Packaging Matters: Preservation of Antioxidant Compounds of Fresh Stinging Nettle Leaves (Urtica dioica L.)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Packaging Conditions
2.3. Characterization of Packaging Materials
2.4. Respiration of Nettle Leaves
2.5. Nettle Leaf Analysis
2.5.1. Water Content
2.5.2. Chromaticity Parameter Analyses
2.5.3. Chemical Analyses
Pigment Compounds
Ascorbic Acid
Phenolic Compounds
Antioxidant Capacity
2.6. Statistical Analysis
3. Results
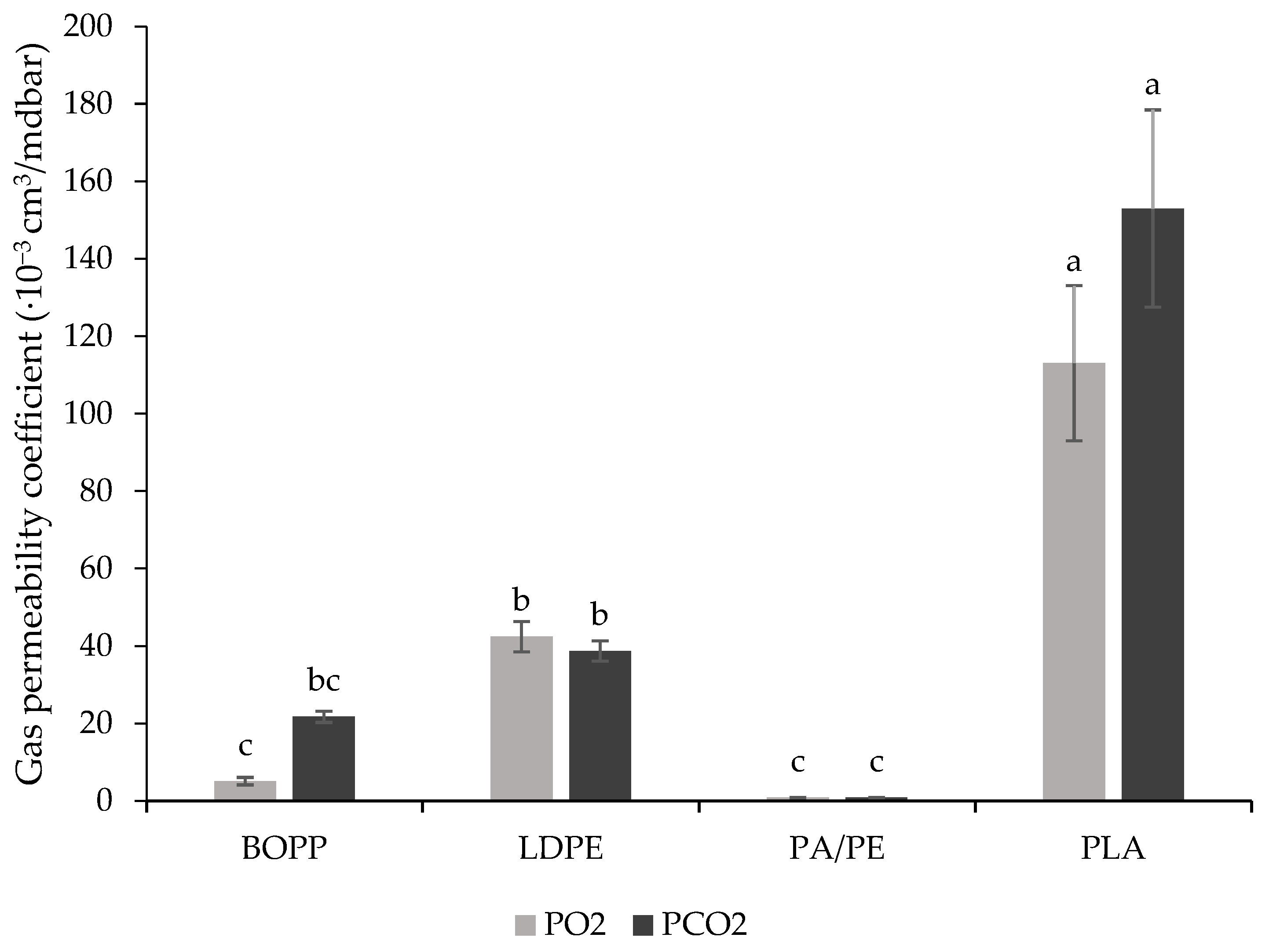
3.1. Physicochemical and Barrier Properties of Used Packaging Materials
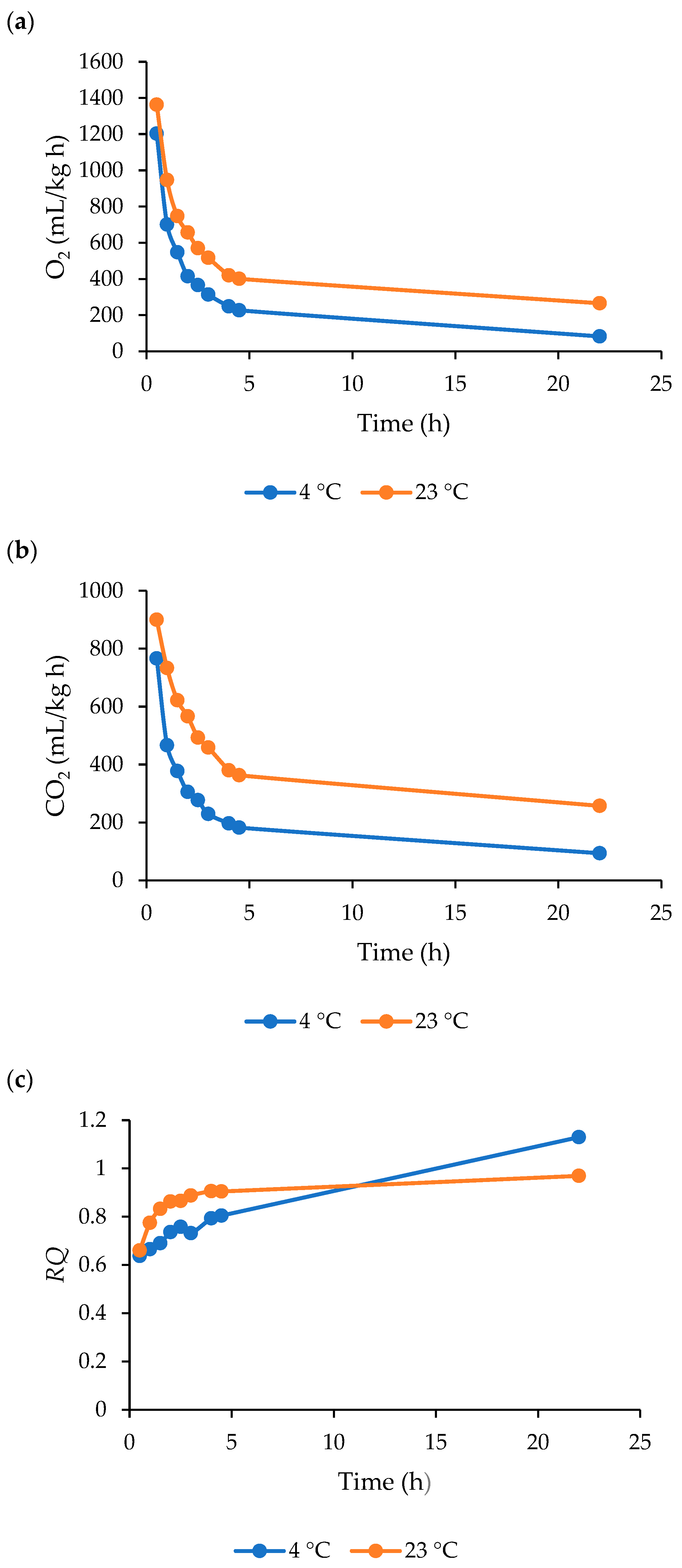
3.2. Respiration of Nettle Leaves
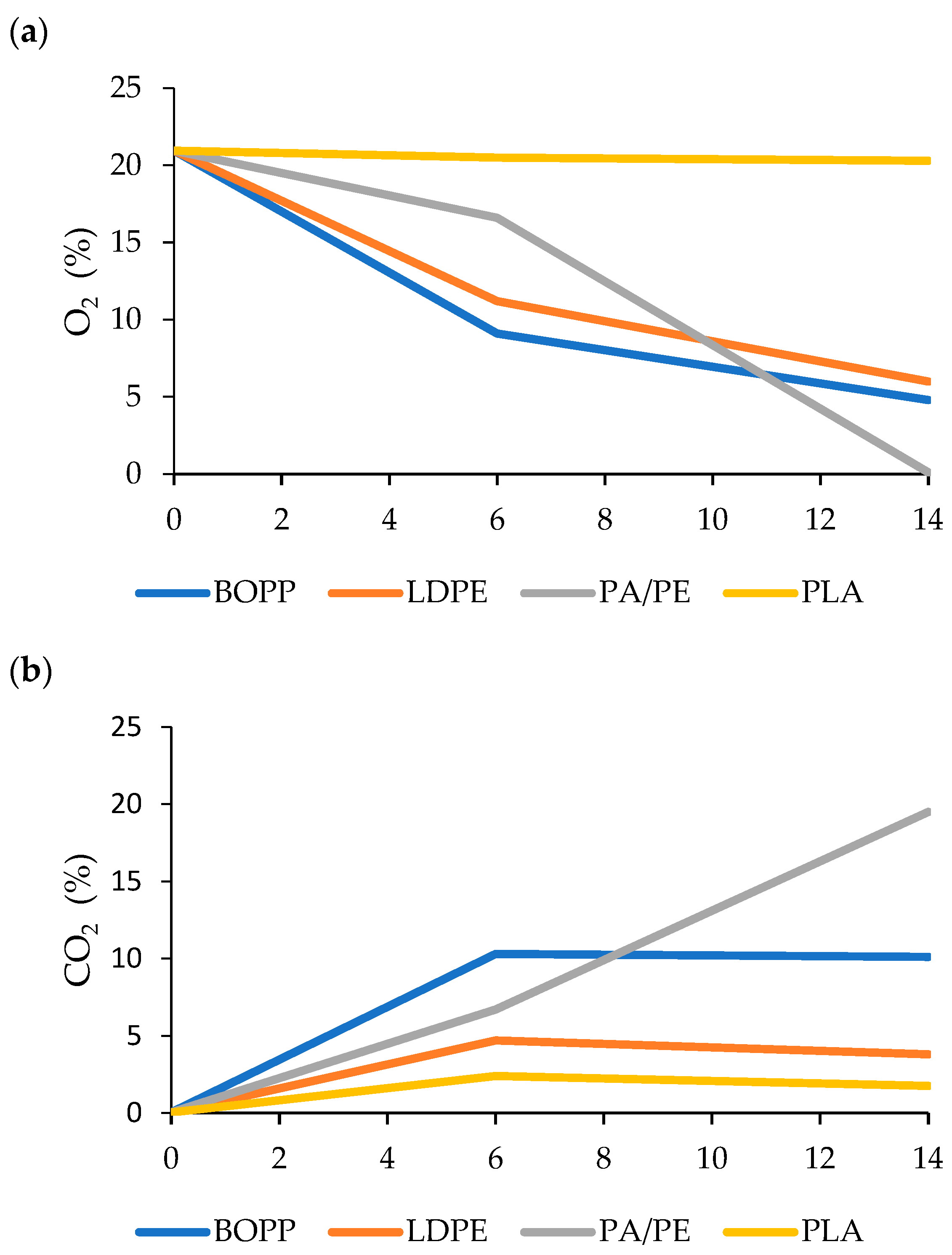
3.3. Gas Composition during the Storage Period
3.4. Characteristics of Fresh and Packed Nettle Leaves
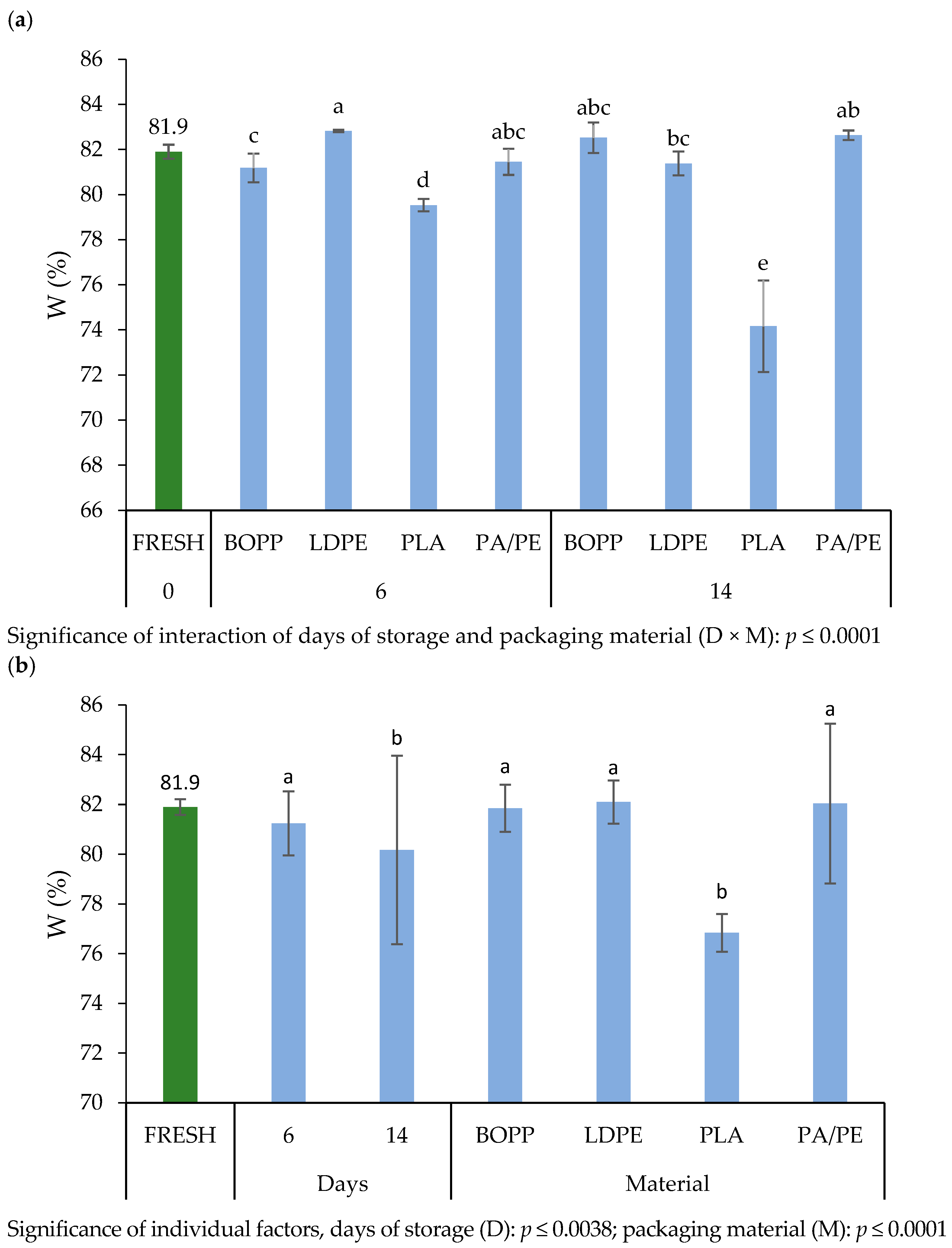
3.4.1. Water Content of Nettle Leaves
3.4.2. Chromaticity Parameters
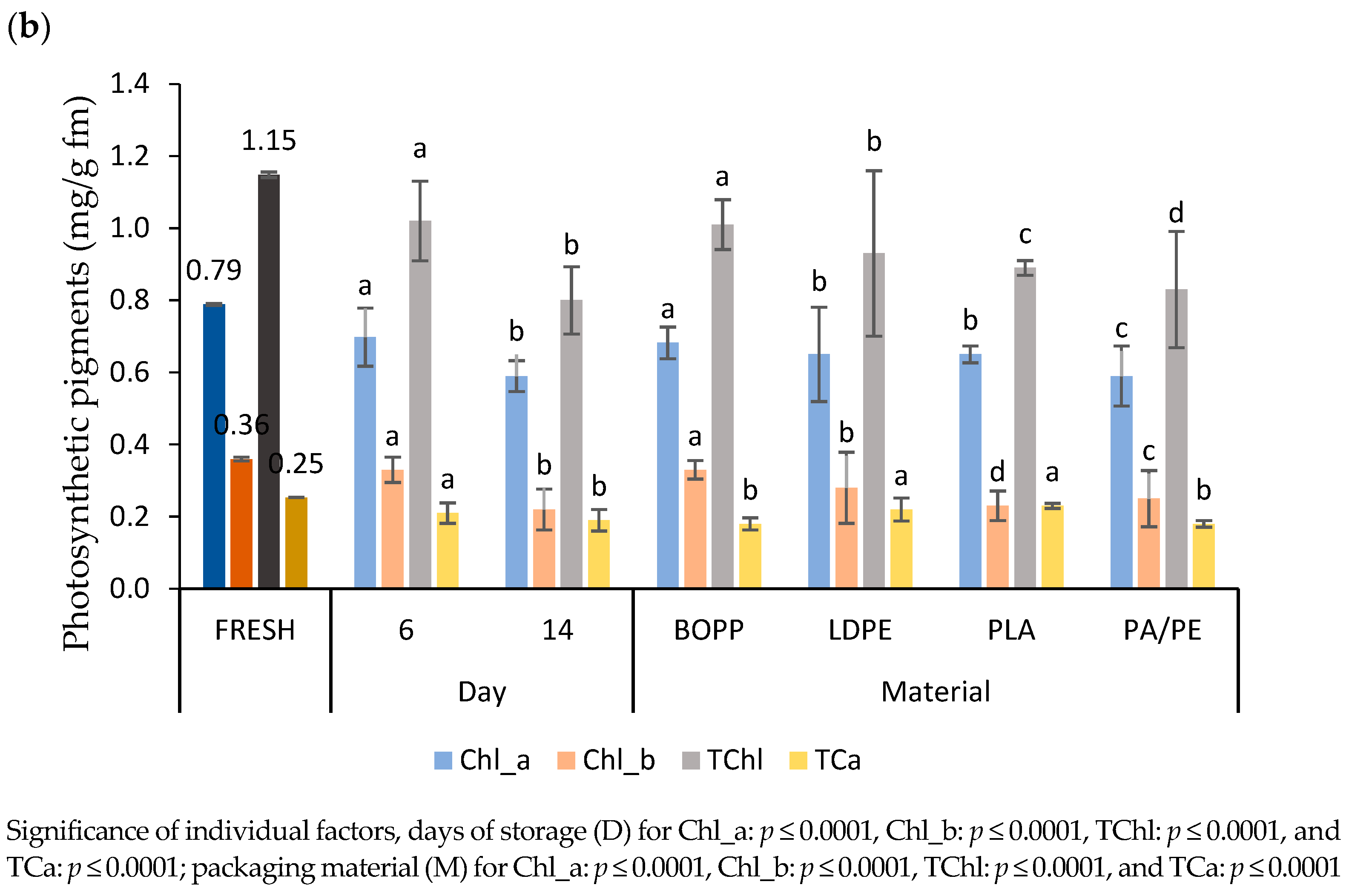
3.4.3. Photosynthetic Pigments
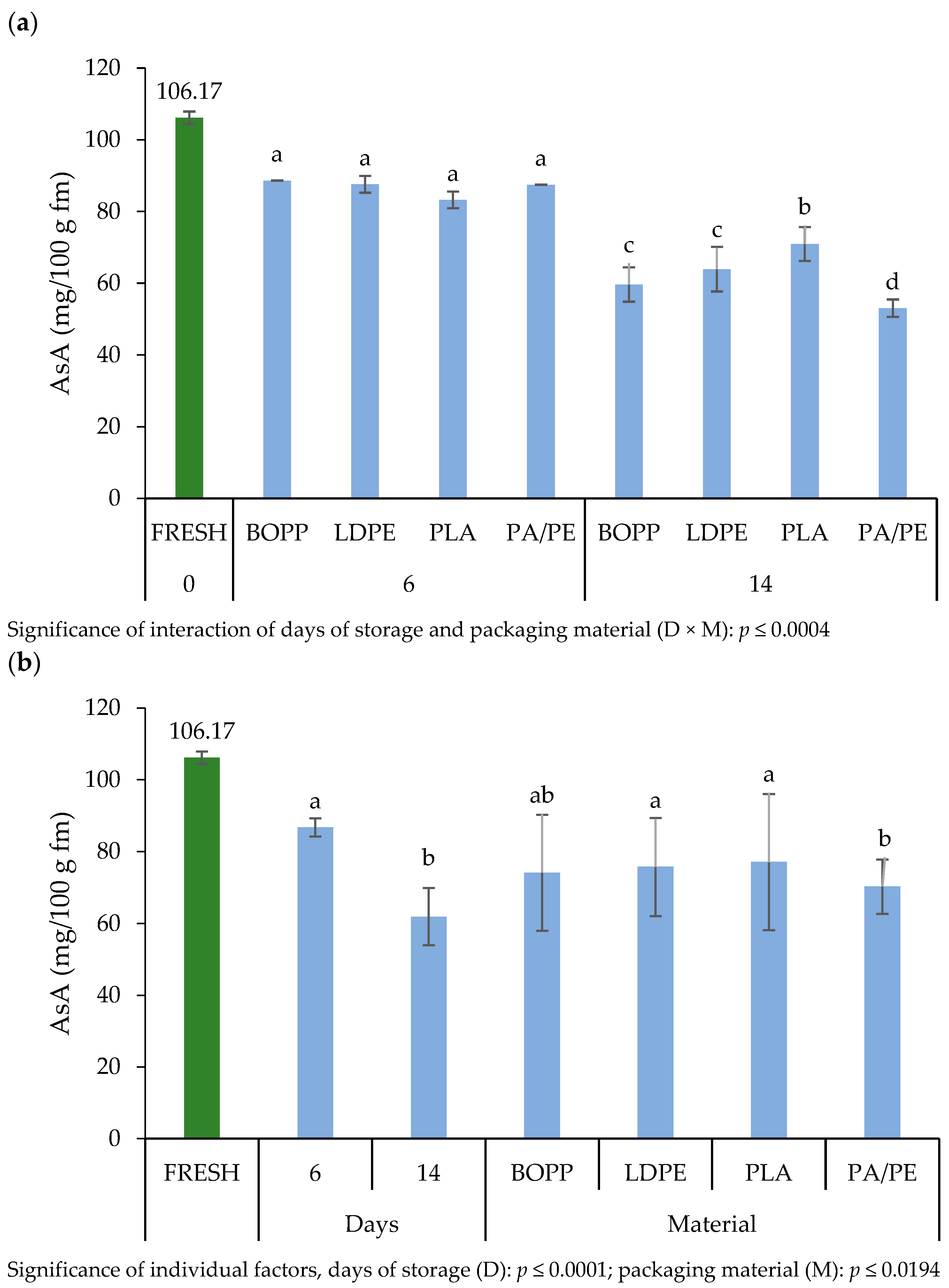
3.4.4. Ascorbic Acid
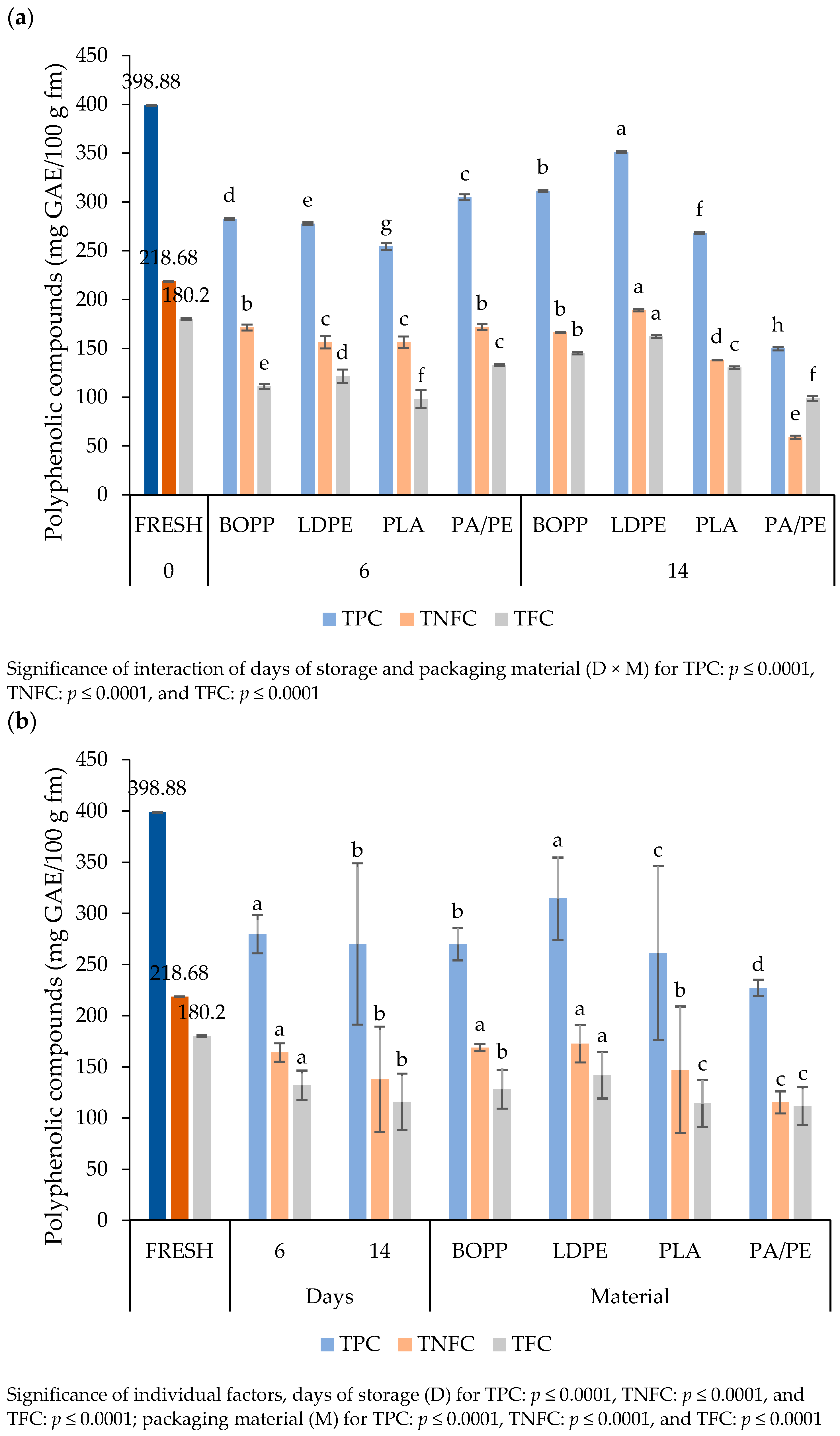
3.4.5. Phenolic Compounds
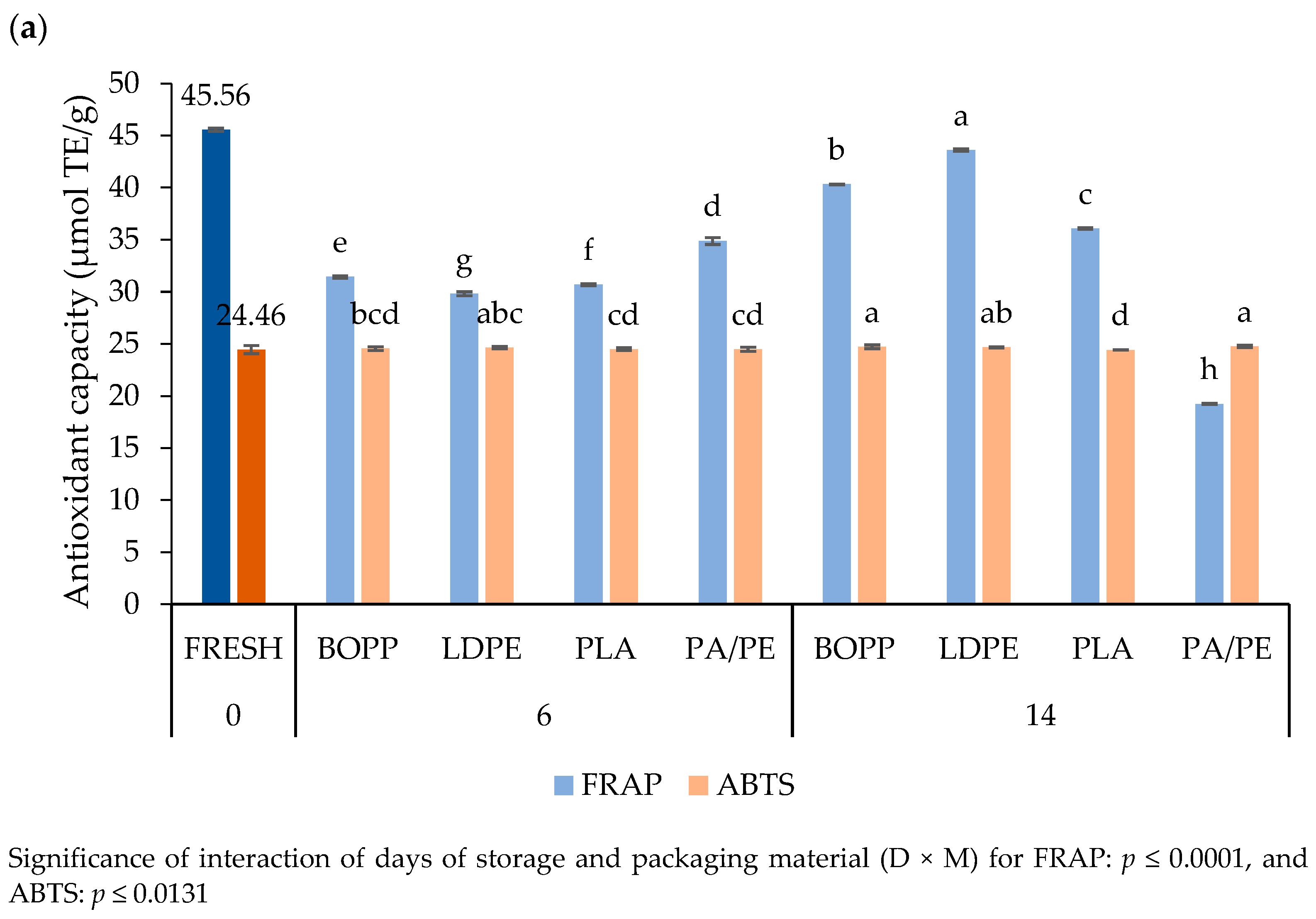
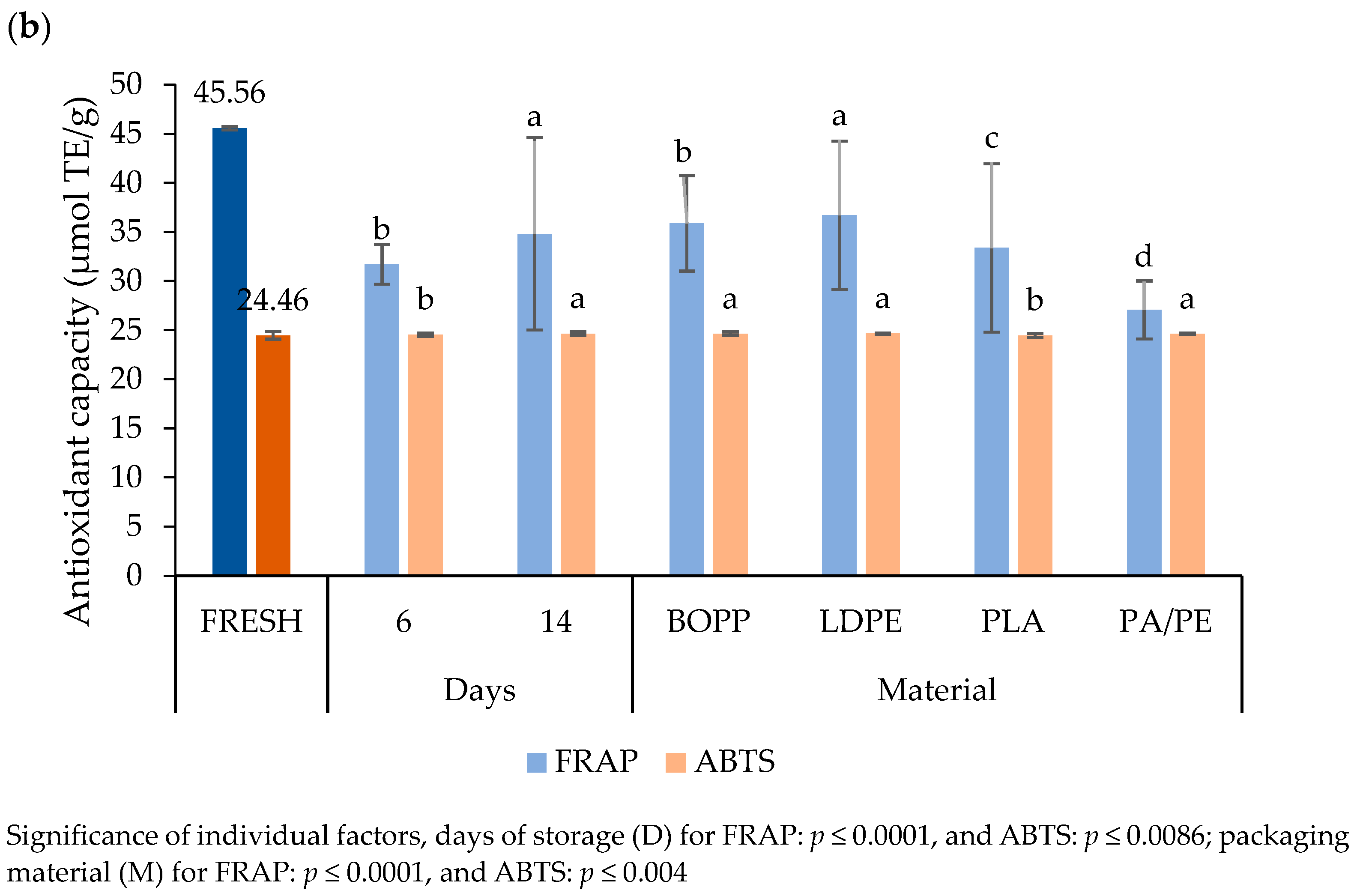
3.4.6. Antioxidant Capacity
4. Discussion
4.1. Physicochemical and Barrier Properties of Used Packaging Materials
4.2. Respiration of Nettle Leaves
4.3. Gas Composition during the Storage Period
4.4. Characteristics of Fresh and Packed Nettle Leaves
4.4.1. Water Content of Nettle Leaves
4.4.2. Chromaticity Parameters
4.4.3. Photosynthetic Pigments
4.4.4. Ascorbic Acid
4.4.5. Phenolic Compounds
4.4.6. Antioxidant Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, M.C.N. Impact of Environmental Conditions on Fruit and Vegetable Quality. Stewart Postharvest Rev. 2008, 4, 1–14. [Google Scholar] [CrossRef]
- Beshai, H.; Sarabha, G.K.; Rathi, P.; Alam, A.U.; Deen, M.J. Freshness Monitoring of Packaged Vegetables. Appl. Sci. 2020, 10, 7937. [Google Scholar] [CrossRef]
- Kanlayanarat, S. Postharvest Technologies for Fresh Leafy Vegetables in Thailand. In Best Practices in Postharvest Management of Leafy Vegetables in Greater Mekong Subregion Countries, 9th ed.; Acedo, A.L., Jr., Weinberger, K., Eds.; AVRDC-World Vegetable Center: Hanoi, Vietnam, 2007; pp. 44–83. [Google Scholar]
- Degl’Innocenti, E.; Pardossi, A.; Tognoni, F.; Guidi, L. Physiological Basis of Sensitivity to Enzymatic Browning in ‘Lettuce’, ‘Escarole’ and ‘Rocket Salad’ When Stored as Fresh-Cut Products. Food Chem. 2007, 104, 209–215. [Google Scholar] [CrossRef]
- Bovi, G.G.; Caleb, O.J.; Linke, M.; Rauh, C.; Mahajan, P.V. Transpiration and Moisture Evolution in Packaged Fresh Horticultural Produce and the Role of Integrated Mathematical Models: A Review. Biosyst. Eng. 2016, 150, 24–39. [Google Scholar] [CrossRef]
- Yu, H.; Sun, M.; Liu, M.; Xiong, M.; Liu, L.; Wang, T.; Guo, Y.; Xie, Y.; Yao, W. Postharvest Shelf Life Simulation for Lettuce (Lactuca sativa L.) Based on Coupling Dynamic Models of Respiration, Gas Exchange, and Pseudomonas fluorescens Growth. Food Packag. Shelf Life 2023, 35, 101021. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Khalil, R.K.S. Effect of Abusive Storage Temperatures on Growth and Survival of Escherichia coli O157:H7 on Leafy Salad Vegetables in Egypt. LWT—Food Sci. Technol. 2016, 65, 954–962. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative Processes and Technologies for Modified Atmosphere Packaging of Fresh and Fresh-Cut Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2017, 59, 411–422. [Google Scholar] [CrossRef]
- Mudau, A.R.; Soundy, P.; Araya, H.T.; Mudau, F.N. Influence of Modified Atmosphere Packaging on Postharvest Quality of Baby Spinach (Spinacia oleracea L.) Leaves. HortScience 2018, 53, 224–230. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A Review on the Modified Atmosphere Preservation of Fruits and Vegetables with Cutting-Edge Technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Petersen, L.; Nielsen, P.V.; Olsen, M.B. Physical and Mechanical Properties of Biobased Materials. Starch 2001, 53, 356–361. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Engineering Evaluation of Thickness and Type of Packaging Materials Based on the Modified Atmosphere Packaging Requirements of Guava (Cv. Baruipur). LWT 2017, 78, 273–280. [Google Scholar] [CrossRef]
- Wójtowicz, A. 2018 Selected Properties of Multilayer Films Applied for Vacuum and Modified Atmosphere Packaging Systems. Agric. Eng. 2018, 22, 89–98. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.B.; Tang, X.; Etxeberria, A.; Chen, E.Y.-X.; Sardon, H. Packaging Materials with Desired Mechanical and Barrier Properties and Full Chemical Recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef]
- Szlachetka, O.; Witkowska-Dobrev, J.; Baryła, A.; Dohojda, M. Low-Density Polyethylene (LDPE) Building Films—Tensile Properties and Surface Morphology. J. Build. Eng. 2021, 44, 103386. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of Polymeric Food Packaging Materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Cannarsi, M.; Sinigaglia, M. Use of Biodegradable Films for Prolonging the Shelf Life of Minimally Processed Lettuce. J. Food Eng. 2008, 85, 317–325. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Parideh, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Torrieri, E.; Immirzi, B.; Di Lorenzo, M.L. Cellulose-Based Sustainable Packaging of Leafy Vegetables: An Experimental Study on the Shelf Life of Baby Spinach. Org. Agric. 2023, 14, 167–180. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2016, 33, 644–663. [Google Scholar] [CrossRef]
- Kowalska, B.; Szczech, M. Differences in Microbiological Quality of Leafy Green Vegetables. Ann. Agric. Environ. Med. 2022, 29, 238–245. [Google Scholar] [CrossRef]
- Opačić, N.; Radman, S.; Fabek Uher, S.; Benko, B.; Voća, S.; Šic Žlabur, J. Nettle Cultivation Practices—From Open Field to Modern Hydroponics: A Case Study of Specialized Metabolites. Plants 2022, 11, 483. [Google Scholar] [CrossRef]
- Dujmović, M.; Opačić, N.; Radman, S.; Fabek Uher, S.; Voća, S.; Šic Žlabur, J. Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress. Agriculture 2023, 13, 1358. [Google Scholar] [CrossRef]
- Dujmović, M.; Opačić, N.; Radman, S.; Fabek Uher, S.; Čoga, L.; Petek, M.; Voća, S.; Šic Žlabur, J. How to Increase the Nutritional Quality of Stinging Nettle Through Controlled Plant Nutrition. Food Technol. Biotechnol. 2023, 16, 451–464. [Google Scholar] [CrossRef]
- ASTM E96-80; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 1980.
- München, G. Gas Permeability Testing Manual; Brugger Feinmechanik GmbH: Munich, Germany, 2008. [Google Scholar]
- Singh, R.; Giri, S.K.; Kulkarni, S.D. Respiratory Behavior of Turning Stage Mature Tomato (Solanum lycopersicum L.) Under Closed System at Different Temperature. Croat. J. Food Sci. Technol. 2013, 5, 78–84. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Holm, G. Chlorophyll Mutations in Barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wines, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- SAS®/STAT 9.4; SAS Institute Inc.: Cary, NC, USA, 2018.
- Shiku, Y.; Hamaguchi, P.Y.; Tanaka, M. Effect of pH on the Preparation of Edible Films Based on Fish Myofibrillar Proteins. Fish. Sci. 2003, 69, 1026–1032. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Di Giuseppe, D.; Kermanizadeh, A.; Crespo, A.M.; Mencattini, A.; Ghibelli, L.; Mancini, V.; Wlodarczyk, K.L.; Hand, D.P.; Martinelli, E.; et al. Polylactic is a Sustainable, Low Absorption, Low Auto-Fluorescence, Alternative to Other Plastics for Microfluidic and Organ-On-Chip Applications. Anal. Chem. 2020, 92, 6693–6701. [Google Scholar] [CrossRef]
- Emblem, A. Plastics Properties for Packaging Materials. In Packaging Technology, 1st ed.; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Cambrige, UK, 2012. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent Semi-Crystalline Polymeric Materials and Their Nanocomposites: A Review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, Water Vapor Barrier and Antimicrobial Properties of PLA/nanoclay Composite Films. Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. On the Use of Plant Cellulose Nanowhiskers to Enhance the Barrier Properties of Polylactic Acid. Cellulose 2010, 17, 987–1004. [Google Scholar] [CrossRef]
- Shogren, R. Water Vapor Permeability of Biodegradable Polymers. J. Environ. Polym. Degr. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Syakir, M.I.; Fazita, M.R.N.; Bhat, A.; Banerjee, A.; Fizree, H.M.; Rizal, S.; Tahir, P.M. Barrier Properties of Biocomposites/Hybrid Films. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites, 1st ed.; Jawaid, M., Sultan, M.T.H., Saba, N., Eds.; Woodhead Publishing: Cambrige, UK, 2019; pp. 241–258. [Google Scholar] [CrossRef]
- Baschetti, M.G.; Minelli, M. Test Methods for the Characterization of Gas and Vapor Permeability in Polymers for Food Packaging Application: A review. Polym. Test. 2020, 89, 106606. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Zhao, M.; Ruan, C.-Q. The Reduce of Water Vapor Permeability of Polysaccharide-Based Films in Food Packaging: A Comprehensive Review. Carbohydr. Polym. 2023, 321, 121267. [Google Scholar] [CrossRef]
- Ščetar, M.; Kurek, M.; Galić, K. Trends in Fruit and Vegetable Packaging—A Review. Croat. J. Food Sci. Technol. 2010, 5, 69–86. [Google Scholar]
- Verde, A.B.; Alvim, I.D.; Luccas, V.; Junior, L.M.; Alves, R.M.V. The Influence of Formulation and Packaging Material on the Rheological Properties of Milk Chocolate. Appl. Food Res. 2022, 2, 100199. [Google Scholar] [CrossRef]
- Gajdoš, J.; Galić, K.; Kurtanjek, Ž.; Ciković, N. Gas Permeability and DSC Characteristics of Polymers Used in Food Packaging. Polym. Test. 2000, 20, 49–57. [Google Scholar] [CrossRef]
- Bodbodak, S.; Moshfeghifar, M. Advances in controlled atmosphere storage of fruits and vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.V., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 127–183. [Google Scholar] [CrossRef]
- Kandasamy, P. Respiration rate of fruits and vegetables for modified atmosphere packaging: A mathematical approach. J. Postharvest Technol. 2022, 10, 88–102. [Google Scholar]
- Mohammadi, M.; Fasihi, M. Polymer Films Characteristics for Modified Atmosphere Packaging: A Brief Review. In Proceedings of the 13th International Conference on Food Industry Science, Organic Agriculture and Food Security, Tibilisi, Georgia, 13 March 2023. [Google Scholar]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling Respiration Rate of Fresh Fruits and Vegetables for Modified Atmosphere Packages: A Review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.; Frias, J.M.; Brecht, J.K.; Chau, K.V. Modelling Respiration Rate of Shredded Galega Kale for Development of Modified Atmosphere Packaging. J. Food Eng. 2002, 54, 299–307. [Google Scholar] [CrossRef]
- Choi, I.-L.; Lee, J.-H.; Choi, D.-H.; Wang, L.-X.; Kang, H.-M. Evaluation of the Storage Characteristics in Maintaining the Overall Quality of Whole and Fresh-Cut Romaine Lettuce during MA Storage. Horticulturae 2021, 7, 461. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Biology and Technology: An Overview. In Postharvest Technology of Horticultural Crops, 3rd ed.; Kader, A.A., Ed.; University of California: Richmond, CA, USA, 2002; pp. 39–47. [Google Scholar]
- Akan, S. Effects of Storage Temperature and Packaging on Physiological and Nutritional Quality Preservation of Minimally Processed Spinach. J. Agric. Sci. Technol. 2022, 24, 679–691. [Google Scholar]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Impact of Exogenous Arginine, Cysteine and Methionine on the Postharvest Senescence of Six Green Leafy Vegetables. J. Hortic. Postharvest Res. 2021, 4, 253–262. [Google Scholar] [CrossRef]
- Kou, L.; Luo, Y.; Park, E.; Turner, E.R.; Barczak, A.; Jurick, W.M. Temperature Abuse Timing Affects the Rate of Quality Deterioration of Commercially Packaged Ready-to-Eat Baby Spinach. Part I: Sensory Analysis and Selected Quality Attributes. Postharvest Biol. Technol. 2014, 91, 96–103. [Google Scholar] [CrossRef]
- Yang, T.-D.; Chen, Y.-L.; Zeng, F.-K.; Ye, M.-Q.; Wang, L.; Luo, Y.; Qi, Y.-W.; Chen, F.-P. Effects of Modified Atmosphere Packaging on the Postharvest Quality of Mulberry Leaf Vegetable. Sci. Rep. 2022, 12, 10893. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Lannard, B. Dry Matter Content Association With Time of on-Tree Maturation, Quality at Harvest, and Changes in Quality After Controlled Atmosphere Storage for ‘Royal Gala’ Apples. Can. J. Plant Sci. 2021, 101, 98–106. [Google Scholar] [CrossRef]
- Schmitz, F.R.W.; Carvalho, L.F.D.; Bertoli, S.L.; de Souza, C.K. Effect of Refrigerated Storage Conditions on Leafy Vegetables. MOJ Food Process Technol. 2019, 7, 75–77. [Google Scholar] [CrossRef]
- Lee, J.S.; Chandra, D. Effects of Different Packaging Materials and Methods on the Physical, Biochemical and Sensory Qualities of Lettuce. J. Food Sci. Technol. 2018, 55, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Cheptoo, G.; Owino, W.; Kenji, G. Effect of Different Packaging Materials on Nutritional Stability of Minimally Processed African Indigenous Leafy Vegetables. J. Med. Act. Plants 2020, 9, 98–110. [Google Scholar] [CrossRef]
- Westwood, C.T.; Mulcock, H. Nutritional Evaluation of Five Species of Forage Brassica. J. N. Z. Grassl. 2012, 74, 31–38. [Google Scholar] [CrossRef]
- Akter, S.; Briste, P.S.; Rabbani, M.G.; Shamsunnahar; Hoshain, S. Effect of Different Levels of Nitrogen (N) and Phosphorus (P) Fertilizer on Growth and Yield of Lettuce in Coastal Zone of Bangladesh. Res. Agric. Livest. Fish. 2022, 9, 125–134. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The Effect of Cultivar Type, Time of Cultivation, and Biostimulant Treatment on the Yield of Spinach (Spinacia oleracea L.). Folia Hortic. Ann. 2010, 22, 9–13. [Google Scholar] [CrossRef]
- Biesiada, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kuś, A. Effect of the Age of Plantation and Harvest Term on Chemical Composition and Antioxidant Activity of Stinging Nettle (Urtica dioica L.). Ecol. Chem. Eng. A 2017, 17, 1061–1068. [Google Scholar]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Žutic, I.; Čoga, L.; Fabek, S.; Benko, B.; Toth, N. Yield and Mineral Content of Stinging Nettle as Affected by Nitrogen Fertilization. J. Agric. Sci. Technol. 2016, 18, 1117–1128. [Google Scholar]
- Eldesouky, A.; Pulido, A.F.; Mesias, F.J. The Role of Packaging and Presentation Format in Consumers’ Preferences for Food: An Application of Projective Techniques. J. Sens. Stud. 2015, 30, 360–369. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Talot, M. Colour Difference ∆E. A Survey. Mach. Graph. Vis. 2021, 20, 383–411. [Google Scholar]
- Zheng, S.; Wang, Q.; Zuo, J. Handling and Processing of Fresh-Cut Leafy Vegetables. Acta Hortic. 2021, 1319, 123–130. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Torales, A.C.; Gutiérrez, D.R.; Rodríguez, S.C. Influence of Passive and Active Modified Atmosphere Packaging on Yellowing and Chlorophyll Degrading Enzymes Activity in Fresh-Cut Rocket Leaves. Food Packag. Shelf Life 2020, 26, 100569. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z. Impact of Elevated Carbon Dioxide on Primary, Secondary Metabolites and Antioxidant Responses of Eleais guineensis Jacq. (oil palm) seedlings. Molecules 2012, 17, 5195–51211. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Sivakumar, D.; Beukes, M.; van Rensburg, W.J. Effect of Modified Atmosphere Packaging on the Quality and Bioactive Compounds of Chinese cabbage (Brasicca rapa L. ssp. chinensis). J. Sci. Food Agric. 2013, 93, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf Senescence: Progression, Regulation, and Application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Mudau, A.R.; Nikomo, M.M.; Soundy, P.; Araya, H.T.; Ngezimana, W.; Mudau, F.N. Influence of Postharvest Storage Temperature and Duration on Quality of Baby Spinach. HortTechnology 2015, 25, 665–670. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.C.; Taoukis, P.S. Effect of Alternative Preservation Steps and Storage on Vitamin C Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Yussif, N.M. Vitamin C. In Vitamin C—An Update on Current Uses and Functions; LeBlanc, J.G., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Oey, I.; Verlinde, P.; Hendrickx, M.; Loey, A.V. Temperature and Pressure Stability of L-ascorbic Acid and/or [6s] 5-methyltetrahydrofolic Acid: A Kinetic Study. Eur. Food Res. Technol. 2006, 223, 71–77. [Google Scholar] [CrossRef]
- Sakaldas, M.; Aslım, A.Ş.; Kuzucu, C.Ö.; Kaynaş, K. The Effects of Modified Atmosphere Packaging and Storage Temperature on Quality and Biochemical Properties of Dill (Anethum graveolens) Leaves. J. Food Agric. Environ. 2010, 8, 21–25. [Google Scholar]
- Cantwell, M.I.; Hong, G.; Schmidt, L.; Ermen, H. Impact of Modified Atmospheres on the Vitamin C content of Salad-Cut Romaine and Other Lettuces. Acta Hortic. 2016, 1141, 167–174. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Rai, D.R.; Paul, S. Quality Changes in Fresh-Cut Spinach (Spinacia oleracea) Under Modified Atmospheres with Perforations. J. Food Qual. 2011, 34, 10–18. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and Salinity are Responsible for Phenolics-Enrichment in Two Differently Pigmented Lettuces. Plant Physiol. Bioch. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Koczkodaj, S.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z.; Baczek, K.B. Intraspecific Variability of Stinging Nettle (Urtica dioica L.). Molecules 2023, 28, 1505. [Google Scholar] [CrossRef] [PubMed]
- Opačić, N.; Radman, S.; Dujmović, M.; Fabek Uher, S.; Benko, B.; Toth, N.; Petek, M.; Čoga, L.; Voća, S.; Šic Žlabur, J. Boosting Nutritional Quality of Urtica dioica L. to Resist Climate Change. Front. Plant Sci. 2024, 15, 1331327. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.B.; da Silva, T.T.C.; Magalhães Couto, S.R.; Sabaa Srur, A.U.O. Total Phenolic Compounds and Antioxidant Activity of Organic Vegetables Consumed in Brazil. Food Nutr. Sci. 2015, 6, 798–804. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Jo, N.Y.; Lee, J.; Byeon, J.E.; Park, H.J.; Ryoo, J.W.; Hwang, S.G. Elevated CO2 Concentration Induces Changes in Plant Growth, Transcriptome, and Antioxidant Activity in Fennel (Foeniculum vulgare Mill.). Front. Plant Sci. 2022, 13, 1067713. [Google Scholar] [CrossRef]

| Phenolic Standard | Equation | R2 |
|---|---|---|
| Caffeoylmalic acid | y = 20,636.8x + 16,807 | 0.9997280 |
| Chlorogenic acid | y = 14511.7x − 66,325.3 | 0.9997641 |
| Coumaric acid | y = 2551.11x + 2349.01 | 0.9998387 |
| Ellagic acid | y = 33,829.1x − 6862.97 | 0.9999998 |
| Ferulic acid | y = 27,461.5x − 90,059.3 | 0.9870226 |
| Gallic acid | y = 60,235.9x − 117,854 | 0.9998908 |
| p-hydroxybenzoic acid | y = 87,853.4x + 2,145,860 | 0.9871377 |
| Protocatechuic acid | y = 90,775.3x − 595,177 | 0.9997176 |
| Vanillic acid | y = 65,980.1x + 143,637 | 0.9999301 |
| Kaempferol | y = 88,470.8x − 1,047,410 | 0.9998243 |
| Naringin | y = 3941.28x − 30,329.7 | 0.9829743 |
| Quercetin | y = 111,886x − 377,503 | 0.9999638 |
| Quercetin-3-glucoside | y = 50,449x − 342,648 | 0.9997252 |
| Rutin trihydrate | y = 34,107.6x – 238,617 | 0.9989752 |
| Packaging Material | l (µm) | Transparency (T600) | Transmittance (%) | WVP × 10−14 (g/m s Pa) | WVTR × 10−5 (g/m2 s) |
|---|---|---|---|---|---|
| BOPP | 21.60 ± 1.17 e | 2.15 ± 0.12 b | 89.74 ± 0.01 b | 9.92 ± 9.92 c | 1.01 ± 0.10 c |
| LDPE | 34.00 ± 6.45 c | 2.18 ± 0.42 a | 84.33 ± 0.01 c | 99.40 ± 58.95 b | 6.44 ± 3.82 b |
| PA/PEr | 168.20 ± 5.69 a | 1.75 ± 0.06 c | 50.70 ± 0.01 e | 77.40 ± 16.97 b | 2.44 ± 0.53 bc |
| PA/PEs | 84.50 ± 3.24 b | 1.04 ± 0.04 e | 81.66 ± 0.01 d | 54.10 ± 2.44 bc | 1.41 ± 0.06 c |
| PLA | 29.30 ± 1.06 d | 1.45 ± 0.05 d | 90.78 ± 0.01 a | 859.00 ± 18.53 a | 64.60 ± 1.39 a |
| L* | a* | b* | ΔE | |
|---|---|---|---|---|
| FRESH | 43.92 ± 0.8 | −14.15 ± 0.05 | 23.12 ± 1.17 | - |
| Results considering combinations of factors | ||||
| 6 days of storage | ||||
| BOPP | 42.96 ± 0.70 abc | −13.52 ± 0.32 b | 21.17 ± 1.76 bc | 2.29 ± 1.85 bc |
| LDPE | 43.37 ± 0.3 abc | −14.28 ± 0.56 b | 23.03 ± 0.13 ab | 0.74 ± 0.28 c |
| PA/PE | 45.41 ± 2.14 a | −13.6 ± 0.81 b | 23.93 ± 1.75 a | 2.81 ± 1.09 bc |
| PLA | 40.49 ± 0.96 c | −13.54 ± 1.29 b | 20.36 ± 1.35 c | 4.66 ± 1.18 b |
| 14 days of storage | ||||
| BOPP | 43 ± 0.92 abc | −14.42 ± 0.65 b | 24.12 ± 1.30 a | 1.93 ± 0.49 c |
| LDPE | 44.49 ± 2.14 ab | −12.96 ± 0.89 b | 22.61 ± 1.85 abc | 2.74 ± 0.71 bc |
| PA/PE | 35.85 ± 2.22 d | −9.39 ± 1.63 a | 15.4 ± 1.44 d | 12.15 ± 3.01 a |
| PLA | 42.21 ± 1.92 bc | −13.12 ± 0.82 b | 21.34 ± 0.98 bc | 3.14 ± 1.09 bc |
| Results considering individual factors | ||||
| Days of storage | ||||
| 6 | 43.06 ± 2.11 a | −13.73 ± 0.78 b | 22.12 ± 1.92 a | 2.63 ± 1.80 b |
| 14 | 41.39 ± 3.80 b | −12.48 ± 2.15 a | 20.87 ± 3.66 b | 4.99 ± 4.56 a |
| Packaging material | ||||
| BOPP | 42.98 ± 0.73 ab | −13.97 ± 0.68 b | 22.65 ± 2.13 a | 2.11 ± 1.23 bc |
| LDPE | 43.93 ± 1.50 a | −13.62 ± 0.98 b | 22.82 ± 1.19 a | 1.74 ± 1.19 c |
| PA/PE | 40.63 ± 5.58 c | −11.5 ± 2.57 a | 19.67 ± 4.89 b | 7.48 ± 5.50 a |
| PLA | 41.35 ± 1.65 bc | −13.33 ± 1.00 b | 20.85 ± 1.19 b | 3.9 ± 1.31 b |
| Significance of varied factors | ||||
| D | 0.0243 | 0.0036 | 0.0492 | 0.0016 |
| M | 0.0127 | 0.0011 | 0.0045 | ≤0.0001 |
| D × M | ≤0.0001 | 0.0013 | ≤0.0001 | ≤0.0001 |
| mg/100 g | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeoylmalic Acid | Chlorogenic Acid | Coumaric Acid | Ellagic Acid | Ferulic Acid | Gallic Acid | p-Hidroxybenyoic Acid | Protocatechuic Acid | Vanillic Acid | Kaempferol | Naringin | Quercetin | Quercetin-3-Glucoside | Rutin Trihydrate | |
| FRESH | 308.56 ± 11.20 | 168.20 ± 9.99 | 0.66 ± 0.17 | 0.40 ± 0.13 | 19.55 ± 2.51 | 3.22 ± 0.01 | 1.15 ± 0.02 | 11.19 ± 0.39 | 4.53 ± 0.46 | 11.91 ± 0.01 | 8.14 ± 0.01 | 3.40 ± 0.01 | 13.97 ± 2.01 | 7.32 ± 0.01 |
| Results considering combinations of factors | ||||||||||||||
| 6 days of storage | ||||||||||||||
| BOPP | 225.13 ± 2.44 b | 141.11 ± 0.01 c | 0.65 ± 0.01 a | 0.25 ± 0.01 d | 19.58 ± 0.11 a | 2.94 ± 0.01 g | 0.87 ± 0.01 e | 10.36 ± 0.11 bc | 3.71 ± 0.49 bc | 11.91 ± 0.01 e | 7.98 ± 0.01 c | 3.38 ± 0.01 cd | 7.14 ± 0.07 b | 7.10 ± 0.01 h |
| LDPE | 258.70 ± 1.45 a | 162.12 ± 0.03 a | 0.17 ± 0.03 d | 0.42 ± 0.01 bc | 16.06 ± 0.04 c | 3.26 ± 0.15 f | 0.95 ± 0.01 d | 10.18 ± 0.04 cd | 4.96 ± 0.02 a | 11.91 ± 0.01 e | 7.91 ± 0.07 d | 3.40 ± 0.01 a | 6.92 ± 0.01 e | 7.24 ± 0.01 e |
| PA/PE | 81.27 ± 0.56 g | 75.91 ± 0.22 f | 0.32 ± 0.01 c | 0.46 ± 0.01 a | 17.38 ± 0.05 b | 5.18 ± 0.04 b | 1.05 ± 0.04 c | 11.43 ± 0.06 a | 4.03 ± 0.02 b | 11.91 ± 0.02 e | 8.09 ± 0.01 b | 3.39 ± 0.01 bc | 7.08 ± 0.01 c | 7.29 ± 0.01 c |
| PLA | 147.02 ± 0.72 f | 114.22 ± 0.01 e | 0.42 ± 0.01 b | 0.43 ± 0.01 b | 3.68 ± 0.01 e | 4.91 ± 0.16 c | 0.78 ± 0.01 f | 10.65 ± 0.46 b | 3.44 ± 0.53 cd | 11.90 ± 0.01 e | 7.86 ± 0.01 e | 3.39 ± 0.01 bc | 7.17 ± 0.05 ab | 7.26 ± 0.01 d |
| 14 days of storage | ||||||||||||||
| BOPP | 214.28 ± 0.24 c | 114.01 ± 0.05 e | 0.00 ± 0.00 e | 0.26 ± 0.01 d | 3.78 ± 0.01 d | 4.34 ± 0.02 d | 1.07 ± 0.01 bc | 9.97 ± 0.04 d | 3.19 ± 0.01 d | 11.99 ± 0.01 c | 8.01 ± 0.01 c | 3.38 ± 0.01 cd | 7.15 ± 0.01 ab | 7.17 ± 0.01 g |
| LDPE | 196.55 ± 0.44 d | 131.36 ± 0.02 d | 0.00 ± 0.00 e | 0.46 ± 0.01 a | 3.38 ± 0.01 g | 5.18 ± 0.01 b | 1.10 ± 0.01 b | 9.99 ± 0.03 d | 3.60 ± 0.08 c | 12.02 ± 0.01 b | 8.13 ± 0.02 b | 3.39 ± 0.01 b | 7.02 ± 0.01 d | 7.33 ± 0.01 a |
| PA/PE | 55.99 ± 1.19 h | 33.92 ± 0.01 g | 0.00 ± 0.00 e | 0.40 ± 0.01 c | 3.57 ± 0.01 f | 3.82 ± 0.02 e | 0.66 ± 0.02 g | 9.09 ± 0.14 e | 1.74 ± 0.01 e | 11.94 ± 0.01 d | 8.29 ± 0.01 a | 3.38 ± 0.01 d | 7.16 ± 0.01 ab | 7.19 ± 0.01 f |
| PLA | 173.73 ± 0.27 e | 158.16 ± 0.02 b | 0.00 ± 0.00 e | 0.45 ± 0.04 a | 3.78 ± 0.01 d | 5.77 ± 0.02 a | 1.40 ± 0.01 a | 8.99 ± 0.02 e | 4.59 ± 0.03 a | 12.09 ± 0.01 a | 8.27 ± 0.01 a | 3.39 ± 0.01 b | 7.20 ± 0.01 a | 7.31 ± 0.01 b |
| Results considering individual factors | ||||||||||||||
| 6 | 178.03 ± 72.09 a | 123.34 ± 33.65 a | 0.39 ± 0.18 a | 0.39 ± 0.09 a | 14.17 ± 6.46 a | 4.07 ± 1.03 b | 0.91 ± 0.11 b | 10.66 ± 0.54 a | 4.04 ± 0.67 a | 11.91 ± 0.01 b | 7.96 ± 0.10 b | 3.39 ± 1.53 a | 7.08 ± 0.11 b | 7.22 ± 0.08 b |
| 14 | 160.14 ± 64.57 b | 109.36 ± 48.37 b | 0.00 ± 0.00 b | 0.39 ± 0.09 a | 3.63 ± 0.17 b | 4.78 ± 0.78 a | 1.06 ± 0.28 a | 9.51 ± 0.50 b | 3.28 ± 1.07 b | 12.01 ± 0.06 a | 8.17 ± 0.12 a | 3.39 ± 0.01 b | 7.13 ± 0.07 a | 7.25 ± 0.08 a |
| BOPP | 219.70 ± 6.14 b | 127.56 ± 14.84 c | 0.32 ± 0.35 a | 0.25 ± 0.01 b | 11.68 ± 8.66 a | 3.64 ± 0.77 d | 0.97 ± 0.11 c | 10.17 ± 0.23 a | 3.45 ± 0.42 b | 11.95 ± 0.05 b | 7.99 ± 0.02 c | 3.38 ± 1.85 a | 7.14 ± 0.05 b | 7.13 ± 0.04 c |
| LDPE | 227.63 ± 34.05 a | 146.74 ± 16.84 a | 0.08 ± 0.09 d | 0.44 ± 0.02 a | 9.72 ± 6.94 c | 4.22 ± 1.05 c | 1.03 ± 0.08 b | 10.08 ± 0.11 a | 4.28 ± 0.74 a | 11.96 ± 0.06 b | 8.02 ± 0.13 c | 3.39 ± 0.01 b | 6.97 ± 0.05 c | 7.29 ± 0.05 a |
| PA/PE | 68.63 ± 13.87 d | 54.91 ± 23.00 d | 0.16 ± 0.17 c | 0.43 ± 0.04 a | 10.48 ± 7.56 b | 4.50 ± 0.74 b | 0.86 ± 0.22 d | 10.26 ± 1.29 a | 2.89 ± 1.26 c | 11.93 ± 0.02 c | 8.19 ± 0.11 a | 3.38 ± 0.01 d | 7.12 ± 0.04 b | 7.24 ± 0.05 b |
| PLA | 160.37 ± 14.64 c | 136.19 ± 24.06 b | 0.21 ± 0.23 b | 0.44 ± 0.03 a | 3.73 ± 0.06 d | 5.34 ± 0.48 a | 1.09 ± 0.34 a | 9.82 ± 0.96 b | 4.02 ± 0.72 a | 11.99 ± 0.11 a | 8.06 ± 0.22 b | 3.39 ± 0.01 c | 7.18 ± 0.03 a | 7.28 ± 0.03 a |
| Significance of varied factors | ||||||||||||||
| D | ≤0.0001 | ≤0.0001 | ≤0.0001 | ns | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | 0.0006 | ≤0.0001 |
| M | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | 0.0041 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| D × M | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ns | ≤0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujmović, M.; Kurek, M.; Mlinar, Z.; Radman, S.; Opačić, N.; Pišonić, P.; Voća, S.; Šic Žlabur, J. Packaging Matters: Preservation of Antioxidant Compounds of Fresh Stinging Nettle Leaves (Urtica dioica L.). Appl. Sci. 2024, 14, 6563. https://doi.org/10.3390/app14156563
Dujmović M, Kurek M, Mlinar Z, Radman S, Opačić N, Pišonić P, Voća S, Šic Žlabur J. Packaging Matters: Preservation of Antioxidant Compounds of Fresh Stinging Nettle Leaves (Urtica dioica L.). Applied Sciences. 2024; 14(15):6563. https://doi.org/10.3390/app14156563
Chicago/Turabian StyleDujmović, Mia, Mia Kurek, Zdenko Mlinar, Sanja Radman, Nevena Opačić, Petra Pišonić, Sandra Voća, and Jana Šic Žlabur. 2024. "Packaging Matters: Preservation of Antioxidant Compounds of Fresh Stinging Nettle Leaves (Urtica dioica L.)" Applied Sciences 14, no. 15: 6563. https://doi.org/10.3390/app14156563
APA StyleDujmović, M., Kurek, M., Mlinar, Z., Radman, S., Opačić, N., Pišonić, P., Voća, S., & Šic Žlabur, J. (2024). Packaging Matters: Preservation of Antioxidant Compounds of Fresh Stinging Nettle Leaves (Urtica dioica L.). Applied Sciences, 14(15), 6563. https://doi.org/10.3390/app14156563










