Bridging the Gap: A Literature Review of Advancements in Obesity and Diabetes Mellitus Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Data Extraction and Synthesis
3. Diagnostic Advancements
3.1. Obesity Diagnostics
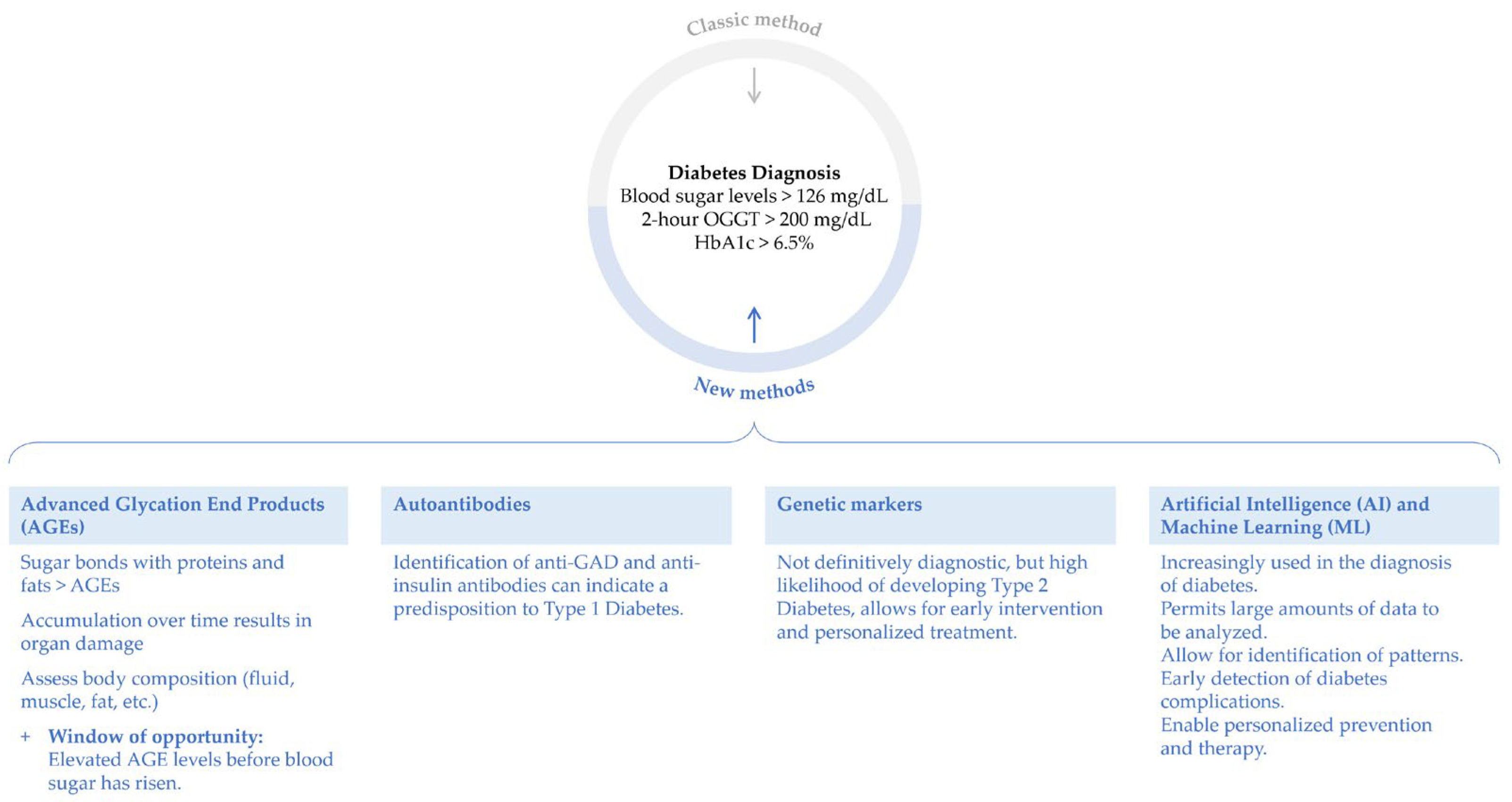
3.2. Diabetes Diagnostics
4. Treatment Advancements
4.1. Obesity Treatments
4.2. Diabetes Treatments
5. Managing Obesity and Type 2 Diabetes
6. Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2024; CDC: Atlanta, GA, USA, 2024.
- Lin, J.; Thompson, T.J.; Cheng, Y.J.; Zhuo, X.; Zhang, P.; Gregg, E.; Rolka, D.B. Projection of the Future Diabetes Burden in the United States through 2060. Popul. Health Metr. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Corrigendum WHO European Regional Obesity Report 2022; WHO: Geneva, Switzerland, 2022; p. 220. [Google Scholar]
- Frühbeck, G.; Luca, B.; Federico, C. Obesity Syndemic in the European Community: Towards a Systems Thinking Approach for Preventive Policies. Eur. Hear. J. 2024, 45, 2181–2182. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Torbeyns, B.; Mathieu, C.; on behalf of the European Diabetes Forum Board. 2024: The Year to Take European Action on Diabetes to the next Level. Diabetologia 2024, 67, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 June 2024).
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Forhan, M.; Gill, S.V. Obesity, Functional Mobility and Quality of Life. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M.; Sandberg, M.; Ahlström, G. The Complexity of Reaching and Maintaining a Healthy Body Weight—The Experience from Adults with a Mobility Disability. BMC Obes. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Bayartai, M.-E.; Luomajoki, H.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Sartorio, A. Differences in Spinal Posture and Mobility between Adults with Obesity and Normal Weight Individuals. Sci. Rep. 2023, 13, 13409. [Google Scholar] [CrossRef] [PubMed]
- Felber, J.P.; Golay, A.; Jéquier, E.; Curchod, B.; Temler, E.; DeFronzo, R.A.; Ferrannini, E. The Metabolic Consequences of Long-Term Human Obesity. Int. J. Obes. 1988, 12, 377–389. [Google Scholar] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic Impacts of Overweight and Obesity: Current and Future Estimates for 161 Countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of Fat-Free Mass Using Bioelectrical Impedance Measurements of the Human Body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Sacco, A.M.; Pasanisi, F.; Scalfi, L. Bioelectrical Impedance (BIA)-Derived Phase Angle in Adults with Obesity: A Systematic Review. Clin. Nutr. 2021, 40, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Anwer, R.; Baig, L.A.; Musharraf, M. Validation of HF-Bioelectrical Impedance Analysis versus Body Mass Index in Classifying Overweight and Obese Pakistani Adults. J. Multidiscip. Healthc. 2023, 16, 983–996. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.M.; Biesek, S.; Vojciechowski, A.S.; Borba, V.Z.C.; Rabito, E.I.; Gomes, A.R.S.; Oliveira, L.A. Estimations of Body Fat by Anthropometry or Bioelectrical Impedance Differ from Those by Dual-Energy X-Ray Absorptiometry in Prefrail Community-Dwelling Older Women. Nutr. Res. 2021, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of Body Composition Assessment by DXA and BIA According to the Body Mass Index: A Retrospective Study on 3655 Measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef]
- Aldobali, M.; Pal, K. Bioelectrical Impedance Analysis for Evaluation of Body Composition: A Review. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Virtual, 4–5 July 2021; pp. 1–10. [Google Scholar]
- Liao, Y.-S.; Li, H.-C.; Lu, H.-K.; Lai, C.-L.; Wang, Y.-S.; Hsieh, K.-C. Comparison of Bioelectrical Impedance Analysis and Dual Energy X-ray Absorptiometry for Total and Segmental Bone Mineral Content with a Three-Compartment Model. Int. J. Environ. Res. Public Health 2020, 17, 2595. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Emerging Role and Recent Applications of Metabolomics Biomarkers in Obesity Disease Research. RSC Adv. 2017, 7, 14966–14973. [Google Scholar] [CrossRef]
- Tsave, O.; Kavakiotis, I. Biomarkers and Machine Learning Applications in Obesity. In Obesity and Diabetes: Scientific Advances and Best Practice; Faintuch, J., Faintuch, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 883–892. ISBN 978-3-030-53370-0. [Google Scholar]
- Aleksandrova, K.; Egea Rodrigues, C.; Floegel, A.; Ahrens, W. Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention. Curr. Obes. Rep. 2020, 9, 219–230. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Mozaffarian, D.; Pischon, T. Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk. Clin. Chem. 2018, 64, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Jędrysik, M.; Wyszomirski, K.; Różańska-Walędziak, A.; Grosicka-Maciąg, E.; Walędziak, M.; Chełstowska, B. The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity. Biomedicines 2024, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, J.; Jiang, M.; Wang, K. The Efficacy and Safety of the Combination Therapy with GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 838277. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, E.; Kalavrizioti, D.; Davoulou, P.; Papachristou, E.; Sinopidis, X.; Fouzas, S.; Dassios, T.; Gkentzi, D.; Kyriakou, S.I.; Karatza, A.; et al. Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus. Diagnostics 2024, 14, 450. [Google Scholar] [CrossRef] [PubMed]
- Czogała, W.; Strojny, W.; Tomasik, P.; Multanowski, M.B.; Wójcik, M.; Miklusiak, K.; Krzysztofik, E.; Wróbel, A.; Miklusiak, K.; Skoczeń, S. The Insight into Insulin-Like Growth Factors and Insulin-Like Growth-Factor-Binding Proteins and Metabolic Profile in Pediatric Obesity. Nutrients 2021, 13, 2432. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.; Machowiak, A.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Accumulation of Advanced Glycation End-Products in the Body and Dietary Habits. Nutrients 2022, 14, 3982. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E.; Oldfield, M.D.; Thomas, M.C. Role of Advanced Glycation End Products in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, S254–S258. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Ziegler, A.-G.; Bonifacio, E. Why Is the Presence of Autoantibodies against GAD Associated with a Relatively Slow Progression to Clinical Diabetes? Diabetologia 2020, 63, 1665–1666. [Google Scholar] [CrossRef]
- Udler, M.S. Type 2 Diabetes: Multiple Genes, Multiple Diseases. Curr. Diabetes Rep. 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L. The Search for Type 2 Diabetes Genes. Ageing Res. Rev. 2003, 2, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Zhuhadar, L.P.; Lytras, M.D. The Application of AutoML Techniques in Diabetes Diagnosis: Current Approaches, Performance, and Future Directions. Sustainability 2023, 15, 13484. [Google Scholar] [CrossRef]
- Nomura, A.; Noguchi, M.; Kometani, M.; Furukawa, K.; Yoneda, T. Artificial Intelligence in Current Diabetes Management and Prediction. Curr. Diabetes Rep. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Khera, R. Machine Learning in Precision Diabetes Care and Cardiovascular Risk Prediction. Cardiovasc. Diabetol. 2023, 22, 259. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, G.M.; Cereda, A.; Porchetta, N.; Benedetto, D.; Matteucci, A.; Bonanni, M.; Chiricolo, G.; De Lorenzo, A. Endovascular Bariatric Surgery as Novel Minimally Invasive Technique for Weight Management in the Morbidly Obese: Review of Literature. Nutrients 2021, 13, 2541. [Google Scholar] [CrossRef] [PubMed]
- Batchelder, A.J.; Williams, R.; Sutton, C.; Khanna, A. The Evolution of Minimally Invasive Bariatric Surgery. J. Surg. Res. 2013, 183, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miao, L.; Ren, Z.; Li, Y. Robotic Bariatric Surgery for the Obesity: A Systematic Review and Meta-Analysis. Surg. Endosc. 2021, 35, 2440–2456. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.F.; Baazaoui, J.; Shahid, F.; Singh, R.; Torres, A.J.; Bashah, M.M. Comparative Analysis of 5-Year Efficacy and Outcomes of Single Anastomosis Procedures as Revisional Surgery for Weight Regain Following Sleeve Gastrectomy. Surg. Endosc. 2023, 37, 7548–7555. [Google Scholar] [CrossRef]
- Hu, L.; Wang, L.; Li, S.; Liu, Y.; Zhang, Z.; Xiao, M.; Zhang, Z.; Wei, Z.; Cui, L.; Jiang, T. Evaluation Study of Single-Anastomosis Duodenal-Ileal Bypass with Sleeve Gastrectomy in the Treatment of Chinese Obese Patients Based on Efficacy and Nutrition. Sci. Rep. 2024, 14, 6522. [Google Scholar] [CrossRef]
- Sánchez-Pernaute, A.; Herrera, M.Á.R.; Ferré, N.P.; Rodríguez, C.S.; Marcuello, C.; Pañella, C.; Antoñanzas, L.L.; Torres, A.; Pérez-Aguirre, E. Long-Term Results of Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S). Obes. Surg. 2022, 32, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Admella, V.; Lazzara, C.; Sobrino, L.; Acrich, E.; Biondo, S.; Pujol-Gebellí, J.; Osorio, J. Patient-Reported Outcomes and Quality of Life After Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S): A Cross-Sectional Study with 283 Patients from a Single Institution. Obes. Surg. 2023, 33, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Shoar, S.; Poliakin, L.; Rubenstein, R.; Saber, A.A. Single Anastomosis Duodeno-Ileal Switch (SADIS): A Systematic Review of Efficacy and Safety. Obes. Surg. 2018, 28, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Mehta, M.; Sheth, D.R.; Hogan, G. Four-Year Nutritional Outcomes in Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy Patients: An Australian Experience. Obes. Surg. 2023, 33, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Neto, M.G. Intragastric Balloon. Minim. Invasive Ther. Allied Technol. 2022, 31, 505–514. [Google Scholar] [CrossRef]
- Tate, C.M.; Geliebter, A. Intragastric Balloon Treatment for Obesity: Review of Recent Studies. Adv. Ther. 2017, 34, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The Critical Role of Gut Microbiota in Obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhou, P.; Wang, J.; Lu, X.; Chen, Y. The Characteristics, Mechanisms and Therapeutics: Exploring the Role of Gut Microbiota in Obesity. Diabetes Metab. Syndr. Obes. 2023, 16, 3691–3705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Qiu, B.; Liang, J.; Li, C. Effects of Fecal Microbiota Transplantation in Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2023, 18, e0288718. [Google Scholar] [CrossRef]
- Sharretts, J.; Galescu, O.; Gomatam, S.; Andraca-Carrera, E.; Hampp, C.; Yanoff, L. Cancer Risk Associated with Lorcaserin—The FDA’s Review of the CAMELLIA-TIMI 61 Trial. N. Engl. J. Med. 2020, 383, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Idrees, Z.; Cancarevic, I.; Huang, L. FDA-Approved Pharmacotherapy for Weight Loss Over the Last Decade. Cureus 2022, 14, e29262. [Google Scholar] [CrossRef] [PubMed]
- Papamargaritis, D.; le Roux, C.W.; Holst, J.J.; Davies, M.J. New Therapies for Obesity. Cardiovasc. Res. 2023, 119, 2825–2842. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Naltrexone/Bupropion for the Treatment of Obesity and Obesity with Type 2 Diabetes. Future Cardiol. 2016, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.B.; Yahya, T.; Satish, P.; Laird, R.; Agatston, A.S.; Cainzos-Achirica, M.; Patel, K.V.; Nasir, K. Glucagon-Like Peptide 1 Receptor Agonists: A Medication for Obesity Management. Curr. Atheroscler. Rep. 2022, 24, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-Term Weight Loss Effects of Semaglutide in Obesity without Diabetes in the SELECT Trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.R.; Andersen, A.; Knop, F.K.; Vilsbøll, T. How Glucagon-like Peptide 1 Receptor Agonists Work. Endocr. Connect. 2021, 10, R200–R212. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-Obesity Drug Discovery: Advances and Challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-Year Effects of Semaglutide in Adults with Overweight or Obesity: The STEP 5 Trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- De Block, C.; Bailey, C.; Wysham, C.; Hemmingway, A.; Allen, S.E.; Peleshok, J. Tirzepatide for the Treatment of Adults with Type 2 Diabetes: An Endocrine Perspective. Diabetes Obes. Metab. 2023, 25, 3–17. [Google Scholar] [CrossRef]
- Sinha, R.; Papamargaritis, D.; Sargeant, J.A.; Davies, M.J. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. J. Obes. Metab. Syndr. 2023, 32, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Formolo, D.A.; Gaspar, J.M.; Melo, H.M.; Eichwald, T.; Zepeda, R.J.; Latini, A.; Okun, M.S.; Walz, R. Deep Brain Stimulation for Obesity: A Review and Future Directions. Front. Neurosci. 2019, 13, 323. [Google Scholar] [CrossRef]
- Ho, A.L.; Sussman, E.S.; Zhang, M.; Pendharkar, A.V.; Azagury, D.E.; Bohon, C.; Halpern, C.H. Deep Brain Stimulation for Obesity. Cureus 2015, 7, e259. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Pietrabissa, G.; Manzoni, G.M.; Cattivelli, R.; Rossi, A.; Novelli, M.; Varallo, G.; Molinari, E. Cognitive Behavioral Therapy to Aid Weight Loss in Obese Patients: Current Perspectives. Psychol. Res. Behav. Manag. 2017, 10, 165–173. [Google Scholar] [CrossRef]
- Moraes, A.d.S.; Padovani, R.d.C.; La Scala Teixeira, C.V.; Cuesta, M.G.S.; Gil, S.d.S.; de Paula, B.; dos Santos, G.M.; Gonçalves, R.T.; Dâmaso, A.R.; Oyama, L.M.; et al. Cognitive Behavioral Approach to Treat Obesity: A Randomized Clinical Trial. Front. Nutr. 2021, 8, 611217. [Google Scholar] [CrossRef]
- Hong, J.S.; Wasden, C.; Han, D.H. Introduction of Digital Therapeutics. Comput. Methods Programs Biomed. 2021, 209, 106319. [Google Scholar] [CrossRef] [PubMed]
- Shafai, G.; Aungst, T.D. Prescription Digital Therapeutics: A New Frontier for Pharmacists and the Future of Treatment. J. Am. Pharm. Assoc. 2023, 63, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Brezing, C.A.; Brixner, D.I. The Rise of Prescription Digital Therapeutics in Behavioral Health. Adv. Ther. 2022, 39, 5301–5306. [Google Scholar] [CrossRef]
- Ramadi, K.B.; Srinivasan, S.S.; Traverso, G. Electroceuticals in the Gastrointestinal Tract. Trends Pharmacol. Sci. 2020, 41, 960–976. [Google Scholar] [CrossRef]
- Long, Y.; Li, J.; Yang, F.; Wang, J.; Wang, X. Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Adv. Sci. 2021, 8, 2004023. [Google Scholar] [CrossRef] [PubMed]
- Benția, D.; Saceleanu, M.V.; Marinescu, A.A.; Ciurea, A.V. Centenary of Insulin Discovery (1921–2021): Nicolae Paulescu’s Original Contributions. Acta Endocrinol 2021, 17, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tirgoviste, C.; Buda, O. Nicolae Constantin Paulescu: The first explicit description of the internal secretion of the pancreas. Acta Med.-Hist. Adriat. 2017, 15, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar]
- Selivanova, O.M.; Grishin, S.Y.; Glyakina, A.V.; Sadgyan, A.S.; Ushakova, N.I.; Galzitskaya, O.V. Analysis of Insulin Analogs and the Strategy of Their Further Development. Biochemistry 2018, 83, S146–S162. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, J.M. Evolution of Insulin: From Human to Analog. Am. J. Med. 2014, 127, S25–S38. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Zinman, B.; Campos, R.V.; Strack, T.; Canadian Lispro Study Group. A Comparative Study of Insulin Lispro and Human Regular Insulin in Patients with Type 2 Diabetes Mellitus and Secondary Failure of Oral Hypoglycemic Agents. Clin. Invest. Med. 2001, 24, 292–298. [Google Scholar] [PubMed]
- Becker, R.H.A. Insulin Glulisine Complementing Basal Insulins: A Review of Structure and Activity. Diabetes Technol. Ther. 2007, 9, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Spencer, C.M. Insulin Aspart. Drugs 1999, 57, 759–765. [Google Scholar] [CrossRef]
- Lindholm, A.; Jacobsen, L.V. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Aspart. Clin. Pharmacokinet. 2001, 40, 641–659. [Google Scholar] [CrossRef]
- Wang, F.; Carabino, J.M.; Vergara, C.M. Insulin Glargine: A Systematic Review of a Long-Acting Insulin Analogue. Clin. Ther. 2003, 25, 1541–1577. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.J.; Plosker, G.L.; Keating, G.M.; McKeage, K.; Scott, L.J. Insulin Glargine. Drugs 2003, 63, 1743–1778. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Kurtzhals, P. Insulin Detemir: From Concept to Clinical Experience. Expert Opin. Pharmacother. 2006, 7, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Havelund, S.; Plum, A.; Ribel, U.; Jonassen, I.; Vølund, A.; Markussen, J.; Kurtzhals, P. The Mechanism of Protraction of Insulin Detemir, a Long-Acting, Acylated Analog of Human Insulin. Pharm. Res. 2004, 21, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, D.; Babazono, T.; Cailleteau, R.; Engberg, S.; Irace, C.; Kjaersgaard, M.I.S.; Mathieu, C.; Rosenstock, J.; Woo, V.; Klonoff, D.C. Once-Weekly Insulin Icodec versus Once-Daily Insulin Degludec as Part of a Basal-Bolus Regimen in Individuals with Type 1 Diabetes (ONWARDS 6): A Phase 3a, Randomised, Open-Label, Treat-to-Target Trial. Lancet 2023, 402, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.Z.; Fareed, A.; Akhtar, S.M.M.; Farhat, S.; Taha, A.M.; Akilimali, A. Efficacy and Safety of Once-Weekly Insulin Icodec Compared to Once-Daily Insulin g U-100 in Patients with Type II Diabetes: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2024, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Bajaj, H.S.; Begtrup, K.; Cailleteau, R.; Gowda, A.; Lingvay, I.; Mathieu, C.; Russell-Jones, D.; Rosenstock, J. Rationale and Design of the Phase 3a Development Programme (ONWARDS 1–6 Trials) Investigating Once-Weekly Insulin Icodec in Diabetes. Diabetes Obes. Metab. 2023, 25, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Asong, M.; Desouza, C.; Gourdy, P.; Kar, S.; Vianna, A.; Vilsbøll, T.; Vinther, S.; Mu, Y. Once-Weekly Insulin Icodec vs. Once-Daily Insulin Degludec in Adults with Insulin-Naive Type 2 Diabetes: The ONWARDS 3 Randomized Clinical Trial. JAMA 2023, 330, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jones, N.; Hohenwarter, L.; Zhao, F.; Chan, V.; Tan, Z.; Carlaw, T.; Morin, T.; Li, J.; Kaur, T.; et al. Systemic Delivery of Proteins Using Novel Peptides via the Sublingual Route. J. Control. Release 2024, 368, 290–302. [Google Scholar] [CrossRef]
- Wu, J.; Roesger, S.; Jones, N.; Hu, C.-M.J.; Li, S.-D. Cell-Penetrating Peptides for Transmucosal Delivery of Proteins. J. Control. Release 2024, 366, 864–878. [Google Scholar] [CrossRef]
- Hunt, N.J.; Lockwood, G.P.; Heffernan, S.J.; Daymond, J.; Ngu, M.; Narayanan, R.K.; Westwood, L.J.; Mohanty, B.; Esser, L.; Williams, C.C.; et al. Oral Nanotherapeutic Formulation of Insulin with Reduced Episodes of Hypoglycaemia. Nat. Nanotechnol. 2024, 19, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Ren, S.; Pan, J.; Wang, Y.; Shen, Y.; Zeng, Z.; Cui, H.; Zhao, X. Versatile Oral Insulin Delivery Nanosystems: From Materials to Nanostructures. Int. J. Mol. Sci. 2022, 23, 3362. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gu, Z.; Yu, J. Smart Oral Insulin Therapy. Matter 2021, 4, 3790–3791. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, Metabolic and Cardiovascular Considerations of SGLT2 Inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Solini, A. SGLT2 Inhibition in Diabetes Mellitus: Rationale and Clinical Prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502. [Google Scholar] [CrossRef]
- Chao, E.C.; Henry, R.R. SGLT2 Inhibition—A Novel Strategy for Diabetes Treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Blau, J.E.; Rother, K.I. SGLT2 Inhibitors May Predispose to Ketoacidosis. J. Clin. Endocrinol. Metab. 2015, 100, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Prasad-Reddy, L.; Isaacs, D. A Clinical Review of GLP-1 Receptor Agonists: Efficacy and Safety in Diabetes and Beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W. GLP-1 Receptor Agonists for Type 2 Diabetes Mellitus: Recent Developments and Emerging Agents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014, 34, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and Glucagon Receptor Agonist, for People with Type 2 Diabetes: A Randomised, Double-Blind, Placebo and Active-Controlled, Parallel-Group, Phase 2 Trial Conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Gutgesell, R.M.; Nogueiras, R.; Tschöp, M.H.; Müller, T.D. Dual and Triple Incretin-Based Co-Agonists: Novel Therapeutics for Obesity and Diabetes. Diabetes Ther. 2024, 15, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A Rationally Designed Monomeric Peptide Triagonist Corrects Obesity and Diabetes in Rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous Glucose Monitoring and Metrics for Clinical Trials: An International Consensus Statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Aleppo, G. Continuous Glucose Monitoring: The Achievement of 100 Years of Innovation in Diabetes Technology. Diabetes Res. Clin. Pract. 2020, 170, 108502. [Google Scholar] [CrossRef]
- Joseph, J.I. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2021, 15, 167–173. [Google Scholar] [CrossRef]
- Irace, C.; Cutruzzolà, A.; Nuzzi, A.; Assaloni, R.; Brunato, B.; Pitocco, D.; Tartaglione, L.; Di Molfetta, S.; Cignarelli, A.; Laviola, L.; et al. Clinical Use of a 180-Day Implantable Glucose Sensor Improves Glycated Haemoglobin and Time in Range in Patients with Type 1 Diabetes. Diabetes Obes. Metab. 2020, 22, 1056–1061. [Google Scholar] [CrossRef]
- Cowart, K. A Review of the First Long-Term Implantable Continuous Glucose Monitoring System Available in the United States. J. Diabetes Sci. Technol. 2021, 15, 160–166. [Google Scholar] [CrossRef]
- Joy, A.; Hafsiya, T.H.; King, G. A Review on Glucose Monitoring Using Enabling Technologies of Internet of Things. In Proceedings of the 2021 7th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 19–20 March 2021; Volume 1, pp. 270–273. [Google Scholar]
- Abubeker, K.M.; Ramani, R.; Krishnamoorthy, R.; Gogula, S.; Baskar, S.; Muthu, S.; Chellamuthu, G.; Subramaniam, K. Internet of Things Enabled Open Source Assisted Real-Time Blood Glucose Monitoring Framework. Sci. Rep. 2024, 14, 6151. [Google Scholar] [CrossRef]
- Peacock, S.; Frizelle, I.; Hussain, S. A Systematic Review of Commercial Hybrid Closed-Loop Automated Insulin Delivery Systems. Diabetes Ther. 2023, 14, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Templer, S. Closed-Loop Insulin Delivery Systems: Past, Present, and Future Directions. Front. Endocrinol. 2022, 13, 919942. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, E.; Hebrok, M. Stem Cell-Based Clinical Trials for Diabetes Mellitus. Front. Endocrinol. 2021, 12, 631463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Feng, B.; Cao, H. The Clinical Efficacy and Safety of Stem Cell Therapy for Diabetes Mellitus: A Systematic Review and Meta-Analysis. Aging Dis. 2020, 11, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Memon, B.; Abdelalim, E.M. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Du, K.; Zou, C. Current Progress in Stem Cell Therapy for Type 1 Diabetes Mellitus. Stem Cell Res. Ther. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yi, Q.; Wang, Y.; Wang, J.; Yu, H.; Zhang, J.; Hu, M.; Xu, J.; Wu, Z.; Hou, L.; et al. Long-Term Glycemic Variability and Risk of Adverse Health Outcomes in Patients with Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Diabetes Res. Clin. Pract. 2022, 192, 110085. [Google Scholar] [CrossRef]
- Sun, B.; Luo, Z.; Zhou, J. Comprehensive Elaboration of Glycemic Variability in Diabetic Macrovascular and Microvascular Complications. Cardiovasc. Diabetol. 2021, 20, 9. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Konishi, K.; Tsunoda, T.; Koyama, H. Significance of Glycemic Variability in Diabetes Mellitus. Intern. Med. 2022, 61, 281–290. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Chan, S.Y.; Du, Z.; Yan, Y.; Wang, T.; Li, P.; Huang, W. Hydrogel-Based Flexible Materials for Diabetes Diagnosis, Treatment, and Management. NPJ Flex. Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial Adhesive Self-Healing Hydrogels to Promote Diabetic Wound Healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional Hydrogels for Diabetic Wound Management. APL Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent Advances in Responsive Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef] [PubMed]
- Tyler, N.S.; Mosquera-Lopez, C.M.; Wilson, L.M.; Dodier, R.H.; Branigan, D.L.; Gabo, V.B.; Guillot, F.H.; Hilts, W.W.; El Youssef, J.; Castle, J.R.; et al. An Artificial Intelligence Decision Support System for the Management of Type 1 Diabetes. Nat. Metab. 2020, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Chaki, J.; Thillai Ganesh, S.; Cidham, S.K.; Ananda Theertan, S. Machine Learning and Artificial Intelligence Based Diabetes Mellitus Detection and Self-Management: A Systematic Review. J. King Saud. Univ. Comput. Inf. Sci. 2022, 34, 3204–3225. [Google Scholar] [CrossRef]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, S.C.; Sainsbury, C.A.R.; Wake, D.J. Diabetes and Artificial Intelligence beyond the Closed Loop: A Review of the Landscape, Promise and Challenges. Diabetologia 2024, 67, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Cotero, V.; Graf, J.; Miwa, H.; Hirschstein, Z.; Qanud, K.; Huerta, T.S.; Tai, N.; Ding, Y.; Jimenez-Cowell, K.; Tomaio, J.N.; et al. Stimulation of the Hepatoportal Nerve Plexus with Focused Ultrasound Restores Glucose Homoeostasis in Diabetic Mice, Rats and Swine. Nat. Biomed. Eng. 2022, 6, 683–705. [Google Scholar] [CrossRef]
- Ashe, J.; Graf, J.; Madhavan, R.; Wallace, K.; Cotero, V.; Abate, S.; Pandey, R.K.; Herzog, R.; Porindla, S.N.; Shoudy, D.; et al. Investigation of Liver-Targeted Peripheral Focused Ultrasound Stimulation (pFUS) and Its Effect on Glucose Homeostasis and Insulin Resistance in Type 2 Diabetes Mellitus: A Proof of Concept, Phase 1 Trial. QJM 2023, 116, 667–685. [Google Scholar] [CrossRef]
- Cotero, V.; Miwa, H.; Graf, J.; Ashe, J.; Loghin, E.; Di Carlo, D.; Puleo, C. Peripheral Focused Ultrasound Neuromodulation (pFUS). J. Neurosci. Methods 2020, 341, 108721. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, G.N.; Manole, F.; Buleu, F.; Motofelea, A.C.; Bircea, S.; Popa, D.; Motofelea, N.; Pirvu, C.A. Bridging the Gap: A Literature Review of Advancements in Obesity and Diabetes Mellitus Management. Appl. Sci. 2024, 14, 6565. https://doi.org/10.3390/app14156565
Pop GN, Manole F, Buleu F, Motofelea AC, Bircea S, Popa D, Motofelea N, Pirvu CA. Bridging the Gap: A Literature Review of Advancements in Obesity and Diabetes Mellitus Management. Applied Sciences. 2024; 14(15):6565. https://doi.org/10.3390/app14156565
Chicago/Turabian StylePop, Gheorghe Nicusor, Felicia Manole, Florina Buleu, Alexandru Catalin Motofelea, Silviu Bircea, Daian Popa, Nadica Motofelea, and Catalin Alexandru Pirvu. 2024. "Bridging the Gap: A Literature Review of Advancements in Obesity and Diabetes Mellitus Management" Applied Sciences 14, no. 15: 6565. https://doi.org/10.3390/app14156565
APA StylePop, G. N., Manole, F., Buleu, F., Motofelea, A. C., Bircea, S., Popa, D., Motofelea, N., & Pirvu, C. A. (2024). Bridging the Gap: A Literature Review of Advancements in Obesity and Diabetes Mellitus Management. Applied Sciences, 14(15), 6565. https://doi.org/10.3390/app14156565







