Exploring the Potential of TNT and Aniline Coexistence to Enhance Their Transports in Saturated Chinese Loess
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chinese Loess Characterization
3.2. Batch Tests
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
3.2.3. Thermodynamic Analysis
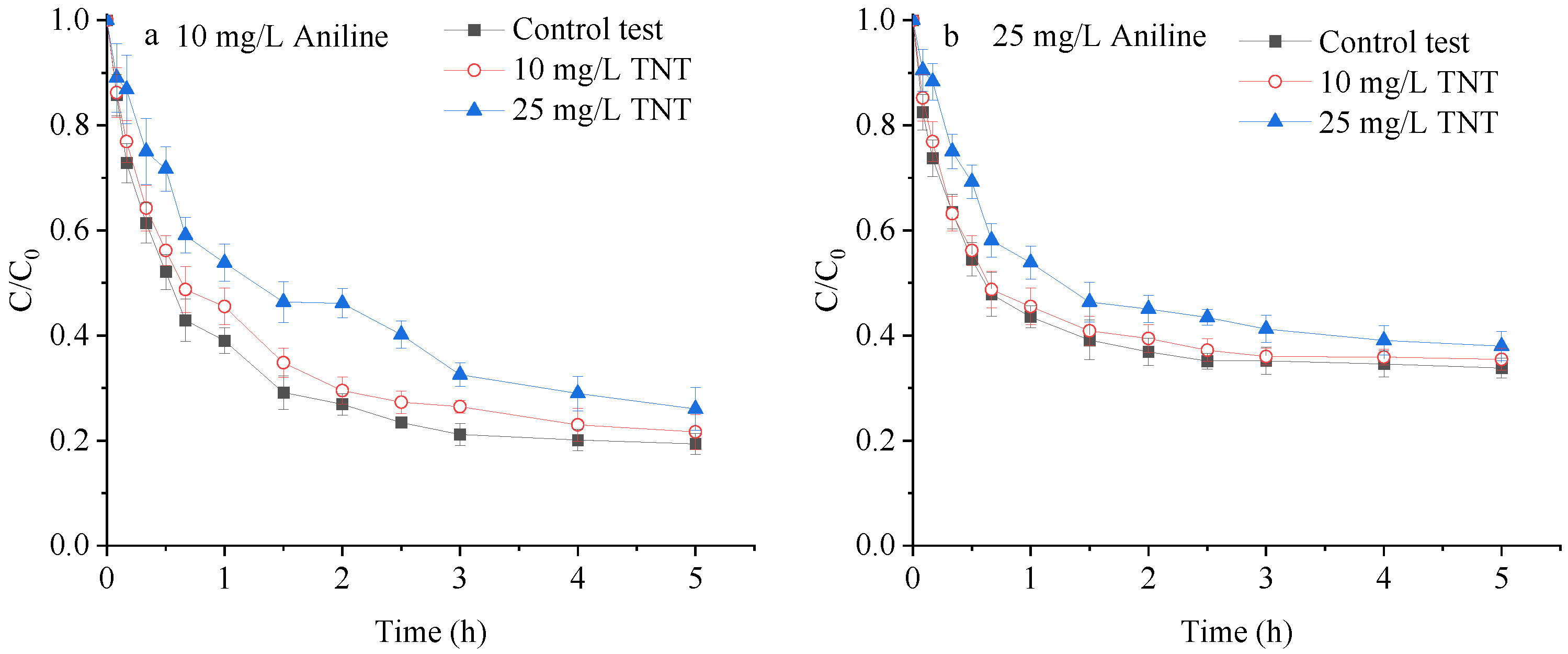
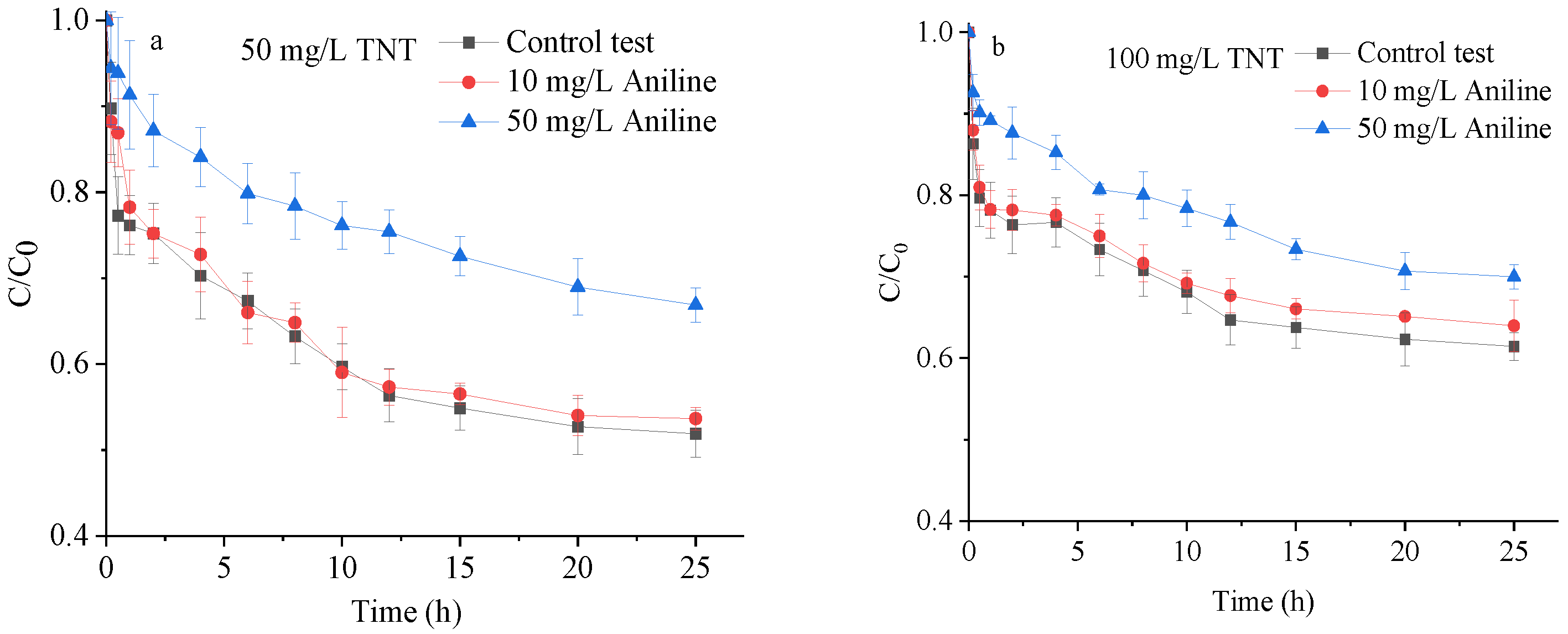
3.2.4. Interaction between TNT and Aniline Adsorption on the Saturated Loess
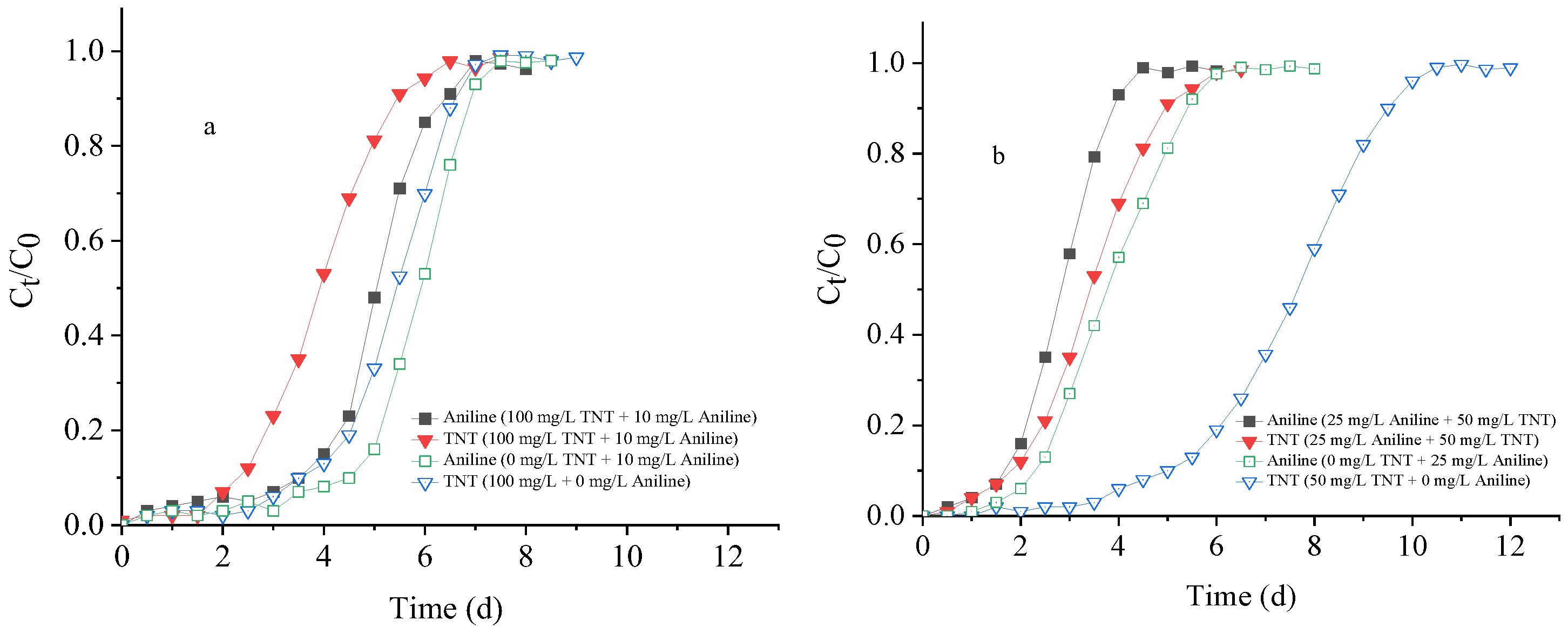
3.3. Column Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Hua, Z.; Cai, Y.; Shen, X.; Li, C.; Liu, X. Effects of hydrodynamic conditions on the sorption behaviors of aniline on sediment with coexistence of nitrobenzene. Environ. Sci. Pollut. Res. 2015, 22, 11595–11605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, R.; Hu, S.; Zhong, Y.; Wu, Y. Aromatic compounds releases aroused by sediment resuspension alter nitrate transformation rates and pathways during aerobic-anoxic transition. J. Hazard. Mater. 2022, 424, 127365. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Liu, H.; Cai, Y.; Qi, X.; Dang, Z. Multicomponent adsorption of heavy metals on biogenic hydroxyapatite: Surface functional groups and inorganic mineral facilitating stable adsorption of Pb(II). J. Hazard. Mater. 2023, 443, 130167. [Google Scholar] [CrossRef]
- Pintor, A.; Brandao, C.C.; Boaventura, R.A.; Botelho, C.M.S. Multicomponent adsorption of pentavalent As, Sb and P on iron-coated cork granulates. J. Hazard. Mater. 2021, 406, 124339. [Google Scholar] [CrossRef]
- Forgionny, A.; Acelas, N.Y.; Ocampo-Pérez, R.; Padilla-Ortega, E.; Leyva-Ramos, R.; Flórez, E. Understanding mechanisms in the adsorption of lead and copper ions on chili seed waste in single and multicomponent systems: A combined experimental and computational study. Environ. Sci. Pollut. Res. 2021, 28, 23204–23219. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Ong, S.K.; Sutthirat, C.; Osathaphan, K. Competitive modeling of sorption and transport of Pb2+, Ni2+, Mn2+ and Zn2+ under binary and multi-metal systems in lateritic soil columns. Geoderma 2012, 189, 278–287. [Google Scholar] [CrossRef]
- Padilla, J.T.; Selim, H.M.; Gaston, L.A. Modeling the competitive sorption and transport of Ni(II) and Zn(II) in soils: Comparing two multicomponent approaches. J. Contam. Hydrol. 2023, 252, 104108. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Frankki, S.; Shchukarev, A.; Skyllberg, U. Binding of 2,4,6-Trinitrotoluene, aniline, and nitrobenzene to dissolved and particulate soil organic matter. Environ. Sci. Technol. 2004, 38, 3074–3080. [Google Scholar] [CrossRef]
- Rosen, G.; Lotufo, G.R.; Belden, J.B.; George, R.D. Environmental characterization of underwater munitions constituents at a former military training range. Environ. Toxicol. Chem. 2022, 41, 275–286. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Wang, S.; Zhao, S.; Nie, Y.; Liao, X.; Cao, H.; Yin, H.; Liu, X. Contamination characteristics of energetic compounds in soils of two different types of military demolition range in China. Environ. Pollut. 2022, 295, 118654. [Google Scholar] [CrossRef]
- Spalding, R.F.; Fulton, J.W. Groundwater munition residues and nitrate near Grand Island, Nebraska, U.S.A. J. Contam. Hydrol. 1988, 2, 139–153. [Google Scholar] [CrossRef]
- Craig, H.D.; Taylor, S. Framework for evaluating the fate, transport, and risks from conventional munitions compounds in underwater environments. Mar. Technol. Soc. J. 2011, 45, 35–46. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.W.; Chen, Y.M.; Zhan, L.T.; Li, Z.Z.; Tang, Q. Adsorption behavior and mechanism of Cd(II) on loess soil from China. J. Hazard. Mater. 2009, 172, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Burrf, G.S.; Zhou, W.J.; Chen, N.; Hou, Y.Y.; Du, H.; Fu, Y.C.; Lu, X.F. The deficiency of organic matter 14C dating in Chinese Loess-paleosol sample. Quat. Geochronol. 2020, 56, 101051. [Google Scholar] [CrossRef]
- Sharma, P.; Mayes, M.A.; Tang, G. Role of soil organic carbon and colloids in sorption and transport of TNT, RDX and HMX in training range soils. Chemosphere 2013, 92, 993–1000. [Google Scholar] [CrossRef]
- Shang, H.T.; Wang, J.L.; Wu, T.; Lin, J.; Mao, B.C. Adsorption of naphthalene on loess soil of Northwestern China. Energy Environ. 2020, 31, 1335–1349. [Google Scholar] [CrossRef]
- Meng, Z.H.; Hu, S.; Sun, R.; Meng, C.Z.; Wu, Y.; Sun, X. Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns, Mechanism and Processes. Water 2024, 16, 180. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, J.W.; Jiang, J.Q.; Masum, H.; Xie, H.J. Lead adsorption on loess under high ammonium environment. Environ. Sci. Pollut. Res. 2021, 28, 4488–4502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, Y.; Chan, J.; Wang, S.C.; Hu, S.H. Batch adsorption and column transport studies of 2,4,6-trinitrotoluene in Chinese loess. Bull. Environ. Contam. Tox. 2019, 103, 75–81. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Z.H.; Wang, S.C.; Wu, Y.; Hu, S.H.; Sun, R. Batch adsorption and column leaching studies of aniline in Chinese loess under different hydrochemical conditions. Bull. Environ. Contam. Tox. 2020, 104, 511–519. [Google Scholar] [CrossRef]
- Hu, S.H.; Lu, C.; Zhang, C.J.; Zhang, Y.J.; Yao, H.R.; Wu, Y. Effects of fresh and degraded dissolved organic matter derived from maize straw on copper sorption on farmland loess. J. Soils Sediments 2016, 16, 327–338. [Google Scholar] [CrossRef]
- Qiao, Z.X.; Sun, R.; Wu, Y.; Hu, S.H.; Liu, X.Y.; Chan, J.W.; Mi, X. Characteristics and metabolic pathway of the bacteria for heterotrophic nitrification and aerobic denitrification in aquatic ecosystems. Environ. Res. 2020, 191, 110069. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Sheng, G.Y.; Manes, M. A partition-limited model for the plant uptake of organic contaminants from soil and water. Environ. Sci. Technol. 2001, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.D.; Mark, N.W.; Taylor, S. Batch soil adsorption and column transport studies of 2,4-dinitroanisole (DNAN) in soils. J. Contam. Hydrol. 2017, 199, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.L.; Qiao, H.; Zhou, C.Z.; Peng, W.; Zhang, K.; Lu, S. Effects of Fulvic Acid on TNT Adsorption in Soil. Soil Sediment Contam. 2018, 27, 186–199. [Google Scholar] [CrossRef]
- Dontsova, K.M.; Yost, S.L.; Simunek, J.; Pennington, J.C.; Williford, C.W. Dissolution and transport of TNT, RDX, and composition B in saturated soil columns. J. Environ. Qual. 2006, 35, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Do, D.D. Adsorption Analysis: Equilibrium and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Trans. Inst. Chem. Eng. B. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Jaramillo, A.M.; Douglas, T.A.; Walsh, M.E.; Trainor, T.P. Dissolution and sorption of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and 2,4,6-trinitrotoluene (TNT) residues from detonated mineral surfaces. Chemosphere 2011, 84, 1058–1065. [Google Scholar] [CrossRef]
- Rawat, A.P.; Kumar, V.; Singh, P.; Shukla, A.C.; Singh, D.P. Kinetic behavior and mechanism of arsenate adsorption by loam and sandy loam soil. Soil Sediment Contam. 2022, 31, 15–39. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Srivastava, V.C. Multicomponent adsorption isotherm modeling using thermodynamically inconsistent and consistent models. AIChE J. 2019, 65, e16727. [Google Scholar] [CrossRef]
- Hefne, J.A.; Mekhemer, W.K.; Alandis, N.M.; Aldayel, O.A.; Alajyan, T. Kinetic and thermodynamic study of the adsorption of Pb(II) from aqueous solution to the natural and treated bentonite. Int. J. Phys. Sci. 2008, 3, 281–288. [Google Scholar]
- Todde, G.; Jha, S.K.; Subramanian, G.; Shukla, M.K. Adsorption of TNT, DNAN, NTO, FOX7, and NQ on cellulose, chitin, and cellulose triacetate. Insights from density functional theory calculations. Surf. Sci. 2018, 668, 54–60. [Google Scholar] [CrossRef]
- Polyakov, V.; Kadoya, W.; Beal, S.; Morehead, H.; Hunt, E.; Cubello, F.; Meding, S.M.; Dontsova, K. Transport of insensitive munitions constituents, NTO, DNAN, RDX, and HMX in runoff and sediment under simulated rainfall. Sci. Total Environ. 2023, 866, 161434. [Google Scholar] [CrossRef]
- Kumar, K.V.; de Castro, M.M.; Martinez-Escandell, M.; Molina-Sabio, M.; Silvestre-Albero, J.; Rodriguez-Reinoso, F. A continuous site energy distribution function from Redlich–Peterson isotherm for adsorption on heterogeneous surfaces. Chem. Phys. Lett. 2010, 492, 187–192. [Google Scholar] [CrossRef]
- Xie, H.J.; Wang, S.Y.; Qiu, Z.H.; Jiang, J.Q. Adsorption of NH4+-N on Chinese loess: Non-equilibrium and equilibrium investigations. J. Environ. Manag. 2017, 202, 46–54. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Interaction of metal ions with clays: I. A case study with Pb (II). Appl. Clay Sci. 2005, 30, 199–208. [Google Scholar] [CrossRef]
- Ghasemi, J.; Asadpour, S. Thermodynamics’ study of the adsorption process of methylene blue on activated carbon at different ionic strengths. J. Chem. Thermodyn. 2007, 39, 967–971. [Google Scholar] [CrossRef]
- Kumar, M.; Tamilarasan, R. Kinetics, equilibrium data and modeling studies for the sorption of chromium by Prosopis juliflora bark carbon. Arab. J. Chem. 2017, 10, 1567–1577. [Google Scholar] [CrossRef]
- Koyuncu, H.; Kul, A.R. Removal of aniline from aqueous solution by activated kaolinite: Kinetic, equilibrium and thermodynamic studies. Colloid Surf. A 2019, 569, 59–66. [Google Scholar] [CrossRef]
- Acosta, J.; Jansen, A.; Kalbitz, B.; Faz, K.; Martinez-Martinez, A.S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, T.; Wei, C.H. Competitive Adsorption Between Phenol, Aniline and n-Heptane in Tailrace Coking Wastewater. Water Air Soil Pollut. 2013, 224, 1362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Guo, Q.; Zhang, Z.; Meng, C.; Sun, R.; Hu, S.; Shen, J.; Sun, C. Exploring the Potential of TNT and Aniline Coexistence to Enhance Their Transports in Saturated Chinese Loess. Appl. Sci. 2024, 14, 6548. https://doi.org/10.3390/app14156548
Wu Y, Guo Q, Zhang Z, Meng C, Sun R, Hu S, Shen J, Sun C. Exploring the Potential of TNT and Aniline Coexistence to Enhance Their Transports in Saturated Chinese Loess. Applied Sciences. 2024; 14(15):6548. https://doi.org/10.3390/app14156548
Chicago/Turabian StyleWu, Yaoguo, Qian Guo, Zherui Zhang, Chengzhen Meng, Ran Sun, Sihai Hu, Jiaru Shen, and Changyu Sun. 2024. "Exploring the Potential of TNT and Aniline Coexistence to Enhance Their Transports in Saturated Chinese Loess" Applied Sciences 14, no. 15: 6548. https://doi.org/10.3390/app14156548




