Reservoir Characteristics of Marine–Continental Transitional Taiyuan Formation Shale and Its Influence on Methane Adsorption Capacity: A Case Study in Southern North China Basin
Abstract
:1. Introduction
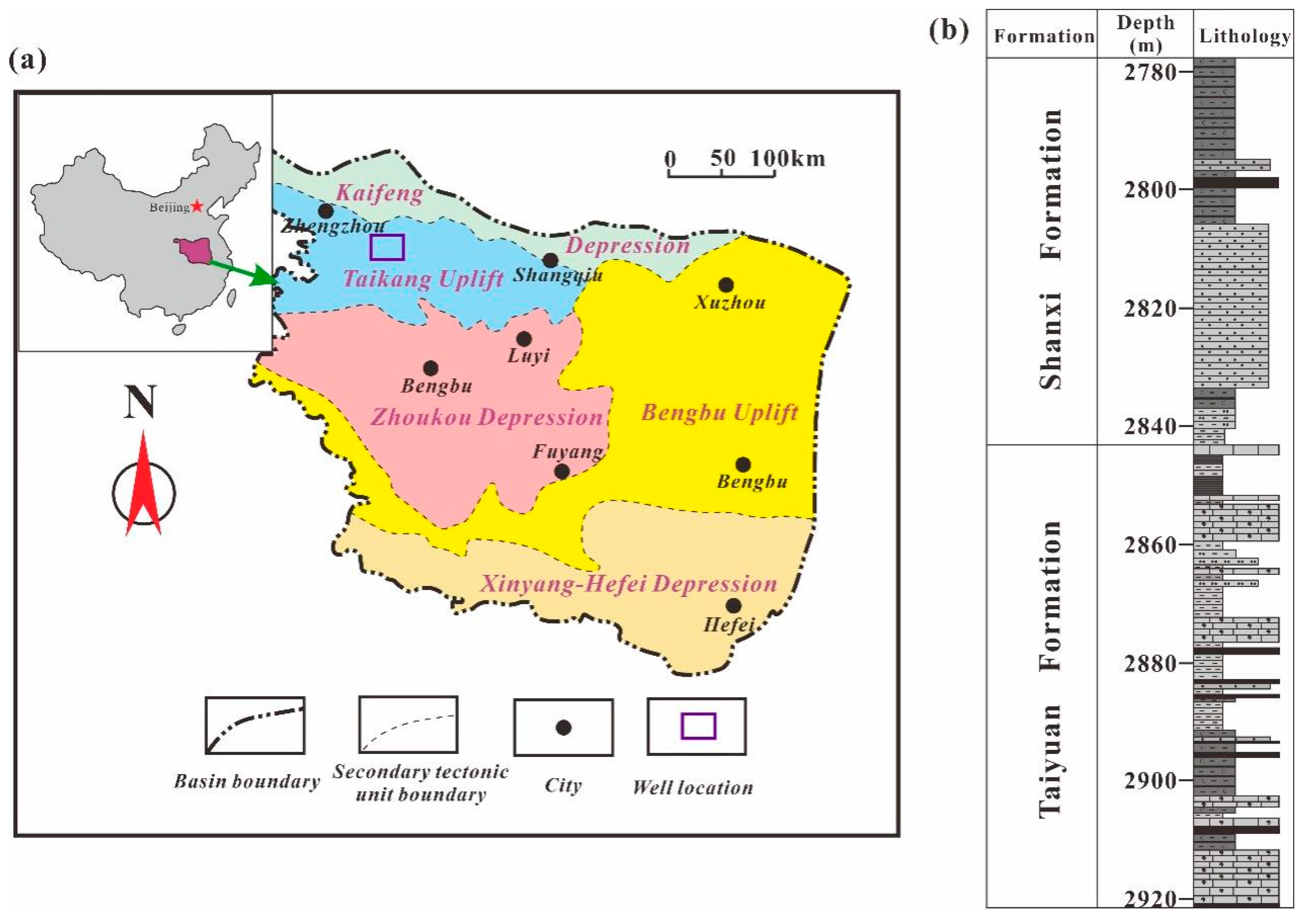
2. Geological Background
3. Samples and Experiments
3.1. Samples
3.2. Experimental Methods
4. Results
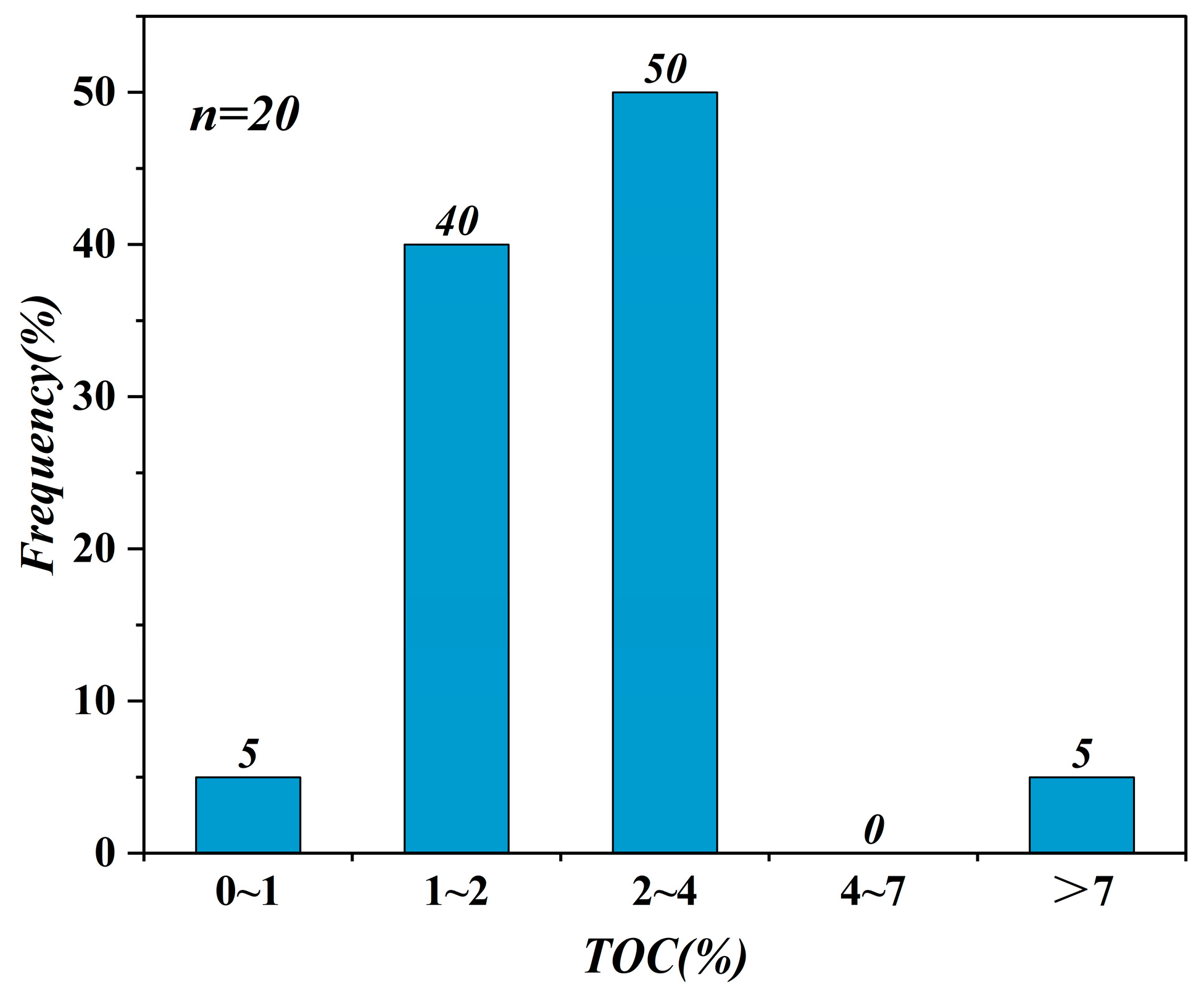
4.1. Organic Geochemical Characters
4.2. Sedimentary Environmental Characteristics
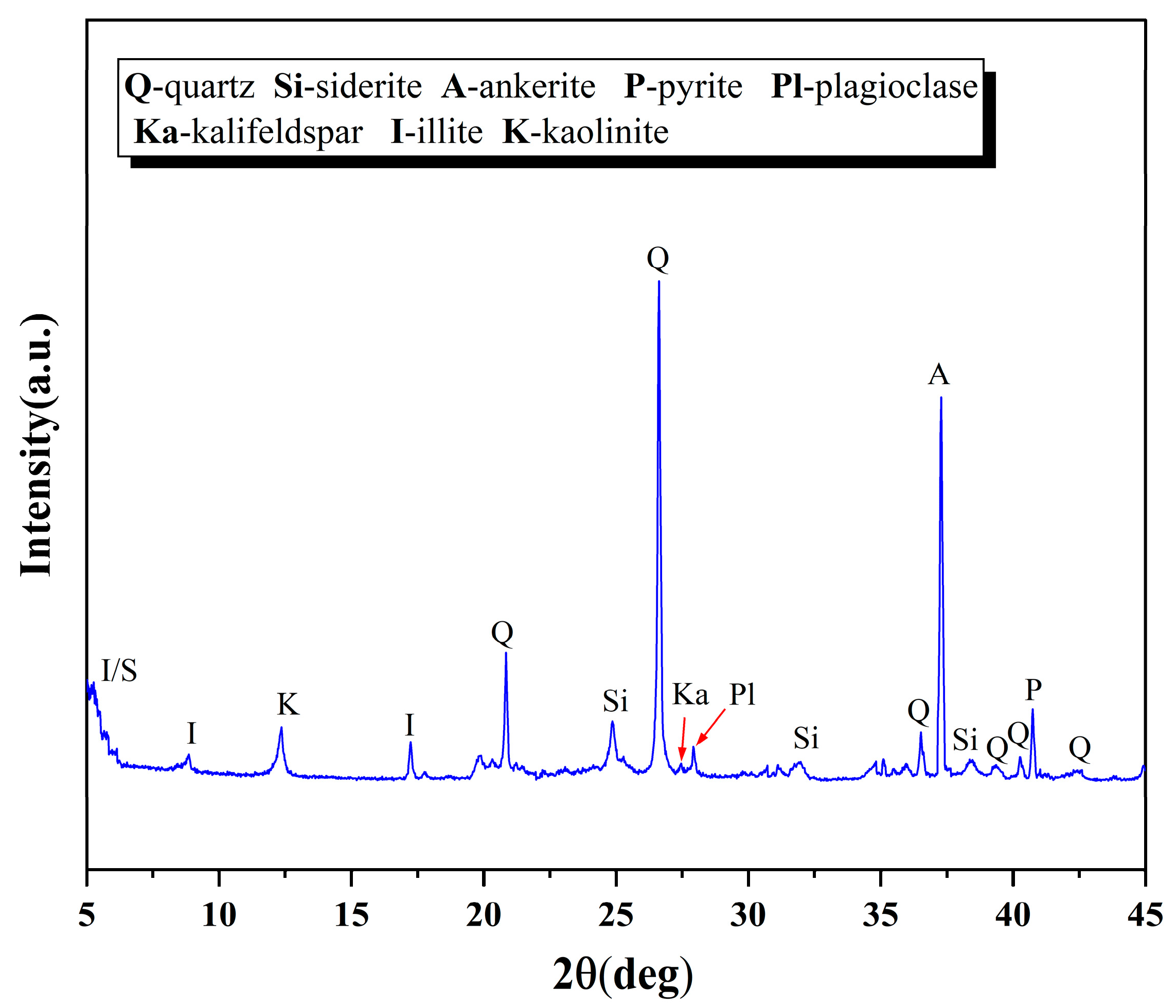
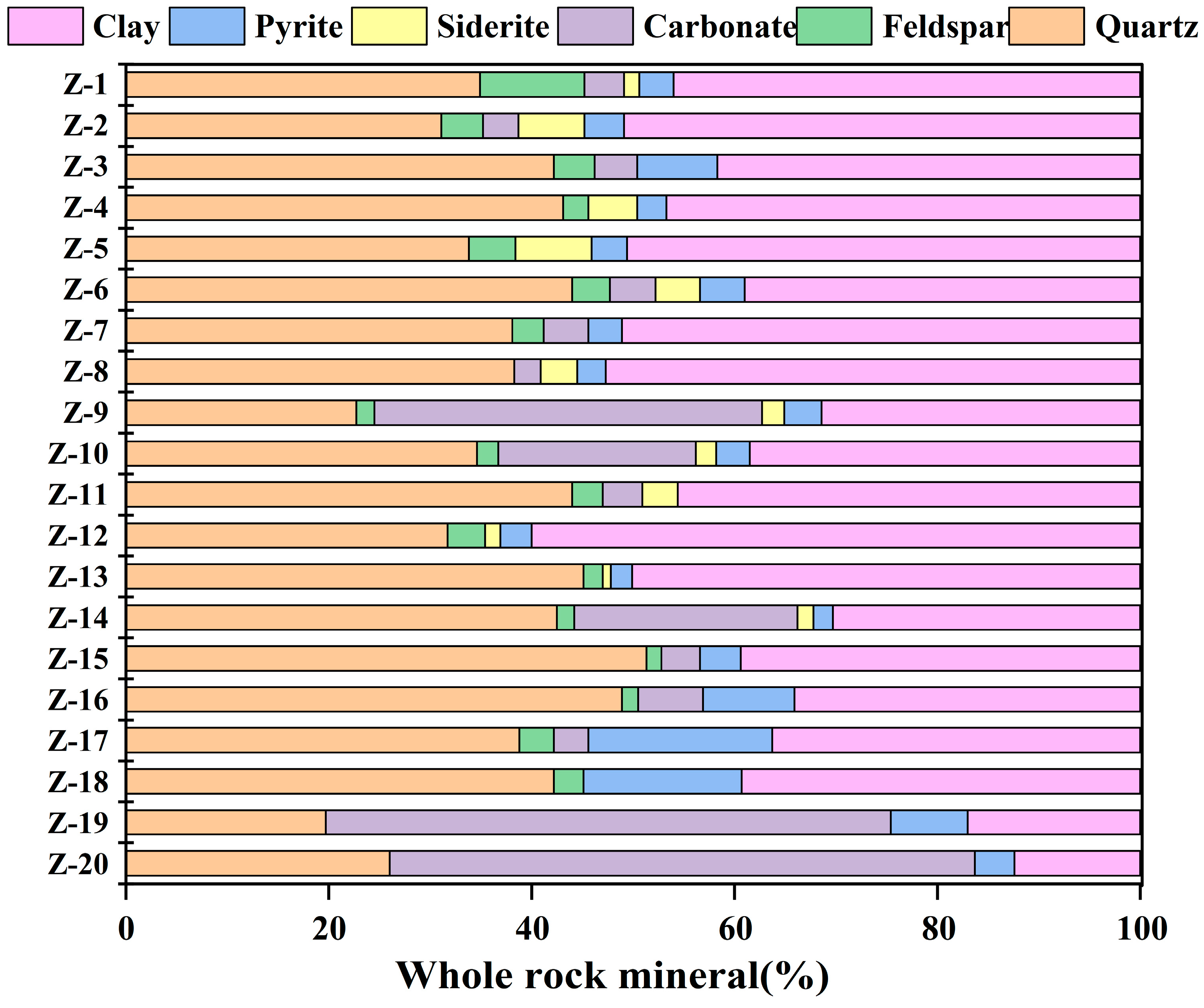
4.3. Petrological and Mineralogical Characteristics
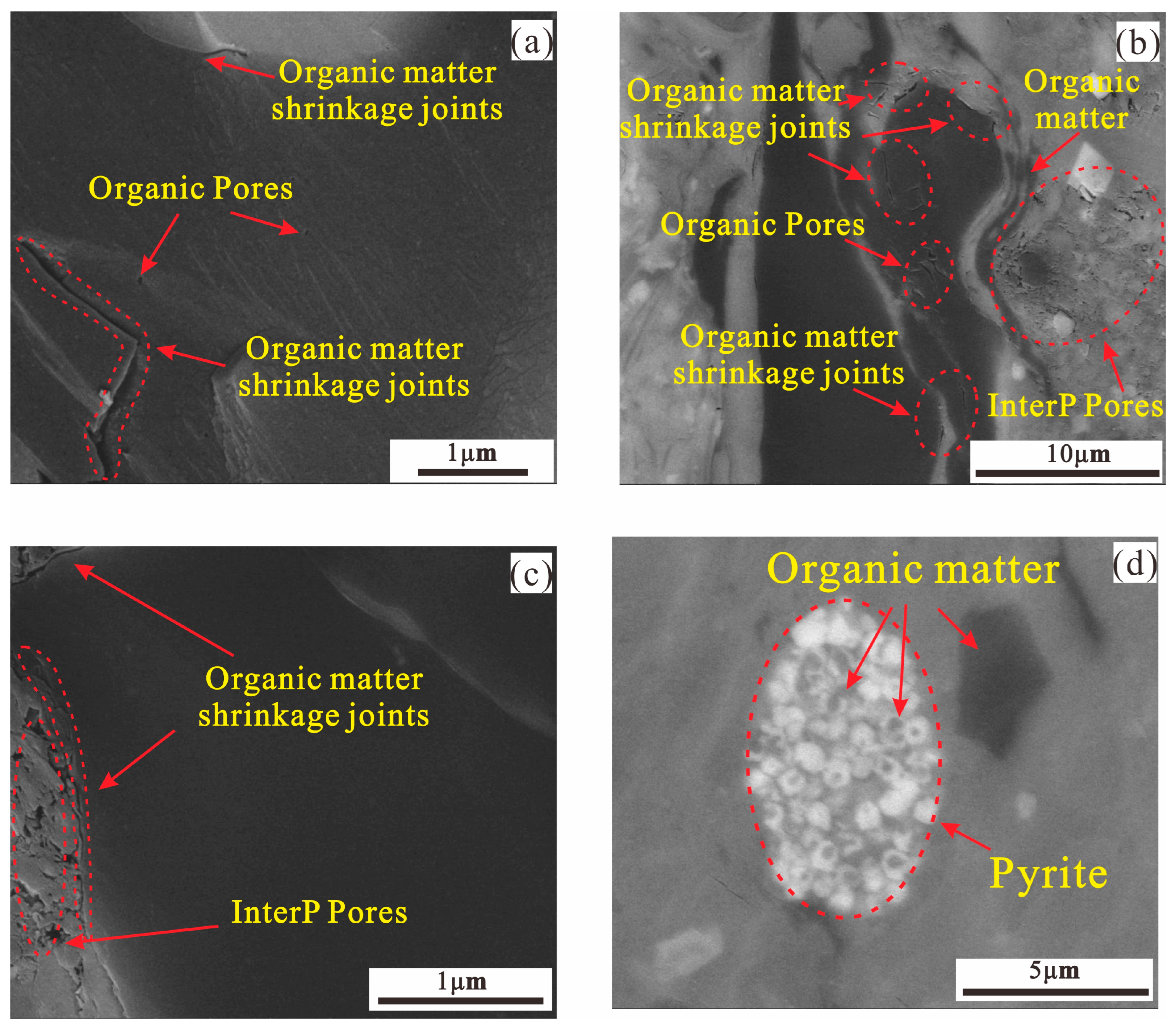
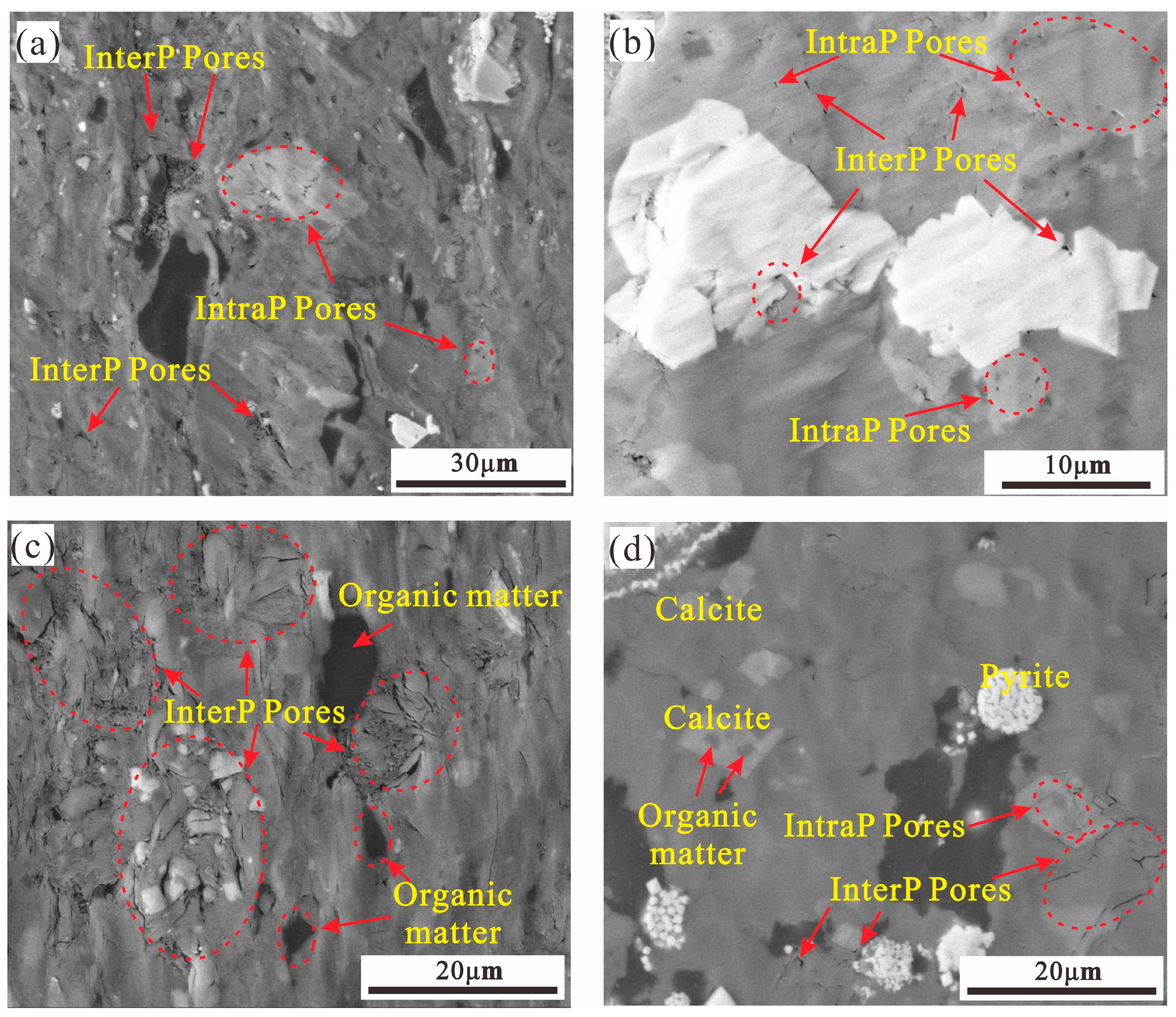
4.4. Pore Type and Morphological Characteristics
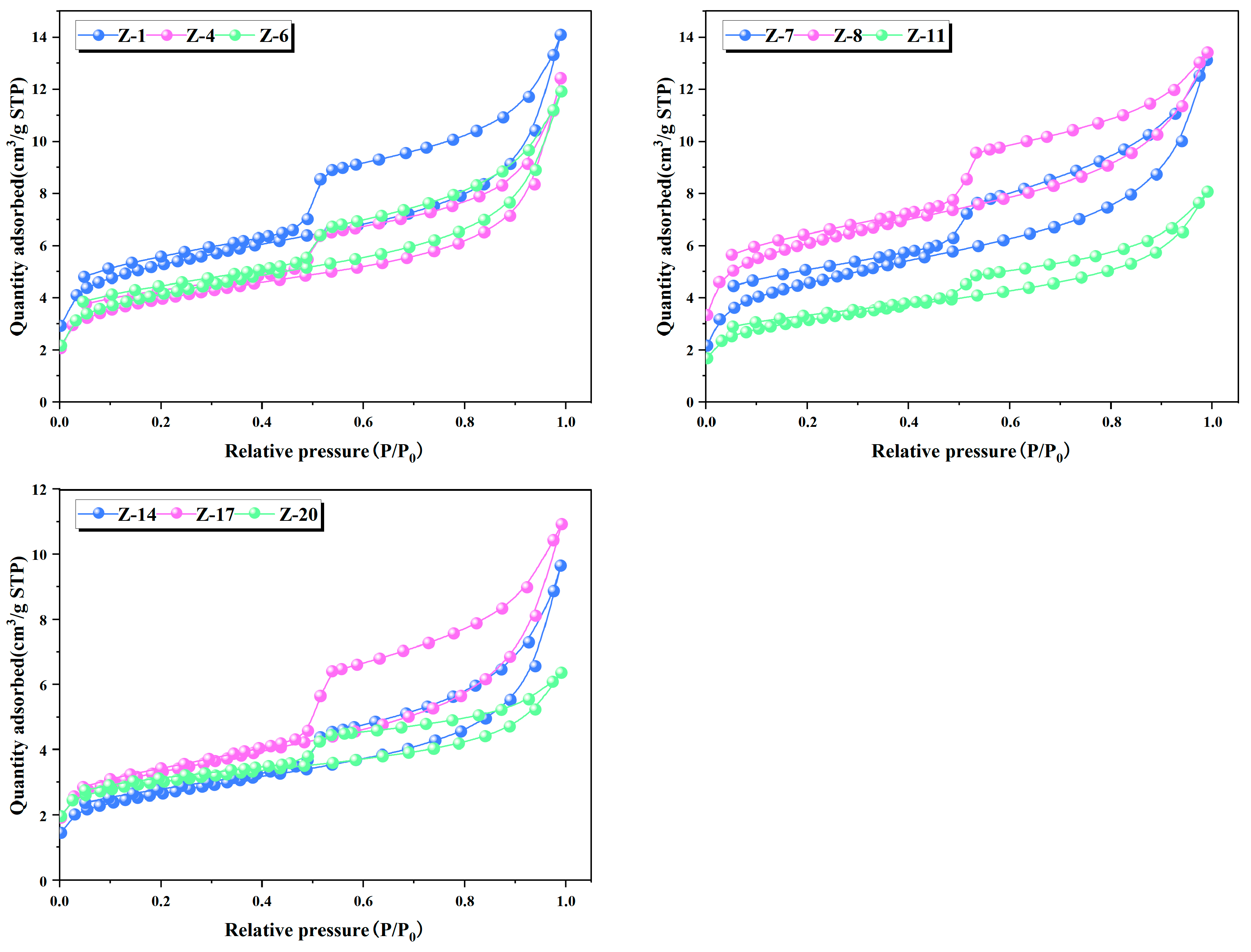
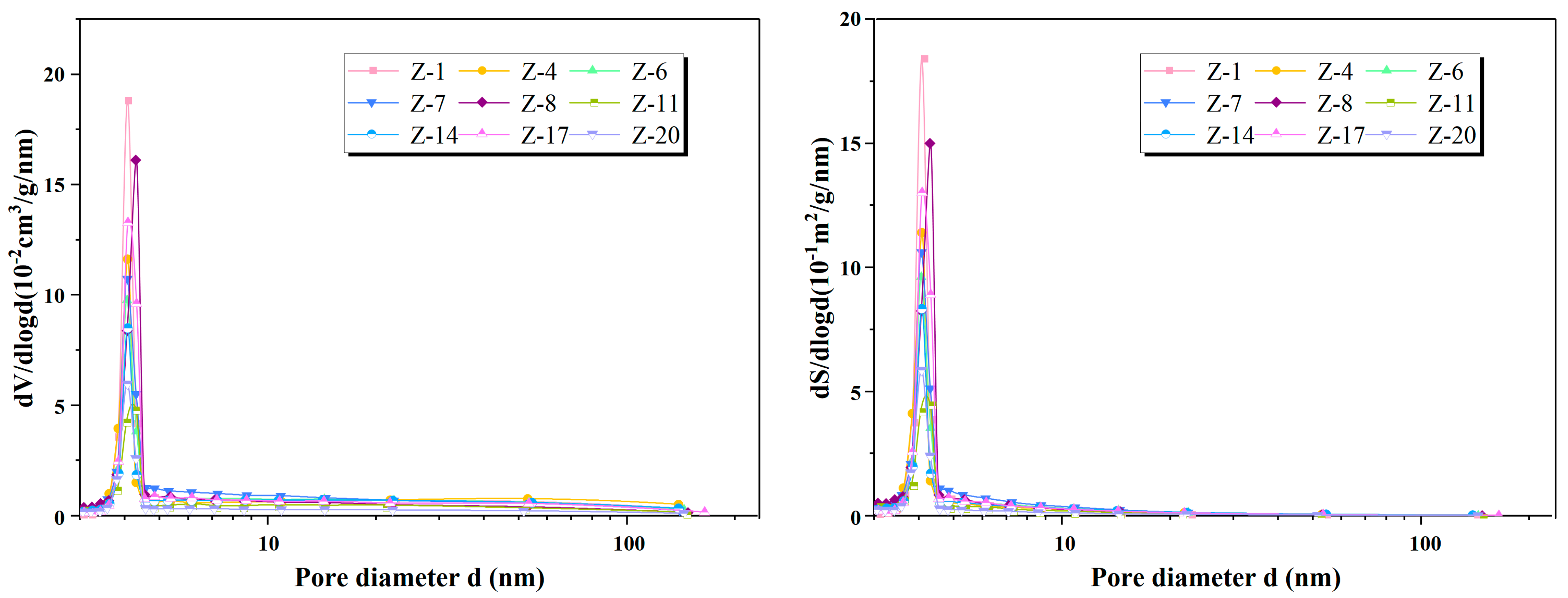
4.5. Pore Structure Characteristics
4.6. Methane Adsorption Characteristics
5. Discussion
5.1. Effect of Organic Matter Characteristics on Shale Adsorption Capacity
5.2. Effect of Pore Structure on Shale Adsorption Capacity
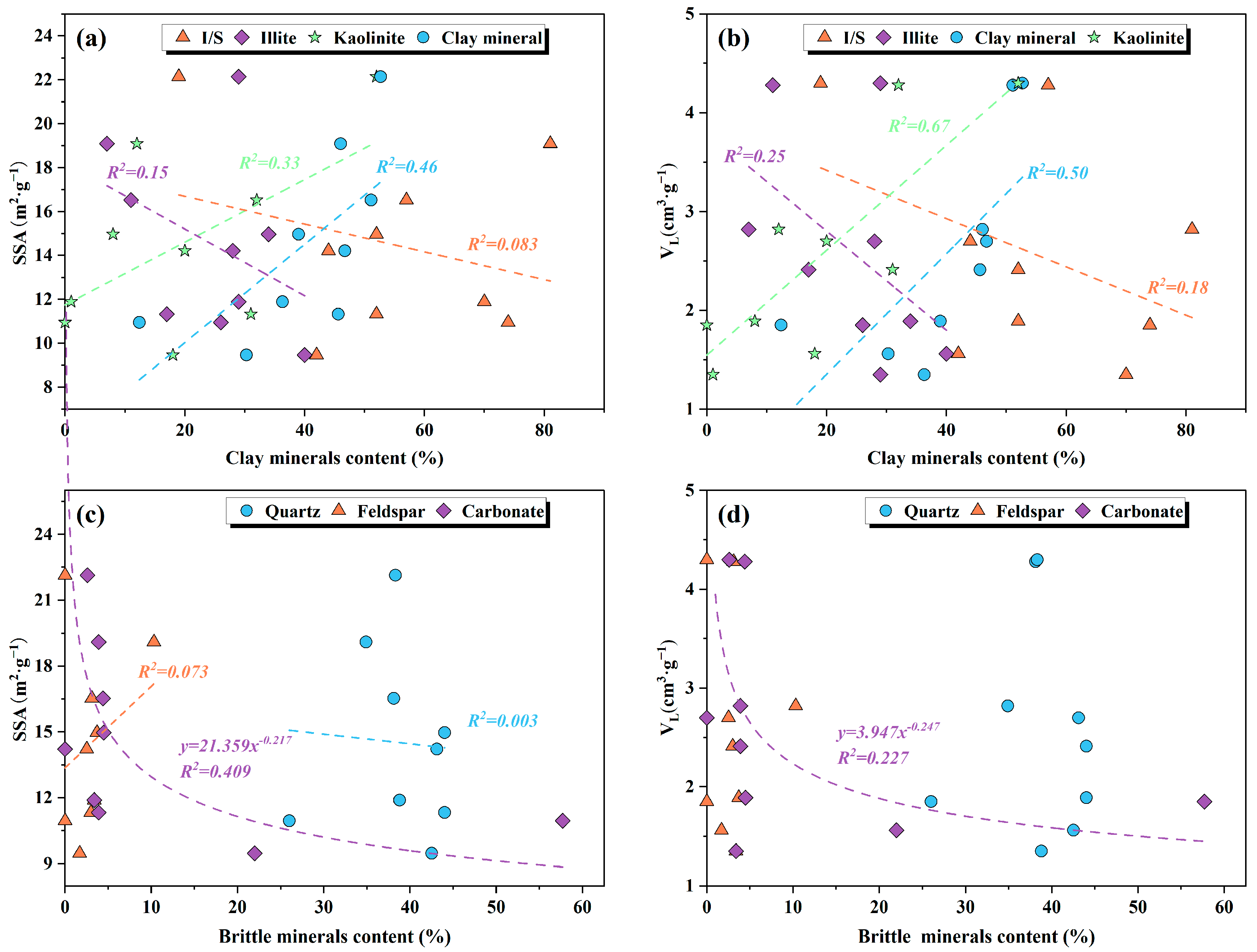
5.3. Effect of Mineral Composition on Shale Adsorption Capacity
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, J.; Qin, S.; Hu, G.; Ni, Y.; Gan, L.; Huang, S.; Hong, F. Major progress in the natural gas exploration and development in the past seven decades in China. Pet. Explor. Dev. 2019, 46, 1100–1110. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Jin, Z.; Gao, J.; Yang, Z.; Wu, S.; Zhao, Q. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12. [Google Scholar]
- Qiu, Z.; Zou, C.; Mills, B.J.W.; Xiong, Y.; Tao, H.; Lu, B.; Liu, H.; Xiao, W.; Simon, W.P. A nutrient control on expanded anoxia and global cooling during the Late Ordovician mass extinction. Commun. Earth Environ. 2022, 39, 82. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, X.; Cai, D.; Xue, G.; He, Z.; Long, S.; Peng, Y.; Gao, B.; Xu, Z.; Dahdah, N. Comparison of marine, transitional, and lacustrine shales: A case study from the Sichuan Basin in China. J. Pet. Sci. Eng. 2017, 150, 334–347. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Li, X.; Zou, C.; Guan, Q.; Zhang, C.; Huang, J.; Wang, S.; Wang, H.; Liu, H.; et al. Breakthrough and prospect of shale gas exploration and development in China. Nat. Gas Ind. B 2016, 3, 12–26. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Li, X.; Li, K.; He, J.; Zhang, Z.; Guo, J.; Chen, Y.; Liu, W. Shale gas exploration and development in the Sichuan Basin: Progress, challenge and countermeasures. Nat. Gas Ind. B 2021, 41, 143–152. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, M.; Wang, D.; Tong, Z.; Hou, X.; Niu, J.; Li, X.; Li, Z.; Zhang, P.; Huang, Y. Fields and directions for shale gas exploration in China. Nat. Gas Ind. B 2022, 9, 20–32. [Google Scholar] [CrossRef]
- Dong, D.; Gao, S.; Huang, J. A discussion on the shale gas exploration and development prospect in the Sichuan Basin. Nat. Gas Ind. B 2014, 34, 12. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Dong, D.; Yang, Z.; Qiu, Z.; Liang, F.; Wang, N.; Huang, Y.; Duan, A.; Zhang, Q.; et al. Geological characteristics, main challenges and future prospect of shale gas. Nat. Gas Geosci. 2017, 28, 1781–1796. [Google Scholar] [CrossRef]
- Zhang, Q.; Grohmann, S.; Xu, X.; Littke, R. Depositional environment and thermal maturity of the coal-bearing Longtan Shale in southwest Guizhou, China: Implications for shale gas resource potential. Int. J. Coal Geol. 2020, 231, 103607. [Google Scholar] [CrossRef]
- Deng, E.; Zhang, Q.; Jin, Z.; Zhu, R.; Yan, Z.; Jiang, B.; Littke, R. Non-overmature equivalents confirmed a high initial hydrocarbon generation potential of the Permian Longtan Shale in southern China. Int. J. Coal Geol. 2022, 259, 104043. [Google Scholar] [CrossRef]
- Bowker, K.A. Barnett Shale gas production, Fort Worth Basin: Issues and discussion. AAPG Bull. 2007, 91, 523–533. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. Shale Gas-in-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2022, 86, 1921–1938. [Google Scholar]
- Montgomery, S.L.; Jarvie, D.M.; Bowker, K.A.; Pollastro, R.M. Mississippian Barnett shale, Fort Worth Basin, north-central Texasa: Gas-shale play with multitrillion cubic foot potential. AAPG Bull. 2005, 89, 155–175. [Google Scholar] [CrossRef]
- Yang, F.; Xie, C.J.; Ning, Z.; Krooss, B.M. High-pressure methane sorption on dry and moisturee-quilibrated shales. Energy Fuels 2017, 31, 482–492. [Google Scholar] [CrossRef]
- Pan, L.; Chen, L.; Cheng, P.; Gai, H. Methane storage capacity of Permian shales with type III kerogen in the Lower Yangtze area, Eastern China. Energies 2022, 15, 1875. [Google Scholar] [CrossRef]
- Yang, W.; He, S.; Su, A.; Zhai, G.; Zhou, Z.; Dong, T.; Tao, Z. Methane adsorption capacities investigation of the Ediacaran organic-rich Doushantuo shale in the Middle Yangtze Platform, South China. Energy Fuels 2021, 35, 16452–16464. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.; Ruppel, S.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Lu, S.; Guo, T.; Wang, M.; Xue, Q.; Zhang, T.; Li, Z.; Sun, Y.; Liu, J.; et al. Critical factors controlling adsorption capacity of shale gas in Wufeng-Longmaxi formation, Sichuan Basin: Evidences from both experiments and molecular simulations. J. Nat. Gas Sci. Eng. 2021, 88, 103774. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X. Influence of reservoir properties on the adsorption capacity and fractal features of shales from Qinshui coalfield. J. Petro. Sci. Eng. 2019, 177, 650–662. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S. Characterization of whole-aperture pore structure and its effect on methane adsorption capacity for transitional shales. Energy Fuels 2018, 32, 3176–3188. [Google Scholar] [CrossRef]
- Xing, Y.; Xiao, X.; Zhou, Q.; Liu, W.; Zhao, Y. Influence of water on the methane adsorption capacity of organic-rich shales and its controlling factors: A review. Energies 2023, 16, 3305. [Google Scholar] [CrossRef]
- Ju, Y.; Qi, Y.; Fang, L.; Zhu, H.; Wang, G.; Wang, G. Chinese shale gas reservoir types ant their controlling factors. Adv. Earth Sci. 2016, 31, 782–799. [Google Scholar]
- Guo, S.; Fu, D.; Gao, D.; Li, H.; Huang, J. Research status and prospects for marine-continental shale gases in China. Petrol. Geol. Exp. 2015, 37, 535–540. [Google Scholar]
- Xu, H.L.; Zhao, Z.J.; Lv, F.L.; Yang, Y.N.; Tang, Z.W.; Sun, G.Z.; Xu, Y.J. Tectonic evolution of the Nanhuabei area and analysis about its petroleum potential. Geotect. Metallog. 2004, 4, 450–463. [Google Scholar]
- Yu, H.Z.; Lv, F.L.; Guo, Q.X.; Lu, W.Z.; Wu, J.Y.; Han, S.H. Proto-sediment basin types and tectonic evolution in the southern edge of North China Plate. Petrol. Geol. Exp. 2005, 27, 111–117. [Google Scholar]
- Liu, Y.; Cheng, D.; Qiu, Q.; Weng, J.; Wang, X. Characteristics of pores and controlling factors of Lower Permian shales in Southern North China Basin. Nat. Gas Geosci. 2020, 31, 1501–1513. [Google Scholar]
- Wang, Y.; Cheng, X.; Fan, K.; Huo, Z.; Wei, L. The Paleoenvironment and Mechanisms of Organic Matter Enrichment of Shale in the Permian Taiyuan and Shanxi Formations in the Southern North China Basin. J. Mar. Sci. Eng. 2023, 11, 992. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.C.; Tang, X.; Huo, Z.P.; Li, Z.; Luo, K.Y.; Li, Z.M. Assessment of shale gas potential of the lower Permian transitional Shanxi-Taiyuan shales in the southern North China Basin. Aust. J. Earth Sci. 2021, 68, 262–284. [Google Scholar] [CrossRef]
- SY/T 6414-2014; Maceral Identification and Statistical Methods on Polished Surfaces of Whole Rocks. China Standard Press: Beijing, China, 2014.
- SY/T 5124-2012; Method of Determining Microscopically the Reflectance of Vitrinite in Sedimentary. China Standard Press: Beijing, China, 2012.
- SY/T 5163-2018; Analysis Method for Clay Minerals and Ordinary Non-Clay Minerals in Sedimentary Rocks by the X-ray Diffraction. China Standard Press: Beijing, China, 2018.
- Maslov, A.V.; Podkovyrov, V.N. Ocean redox state at 2500-500 Ma: Modern concepts. Lithol. Miner. Resour. 2018, 53, 190–211. [Google Scholar] [CrossRef]
- Alberdi-Genolet, M.; Tocco, R. Trace metals and organic geochemistry of the machiques member (aptian–albian) and la luna formation (cenomanian–campanian), venezuela. Chem. Geol. 1999, 160, 19–38. [Google Scholar] [CrossRef]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Fan, Q.; Xia, G.; Li, G.; Yi, H. Analytical Methods and Research Progress of Redox Conditions in the Paleo-Ocean. Acta Sedimentol. Sin. 2022, 40, 1151–1171. [Google Scholar]
- Yan, J.; Xu, S.; Li, F. Geochemistry of the dysaerobic sedimentary environments of the Qixia Formationin Badong, Hubei. Sediment. Facies Palaeogeogr. 1998, 18, 27–32. [Google Scholar]
- Ma, B.; Xu, S.; Chen, M.; Zhang, J. An Overview of Influence Factors of Methane Adsorption Capacity in Shale. Mar. Orig. Pet. Geol. 2018, 23, 31–38. [Google Scholar]
- Ge, T.; Pan, J.; Wang, K.; Liu, W.; Mou, P.; Wang, X. Heterogeneity of pore structure of late Paleozoic transitional facies coal-bearing shale in the Southern North China and its main controlling factors. Mar. Petrol. Geol. 2020, 122, 104710. [Google Scholar] [CrossRef]
- Chen, K.; Chen, K.; Liu, X.; Liu, J.; Zhang, C.; Guan, M.; Zhou, S. Lithofacies and pore characterization of continental shale in the second Member of the Kongdian Formation in the Cangdong Sag, Bohai Bay Basin, China. J. Petrol. Sci. Eng. 2019, 177, 154–166. [Google Scholar] [CrossRef]
- Yang, W.; Zuo, R.; Jiang, Z.; Chen, D.; Song, Y.; Luo, Q.; Wang, Q.; Zhu, H. Effect of lithofacies on pore structure and new insights into pore-preserving mechanisms of the over-mature Qiongzhusi marine shales in Lower Cambrian of the southern Sichuan Basin, China. Mar. Petrol. Geol. 2018, 98, 746–762. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Bustin, A.M.M.; Chalmers, G.R.L.; He, L.; Melnichenko, Y.B.; Radliński, A.P.; Blach, T.P. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.; Liu, Q.; Jiang, S.; Liu, K.; Tan, J.; Gao, F. Mechanism of shale gas occurrence: Insights from comparative study on pore structures of marine and lacustrine shales. Mar. Petrol. Geol. 2019, 104, 200–216. [Google Scholar] [CrossRef]
- Wang, E.; Guo, T.; Li, M.; Li, C.; Dong, X.; Zhang, N.; Feng, Y. Exploration potential of different lithofacies of deep marine shale gas systems: Insight into organic matter accumulation and pore formation mechanisms. J. Nat. Gas Sci. Eng. 2022, 102, 104563. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rexer, T.F.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane adsorption on shale under simulated geological temperature and pressure conditions. Energy Fuels 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Yang, Y. Application of bitumen and graptolite reflectance in the Silurian Longmaxi shale, southeastern Sichuan Basin. Petrol. Geol. Exp. 2016, 38, 466–472. [Google Scholar]
- He, Q.; Dong, T.; He, S.; Zhai, G. Methane adsorption capacity of marine-continental transitional facies shales: The case study of the Upper Permian Longtan Formation, northern Guizhou Province, Southwest China. J. Petrol. Sci. Eng. 2019, 183, 106406. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.C.; Han, S.B.; Xue, B.; Zhao, Q.R. Classification and the developmental regularity of organic-associated pores (OAP) through a comparative study of marine, transitional, and terrestrial shales in China. J. Nat. Gas. Sci. Eng. 2016, 36, 358–368. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, Z.; Jiang, S.; Wang, P.; Xiang, C. Effect of organic matter and maturity on pore size distribution and gas storage capacity in high-mature to post-mature shales. Energy Fuels 2016, 30, 8985–8996. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Chen, F.; Zhang, K.; Tang, L. Behavior and controlling factors of methane adsorption in Jurassic continental shale, northeastern Sichuan Basin. Energy Geosci. 2023, 4, 83–92. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.; Tian, H.; Zhou, Q.; Cheng, P. Geological models of gas in place of the Longmaxi shale in Southeast Chongqing, South China. Mar. Petrol. Geol. 2016, 73, 433–444. [Google Scholar] [CrossRef]
- Li, Q.; Pang, X.; Ling, T.; Chen, G.; Shao, X.; Jia, N. Occurrence features and gas content analysis of marine and continental shales: A comparative study of Longmaxi Formation and Yanchang Formation. J. Nat. Gas. Sci. Eng. 2018, 56, 504–522. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Petrol. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, Z.; Gao, B.; Zhao, J. Controlling factors on the enrichment and high productivity of shale gas in the Wufeng-Longmaxi Formations, southeastern Sichuan Basin. Geosci. Front. 2020, 23, 1–10. [Google Scholar]


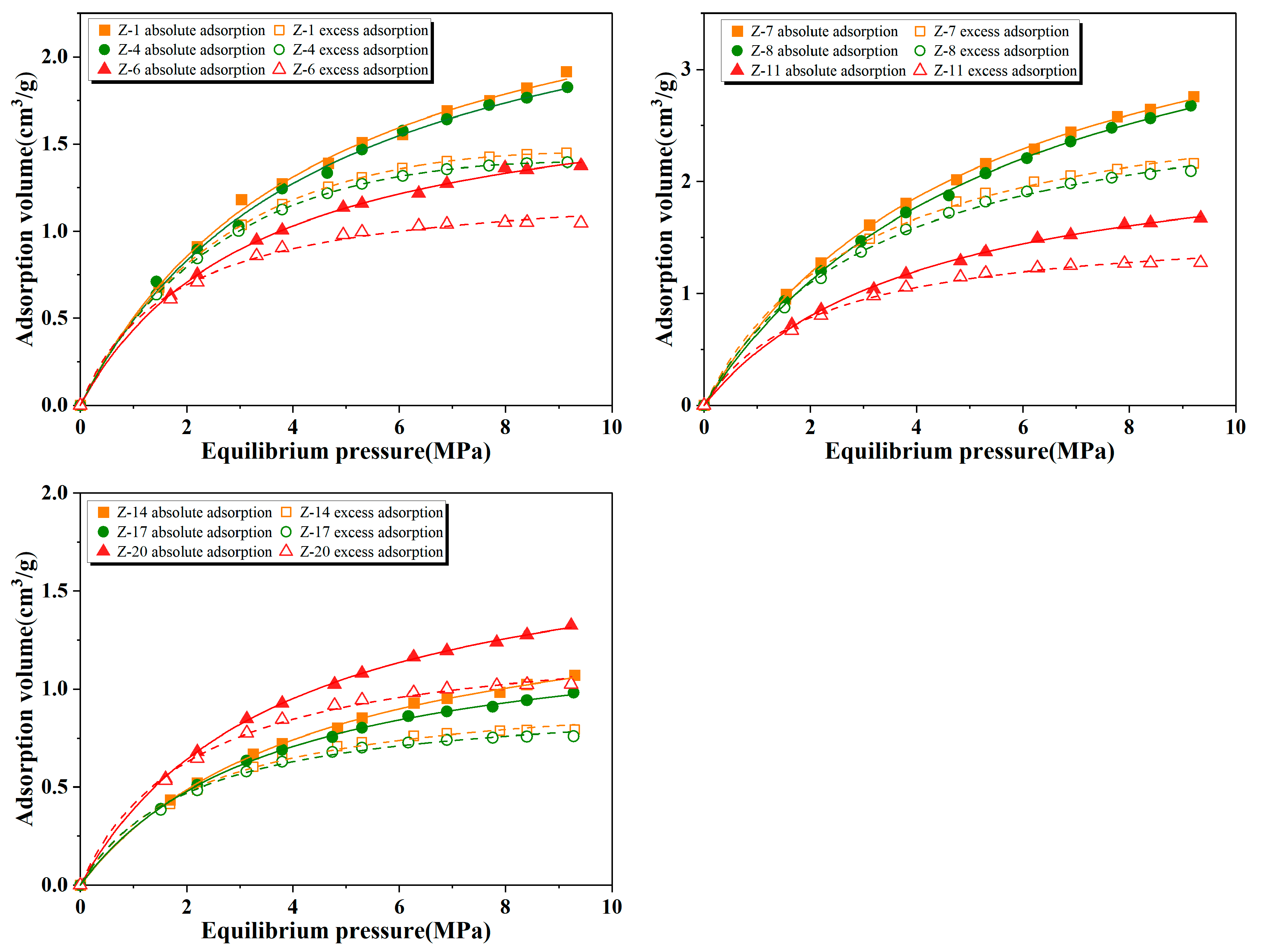
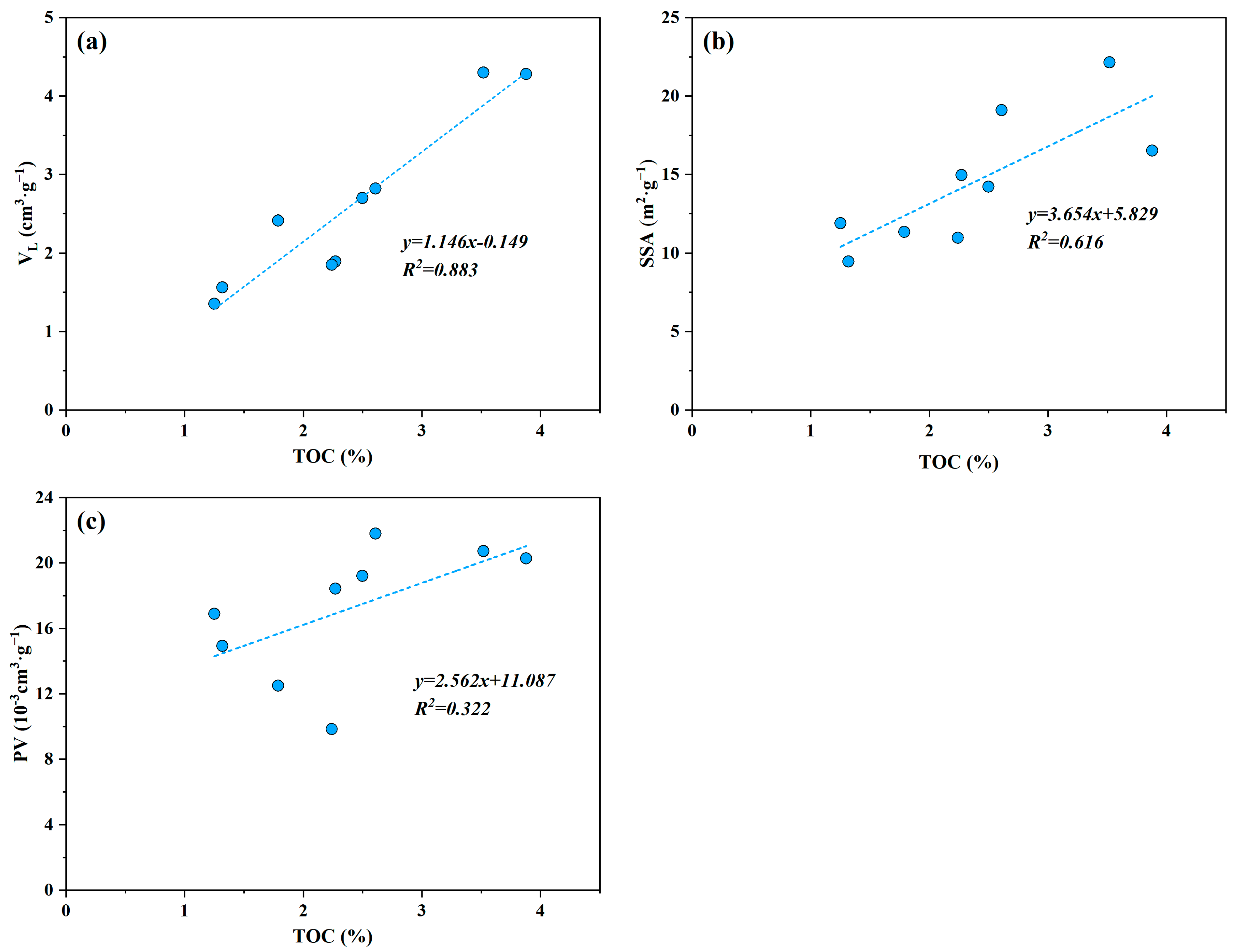
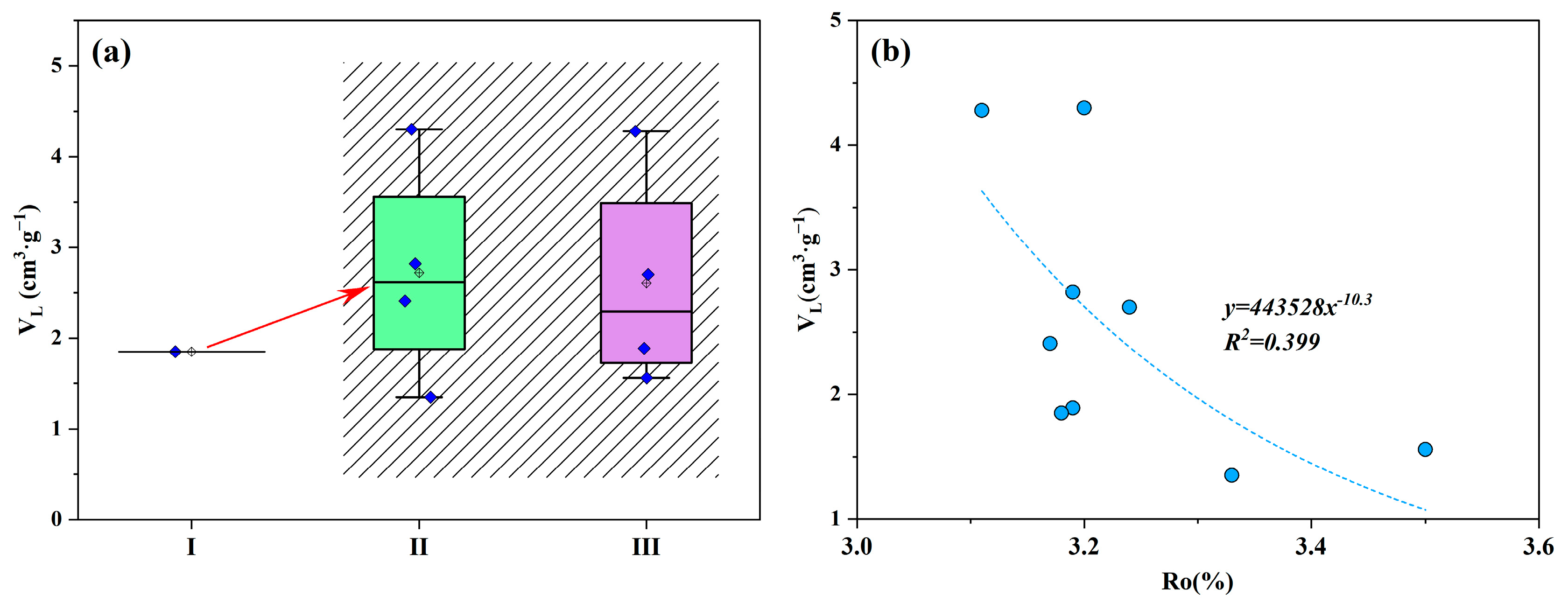
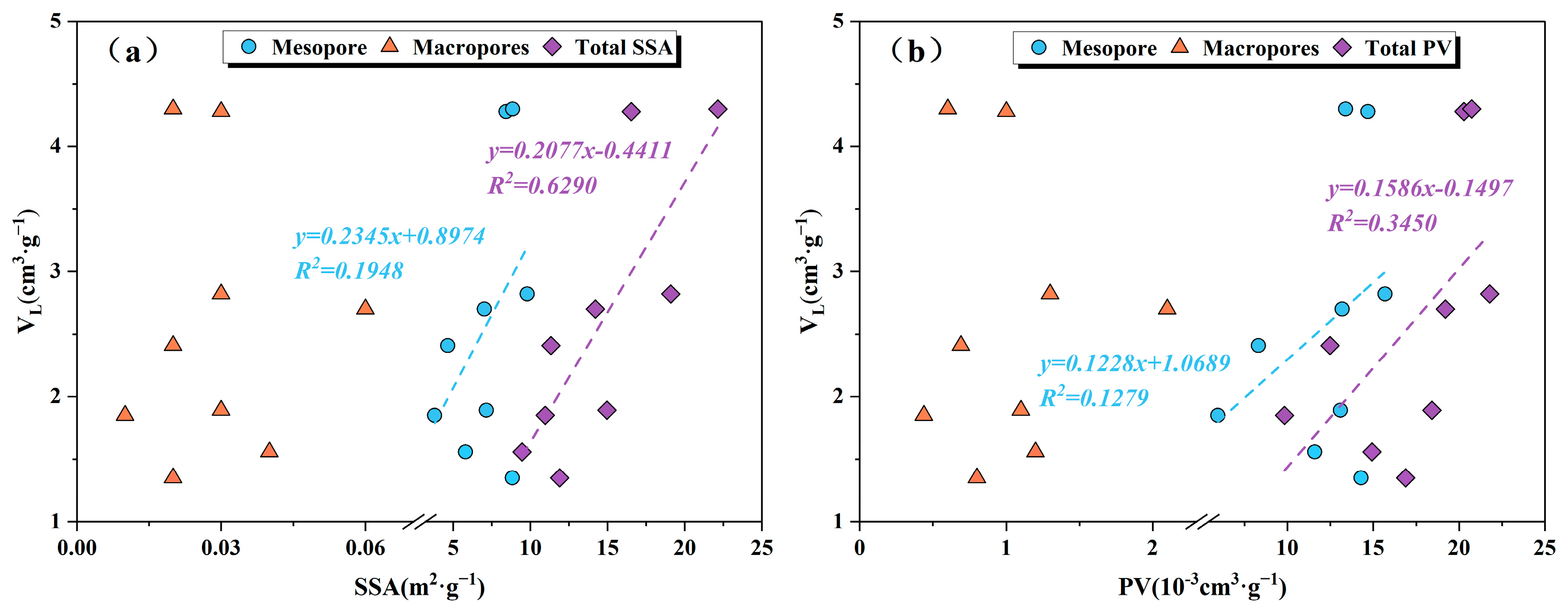
| Sample ID | Depth (m) | TOC (%) | Ro (%) | Sapropel-Inite (%) | Exinite (%) | Vitrinite (%) | Inertinite (%) | TI | OM Type |
|---|---|---|---|---|---|---|---|---|---|
| Z-1 | 2845.0 | 2.61 | 3.19 | 84 | 6 | 6 | 4 | 78.5 | II1 |
| Z-4 | 2861.5 | 2.50 | 3.24 | 20 | 10 | 42 | 28 | −34.5 | III |
| Z-6 | 2868.8 | 2.27 | 3.19 | 28 | 12 | 40 | 20 | −16.0 | III |
| Z-7 | 2876.4 | 3.88 | 3.11 | 34 | 14 | 36 | 16 | −2.0 | III |
| Z-8 | 2880.0 | 3.52 | 3.20 | 50 | 16 | 24 | 10 | 30.0 | II2 |
| Z-11 | 2886.6 | 1.79 | 3.17 | 82 | 8 | 8 | 2 | 78.0 | II1 |
| Z-14 | 2897.1 | 1.32 | 3.50 | 25 | 8 | 44 | 23 | −27.0 | III |
| Z-17 | 2905.7 | 1.25 | 3.33 | 84 | 2 | 8 | 6 | 73.0 | II1 |
| Z-20 | 2910.6 | 2.24 | 3.18 | 92 | 4 | 2 | 2 | 90.5 | I |
| Sample ID | Element Content/10−6 | Redox Proxies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| V | Ni | Cu | Zn | La | Ce | V/(V + Ni) | Cu/Zn | Ce/La | |
| Z-1 | 120 | 18.4 | 16.1 | 73.3 | 49.32 | 98.56 | 0.87 | 0.22 | 2.00 |
| Z-3 | 95 | 24.0 | 21.5 | 92.4 | 50.28 | 87.98 | 0.80 | 0.23 | 1.75 |
| Z-5 | 164 | 28.0 | 22.8 | 99.2 | 47.52 | 88.32 | 0.85 | 0.23 | 1.86 |
| Z-7 | 56 | 12.1 | 19.4 | 120.2 | 58.08 | 91.31 | 0.82 | 0.16 | 1.57 |
| Z-13 | 99 | 30.5 | 14.1 | 60.4 | 26.40 | 49.80 | 0.76 | 0.23 | 1.89 |
| Sample ID | BET SSA (m2/g) | PV (10−3m3/g) | APS (nm) | Langmuir Volume (m3/t) | Langmuir Pressure (MPa) |
|---|---|---|---|---|---|
| Z-1 | 19.10 | 21.79 | 4.57 | 2.82 | 4.61 |
| Z-4 | 14.21 | 19.21 | 5.41 | 2.70 | 4.45 |
| Z-6 | 14.97 | 18.43 | 4.93 | 1.89 | 3.34 |
| Z-7 | 16.53 | 20.28 | 4.91 | 4.28 | 5.21 |
| Z-8 | 22.14 | 20.73 | 3.75 | 4.30 | 5.70 |
| Z-11 | 11.33 | 12.48 | 4.41 | 2.41 | 4.03 |
| Z-14 | 9.47 | 14.92 | 6.30 | 1.56 | 4.42 |
| Z-17 | 11.89 | 16.89 | 5.68 | 1.35 | 3.63 |
| Z-20 | 10.96 | 9.827 | 3.59 | 1.85 | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Hu, Y. Reservoir Characteristics of Marine–Continental Transitional Taiyuan Formation Shale and Its Influence on Methane Adsorption Capacity: A Case Study in Southern North China Basin. Appl. Sci. 2024, 14, 6577. https://doi.org/10.3390/app14156577
Jiang W, Hu Y. Reservoir Characteristics of Marine–Continental Transitional Taiyuan Formation Shale and Its Influence on Methane Adsorption Capacity: A Case Study in Southern North China Basin. Applied Sciences. 2024; 14(15):6577. https://doi.org/10.3390/app14156577
Chicago/Turabian StyleJiang, Wei, and Yang Hu. 2024. "Reservoir Characteristics of Marine–Continental Transitional Taiyuan Formation Shale and Its Influence on Methane Adsorption Capacity: A Case Study in Southern North China Basin" Applied Sciences 14, no. 15: 6577. https://doi.org/10.3390/app14156577
APA StyleJiang, W., & Hu, Y. (2024). Reservoir Characteristics of Marine–Continental Transitional Taiyuan Formation Shale and Its Influence on Methane Adsorption Capacity: A Case Study in Southern North China Basin. Applied Sciences, 14(15), 6577. https://doi.org/10.3390/app14156577





