene—Air oxidation and skin sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- McGraw, G.W.; Hemingway, R.W.; Ingram, L.L., Jr.; Canady, C.S.; McGraw, W.B. Thermal Degradation of Terpenes: Camphene, Δ3-Carene, Limonene, and α-Terpinene. Environ. Sci Technol. 1999, 33, 4029–4033. [Google Scholar] [CrossRef]
- Rettberg, N.; Thorner, S.; Garbe, L.A. Bugging hop analysis—On the isomerization and oxidation of terpene alcohols during steam distillation. Brew. Sci.—Monatsschrift Für Brauwiss 2012, 65, 112–117. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bueno, J.; Leuer, E.; Kearney, M., Jr.; Green, E.H.; Greenbaum, E.A. The preservation and augmentation of volatile terpenes in cannabis inflorescence. J. Cannabis Res. 2020, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.C. Method Validation of Analytical Procedures. PharmaTutor Mag. 2015, 3, 32–39. [Google Scholar]
- Egon, S. Chemical varieties of plants containing terpenoids. Essenze E Deriv. Agrum. 1957, 27, 188–220. [Google Scholar]
- Martin, L.; Smith, D.M.; Farmilo, C.G. Essential oil from fresh Cannabis sativa and its use in identification. Nature 1961, 191, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.C.; Handa, K.L.; Nigam, I.C.; Levi, L. Essential oils and their constituents XXIX. The essential oil of marihuana: Composition of genuine Indian Cannabis sativa L. Can. J. Chem. 1965, 43, 3372–3376. [Google Scholar] [CrossRef]
- Hood, L.V.S.; Dames, M.E.; Barry, G.T. Headspace volatiles of marijuana. Nature 1973, 242, 402–403. [Google Scholar] [CrossRef]
- Hood, L.V.S.; Barry, G.T. Headspace volatiles of marihuana and hashish: Gas chromatographic analysis of samples of different geographic origin. J. Chromatogr. 1978, 166, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Shapira, A.; Berman, P.; Futoran, K.; Guberman, O.; Meiri, D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 11425–11432. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/terpenoids in Cannabis—Are they important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Satterfield-Doerr, M.; Parikh, A.; Brodbelt, J.S. Determination of Cannabinoids in Water and Human Saliva by Solid-Phase Microextraction and Quadrupole Ion Trap Gas Chromatography/Mass Spectrometry. Anal. Chem. 1998, 70, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Ilias, Y.; Rudaz, S.; Mathieu, P.; Christen, P.; Veuthey, J.L. Extraction and analysis of different Cannabis samples by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. J. Sep. Sci. 2005, 28, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Long, C.; Zhong, C.; Sun, C.; Mao, H. Analysis of chemical constituents of the volatile oil from Cannabis sativa by SPME-GC-MS. Zhongguo Yao Fang (J. China Pharm.) 2008, 19, 2613–2614. [Google Scholar]
- Krill, C.; Rochfort, S.; Spangenberg, G. A High-Throughput Method for the Comprehensive Analysis of Terpenes and Terpenoids in Medicinal Cannabis Biomass. Metabolites 2020, 10, 276. [Google Scholar] [CrossRef]
- Myers, C.; Herrington, J.S.; Hamrah, P.; Anderson, K. Accelerated Solvent Extraction of Terpenes in Cannabis Coupled with Various Injection Techniques for GC-MS Analysis. Front. Chem. 2021, 9, 619770. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]


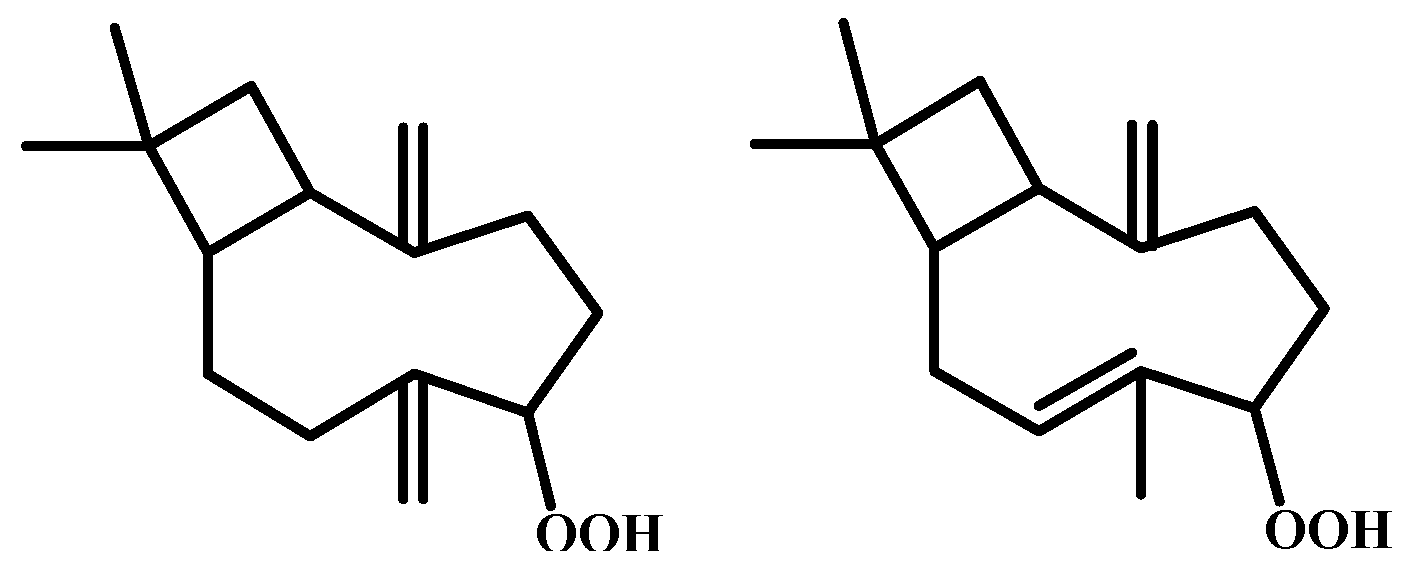
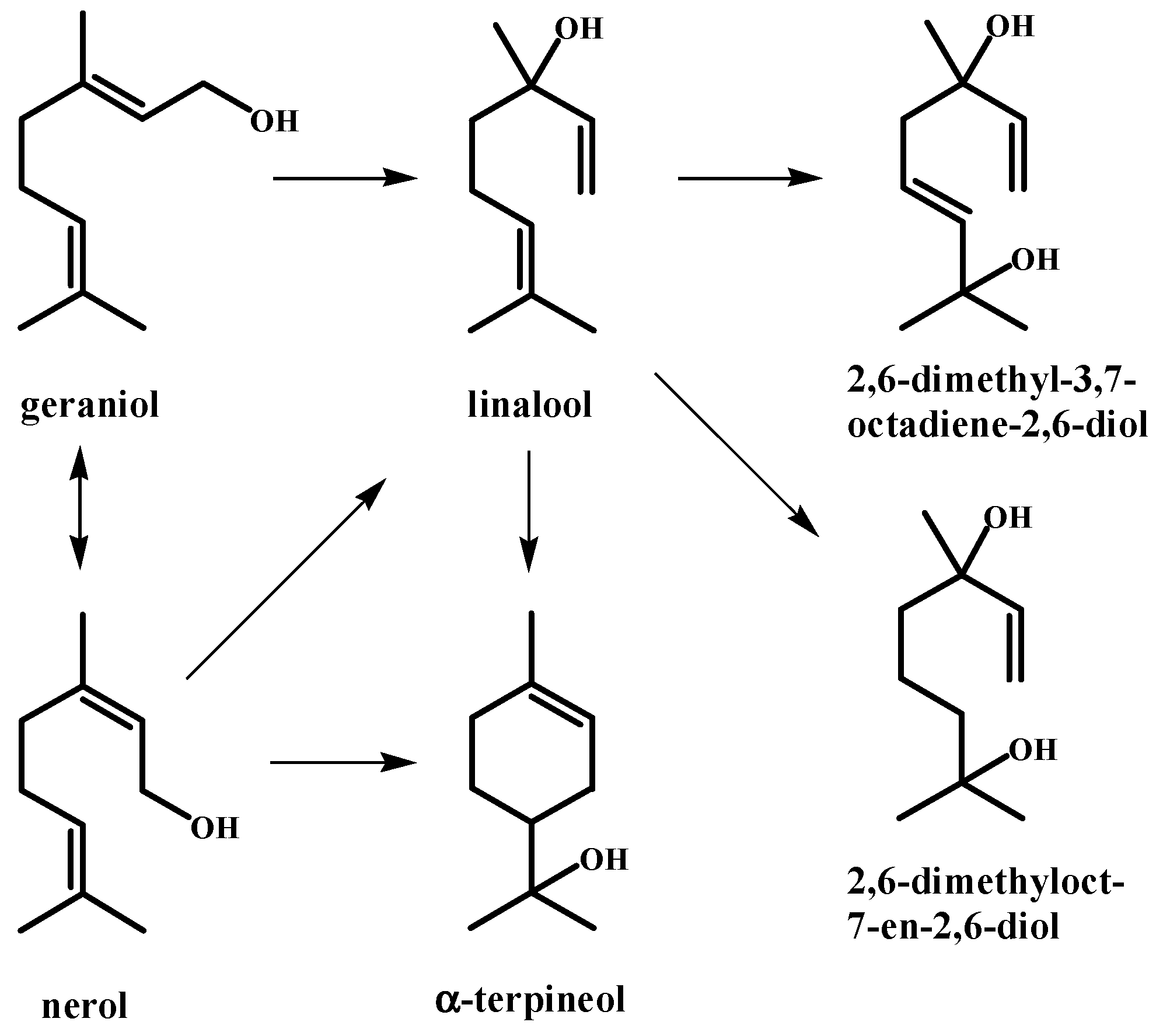

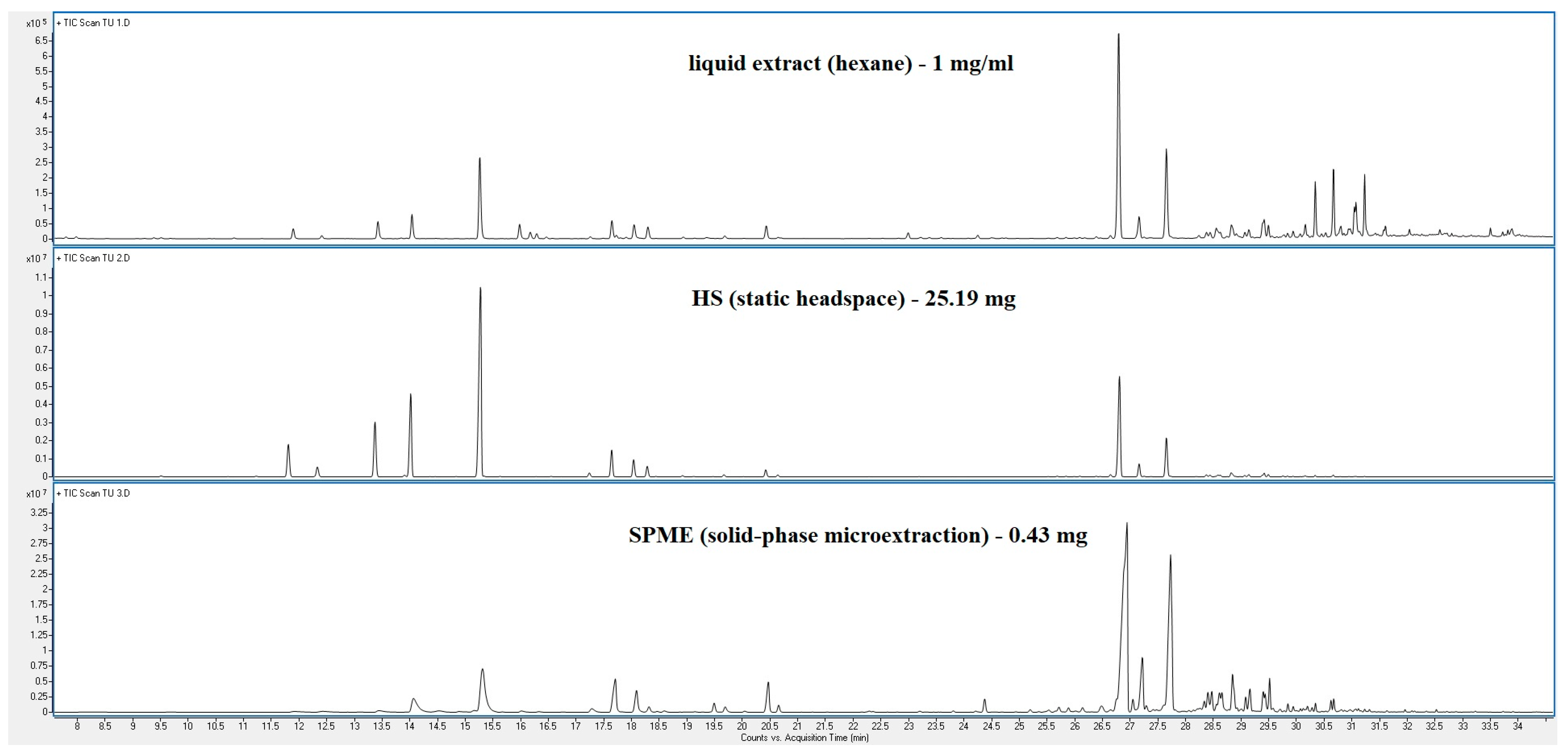
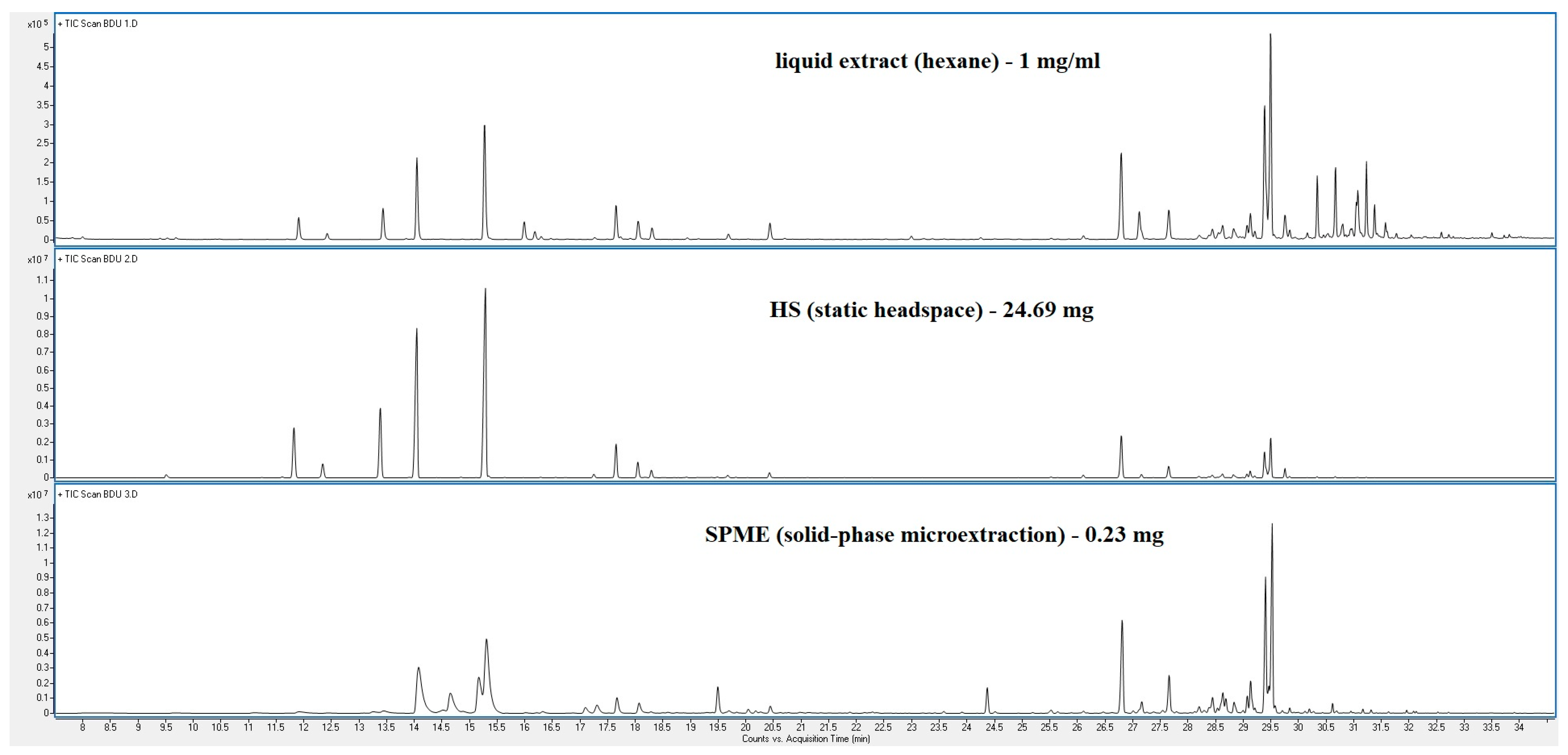
| Peak | RT | % (Liq) | % (HS) | % (SPME) | Compounds | Type | RI |
|---|---|---|---|---|---|---|---|
| 1 | 6.429 | traces | 2,3-butanediol | glycol | 788 | ||
| 2 | 7.789 | traces | 2,4-dimethylheptane | hydrocarbon | 821 | ||
| 3 | 9.212 | traces | Ethylbenzene | aromatic hydrocarbon | 855 | ||
| 4 | 9.379 | traces | 4-methyloctane | hydrocarbon | 863 | ||
| 5 | 9.509 | traces | p-xylene | aromatic hydrocarbon | 865 | ||
| 6 | 9.524 | traces | 1-hexanol | organic alcohol | 868 | ||
| 7 | 10.718 | traces | traces | Heptanal | alkyl aldehyde | 901 | |
| 8 | 11.223 | 0.09 | 0.63 | 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 | |
| 9 | 11.600 | traces | α-thujene | bicyclic monoterpene | 929 | ||
| 10 | 11.808 | 0.47 | 2.71 | traces | α-pinene | bicyclic monoterpene | 937 |
| 11 | 12.329 | 0.14 | 0.74 | traces | Camphene | bicyclic monoterpene | 952 |
| 12 | 12.810 | traces | Benzaldehyde | aromatic aldehyde | 962 | ||
| 13 | 13.372 | 0.86 | 4.93 | 0.20 | β-pinene | bicyclic monoterpene | 979 |
| 14 | 13.893 | traces | 6-methyl-5-hepten-2-one | unsaturated methylated ketone | 986 | ||
| 15 | 14.053 | 9.80 | 48.94 | 11.81 | β-myrcene | acyclic monoterpene | 991 |
| 16 | 14.887 | traces | α-terpinene | monocyclic monoterpene | 1017 | ||
| 17 | 15.111 | traces | traces | p-cymene | monocyclic monoterpene | 1025 | |
| 18 | 15.247 | 3.28 | 15.12 | 4.18 | Limonene | monocyclic monoterpene | 1030 |
| 19 | 15.328 | traces | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 20 | 15.624 | traces | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 21 | 15.969 | traces | traces | trans-β-ocimene | acyclic monoterpene | 1049 | |
| 22 | 16.273 | traces | traces | γ-terpinene | monocyclic monoterpene | 1060 | |
| 23 | 16.554 | traces | sabinene hydrate | bicyclic monoterpenoid | 1068 | ||
| 24 | 17.243 | 0.16 | 0.15 | Terpinolene | monocyclic monoterpene | 1088 | |
| 25 | 17.636 | 0.65 | 1.42 | 0.39 | Linalool | acyclic monoterpenoid | 1099 |
| 26 | 18.037 | 0.48 | 0.63 | 0.47 | fenchyl alcohol | bicyclic monoterpenoid | 1113 |
| 27 | 18.286 | 0.31 | 0.35 | cis-pinene hydrate | bicyclic monoterpenoid | 1121 | |
| 28 | 18.590 | traces | neo-allo-ocimene | acyclic monoterpene | 1131 | ||
| 29 | 19.079 | traces | Ipsdienol | acyclic monoterpenoid | 1147 | ||
| 30 | 19.672 | 0.10 | traces | traces | Borneol | bicyclic monoterpenoid | 1166 |
| 31 | 20.025 | 0.11 | 0.10 | 0.10 | terpinen-4-ol | monocyclic monoterpenoid | 1177 |
| 32 | 20.426 | 0.35 | 0.19 | 0.12 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 33 | 20.706 | traces | Dodecane | alkane hydrocarbon | 1200 | ||
| 34 | 24.931 | traces | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 35 | 25.516 | traces | traces | 0.16 | Ylangene | tricyclic sesquiterpene | 1372 |
| 36 | 25.637 | traces | traces | 0.09 | α-copaene | tricyclic sesquiterpene | 1376 |
| 37 | 25.813 | traces | β-patchoulene | tricyclic sesquiterpene | 1381 | ||
| 38 | 25.997 | traces | traces | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 39 | 26.206 | traces | Tetradecane | alkane hydrocarbon | 1400 | ||
| 40 | 26.462 | traces | 0.21 | cis-β-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 41 | 26.639 | 0.11 | 0.09 | 0.45 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 |
| 42 | 26.791 | 10.23 | 6.23 | 13.63 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 43 | 26.879 | traces | γ-maaliene | tricyclic sesquiterpene | 1430 | ||
| 44 | 27.024 | traces | β-copaene | tricyclic sesquiterpene | 1433 | ||
| 45 | 27.144 | 7.87 | 2.00 | γ-elemene | monocyclic sesquiterpene | 1434 | |
| 46 | 27.160 | 0.57 | 0.66 | 2.09 | α-bergamotene | bicyclic sesquiterpene | 1436 |
| 47 | 27.248 | 1.95 | 1.20 | 3.53 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 48 | 27.376 | traces | traces | 0.19 | guaia-6,9-diene | bicyclic sesquiterpene | 1443 |
| 49 | 27.585 | 0.32 | humulen-(v1) | bicyclic sesquiterpene | 1455 | ||
| 50 | 27.649 | 5.64 | 2.72 | 8.35 | α-humulene | monocyclic sesquiterpene | 1454 |
| 51 | 28.042 | traces | traces | 0.12 | 4,5-di-epi-aristolochene | bicyclic sesquiterpene | 1467 |
| 52 | 28.194 | 0.64 | 0.19 | 0.91 | γ-muurolene | bicyclic sesquiterpene | 1477 |
| 53 | 28.282 | traces | α-amorphene | bicyclic sesquiterpene | 1485 | ||
| 54 | 28.370 | 0.44 | 0.18 | 0.77 | 4a,8-Dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 |
| 55 | 28.434 | 1.34 | 0.45 | 2.25 | β-selinene | bicyclic sesquiterpene | 1486 |
| 56 | 28.555 | 0.51 | δ-selinene | bicyclic sesquiterpene | 1488 | ||
| 57 | 28.627 | 1.65 | 0.59 | 3.20 | α-selinene | bicyclic sesquiterpene | 1494 |
| 58 | 28.835 | 5.80 | 2.15 | 7.70 | α-bulnesene | bicyclic sesquiterpene | 1505 |
| 59 | 28.988 | traces | 0.25 | γ-cadinene | bicyclic sesquiterpene | 1513 | |
| 60 | 29.124 | 1.40 | 0.42 | 2.22 | δ-amorphene | bicyclic sesquiterpene | 1519 |
| 61 | 29.380 | 7.06 | 1.79 | 9.24 | γ-selinene | bicyclic sesquiterpene | 1544 |
| 62 | 29.493 | 7.51 | 2.13 | 12.16 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 63 | 29.757 | 3.18 | 2.15 | traces | germacrene B | monocyclic sesquiterpene | 1557 |
| 64 | 30.158 | 0.36 | traces | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 65 | 31.312 | traces | Cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 66 | 31.575 | 0.47 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 67 | 32.378 | traces | Guaiazulene | bicyclic sesquiterpene | 1775 | ||
| 73.23% | 98.03% | 88.34% | |||||
| 38 cpd | 51 cpd | 46 cpd |
| Peak | RT | % (Liq) | % (HS) | % (SPME) | Compound | Type | RI |
|---|---|---|---|---|---|---|---|
| 1 | 2.966 | traces | 2-methylbutanal | saturated fatty aldehyde | 662 | ||
| 2 | 4.578 | traces | 2-methyl-1-butanol | alcohol | 739 | ||
| 3 | 6.373 | 0.22 | DL-2,3-butanediol | glycol | 773 | ||
| 4 | 6.510 | traces | 2,3-butanediol | glycol | 788 | ||
| 5 | 7.799 | 0.12 | 2,4-dimethyl-heptane | hydrocarbon | 821 | ||
| 6 | 9.508 | 0.17 | 1-hexanol | alcohol | 868 | ||
| 7 | 10.718 | traces | heptanal | saturated fatty aldehyde | 901 | ||
| 8 | 11.231 | 0.10 | 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 | ||
| 9 | 11.608 | traces | α-thujene | bicyclic monoterpene | 929 | ||
| 10 | 11.808 | 0.99 | 5.13 | 0.28 | α-pinene | bicyclic monoterpene | 937 |
| 11 | 12.33 | 0.30 | 1.58 | 0.30 | camphene | bicyclic monoterpene | 953 |
| 12 | 12.811 | traces | benzaldehyde | aromatic aldehyde | 962 | ||
| 13 | 13.372 | 1.69 | 8.27 | 0.37 | β-pinene | bicyclic monoterpene | 979 |
| 14 | 13.901 | 0.24 | 2,2,4,6,6-pentamethylheptane | hydrocarbon | 991 | ||
| 15 | 14.013 | 2.23 | 12.11 | 2.64 | β-myrcene | acyclic monoterpene | 991 |
| 16 | 14.358 | traces | ethyl hexanoate | fatty acid ester | 1000 | ||
| 17 | 14.839 | traces | 0.16 | α-terpinene | monocyclic monoterpene | 1017 | |
| 18 | 14.983 | traces | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 19 | 15.111 | traces | 0.22 | p-cymene | monocyclic monoterpene | 1025 | |
| 20 | 15.272 | 7.97 | 32.44 | 7.09 | limonene | monocyclic monoterpene | 1030 |
| 21 | 15.336 | 0.10 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 22 | 15.624 | traces | traces | cis-β-ocimene | acyclic monoterpene | 1038 | |
| 23 | 16.017 | 0.20 | trans-β-ocimene | acyclic monoterpene | 1049 | ||
| 24 | 16.282 | traces | 0.09 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 25 | 16.546 | traces | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 26 | 16.739 | traces | linalool oxide | monocyclic monoterpenoid | 1086 | ||
| 27 | 17.283 | 0.51 | terpinolene | monocyclic monoterpene | 1088 | ||
| 28 | 17.644 | 1.73 | 3.69 | 3.45 | linalool | acyclic monoterpenoid | 1099 |
| 29 | 17.730 | 0.33 | undecane | hydrocarbon | 1100 | ||
| 30 | 17.789 | traces | nonanal | saturated fatty aldehyde | 1104 | ||
| 31 | 18.037 | 1.57 | 2.36 | 2.03 | fenchol | bicyclic monoterpenoid | 1113 |
| 32 | 18.286 | 1.23 | 1.50 | 0.49 | cis-pinene hydrate | bicyclic monoterpenoid | 1121 |
| 33 | 18.438 | traces | traces | methyl octanoate | fatty acid ester | 1126 | |
| 34 | 18.590 | 0.15 | neo-allo-ocimene | acyclic monoterpene | 1131 | ||
| 35 | 18.919 | 0.15 | 0.17 | trans-pinene hydrate | bicyclic monoterpenoid | 1140 | |
| 36 | 19.015 | traces | camphor | bicyclic monoterpenoid | 1145 | ||
| 37 | 19.119 | traces | traces | camphene hydrate | bicyclic monoterpenoid | 1148 | |
| 38 | 19.408 | traces | isoborneol | bicyclic monoterpenoid | 1157 | ||
| 39 | 19.665 | 0.31 | 0.30 | 0.51 | borneol | bicyclic monoterpenoid | 1166 |
| 40 | 20.025 | traces | 0.10 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 41 | 20.418 | 1.29 | 0.98 | 2.41 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 42 | 20.635 | 0.19 | 0.25 | 0.49 | ethyl octanoate | fatty acid ester | 1196 |
| 43 | 23.192 | traces | 0.10 | bornyl acetate | bicyclic monoterpenoid | 1286 | |
| 44 | 23.801 | traces | 0.10 | (E)-4-decenoic acid methyl ester | fatty acid ester | 1299 | |
| 45 | 24.202 | traces | 0.10 | methyl decanoate | fatty acid ester | 1325 | |
| 46 | 24.939 | traces | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 47 | 25.525 | traces | 0.24 | ylangene | tricyclic sesquiterpene | 1372 | |
| 48 | 25.685 | 0.11 | 0.48 | ethyl trans-4-decenoate | fatty acid ester | 1375 | |
| 49 | 25.837 | 0.09 | 0.36 | hexyl hexanoate | fatty acid ester | 1384 | |
| 50 | 25.990 | traces | 0.13 | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 51 | 26.086 | 0.10 | 0.38 | ethyl decanoate | fatty acid ester | 1396 | |
| 52 | 26.246 | traces | tetradecane | hydrocarbon | 1400 | ||
| 53 | 26.302 | traces | cyperene | tricyclic sesquiterpene | 1399 | ||
| 54 | 26.390 | 0.17 | 0.09 | sesquithujene | bicyclic sesquiterpene | 1402 | |
| 55 | 26.463 | traces | 0.72 | cis-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 56 | 26.647 | 0.29 | 0.32 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 | |
| 57 | 26.807 | 21.80 | 15.85 | 30.19 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 58 | 27.047 | 0.66 | 10,10-dimethyl-2,6-dimethylenebicyclo [7.2.0]undecane | bicyclic sesquiterpene | 1440 | ||
| 59 | 27.16 | 2.30 | 1.71 | 4.48 | α-bergamotene | bicyclic sesquiterpene | 1435 |
| 60 | 27.248 | 0.14 | traces | 0.35 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 61 | 27.376 | traces | guaia-6,9-diene | bicyclic sesquiterpene | 1444 | ||
| 62 | 27.465 | traces | epi-β-santalene | bicyclic sesquiterpene | 1448 | ||
| 63 | 27.480 | 0.09 | α-himachalene | bicyclic sesquiterpene | |||
| 64 | 27.649 | 9.18 | 5.64 | 16.79 | α-humulene | monocyclic sesquiterpene | 1454 |
| 65 | 27.809 | traces | β-santalene | bicyclic sesquiterpene | 1462 | ||
| 66 | 28.338 | 0.63 | α-curcumene | aromatic sesquiterpene | 1483 | ||
| 67 | 28.370 | 0.47 | 0.28 | selina-4,11-diene | bicyclic sesquiterpene | 1474 | |
| 68 | 28.402 | 1.22 | 4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 | ||
| 69 | 28.443 | 0.50 | 0.22 | 1.22 | β-selinene | bicyclic sesquiterpene | 1486 |
| 70 | 28.587 | 0.27 | 1.26 | valencene | bicyclic sesquiterpene | 1492 | |
| 71 | 28.627 | 0.42 | 0.22 | 1.18 | α-selinene | bicyclic sesquiterpene | 1494 |
| 72 | 28.747 | traces | 0.11 | β-dihydroagarofuran | tricyclic sesquiterpenoid | 1496 | |
| 73 | 28.819 | 1.56 | 0.74 | 2.77 | α-farnesene | acyclic sesquiterpene | 1508 |
| 74 | 28.931 | 0.19 | β-curcumene | monocyclic sesquiterpene | 1514 | ||
| 75 | 28.972 | traces | sesquicineole | bicyclic sesquiterpenoid | 1516 | ||
| 76 | 29.140 | 0.65 | 0.29 | 1.38 | β-sesquiphellandrene | monocyclic sesquiterpene | 1524 |
| 77 | 29.389 | 0.90 | 0.12 | 1.05 | γ-selinene | bicyclic sesquiterpene | 1538 |
| 78 | 29.493 | 0.85 | 0.27 | 1.55 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 79 | 29.757 | 0.09 | germacrene B | monocyclic sesquiterpene | 1557 | ||
| 80 | 29.781 | 0.20 | nerolidol | acyclic sesquiterpenoid | 1544 | ||
| 81 | 30.158 | 1.06 | 0.17 | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 82 | 30.334 | 3.60 | 0.15 | 0.34 | guaiol | bicyclic sesquiterpenoid | 1596 |
| 83 | 30.455 | 0.18 | traces | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | |
| 84 | 30.527 | traces | humulene epoxide II | bicyclic sesquiterpenoid | 1606 | ||
| 85 | 30.663 | 4.97 | 0.18 | 0.58 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 |
| 86 | 30.799 | 1.17 | traces | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 87 | 30.859 | 0.23 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 88 | 31.040 | 1.66 | traces | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 89 | 31.072 | 2.99 | traces | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 90 | 31.224 | 4.25 | traces | 0.11 | bulnesol | bicyclic sesquiterpenoid | 1667 |
| 91 | 31.320 | 0.10 | cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 92 | 31.575 | 0.35 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 93 | 32.523 | 0.09 | α-phellandrene dimer | tricyclic terpene | 1801 | ||
| 94 | 33.733 | traces | hexadecanoic acid | saturated fatty acid | 1968 | ||
| 80.02% | 97.87% | 90.80% | |||||
| 38 cpd | 74 cpd | 57 cpd |
| Peak | RT | % (Liq) | % (HS) | % (SPME) | Compound | Type | RI |
|---|---|---|---|---|---|---|---|
| 1 | 8.987 | traces | 3-hexen-1-ol | 856 | |||
| 2 | 9.398 | traces | 4-methyl-octane | hydrocarbon | 863 | ||
| 3 | 9.528 | 0.10 | p-xylene | aromatic hydrocarbon | 865 | ||
| 4 | 9.508 | 0.14 | 0.48 | 0.22 | 1-hexanol | organic alcohol | 868 |
| 5 | 11.239 | traces | 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 | ||
| 6 | 11.608 | 0.14 | traces | α-thujene | bicyclic monoterpene | 929 | |
| 7 | 11.824 | 1.61 | 6.92 | 0.57 | α-pinene | bicyclic monoterpene | 937 |
| 8 | 12.345 | 0.46 | 2.00 | 0.13 | camphene | bicyclic monoterpene | 952 |
| 9 | 13.388 | 2.33 | 9.41 | 0.68 | β-pinene | bicyclic monoterpene | 979 |
| 10 | 14.045 | 5.58 | 21.60 | 9.79 | β-myrcene | acyclic monoterpene | 991 |
| 11 | 14.406 | traces | α-phellandrene | monocyclic monoterpene | 1005 | ||
| 12 | 14.646 | 4.14 | Δ3-carene | bicyclic monoterpene | 1011 | ||
| 13 | 14.879 | 0.35 | α-terpinene | monocyclic monoterpene | 1017 | ||
| 14 | 14.999 | traces | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 15 | 15.119 | traces | 5.41 | p-cymene | monocyclic monoterpene | 1025 | |
| 16 | 15.288 | 8.60 | 28.09 | 13.92 | limonene | monocyclic monoterpene | 1030 |
| 17 | 15.344 | 0.21 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 18 | 15.632 | traces | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 19 | 15.977 | traces | 0.11 | trans-β-ocimene | acyclic monoterpene | 1049 | |
| 20 | 16.290 | traces | 0.27 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 21 | 16.554 | traces | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 22 | 16.322 | traces | p-cresol | phenol derivative | 1077 | ||
| 23 | 16.875 | 0.79 | m-cymenene | aromatic compound | 1082 | ||
| 24 | 17.252 | 0.47 | terpinolene | monocyclic monoterpene | 1088 | ||
| 25 | 17.308 | 1.24 | p-cymenene | aromatic compound | 1090 | ||
| 26 | 17.652 | 2.45 | 4.08 | 1.64 | linalool | acyclic monoterpenoid | 1099 |
| 27 | 17.730 | 0.20 | undecane | hydrocarbon | 1100 | ||
| 28 | 18.045 | 1.52 | 1.97 | 1.25 | fenchol | bicyclic monoterpenoid | 1113 |
| 29 | 18.286 | 0.91 | 0.96 | trans-pinene hydrate | bicyclic monoterpenoid | 1132 | |
| 30 | 19.127 | traces | traces | camphene hydrate | bicyclic monoterpenoid | 1148 | |
| 31 | 18.871 | traces | 5-methyl-undecane | hydrocarbon | 1156 | ||
| 32 | 19.296 | traces | 2,3-dimethyldecane | hydrocarbon | 1157 | ||
| 33 | 19.673 | 0.43 | 0.38 | 0.35 | borneol | bicyclic monoterpenoid | 1166 |
| 34 | 19.817 | traces | 0.10 | 3-methyl-undecane | hydrocarbon | 1170 | |
| 35 | 20.025 | traces | 0.35 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 36 | 20.178 | 0.19 | p-cymene-8-ol | monocyclic monoterpenoid | 1183 | ||
| 37 | 20.426 | 1.21 | 0.66 | 0.61 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 38 | 20.627 | traces | myrtenal | bicyclic monoterpenoid | 1193 | ||
| 39 | 20.705 | traces | dodecane | hydrocarbon | 1200 | ||
| 40 | 23.192 | traces | bornyl acetate | bicyclic monoterpenoid | 1286 | ||
| 41 | 23.585 | 0.15 | carvacrol | monoterpenoid phenol | 1299 | ||
| 42 | 24.931 | traces | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 43 | 25.525 | traces | 0.10 | 0.33 | ylangene | tricyclic sesquiterpene | 1372 |
| 44 | 25.637 | traces | 0.10 | copaene | tricyclic sesquiterpene | 1376 | |
| 45 | 25.845 | traces | hexyl hexanoate | fatty acid ester | 1384 | ||
| 46 | 25.893 | traces | α-bourbonene | tricyclic sesquiterpene | 1384 | ||
| 47 | 26.182 | traces | tetradecane | hydrocarbon | 1400 | ||
| 48 | 26.471 | 0.10 | cis-β-caryophyllene | bicyclic sesquiterpene | 1406 | ||
| 49 | 26.791 | 6.85 | 5.43 | 7.56 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 50 | 27.121 | 1.90 | 0.22 | γ-elemene | monocyclic sesquiterpene | 1434 | |
| 51 | 27.160 | 0.44 | 0.42 | 0.87 | α-bergamotene | bicyclic sesquiterpene | 1435 |
| 52 | 27.248 | traces | traces | α-guaiene | bicyclic sesquiterpene | 1439 | |
| 53 | 27.376 | traces | 0.12 | guaia-6,9-diene | bicyclic sesquiterpene | 1444 | |
| 54 | 27.545 | 0.24 | humulen-(v1) | bicyclic sesquiterpene | 1455 | ||
| 55 | 27.649 | 2.36 | 1.51 | 3.07 | α-humulene | monocyclic sesquiterpene | 1454 |
| 56 | 28.202 | 0.35 | 0.22 | 0.56 | γ-muurolene | bicyclic sesquiterpene | 1477 |
| 57 | 28.290 | traces | 0.25 | α-amorphene | bicyclic sesquiterpene | 1482 | |
| 58 | 28.386 | 0.41 | 4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 | ||
| 59 | 28.378 | 0.20 | 0.15 | selina-4,11-diene | bicyclic sesquiterpene | 1474 | |
| 60 | 28.443 | 0.79 | 0.38 | 1.27 | β-selinene | bicyclic sesquiterpene | 1486 |
| 61 | 28.531 | traces | δ-selinene | bicyclic sesquiterpene | 1495 | ||
| 62 | 28.627 | 1.20 | 0.63 | 1.89 | α-selinene | bicyclic sesquiterpene | 1494 |
| 63 | 28.749 | 0.12 | β-dihydroagarofuran | tricyclic sesquiterpenoid | 1496 | ||
| 64 | 28.819 | 1.01 | 0.49 | 0.97 | α-farnesene | acyclic sesquiterpene | 1508 |
| 65 | 28.996 | 0.10 | traces | 0.18 | γ-cadinene | bicyclic sesquiterpene | 1513 |
| 66 | 29.132 | 2.52 | δ-amorphene | bicyclic sesquiterpene | 1497 | ||
| 67 | 29.388 | 10.00 | 3.26 | 8.90 | γ-selinene | bicyclic sesquiterpene | 1544 |
| 68 | 29.469 | 1.33 | α-bisabolene | monocyclic sesquiterpene | 1540 | ||
| 69 | 29.501 | 13.54 | 4.27 | 11.69 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 70 | 29.757 | 1.74 | 0.85 | germacrene B | monocyclic sesquiterpene | 1557 | |
| 71 | 30.158 | 0.34 | traces | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 72 | 30.334 | 3.09 | 0.10 | guaiol | bicyclic sesquiterpenoid | 1596 | |
| 73 | 30.450 | 0.18 | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | ||
| 74 | 30.663 | 3.76 | 0.11 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 | |
| 75 | 30.799 | 1.25 | traces | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 76 | 30.859 | 0.20 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 77 | 31.045 | 1.45 | traces | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 78 | 31.072 | 3.15 | traces | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 79 | 31.224 | 3.60 | traces | bulnesol | bicyclic sesquiterpenoid | 1667 | |
| 80 | 31.312 | 0.16 | cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 81 | 31.377 | 2.00 | traces | α-bisabolol | monocyclic sesquiterpenoid | 1684 | |
| 82 | 31.575 | 1.02 | juniper camphor | bicyclic sesquiterpenoid | 1692 | ||
| 83 | 32.523 | traces | α-phellandrene dimer | tricyclic terpene | 1801 | ||
| 84 | 32.719 | 0.23 | selina-4,7-diol | bicyclic sesquiterpenoid | 1826 | ||
| 86.85% | 96.45% | 85.43% | |||||
| 44 cpd | 56 cpd | 49 cpd |
| Peak | RT | % (Liq) | % (HS) | % (SPME) | Compound | Type | RI |
|---|---|---|---|---|---|---|---|
| 1 | 9.398 | 0.06 | 4-methyl-octane | hydrocarbon | 863 | ||
| 2 | 9.515 | 0.22 | 1-hexanol | organic alcohol | 868 | ||
| 3 | traces | o-xylene | aromatic hydrocarbon | 887 | |||
| 4 | 10.341 | traces | 2-heptanone | ketone | 891 | ||
| 5 | 10.734 | traces | heptanal | alkyl aldehyde | 901 | ||
| 6 | 11.239 | 0.29 | 5,5-Dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 | ||
| 7 | 11.600 | 1.33 | 0.10 | 3-methyl-2-butenoic acid ethyl ester | fatty acid ester | 924 | |
| 8 | 11.824 | 0.64 | 4.41 | 0.15 | α-pinene | bicyclic monoterpene | 937 |
| 9 | 12.345 | 0.19 | 1.33 | 0.18 | camphene | bicyclic monoterpene | 952 |
| 10 | 12.818 | traces | benzaldehyde | aromatic aldehyde | 962 | ||
| 11 | 13.379 | 0.91 | 5.99 | β-pinene | bicyclic monoterpene | 979 | |
| 12 | 13.941 | traces | 6-methyl-5-heptene-2-one | unsaturated methylated ketone | 986 | ||
| 13 | 13.917 | 0.17 | 2,2,4,6,6-pentamethylheptane | hydrocarbon | 991 | ||
| 14 | 14.037 | 3.30 | 19.69 | 6.11 | β-myrcene | acyclic monoterpene | 991 |
| 15 | 14.398 | 0.02 | α-phellandrene | monocyclic monoterpene | 1005 | ||
| 16 | 14.846 | traces | traces | α-terpinene | monocyclic monoterpene | 1017 | |
| 17 | 14.999 | traces | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 18 | 15.039 | traces | isomyrcenol | acyclic monoterpenoid | 1022 | ||
| 19 | 15.119 | traces | traces | p-cymene | monocyclic monoterpene | 1025 | |
| 20 | 15.271 | 4.39 | 22.62 | 6.07 | limonene | monocyclic monoterpene | 1030 |
| 21 | 15.343 | 0.12 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 22 | 15.632 | traces | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 23 | 16.025 | 0.11 | trans-β-ocimene | acyclic monoterpene | 1049 | ||
| 24 | 16.289 | traces | traces | γ-terpinene | monocyclic monoterpene | 1060 | |
| 25 | 16.562 | traces | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 26 | 17.291 | 0.26 | terpinolene | monocyclic monoterpene | 1088 | ||
| 27 | 17.644 | 1.28 | 3.27 | 1.93 | linalool | acyclic monoterpenoid | 1099 |
| 28 | 17.813 | traces | nonanal | aldehyde | 1104 | ||
| 29 | 18.045 | 0.64 | 1.25 | 0.72 | fenchol | bicyclic monoterpenoid | 1113 |
| 30 | 18.294 | 0.39 | 0.63 | 0.15 | trans-pinene hydrate | bicyclic monoterpenoid | 1140 |
| 31 | 18.598 | 0.10 | allo-ocimene | acyclic monoterpene | 1144 | ||
| 32 | 18.927 | traces | cis-pinene hydrate | bicyclic monoterpenoid | 1121 | ||
| 33 | 19.135 | traces | camphene hydrate | bicyclic monoterpenoid | 1148 | ||
| 34 | 19.672 | 0.22 | 0.24 | 0.20 | borneol | bicyclic monoterpenoid | 1166 |
| 35 | 20.033 | traces | 0.11 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 36 | 20.426 | 0.59 | 0.48 | 0.80 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 37 | 21.147 | traces | 2,4-dimethyl-benzaldehyde | aromatic aldehyde | 1181 | ||
| 38 | 24.931 | traces | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 39 | 15.196 | 0.10 | clovene | tricyclic sesquiterpene | 1440 | ||
| 40 | 25.524 | traces | 0.15 | ylangene | tricyclic sesquiterpene | 1372 | |
| 41 | 25.645 | traces | traces | copaene | tricyclic sesquiterpene | 1376 | |
| 42 | 25.989 | traces | traces | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 43 | 26.390 | traces | sesquithujene | bicyclic sesquiterpene | 1402 | ||
| 44 | 26.462 | traces | 0.38 | cis-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 45 | 26.647 | 0.17 | 0.24 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 | |
| 46 | 26.807 | 16.37 | 15.56 | 18.65 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 47 | 27.023 | traces | β-copaene | tricyclic sesquiterpene | 1432 | ||
| 48 | 27.168 | 2.23 | 1.64 | 3.86 | trans-α-bergamotene | bicyclic sesquiterpene | 1435 |
| 49 | 27.256 | 2.79 | 2.76 | 4.73 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 50 | 27.464 | traces | epi-β-santalene | bicyclic sesquiterpene | 1448 | ||
| 51 | 27.585 | 0.40 | humulene-(v1) | bicyclic sesquiterpene | 1455 | ||
| 52 | 27.657 | 7.50 | 5.57 | 11.76 | α-humulene | monocyclic sesquiterpene | 1454 |
| 53 | 28.042 | traces | aristolochene | bicyclic sesquiterpene | 1476 | ||
| 54 | 28.050 | 0.12 | drima-7,9-diene | bicyclic sesquiterpene | 1461 | ||
| 55 | 28.330 | 0.58 | α-curcumene | monocyclic sesquiterpene | 1483 | ||
| 56 | 28.378 | 0.26 | 0.88 | 4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 | |
| 57 | 28.442 | 1.26 | 0.67 | 2.99 | β-selinene | bicyclic sesquiterpene | 1486 |
| 58 | 28.627 | 1.54 | 0.79 | 3.85 | α-selinene | bicyclic sesquiterpene | 1494 |
| 59 | 28.835 | 6.89 | 3.31 | 8.51 | α-bulnesene | bicyclic sesquiterpene | 1505 |
| 60 | 29.012 | 0.20 | γ-cadinene | bicyclic sesquiterpene | 1513 | ||
| 61 | 29.380 | 4.15 | 1.42 | 5.06 | γ-selinene | bicyclic sesquiterpene | 1538 |
| 62 | 29.468 | 1.08 | α-bisabolene | monocyclic sesquiterpene | 1540 | ||
| 63 | 29.493 | 5.53 | 2.01 | 7.04 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 64 | 29.757 | 0.82 | 0.30 | germacrene B | monocyclic sesquiterpene | 1557 | |
| 65 | 30.158 | 0.77 | traces | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 66 | 30.342 | 4.59 | 0.18 | traces | guaiol | bicyclic sesquiterpenoid | 1596 |
| 67 | 30.438 | traces | β-atlantol | monocyclic sesquiterpenoid | 1607 | ||
| 68 | 30.454 | 0.35 | traces | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | |
| 69 | 30.663 | 5.02 | 0.19 | 0.19 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 |
| 70 | 30.799 | 1.81 | traces | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 71 | 30.859 | 0.33 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 72 | 31.040 | 2.56 | traces | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 73 | 31.072 | 4.76 | 0.10 | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 74 | 31.232 | 4.82 | 0.09 | traces | bulnesol | bicyclic sesquiterpenoid | 1667 |
| 75 | 31.312 | traces | cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 76 | 31.380 | 0.60 | α-bisabolol | monocyclic sesquiterpenoid | 1684 | ||
| 77 | 31.575 | 0.54 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 88.10% | 97.17% | 88.85% | |||||
| 34 cpd | 57 cpd | 47 cpd |
| GC/MS | LOH LL1 | LOH LL2 | LOH LL3 | LOH LL4 |
|---|---|---|---|---|
| Liquid | 37 | 38 | 45 | 34 |
| HS | 51 | 74 | 55 | 57 |
| SPME | 46 | 57 | 49 | 47 |
| All identified different compounds | 67 | 94 | 84 | 77 |
| Compound | LOH LL1 µg/g | LOH LL2 µg/g | LOH LL3 µg/g | LOH LL4 µg/g |
|---|---|---|---|---|
| α-pinene | 45.6 | 60.0 | 101.6 | 47.0 |
| camphene | 13.5 | 18.33 | 29.38 | 14.4 |
| β-pinene | 83.7 | 102.0 | 147.5 | 62.0 |
| β-myrcene | 2136.2 | 294.3 | 744.1 | 544.2 |
| limonene | 473.3 | 705.9 | 803.7 | 457.1 |
| linalool | 151.5 | 236.1 | 367.6 | 231.3 |
| β-caryophyllene | 1322.1 | 1704.0 | 542.1 | 1599.4 |
| α-humulene | 535.0 | 538.4 | 152.1 | 605.2 |
| caryophyllene oxide | 0.1 | 0.2 | 0.2 | 0.2 |
| guaiol | - | 313.7 | 288.8 | 522.3 |
| α-bisabolol | - | - | 170.0 | 64.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanuš, L.O. Comparison of Three Gas Chromatographic Methods—Identification of Terpenes and Terpenoids in Cannabis sativa L. Appl. Sci. 2024, 14, 6476. https://doi.org/10.3390/app14156476
Hanuš LO. Comparison of Three Gas Chromatographic Methods—Identification of Terpenes and Terpenoids in Cannabis sativa L. Applied Sciences. 2024; 14(15):6476. https://doi.org/10.3390/app14156476
Chicago/Turabian StyleHanuš, Lumír Ondřej. 2024. "Comparison of Three Gas Chromatographic Methods—Identification of Terpenes and Terpenoids in Cannabis sativa L." Applied Sciences 14, no. 15: 6476. https://doi.org/10.3390/app14156476
APA StyleHanuš, L. O. (2024). Comparison of Three Gas Chromatographic Methods—Identification of Terpenes and Terpenoids in Cannabis sativa L. Applied Sciences, 14(15), 6476. https://doi.org/10.3390/app14156476






