Fuel–Water Emulsions as an Alternative Fuel for Gas Turbines: A Project Summary
Abstract
:1. Introduction
2. Subject and Methodology of Research
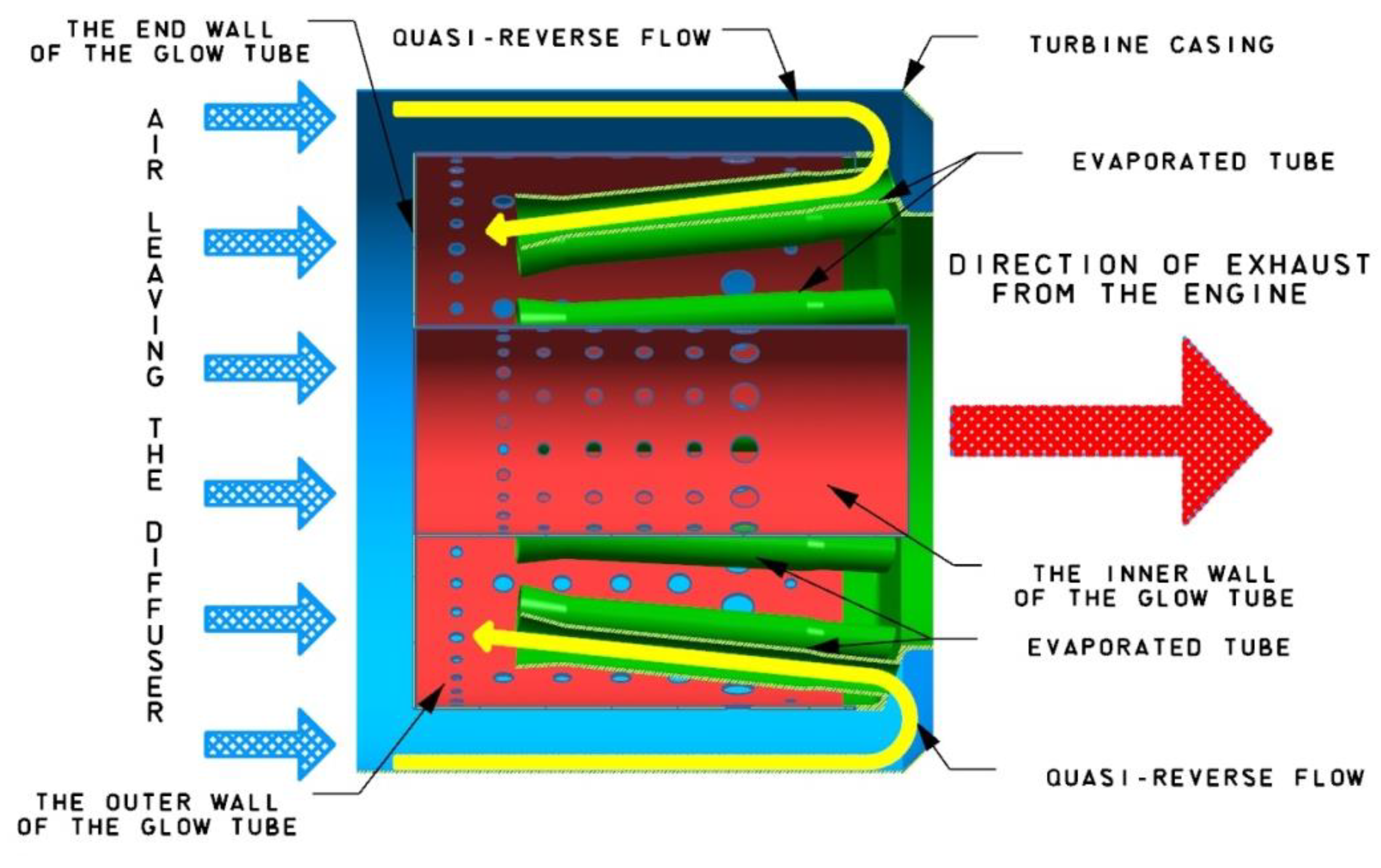
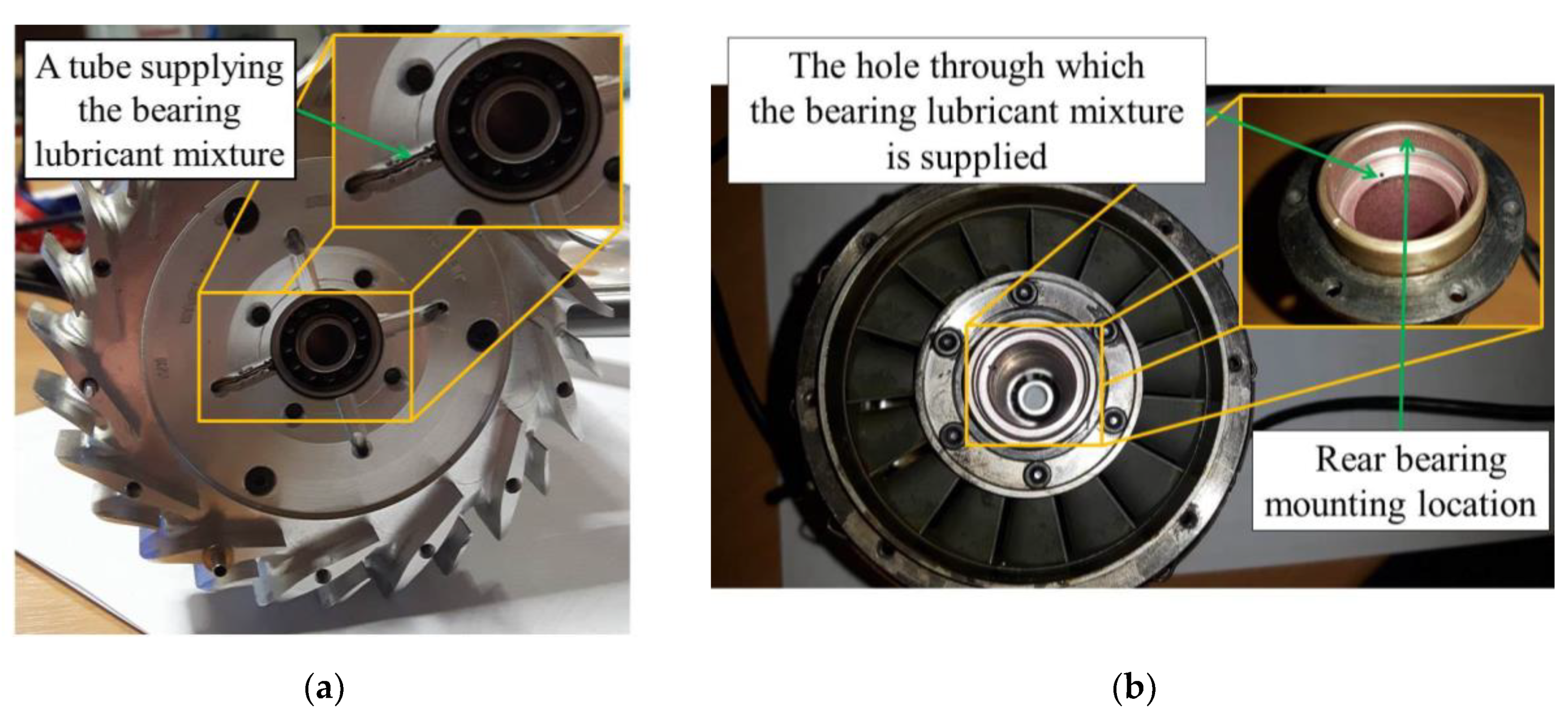
2.1. Miniature Gas Turbine
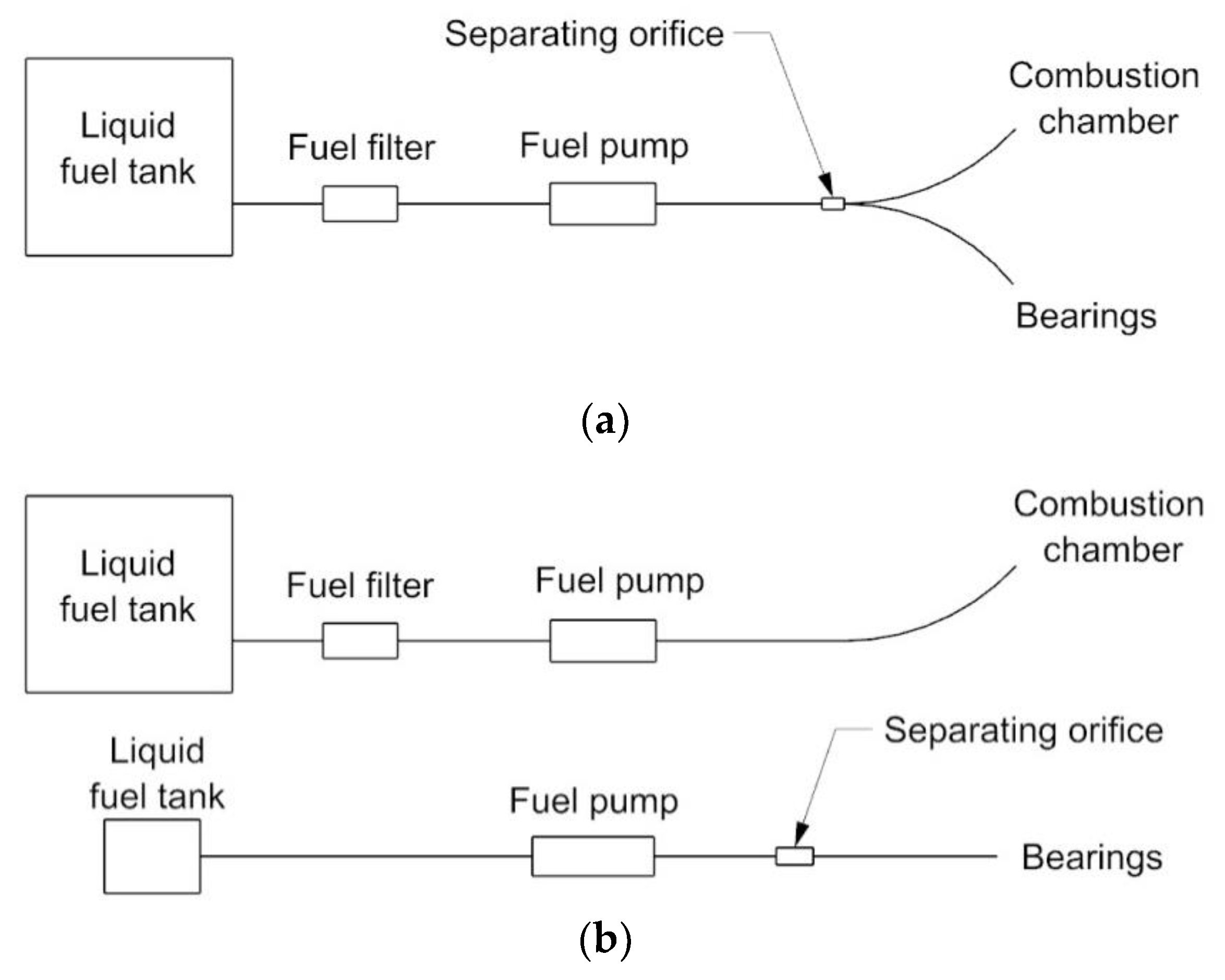
2.2. Research Stand
2.3. The Course of Experiments
2.4. Numerical Calculations
2.4.1. Combustion and Turbulence Model
2.4.2. Geometry
2.4.3. Boundary Conditions
2.4.4. Numerical Grid
2.5. Fuel Mixtures
2.5.1. Surfactant
2.5.2. Fuel–Water Emulsion
3. Research Results and Discussion
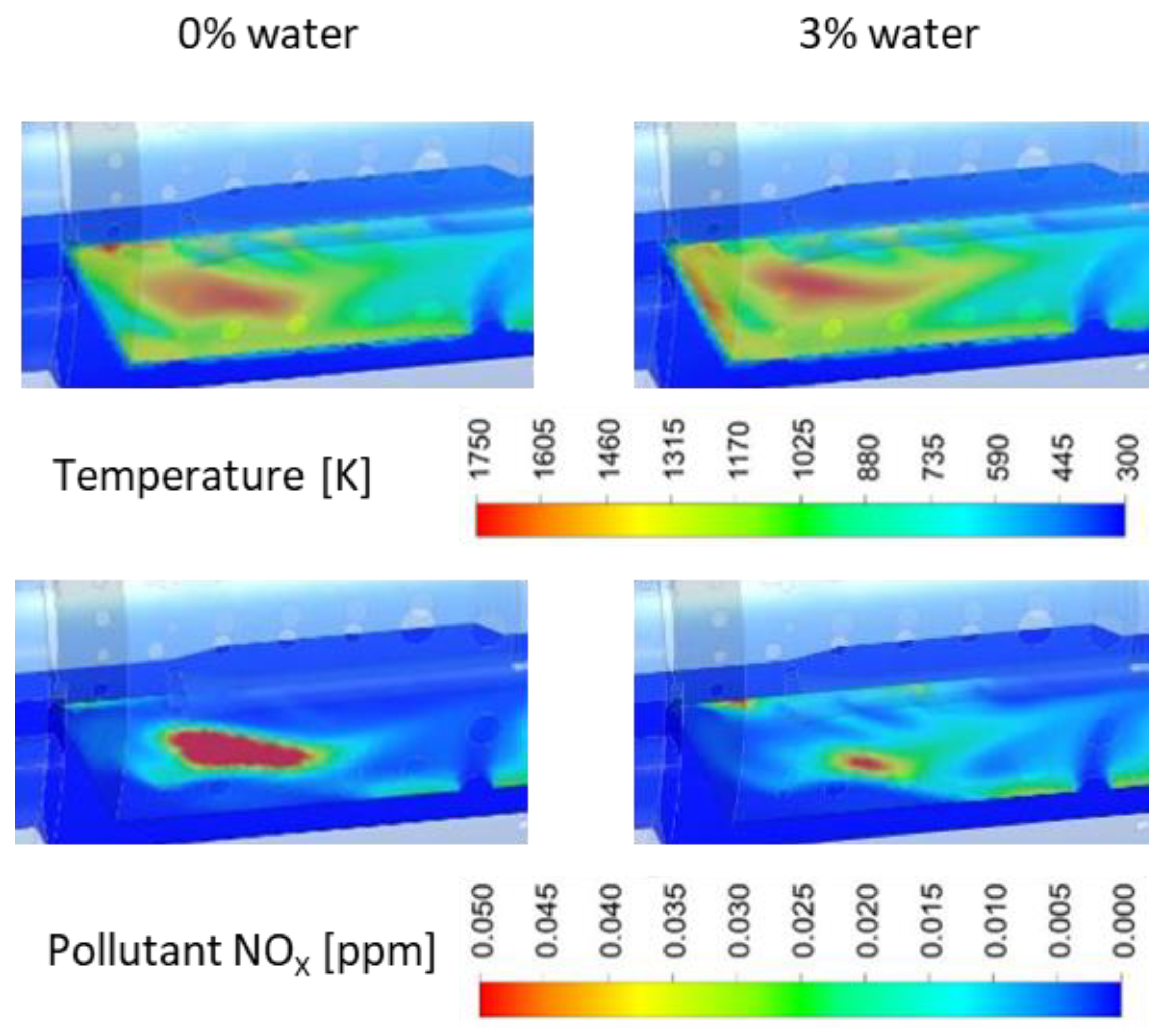
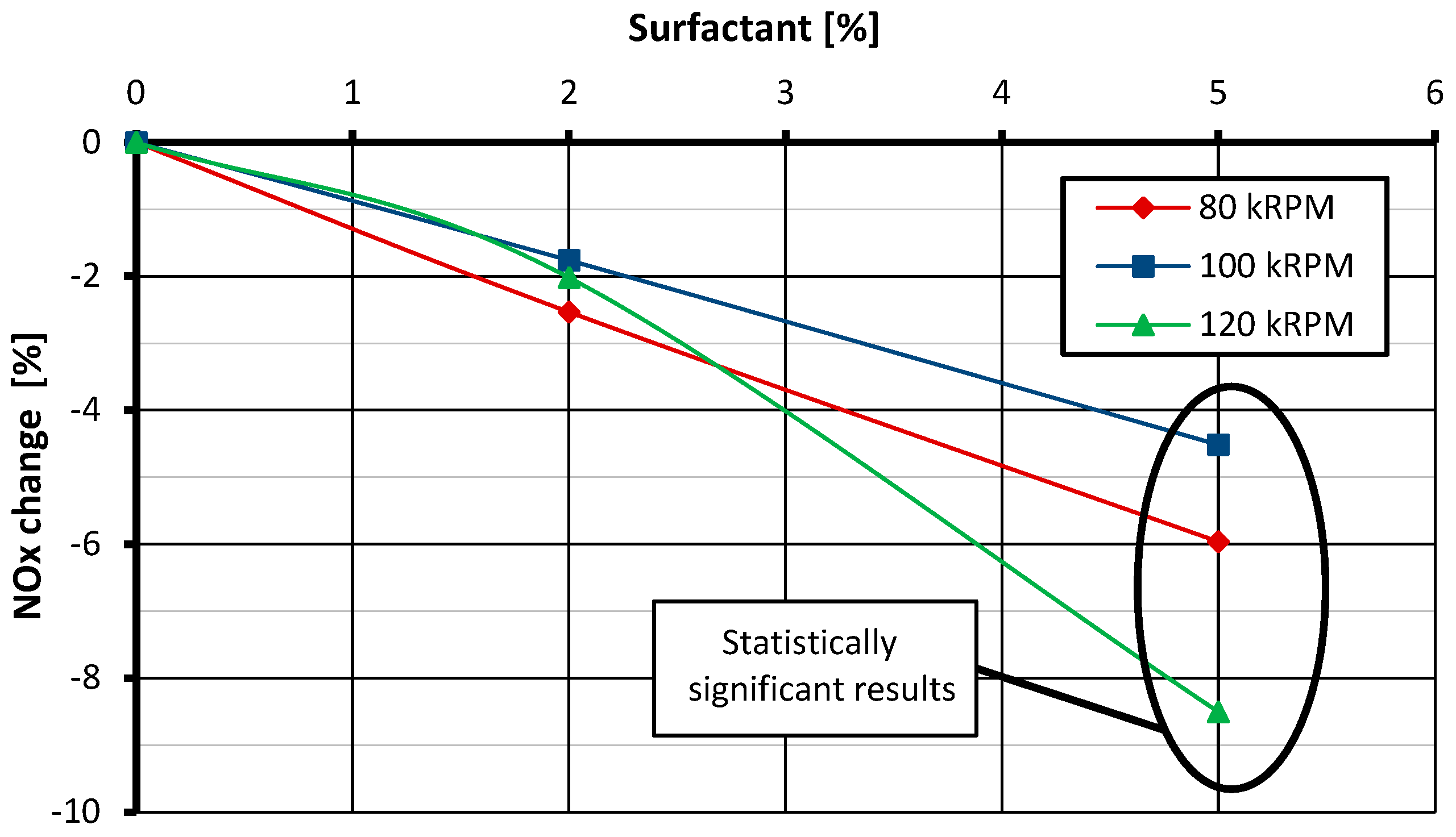
3.1. NOx Emissions
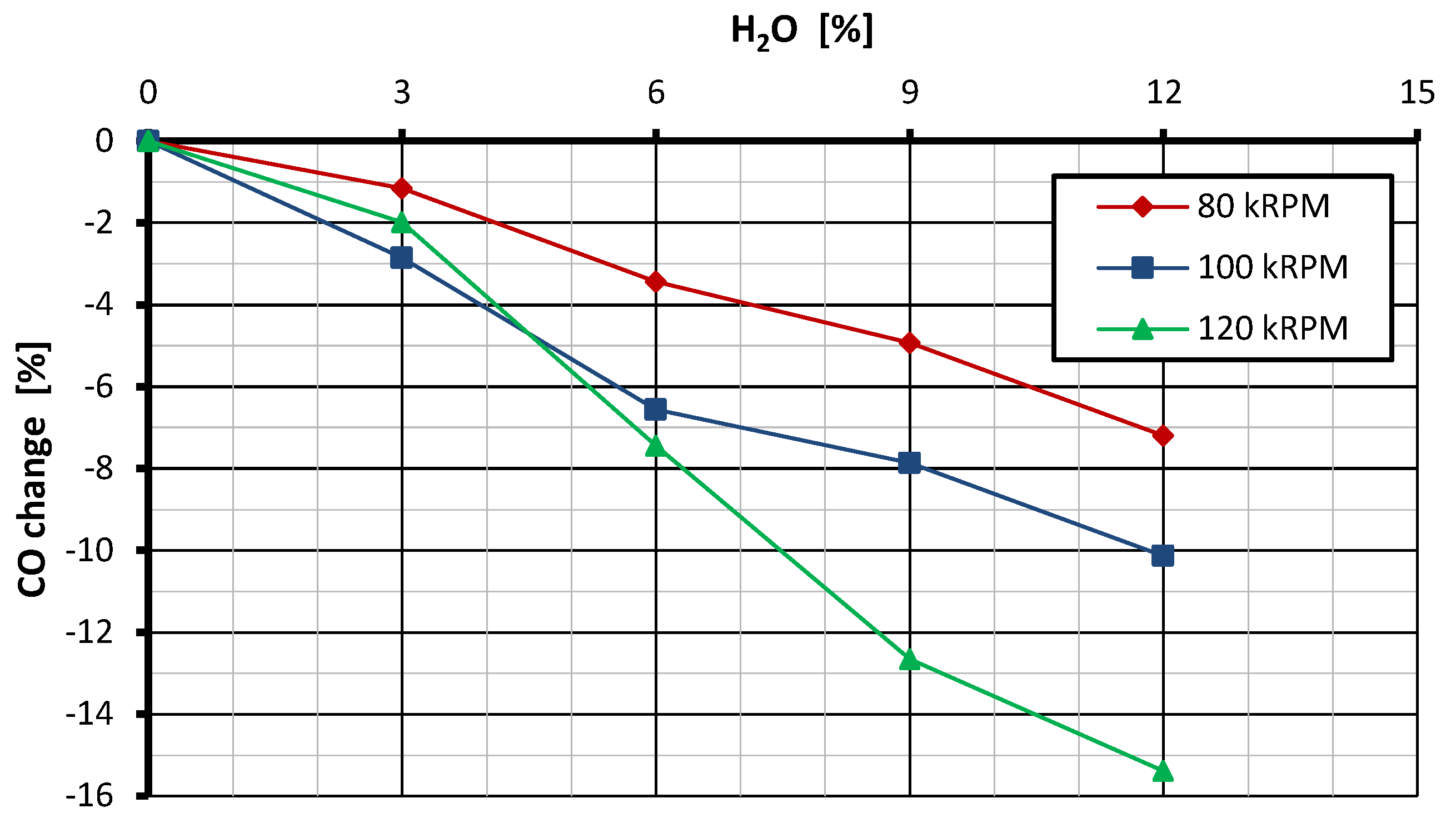
3.2. CO Emissions
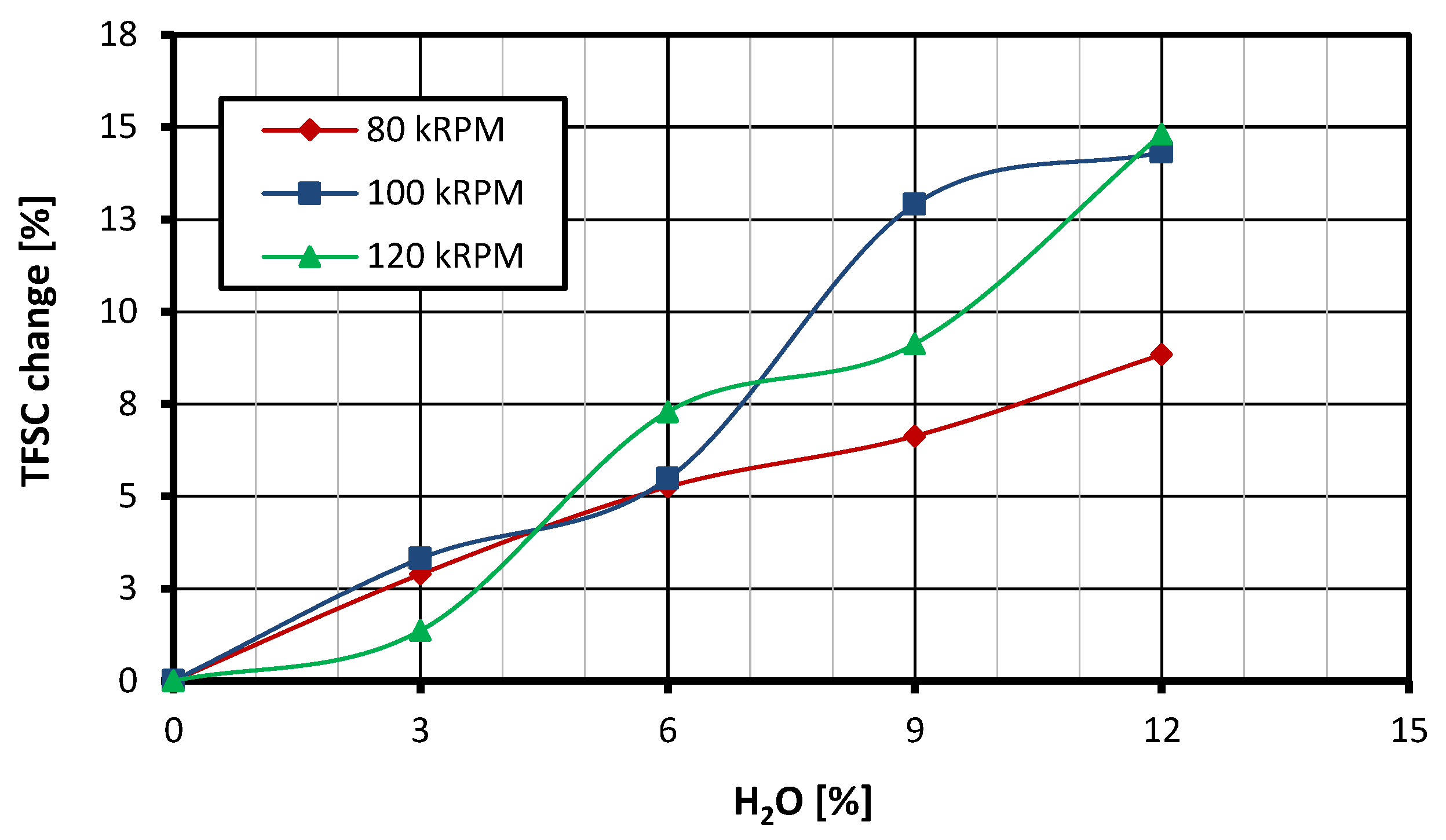
3.3. Fuel Consumption
3.4. The Influence of the Presence of an Emulsifier
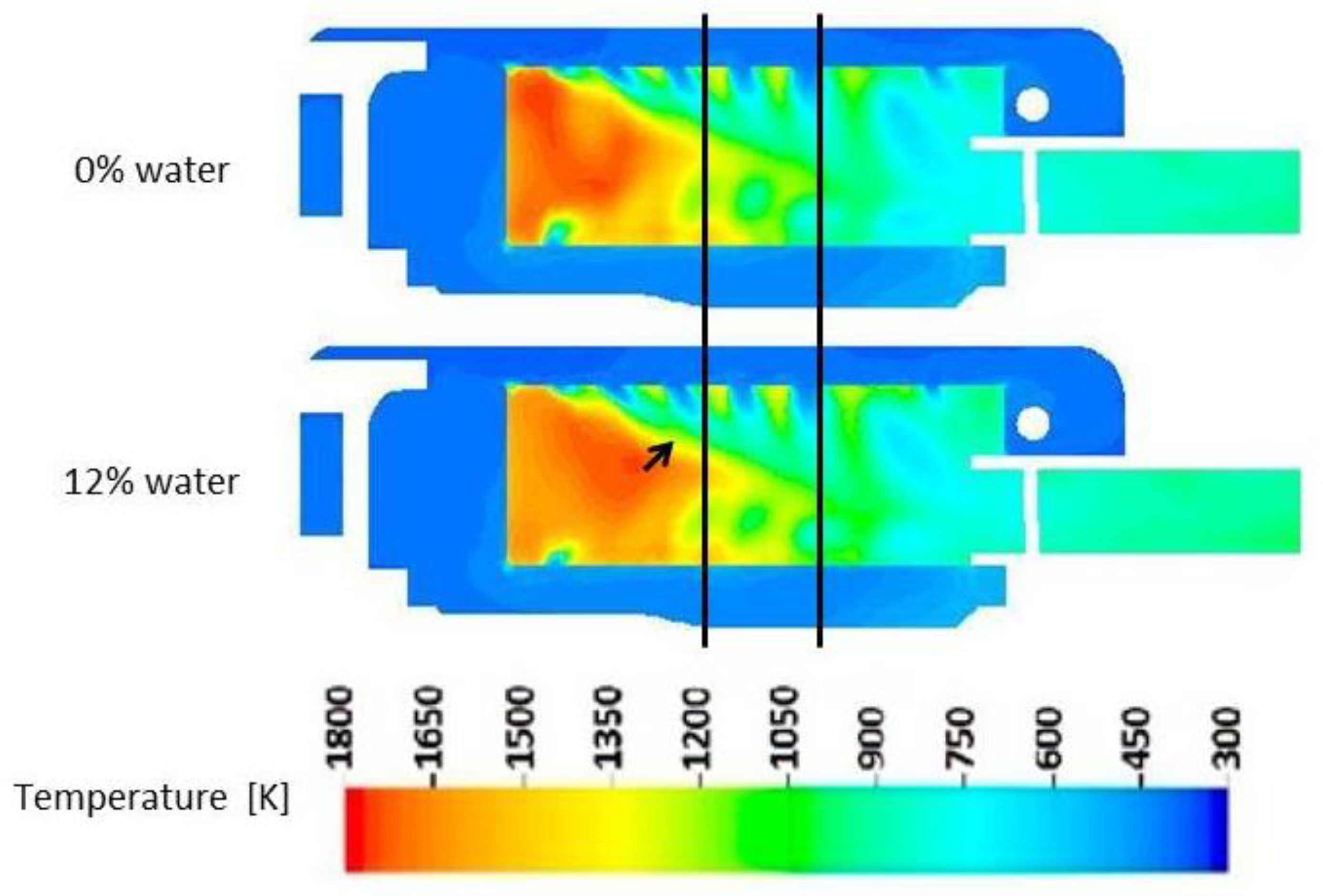
3.4.1. Combustion Process
3.4.2. Deposits in the Engine
4. Conclusions
- The use of a fuel–water emulsion leads to the reduction of NOx emissions in miniature gas turbines and full-size turbines. It occurs as a result of the extinction of hot spots in the primary combustion zone, where NO is produced intensively according to the thermal mechanism. However, it should be borne in mind that the nitrogen contained in the fuel in the case of emulsion fuel counteracts the reduction in NOx emissions, and that this effect is more intense the more water there is in the emulsion.
- Adding water to fuel in miniature gas turbines can lead to a reduction in CO emissions. This effect is caused by changes in the distribution of reaction intensity in the combustion zone, which promote the further oxidation of CO.
- When feeding the turbine with a fuel–water emulsion, the total flow rate of the fuel mixture applied to the combustion chamber increases compared with the case in which the turbine operates at the same speed and is fed with standard fuel. However, the consumption of base fuel (after subtracting the mass of water and emulsifier) is reduced in the GTM120 miniature gas turbine. For a rotational speed of 80 krpm and an emulsion containing 12% water, a reduction of 5.46% in the consumption of base fuel was recorded.
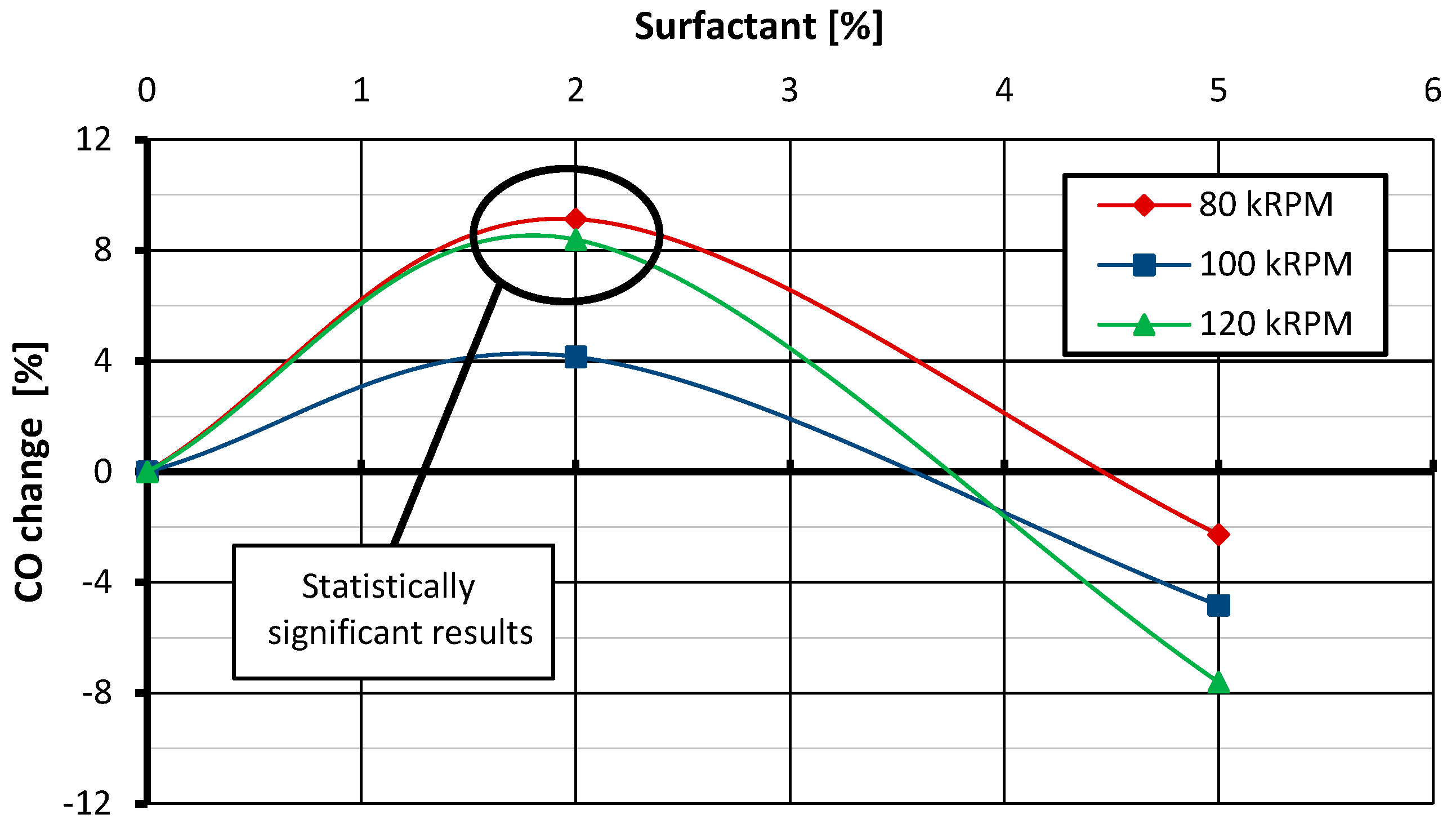
- The surfactant used in the tests, added to the fuel in an amount of 2% of the mixture mass, does not affect NOx emissions but has a negative impact on CO emissions. Increasing its content to 5% reduces NOx emissions compared with the emissions recorded for the reference case and has no impact on CO emissions. Moreover, the addition of emulsifiers in the amount of both 2% and 5% does not have a statistically significant effect on the thrust-specific fuel consumption.
- The use of emulsion fuel may lead to the formation of deposits on the elements of the hot section of the gas turbine. The glassy greenish coatings should be attributed to the surfactant, while the deposits in the form of white dust should be attributed to the water present in the emulsion or the synergistic effect of water and the surfactant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Encyclopedia Britannica. Available online: https://www.britannica.com/science/emulsion-chemistry (accessed on 26 April 2024).
- Yahaya Khan, M.; Abdul Karim, Z.A.; Hagos, F.Y.; Aziz, A.R.A.; Tan, I.M. Current Trends in Water-in-Diesel Emulsion as a Fuel. Sci. World J. 2014, 2014, 527472. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, K.H. The fuel properties of three-phase emulsions as an alternative fuel for diesel engines. Fuel 2003, 82, 1367–1375. [Google Scholar] [CrossRef]
- Niszczota, P.; Chmielewski, M.; Gieras, M. Fuel-Water Emulsion as an Alternative Fuel for Gas Turbines in the Context of Combustion Process Properties—A Review. Energies 2022, 15, 8979. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, L.W. Comparison of fuel properties and emission characteristics of two-and three-phase emulsions prepared by ultrasonically vibrating and mechanically homogenizing emulsification methods. Fuel 2008, 87, 2154–2161. [Google Scholar] [CrossRef]
- Mondal, P.K.; Mandal, B.K. Experimental investigation on the combustion, performance and emission characteristics of a diesel engine using water emulsified diesel prepared by ultrasonication. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 510. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Karmakar, S.; Som, S.K. Puffing and micro-explosion behavior in combustion of butanol/Jet A-1 and acetone-butanolethanol (ABE)/Jet A-1 fuel droplets. Combust. Sci. Technol. 2017, 189, 1796–1812. [Google Scholar] [CrossRef]
- Kadota, T.; Yamasaki, H. Recent advances in the combustion of water fuel emulsion. Prog. Energy Combust. Sci. 2002, 28, 385–404. [Google Scholar] [CrossRef]
- Jeong, I.; Lee, K.H.; Kim, J. Characteristics of auto-ignition and micro-explosion behavior of a single droplet of water-in-fuel. J. Mech. Sci. Technol. 2008, 22, 148–156. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar] [CrossRef]
- Kim, H.; Baek, S.W. Combustion of a single emulsion fuel droplet in a rapid compression machine. Energy 2016, 106, 422–430. [Google Scholar] [CrossRef]
- Califano, V.; Calabria, R.; Massoli, P. Experimental evaluation of the effect of emulsion stability on micro-explosion phenomena for water-in-oil emulsions. Fuel 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Investigation on the conditions leading to the micro-explosion of emulsified fuel droplet using two colors LIF method. Exp. Therm. Fluid Sci. 2020, 116, 110106. [Google Scholar] [CrossRef]
- Antonov, D.V.; Fedorenko, R.M.; Yanovskiy, L.S.; Strizhak, P.A. Physical and Mathematical Models of Micro-Explosions: Achievements and Directions of Improvement. Energies 2023, 16, 6034. [Google Scholar] [CrossRef]
- Fu, W.B.; Hou, L.Y.; Wang, L.; Ma, F.H. A unified model for the micro-explosion of emulsified droplets of oil and water. Fuel Process. Technol. 2002, 79, 107–119. [Google Scholar] [CrossRef]
- Le Clercq, P.; Noll, B.; Aigner, M. Modeling evaporation and microexplosion of water-in-alkane emulsion droplets. In Proceedings of the AIChE 2006 Annual Meeting, San Francisco, CA, USA, 12–17 November 2006. [Google Scholar]
- Klarman, A.F.; Rollo, A.J.; Scott, H.C. Evaluation of the Water/Fuel Emulsion Concept for Test Cell Smoke Abatement; Naval Air Propulsion Center, Propulsion Technology and Project Engineering Dept: Trenton, NJ, USA, 1978. [Google Scholar]
- Molière, M.; Colas, M.; Freimark, M. Des émulsions eau-fuel pour réduire les émissions de NOx de turbines à gaz. Rev. Gén. Therm. 1989, 330–331, 428–435. [Google Scholar]
- Meisl, J.; Lauer, G.; Hoffmann, S. Low NOx emission technology for the VX4. 3A gas turbine series in fuel oil operation. In Proceedings of the ASME Turbo Expo 2002: Power for Land, Sea, and Air, Amsterdam, The Netherlands, 3–6 June 2002; Volume 36061, pp. 665–671. [Google Scholar] [CrossRef]
- Prade, B.; Meisl, J.R.; Berenbrink, P.; Streb, H.; Hoffmann, S. Burner development for flexible engine operation of the newest Siemens gas turbines. In Proceedings of the ASME Turbo Expo 2003, collocated with the 2003 International Joint Power Generation Conference, Atlanta, GA, USA, 16–19 June 2003; Volume 36851, pp. 371–376. [Google Scholar] [CrossRef]
- Lauer, G.; Meisl, J.; Belting, C.; Hoffmann, S. Further development of low-emissions oil combustion in the Vx4. 3A hybrid burner. VGB Powertech. 2002, 82, 51–55. [Google Scholar]
- Aguilar Hernandez, D.; Sullivan-Lewis, E.; McDonell, V. Adaptation of a 60kW Commercial Natural Gas Fired Microturbine for Operation on Diesel and Diesel-Water Emulsions. In Proceedings of the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, Charlotte, NC, USA, 26–30 June 2017; Volume 50848, p. V04AT04A053. [Google Scholar] [CrossRef]
- Spadaccini, L.J.; Pelmas, J. Evaluation of oil/water emulsions for gas turbine engines. Adv. Chem. 1978, 166, 232–244. [Google Scholar] [CrossRef]
- Lee, T.S.; Yang, J.Y. An Experimental Study on the Burning of Water-in-Ol Emulsions. Trans. Aeronaut Astronaut. Soc. China 1999, 31, 252–277. [Google Scholar]
- Gopidesi, R.K.; Selvi Rajaram, P. A review on emulsified fuels and their application in diesel engine. Int. J. Ambient Energy 2022, 43, 732–740. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Study of the droplet size of the discrete phase of a fuel-water emulsion. J. KONBiN 2023, 53, 1–12. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ghobadian, B.; Najafi, G.; Hoseini, S.S.; Mofijur, M.; Mazlan, M. Impact of water–biodiesel–diesel nano-emulsion fuel on performance parameters and diesel engine emission. Fuel 2020, 280, 118576. [Google Scholar] [CrossRef]
- Jhalani, A.; Sharma, D.; Soni, S.L.; Sharma, P.K.; Sharma, S. A comprehensive review on water-emulsified diesel fuel: Chemistry, engine performance and exhaust emissions. Environ. Sci. Pollut. Res. 2019, 26, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Hasannuddin, A.K.; Wira, J.Y.; Srithar, R.; Sarah, S.; Ahmad, M.I.; Aizam, S.A.; Aiman, M.A.B.; Zahari, M.; Watanabe, S.; Azrin, M.A.; et al. Effect of emulsion fuel on engine emissions—A review. Clean Technol. Environ. Policy 2016, 18, 17–32. [Google Scholar] [CrossRef]
- Naegeli, D.W.; Fodor, G.E.; Moses, C.A. Fuel Microemulsions for Jet Engine Smoke Reduction; Tyndall Air Force Base: Panama City, FL, USA, 1980. [Google Scholar]
- Park, K.; Kwak, I.; Oh, S. The effect of water emulsified fuel on a motorway-bus diesel engine. KSME Int. J. 2004, 18, 2049–2057. [Google Scholar] [CrossRef]
- Gürbüz, H.; Akçay, H.; Aldemir, M.; Akçay, İ.H.; Topalcı, Ü. The effect of euro diesel-hydrogen dual fuel combustion on performance and environmental-economic indicators in a small UAV turbojet engine. Fuel 2021, 306, 121735. [Google Scholar] [CrossRef]
- Dutczak, J. Micro turbine engines for drones propulsion. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Scientific Conference on Automotive Vehicles and Combustion Engines (KONMOT 2016), Krakow, Poland, 22–23 September 2016. [Google Scholar] [CrossRef]
- JETPOL Tomasz Kiciński. Available online: https://jetpol.tech (accessed on 26 April 2024).
- Gieras, M. Miniaturowe Silniki Turboodrzutowe, 1st ed.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2016; pp. 41–51. ISBN 978-83-7814-552-3. [Google Scholar]
- Alabaş, H.A.; Çeper, B.A. Effect of the hydrogen/kerosene blend on the combustion characteristics and pollutant emissions in a mini jet engine under CDC conditions. Int. J. Hydrogen Energy 2024, 52, 1275–1287. [Google Scholar] [CrossRef]
- Chmielewski, M.; Gieras, M. Small gas turbine GTM-120 bench testing with emission measurements. J. KONES 2015, 22, 47–54. [Google Scholar] [CrossRef]
- Chmielewski, M. Badanie Wpływu Zmiennej Geometrii Komory Spalania Na Efektywność Procesów Spalania I Emisje Substancji Szkodliwych W Miniaturowej Turbinie Gazowej. Ph.D. Thesis, The Institute of Heat Engineering, Warsaw, Poland, 2016. [Google Scholar]
- Chmielewski, M.; Niszczota, P.; Gieras, M. Combustion efficiency of fuel-water emulsion in a small gas turbine. Energy 2020, 211, 118961. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Impact of the Application of Fuel and Water Emulsion on CO and NOx Emission and Fuel Consumption in a Miniature Gas Turbine. Energies 2021, 14, 2224. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Effect of Adding Emulsifier to Fuel on Work Efficiency and Gas Turbine Emissions. Energies 2021, 14, 5255. [Google Scholar] [CrossRef]
- Peters, N. Laminar diffusion flamelet models in non-premixed turbulent combustion. Prog. Energy Combust. Sci. 1984, 10, 319–339. [Google Scholar] [CrossRef]
- Peters, N. Laminar flamelet concepts in turbulent combustion. Symp. (Int.) Combust. 1988, 21, 1231–1250. [Google Scholar] [CrossRef]
- Dixon-Lewis, G. Structure of laminar flames. Symp. (Int.) Combust. 1991, 23, 305–324. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. Lectures in Mathematical Models of Turbulence; Academic Press: London, UK, 1972. [Google Scholar]
- Chmielewski, M.; Gieras, M. Impact of variable geometry combustor on performance and emissions from miniature gas turbine engine. J. Energy Inst. 2017, 90, 257–264. [Google Scholar] [CrossRef]
- Verstraete, D.; Hendrick, P.; Djanali, V.; Ling, J.; Wong, K.C.; Armfield, S. Micro propulsion activities at the University of Sydney. In Proceeding of the Powermems 2010 Conference, Leuven, Belgium, 30 November–3 December 2010. [Google Scholar]
- Gieras, M.; Stańkowski, T. Computational study of an aerodynamic flow through a micro-turbine engine combustor. J. Power Technol. 2012, 92, 68–79. [Google Scholar]
- Toro, C.A.G.; Wong, K.C.; Armfield, S. Computational study of a micro-turbine engine combustor using large eddy simulation and Reynolds averaged turbulence models. ANZIAM J. 2007, 49, C407–C422. [Google Scholar] [CrossRef]
- White, F.M. Fluid Mechanics, 5th ed.; McGraw-Hill: Boston, MA, USA, 2003; p. 467. [Google Scholar]
- Niszczota, P.; Gieras, M. Photographic data on the influence of the composition, preparation method, time and fuel system on the size of water droplets in a fuel-water emulsion. Data Brief 2022, 43, 108406. [Google Scholar] [CrossRef] [PubMed]
- Niszczota, P.; Gieras, M. Photographic data on the influence of the composition, preparation method, time and fuel system on the size of water droplets in a fuel-water emulsion. Zenodo 2022. [Google Scholar] [CrossRef]
- Park, J.; Nguyen, T.H.; Joung, D.; Huh, K.Y.; Lee, M.C. Prediction of NOx and CO Emissions from an Industrial Lean-Premixed Gas Turbine Combustor Using a Chemical Reactor Network Model. Energy Fuels 2013, 27, 1643–1651. [Google Scholar] [CrossRef]
- Correa, S.M. A review of NOx formation under gas-turbine combustion conditions. Combust. Sci. Technol. 1993, 87, 329–362. [Google Scholar] [CrossRef]
- Gieras, M. Komory Spalania Silników Turbinowych: Organizacja Procesu Spalania, 1st ed.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2010; pp. 120–124. ISBN 978-83-7207-864-3. [Google Scholar]
- Łapucha, R. Komory Spalania Silników Turbinowo-Odrzutowych: Procesy, Obliczenia, Badania; Wydawnictwa Naukowe Instytutu Lotnictwa: Warsaw, Poland, 2004; pp. 128–143. ISBN 83-915995-8-2. [Google Scholar]
- Chmielewski, M.; Gieras, M.; Niszczota, P. Fuel-Water emulsion impact on miniature gas turbine pollutant emission. In Proceedings of the E3S Web of Conferences, Warsaw, Poland, 3–6 December 2019; EDP Sciences: Les Ulis, France, 2019; Volume 137, p. 01046. [Google Scholar] [CrossRef]
- Naegeli, D.W.; Moses, C.A. Fuel Microemulsions for Jet Engine Smoke Reduction. In Proceedings of the ASME 1982 International Gas Turbine Conference and Exhibit, London, UK, 18–22 April 1982; Volume 79580, p. V003T06A009. [Google Scholar] [CrossRef]
- Naegeli, D.W.; Moses, C.A. Fuel microemulsions for jet engine smoke reduction. J. Eng. Power 1983, 105, 18–23. [Google Scholar] [CrossRef]
- Mann, M.; Jainski, C.; Euler, M.; Böhm, B.; Dreizler, A. Transient flame-wall interactions: Experimental analysis using spectroscopic temperature and CO concentration measurements. Combust. Flame 2014, 161, 2371–2386. [Google Scholar] [CrossRef]
- De Giorgi, M.G.; Fontanarosa, D.; Ficarella, A.; Pescini, E. Effects on performance, combustion and pollutants of water emulsified fuel in an aeroengine combustor. Appl. Energy 2020, 260, 114263. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Influence of surfactant addition to emulsion fuels on the hot section of turbine engines. J. KONBiN 2023, 53, 13–25. [Google Scholar] [CrossRef]









| Parameter | Sensor | Uncertainty of Measurement |
|---|---|---|
| Thrust | NS-6 + WDT-1 | ±0.55 N |
| Pressure | Kobold SEN-8701/2 B045 | ±2.50 kPa |
| Pressure | Kobold SEN-8701/2 B055 | ±4.00 kPa |
| Pressure | Dudek P400U | ±34.0 Pa |
| Pressure | Honeywell ASDX030D44R | 4.14 kPa |
| Temperature | Thermocouple NiCr-NiAl | - in the range of −40 ÷ 375 °C: ±1.5 °C - in the range 375 ÷ 1000 °C: ±0.4% of the measured value |
| Parameter | Resolution | Uncertainty of Measurement |
|---|---|---|
| O2 | 0.01 vol. | ±0.2% vol. |
| CO | 1 ppm | ±5% of the measured value |
| NO | 0.1 ppm | ±2 ppm |
| NO2 | 0.1 ppm | ±5 ppm |
| CO2 | 0.01% vol. | ±0.3% vol. + 1% of the measured value |
| Component | Percentage Content |
|---|---|
| Rokwin 80 | 50.00 |
| Rokanol RZ4P11 | 25.00 |
| Rokanol DB3 | 22.50 |
| Rokafenol N8 | 1.67 |
| Water | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niszczota, P.; Gieras, M. Fuel–Water Emulsions as an Alternative Fuel for Gas Turbines: A Project Summary. Appl. Sci. 2024, 14, 6686. https://doi.org/10.3390/app14156686
Niszczota P, Gieras M. Fuel–Water Emulsions as an Alternative Fuel for Gas Turbines: A Project Summary. Applied Sciences. 2024; 14(15):6686. https://doi.org/10.3390/app14156686
Chicago/Turabian StyleNiszczota, Paweł, and Marian Gieras. 2024. "Fuel–Water Emulsions as an Alternative Fuel for Gas Turbines: A Project Summary" Applied Sciences 14, no. 15: 6686. https://doi.org/10.3390/app14156686
APA StyleNiszczota, P., & Gieras, M. (2024). Fuel–Water Emulsions as an Alternative Fuel for Gas Turbines: A Project Summary. Applied Sciences, 14(15), 6686. https://doi.org/10.3390/app14156686






