Abstract
Cerebral aneurysms come in a wide range of shapes and sizes; they can also evolve over time, presenting significant changes. Large aneurysms are generally thought to be more prone to rupture, but rupture has also been observed in small aneurysms, indicating the presence of additional risk factors. The aim of this study was to assess the effects of the aneurysm’s size and wall thickness on its rupture risk, by using fluid–structure interaction simulations. Six patient-specific geometries were studied: four related to the effect of size and two related to the effect of wall thickness. Additional cases in which the aneurysm was removed were included. It was found that thinner walls suffered from significantly greater stresses, whereas an increment in size led, in general, to lower levels of wall shear stress and greater equivalent stress. By removing the aneurysm, the reduction in the time-averaged wall shear stress was 75% at the rupture point. Although the size of an aneurysm has a great impact on its rupture risk, the wall thickness needs to be considered, since with maintenance of its size, an aneurysm can suffer from wall thinning, which can lead to structural failure.
1. Introduction
A cerebral aneurysm is a lesion that presents as a focal bulging in blood vessels and is typically located in a region of the brain known as the circle of Willis [1]. The prevalence of this condition is about 3% among individuals without underlying health conditions [2]. The most severe outcome of these lesions is rupture, leading to subarachnoid hemorrhage (SAH) [3,4], which is associated with a mortality rate that exceeds 40% [5].
The serious consequences that a cerebral aneurysm may produce, along with the high-complexity treatments the patient can receive, such as neurosurgery, necessitate the development of a method to predict the rupture risk of cerebral aneurysms. This would allow better decisions to be made regarding whether or not the patient needs complex treatment. Currently, such a method does not exist, but it is known that the interaction between blood flow and artery walls is the key factor involved in aneurysms’ formation, as well as their development and rupture [6].
It is thought that wall shear stress (WSS) is one of the most important hemodynamic factors to study, as zones with high WSS levels are more prone to aneurysm formation [7,8,9], while zones with low WSS have been related to the development and rupture of existing lesions. Low stress levels have been found at sites where ruptures have been produced at the aneurysm dome [10,11], although high WSS levels combined with other factors can also lead to rupture [12]. Newtonian fluid provides a good approximation for blood flow in large arteries. However, inside the aneurysm, this approximation is incorrect because of the presence of flow regions with lower velocities, in which non-Newtonian properties of viscosity become important. In such cases, blood flow must be simulated using a non-Newtonian model [12].
Once an aneurysm has been initiated, different outcomes can occur: rupture at an early stage, an increase in size, and then, rupture or stability over time [13]. The size of an aneurysm does not evolve at a constant rate [14]. There are periods where it does not change, other periods where it can grow, and different growth rates between ruptured and unruptured aneurysms [15]. It has been shown that aneurysm growth can lead to significant morphological changes from at least 23 weeks [16].
Although it may be intuitive to think that an aneurysm will rupture when it is large, small aneurysms also suffer from ruptures [17], which suggests that size is not the only risk factor. In addition to health conditions, such as hypertension or trauma, the aneurysm wall is thinner than that of the parent artery [18], and contrary to what may be thought, when the aneurysm grows, its wall thickness does not necessarily decrease [19], because it is a living structure that can repair itself. The rupture risk increases to 0.5% per annum for aneurysms with diameters greater than 10 mm [3]. The width of the aneurysm neck and the size ratio of the aneurysm have been reported to affect the rupture risk [16]. Other risk factors are the presence of atherosclerotic plaque, smoking, and hypertension [3].
In several studies, hemodynamic parameters have been modified to reflect different health conditions that might affect the rupture risk of an aneurysm, and a few studies have modified geometrical factors. Sun et al. [20] showed that when an aneurysm grows and its wall thickness decreases, the rupture risk increases due to a reduction in the WSS and an increment in the von Mises stress at the aneurysm. Even at a constant wall thickness, the maximum von Mises stress still increases. Nath et al. [21] studied spherical aneurysm geometries using structural simulations. Initially, aneurysms showed a reduction in the maximum von Mises stress, but this then increased along with the aneurysm size, leading to a higher rupture risk. Other works have used cylindrical/spherical membranes subjected to 1D enlargement to model the development of an aneurysm, and the mechanical response has been attributed to collagen and elastin [22].
These previous works assumed a uniformly distributed wall thickness over the aneurysm dome; however, real aneurysms do not have a uniform wall thickness distribution, and this can be related to stress concentrations [23] or aneurysm expansion when present in the abdomen [24]. Even with current medical technology, wall thickness remains a difficult parameter to measure in patients without performing surgery or removing the aneurysm, especially for cerebral aneurysms. Furthermore, in terms of removing an aneurysm, simulations have shown that a non-uniform wall thickness distribution is associated with higher stress values at the rupture site compared with configurations with a constant wall thickness [25].
In the present work, the effect of geometrical parameters was studied, specifically, the effects of the aneurysm wall thickness and size on the rupture risk. Each parameter was varied separately to better show the individual effects on the rupture risk of cerebral aneurysms. Size changes alter the fluid dynamics and level of structural stress in aneurysms, but a reduction in the aneurysm wall thickness modifies the structural stress. We are interested in both phenomena. We additionally studied the WSS between the pre-aneurysm and aneurysm state in lateral aneurysms. Wall thickness changes produce wall deformation and, therefore, changes in the fluid dynamics in the aneurysms. Finally, there is a change in the WSS, which can increase the rupture risk [23].
Six patient-specific geometries were chosen, and boundary conditions representing healthy conditions for an adult patient were used. Due to the importance of accounting for blood and wall interactions in simulations to accurately predict patient-specific hemodynamics [24,25,26,27], a fluid–structure interaction (FSI) approach was used. Additionally, as differences in displacement of up to 30% have been observed when comparing two-way and one-way FSI simulations [28], the former method was used.
Additionally, cases were included where the aneurysm was virtually removed from the lateral geometries with the aim of simulating the state prior to lesion initiation.
2. Materials and Methods
2.1. Materials and Methods
The geometries were obtained from a cerebral aneurysm database generated with the methodology presented by Valencia et al. [29], who obtained medical images provided by Dr. Alfonso Asenjo (INCA) from the Instituto de Neurocirugía using the tridimensional rotational angiography method with a Philips Integris Allura device. The acquisition process is reported in detail in [29]. All patients involved in this investigation gave their informed consent for their 3D angiography image data to be used for numerical investigations, as reported in [29], and no new geometries were reconstructed for the present study.
Six geometries were studied: three ruptured and three unruptured. For each state, two lateral geometries and one terminal geometry were included in the database, considering different sizes and forms. Geometries with small aneurysms and those with blebs in the aneurysms were excluded.
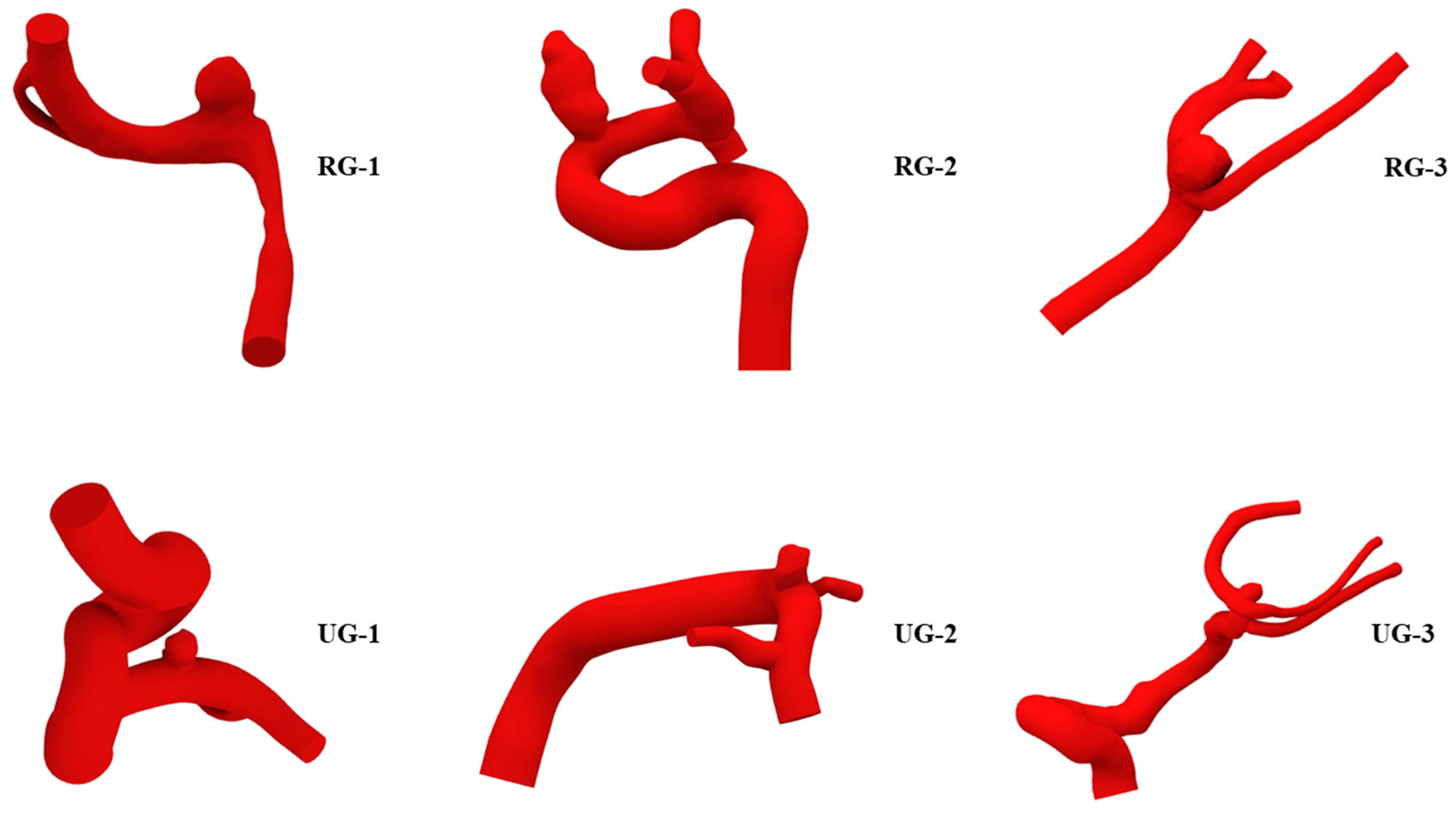
Those geometries can be seen, along with their names, in Figure 1. Those with ruptures were named RG, and those without ruptures were named UG. Table 1 shows each geometry’s name as well as its type, location, and total geometric volume. According to that table, aneurysms were located in the anterior cerebral, anterior communicating, and pericallosal artery (ACA); the internal carotid, ophthalmic, posterior communicating, and anterior choroidal artery (ICA); or the middle cerebral artery (MCA). Both RG-1 and UG-1 were used to study the effect of aneurysm wall thickness. The other geometries were used to study the effect of aneurysm size. Since the worst outcome for an aneurysm is its rupture, geometries that presented with ruptures were modified to reduce their size to study a past state, and those with no previous ruptures were modified to increase their size to simulate a possible future state. The methodology used to modify the size is shown next.
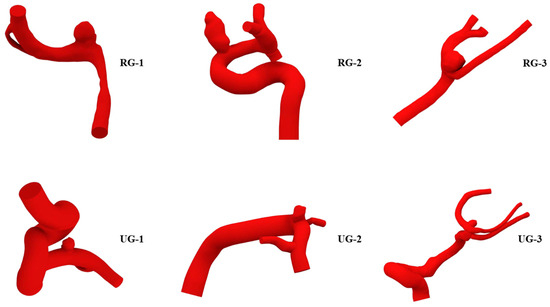
Figure 1.
Geometries selected for the study. With previous rupture: RG-1 to RG-3, at the top; without previous rupture: UG-1 to UG-3, at the bottom.

Table 1.
The rupture status, type, location, and total volume of each geometry. RG: previous rupture geometry; UG: without previous rupture geometry.
2.2. Size Variation
Since there is no definitive method to predict aneurysm growth due to the many factors that can affect the growing process, in this study, we used a method found in the literature. The employed algorithm was an adaptation of that described by Sun in previously cited work. That algorithm was modified for use with T-spline bodies in Autodesk Fusion 360 and to decrease those ruptured aneurysms.
Instead of using offsets, as was performed in the original procedure, here, for the scaling process, a scale factor s was used to expand the aneurysm parallel to its neck plane. s is defined as the ratio between equivalent aneurysm diameters after (Df) and before (Di) scaling:
Df/Di = s
It was assumed that the contours in which the aneurysm was sectioned were similar to the circumference. Accordingly, the following formula was used:
where the approximation is taken as equality. Δ is the offset used by Sun et al., i.e., Δ = (α − 1). Then, the previous expression can be written as Df = Di + 2(α − 1), and s can be defined as follows:
Df ≈ Di + 2Δ
s = (2α − 1)
Expression (3) was used to show the growth of unruptured aneurysms. For ruptured aneurysms, s′ was used instead, defined as s′ = 1/s.
The main steps of the algorithm are listed below:
- From the original geometry, the aneurysm was extracted to convert its main faces into a T-spline body. The neck plane was visually identified, and a point was placed at its center, through which a perpendicular axis V passed.
- A factor αmax = 1.26 was used for all the cases. As in the original algorithm, there is an α parameter that begins at 0 when V = 0 and linearly varies up to αmax for V > Vmax/3. Vmax is the distance between the point at the center of the neck and the highest point of the aneurysm dome. The height of each contour on the T-spline body, approximately parallel to the neck plane, was measured and named Vc.
- Each contour was moved upwards for unruptured aneurysms and downwards for ruptured aneurysms in the quantity Vc (α − 1). Additionally, each contour was scaled with the factor s or s′ depending on the rupture status of the aneurysm, parallel to the neck plane. The moving and scaling processes were repeated for each contour line on the body.
- When the process was completed, the T-spline body was converted into a surface. The top zone of the aneurysm was closed following the curvature of the surrounding faces, and the zone at the bottom of the aneurysm was closed with another surface. Then, the created surface bodies were joined and converted into a solid. Finally, the resultant scaled aneurysm was joined back to the artery. For lateral aneurysms only, a fillet was created on the neck to form an organic transition between the artery and the aneurysm.
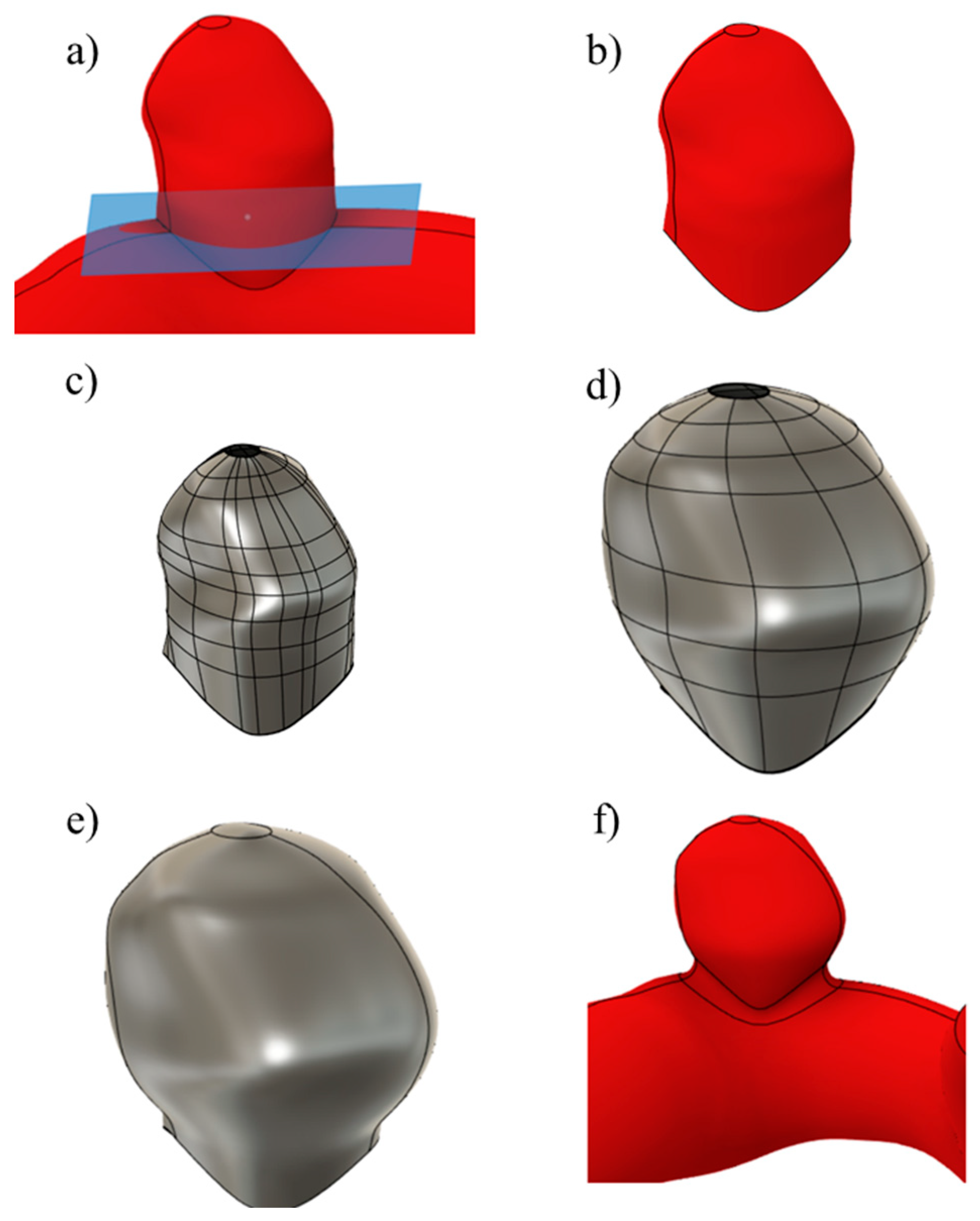
An example of the main steps followed is shown in Figure 2, where the algorithm is applied to UG-2.
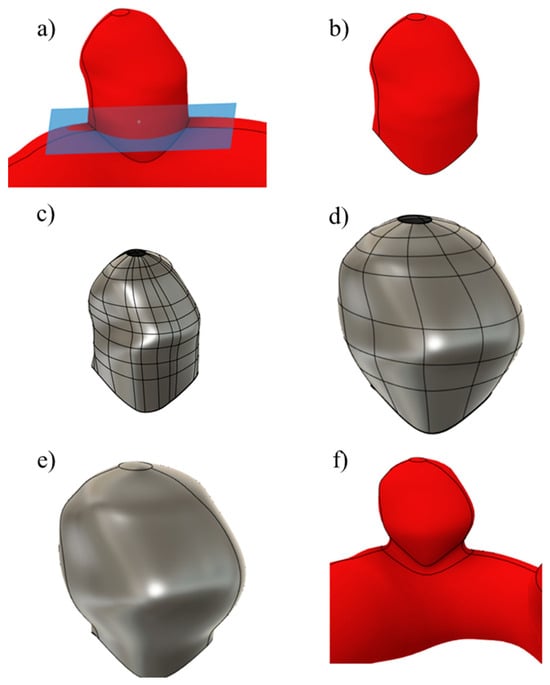
Figure 2.
The main steps involved in the scaling process: (a) neck plane, (b) aneurysm removal, (c) T-spline body, (d) scaled body, (e) solid body, and (f) aneurysm joined to the artery.
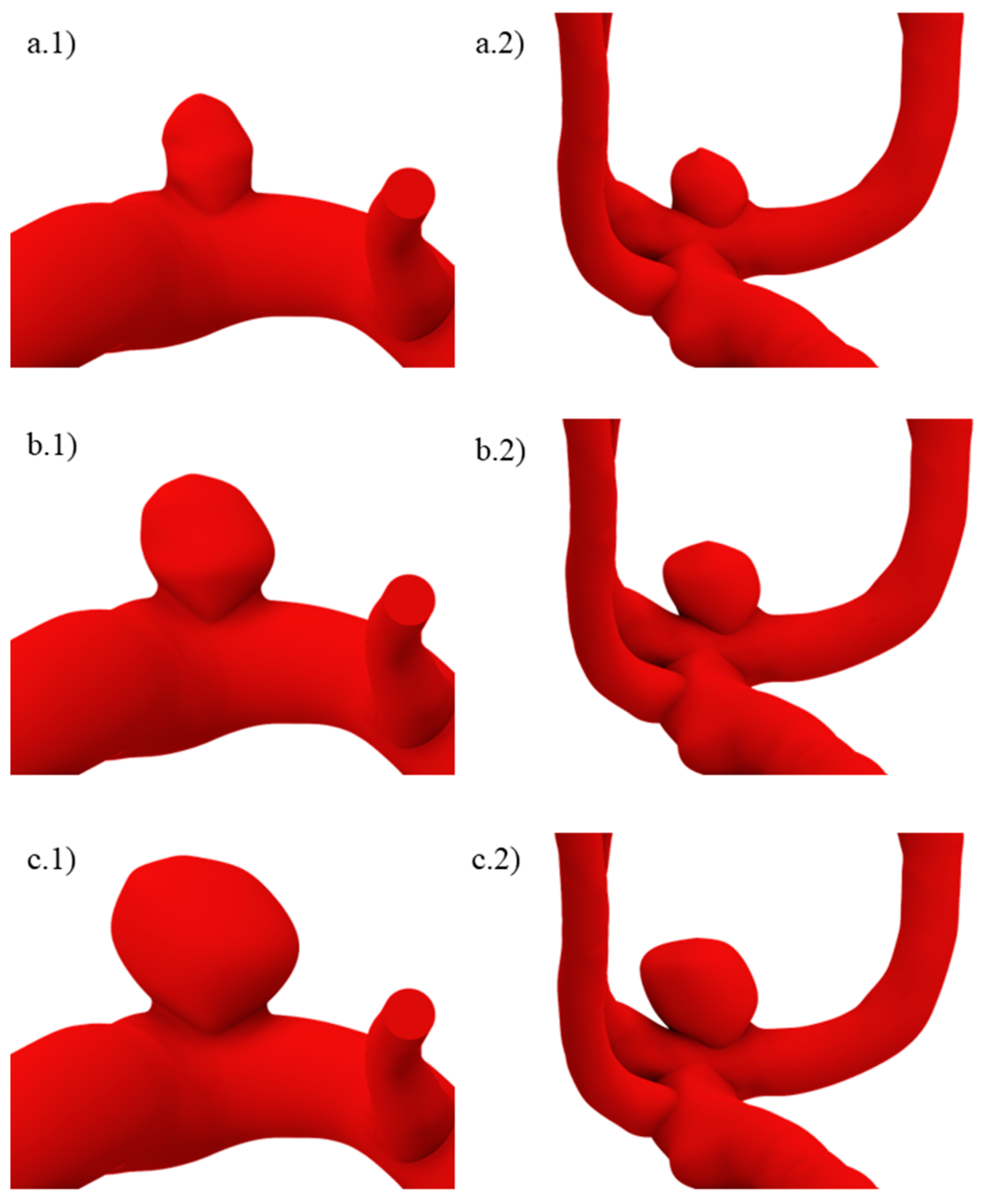
Since for unruptured geometries, neither the size at which the aneurysm would rupture nor how the structural and fluid parameters would change were known before performing the simulations, two larger variations were created. These are shown for both original geometries in Figure 3. In (a), size I geometries are shown, while in (b) and (c), sizes II and III are also shown. Here, and for the ruptured geometries, a lower number indicates a smaller aneurysm size.
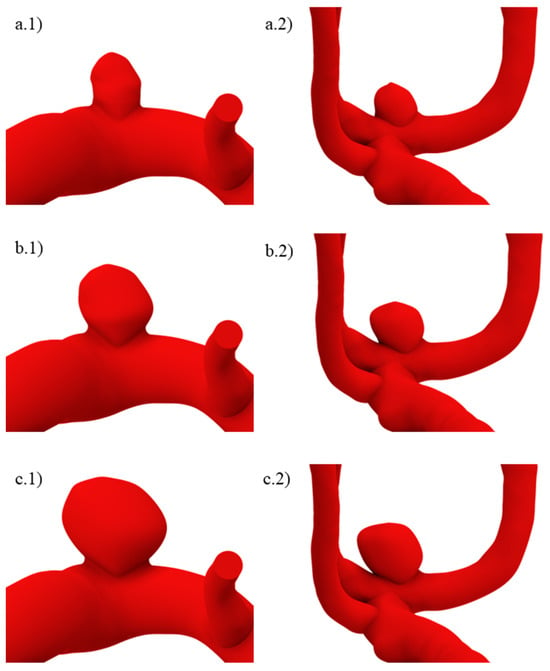
Figure 3.
Unruptured geometries of sizes (a.1,a.2) I, (b.1,b.2) II, and (c.1,c.2) III.
The ruptured geometry variations can be seen in Figure 4, where (a) is size IV, and (b), (c), and (d) are sizes III, II, and I. The algorithm was applied beginning from geometries (a) and (b) to obtain (b) and (c), respectively, whereas (d), which represents a recently initiated aneurysm, had a semi-spherical morphology whose height was approximately the same as that yielded by two consecutive applications of the algorithm starting from (c). For size I ruptured geometries, the aneurysm neck was decreased.
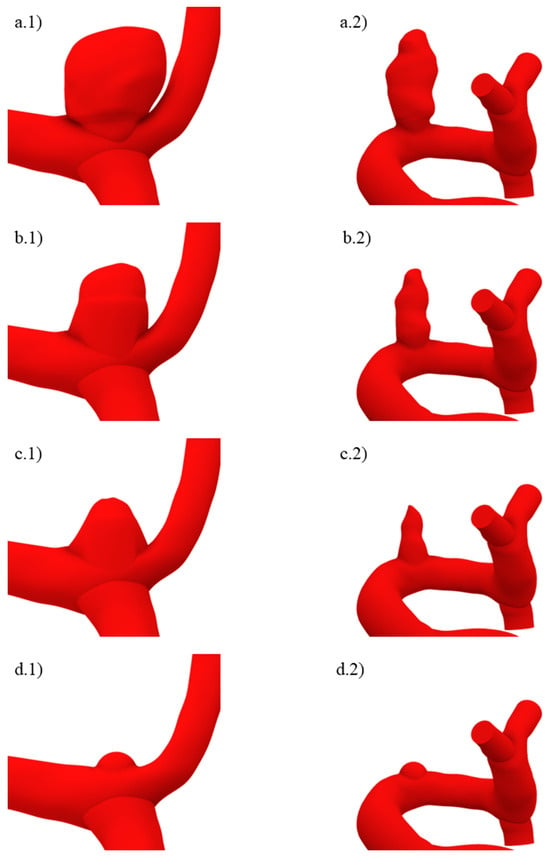
Figure 4.
Ruptured geometries of sizes (a.1,a.2) IV, (b.1,b.2) III, (c.1,c.2) II, and (d.1,d.2) I.
2.3. Aneurysm Wall Thickness Variation
To reflect the effect of aneurysm wall thickness, in one case, that region was set with the same thickness as the artery, 0.35 mm, whereas in the other two cases, the aneurysm thickness was reduced to 0.2 and 0.10 mm, respectively, since aneurysm walls can be as thin as 0.05 mm [23,30]. The wall thickness of an aneurysm dome can be characterized as a thick wall, transition, or thin wall [23]. The wall thickness variation used in this study included thick and transition aneurysm wall thicknesses.
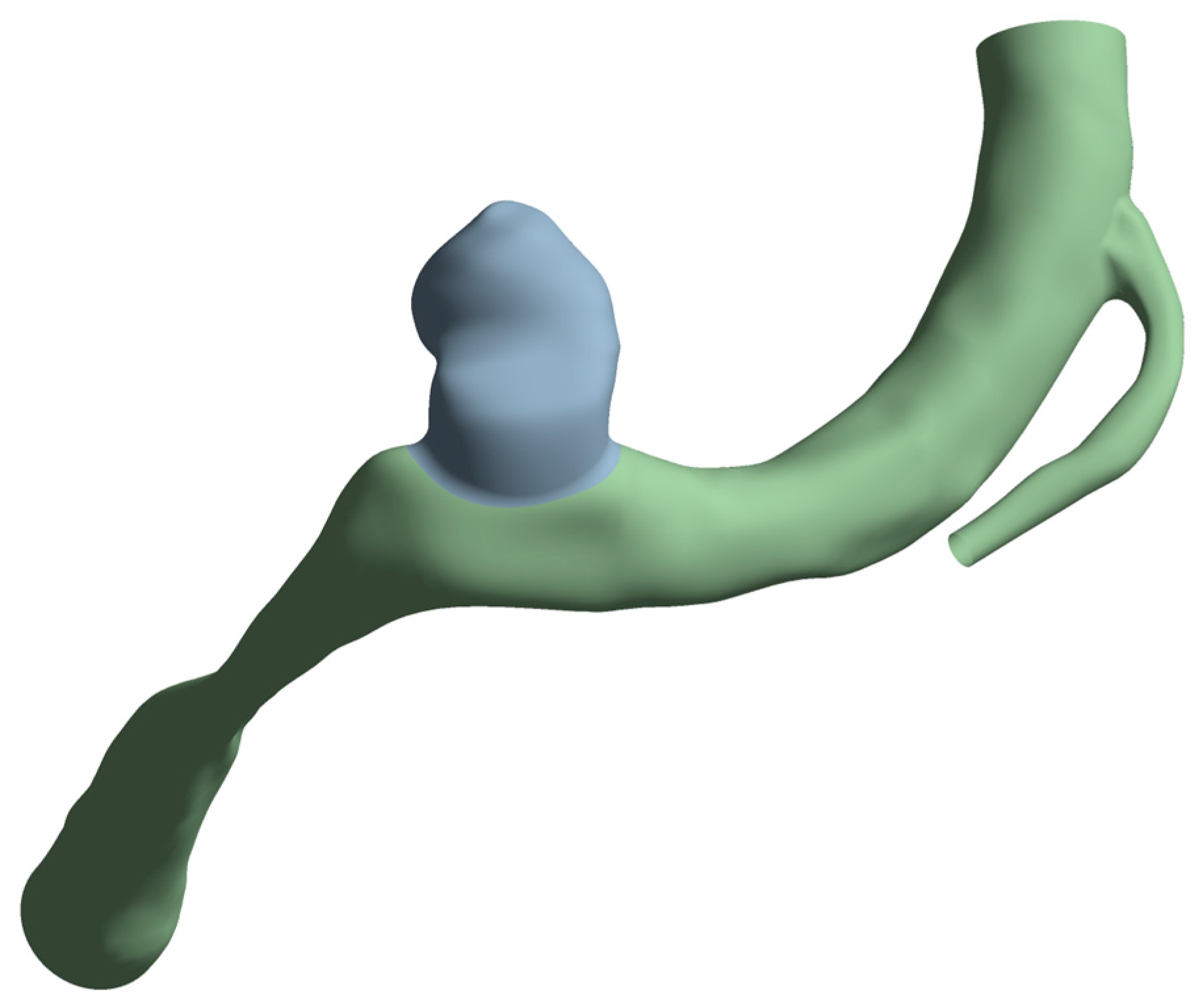
The aneurysm thickness was varied when creating the mesh and set as a structural element property, as mentioned in the following section. From the original geometry, two surface bodies were created. These are shown in gray for the aneurysm and green for the artery in Figure 5. The artery thickness was kept at 0.35 mm for all cases, while the aneurysm thickness was varied, as stated previously. To obtain a compatible mesh between these two zones, the shared topology option from SpaceClaim was activated at their boundary.
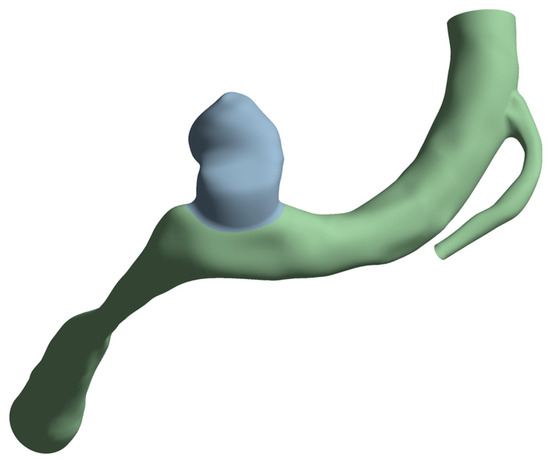
Figure 5.
Artery surfaces (in green) created with a wall thickness of 0.35 mm and the aneurysm (in gray) with a thickness of 0.35, 0.2, or 0.10 mm.
2.4. Numerical Setup
2.4.1. Fluid Model
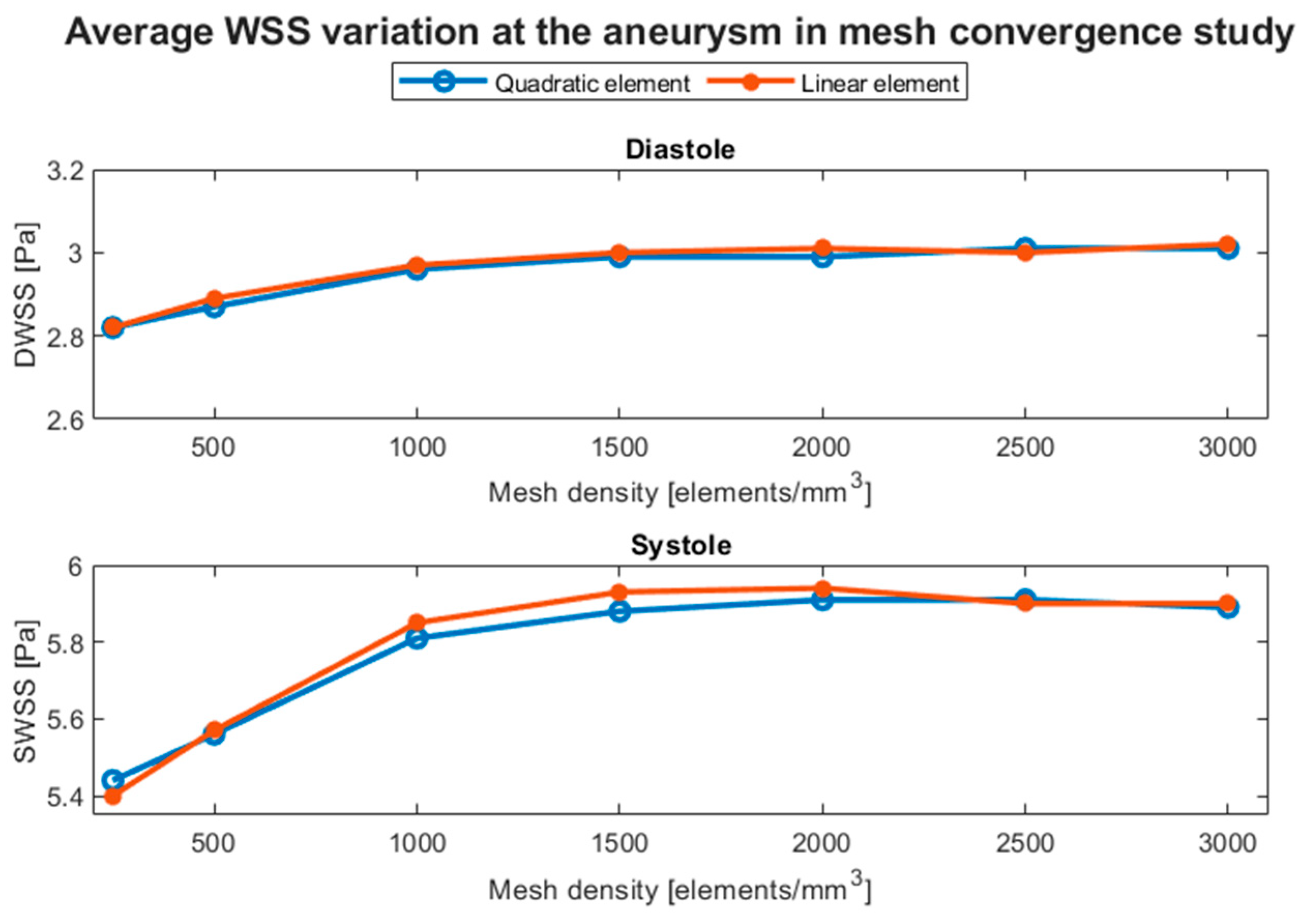
Since there was a wide variety of geometric sizes, we used one with a total volume that was similar to the average volume. RG-2 was selected for the mesh tests for both domains. The fluid mesh test was based on the mesh density, the ratio between the total number of elements, and the geometry’s total volume. It was performed using computational fluid dynamics simulations in Ansys Fluent to solve the equations associated with the fluid, using the finite volume method (FVM). Densities from 250 elements/mm3 up to 3000 elements/mm3 were included, using both linear and quadratic tetrahedral elements. The chosen scheme was PISO, and spatial discretization was performed using the least-squares cell based on the gradient and the second order for pressure and momentum. The transient formulation was first-order implicit with a time step of 0.0005 [s], 3600 time steps, and 200 maximum iterations per time step.
The blood model used was employed in previous work that used geometries from the same database [31]. Blood was modeled as a laminar and incompressible Casson fluid with a density of 1065 kg/m3. The boundary conditions for entry were the Womersley velocity profile, whereas for the exit, a three-element Windkessel model with pressures ranging between 80 and 120 mmHg was chosen. The total simulated time was 1.8 s, slightly more than two complete cardiac cycles, and the considered results were the area-averaged WSS at the diastole (DWSS) and systole (SWSS) from the second cardiac cycle. Simulations were performed using double precision in a machine with an AMD Ryzen 5 5600 G processor and 16 GB of RAM.
Figure 6 shows the evolution of both parameters with respect to the mesh. We chose to use a mesh density of about 700 elements/mm3 with size refinements at the aneurysm dome and at the zone in contact with the artery wall because at sizes between 500 and 1000 elements/mm3, the difference was less than 5% and because two-way FSI simulations require considerable computational resources. Additionally, the differences between quadratic and linear elements are negligible; therefore, the former was chosen.
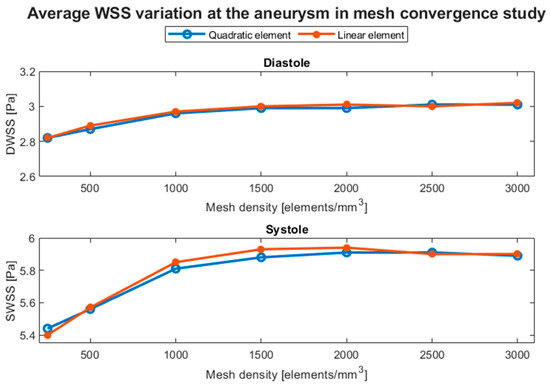
Figure 6.
Average WSS variation in the aneurysm at diastole (DWSS) and systole (SWSS), obtained in the mesh convergence test based on the mesh density with linear and quadratic tetrahedral elements.
2.4.2. Structural Model
Mesh tests for the structural domain were based on the element size. The geometry was the same as that used in previous tests, but computational structural dynamics simulations were performed with Ansys Transient Structural to solve the corresponding associated equations via the finite element method (FEM). The linear shell element was used since the wall thicknesses of the artery and the aneurysm were considerably smaller than the diameter of the artery. The wall thickness is commonly set to 10% of the artery diameter. In this case, considering the average diameter of the selected geometries, the wall thickness was set to 0.35 mm. This thickness was used for both the aneurysm and the artery, and it was kept for all cases to show the size effect.
The element size was 0.15 to 0.50 mm. An external pressure of 1650 Pa, representing the action of cerebrospinal fluid for a healthy adult [32], was used, and the internal pressure was set to 15 kPa, representing the action of the blood flow. The inlet and outlets were assumed to have fixed support. A five-parameter Mooney–Rivlin hyperelastic model was used for both the mesh tests and FSI simulations. Its elastic energy density function W can be seen in Equation (4):
where I1 and I2 are the strain invariants, and c10, c01, c11, c20, and c02 are model constants obtained from the literature [33]. Although the wall of an aneurysm is anisotropic, in this work, it was modeled as an isotropic material, which is common practice since both approaches yield similar results [34].
W = c10(I1 − 3) + c01(I2 − 3) + c11(I1 − 3) (I2 − 3) + c20(I1 − 3)2 + c02(I2 − 3)2
The considered results were the total displacement and the von Mises stress at the aneurysm. The results and percentage differences with respect to the mesh with the smallest element (D015) can be seen in Table 2. For the von Mises stress, differences of greater than 5% were observed, so we chose to use a maximum size element of 0.20 mm, since the differences were below that value.

Table 2.
Displacement and von Mises stress evolution obtained for the mesh tests and their percentage differences with respect to the 0.15 mm element size.
2.4.3. Fluid–Structure Coupling
Two-way FSI simulations were performed using Ansys System Coupling to exchange information between fluent and transient structural boundary conditions. To ensure the compatibility of both domains, the structural and fluid displacement must be compatible (Equation (5)), traction at their boundary must be in balance (Equation (6)), and the fluid must obey the no-slip condition (Equation (7)) [35]:
where δ is the displacement, is the stress tensor, is the normal vector at the boundary, is the velocity vector, is the mobile velocity coordinate, and the subscripts S and F stand for the structural and fluid domains, respectively.
δS = δF
In addition to the settings already mentioned for the fluid domain, dynamic mesh options were activated with smoothing and remeshing methods to create information exchange zones. In the structural domain, the internal pressure was removed, and exchange information zones were also created.
3. Results
A total of 24 FSI simulations were carried out to evaluate the effects of geometrical factors, such as the aneurysm size and wall thickness, and to determine the structural and hemodynamic environment of the lateral arteries prior to lesion initiation. The aneurysm wall thickness effect is presented first, followed by the size effects and, lastly, the aneurysm removal results.
3.1. Aneurysm Wall Thickness Modification
The aneurysm wall thickness modification produced negligible differences in the hemodynamic parameters. For both the average pressure and WSS, the differences were less than 5%. Therefore, in Figure 7, only the temporal evolution of the average WSS at the aneurysm is presented for each wall thickness and geometry in the last part of the simulated cycle, and the curves are almost indistinguishable from each other. That figure and the similar ones following it were created with MATLAB R2023a.
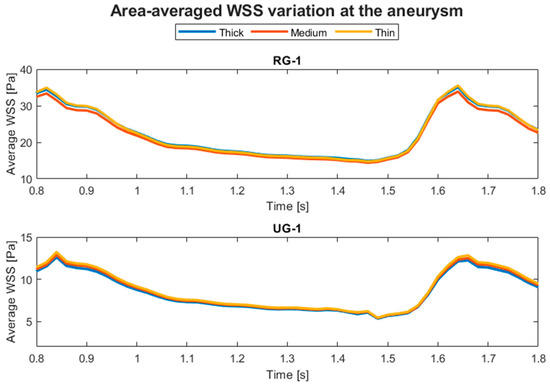
Figure 7.
Temporal evolution of the average WSS at the aneurysm for RG-1 and UG-1 for each wall thickness.
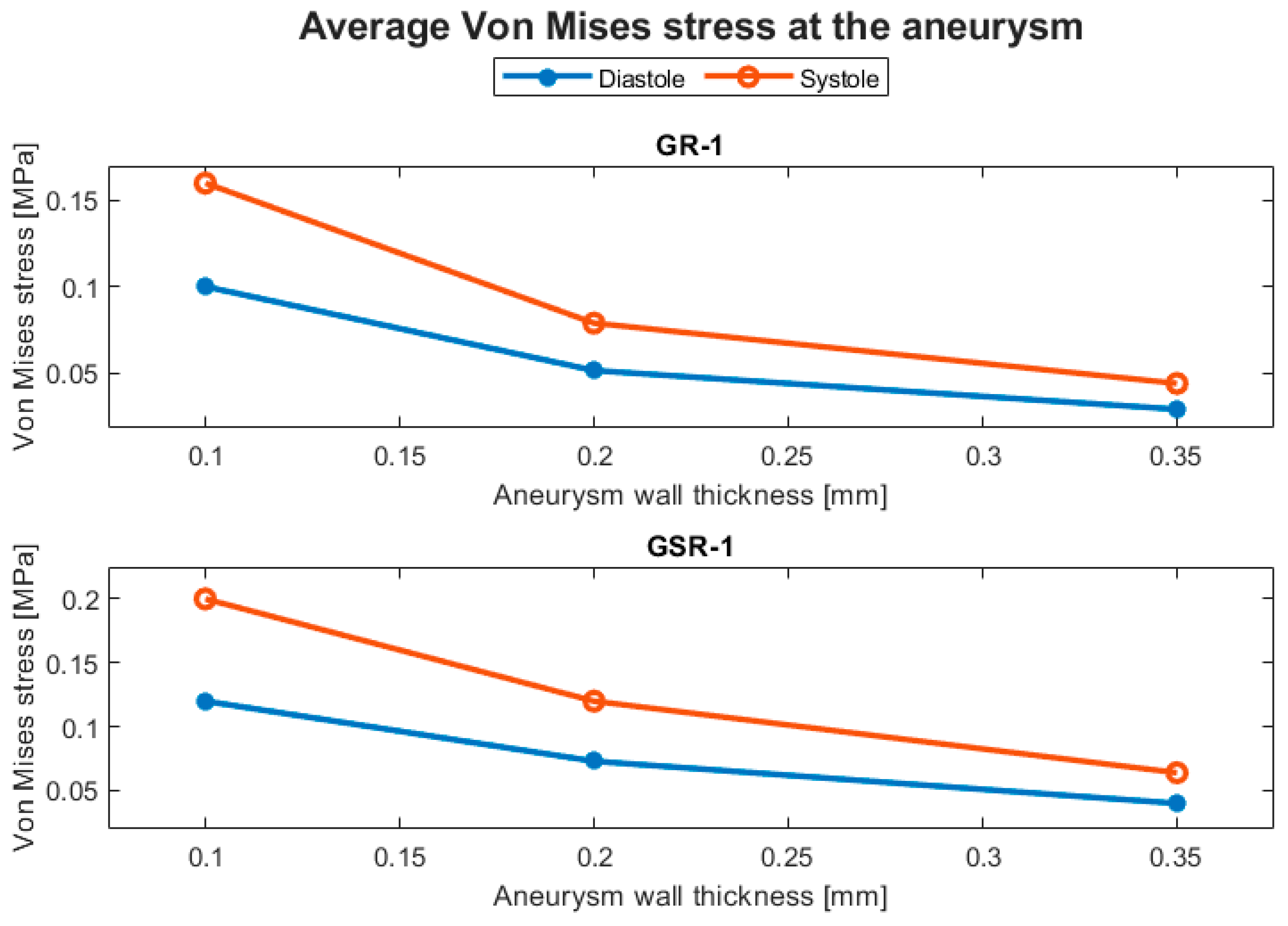
Structural parameters included the displacement and the von Mises stress. The displacement behavior of the ruptured and the unruptured aneurysms was contrasting, as can be seen in Figure 8, where the average displacement in the aneurysm is plotted against the wall thickness for each case. In Figure 9, the same plot is presented but for the von Mises stress. Both geometries had increased average perceived stress as the aneurysm wall thinned. The displacement contours can be seen in Figure 10 and Figure 11.
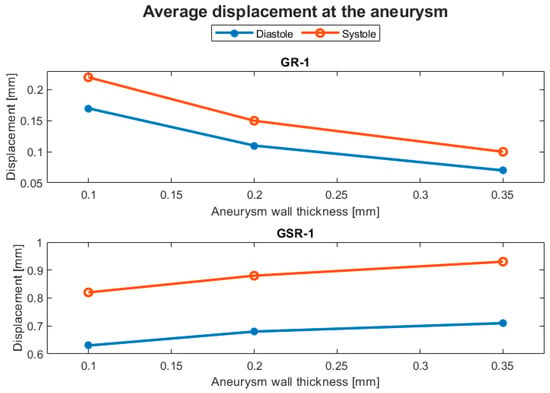
Figure 8.
Evolution of the average displacement at the aneurysm with respect to the aneurysm wall thickness.
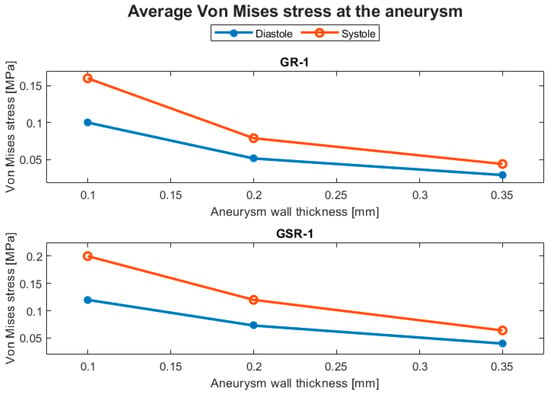
Figure 9.
Evolution of the average von Mises stress at the aneurysm with respect to the aneurysm wall thickness.
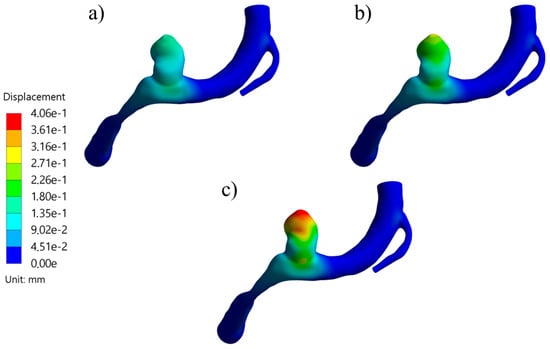
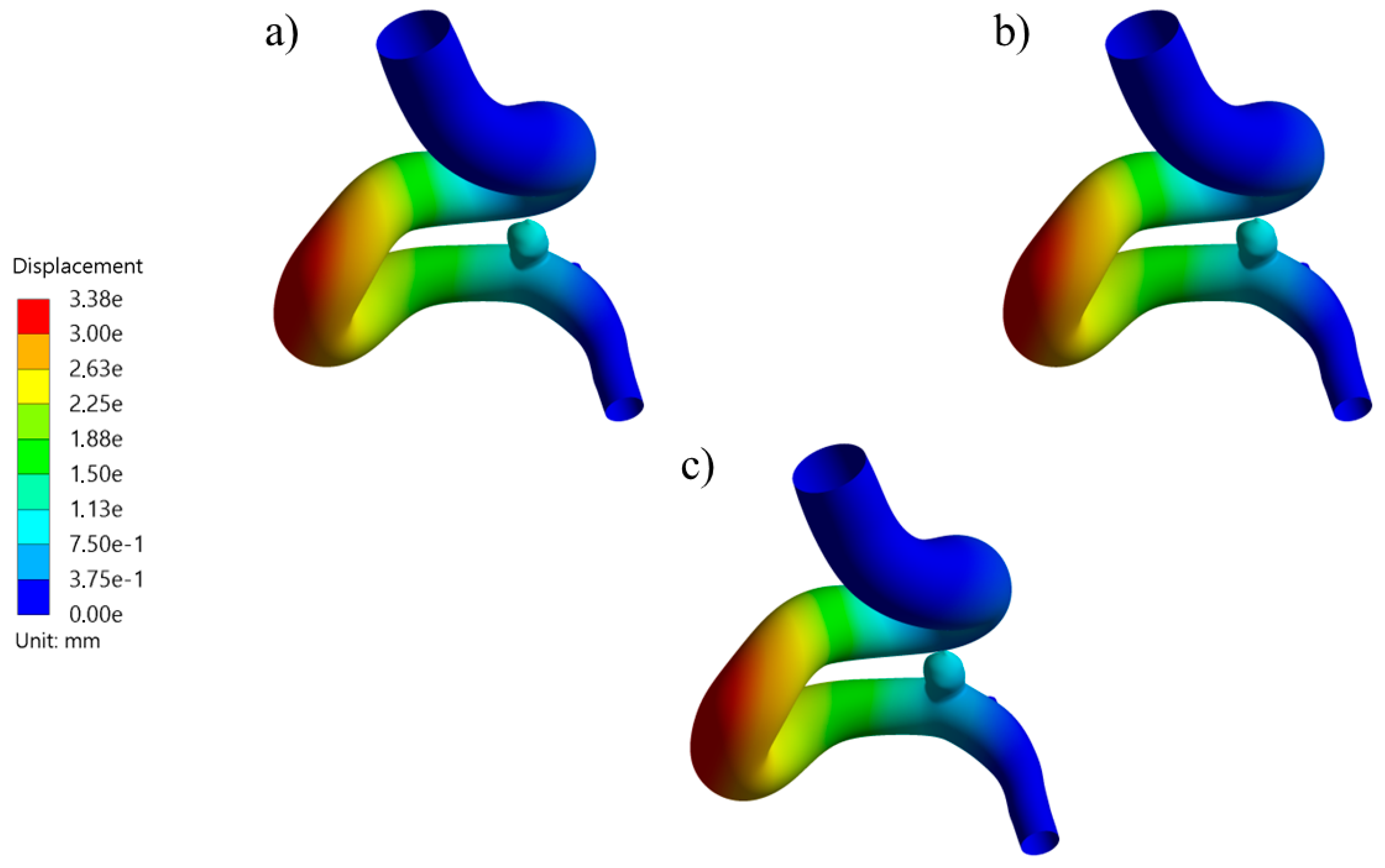
Figure 10.
Displacement contours at systole for RG-1 using (a) thick, (b) medium, and (c) thin aneurysm wall thicknesses.
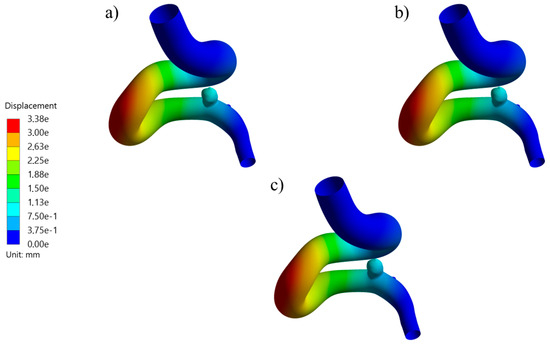
Figure 11.
Displacement contours at systole for UG-1 using (a) thick, (b) medium, and (c) thin aneurysm wall thicknesses.
3.2. Aneurysm Size Modification
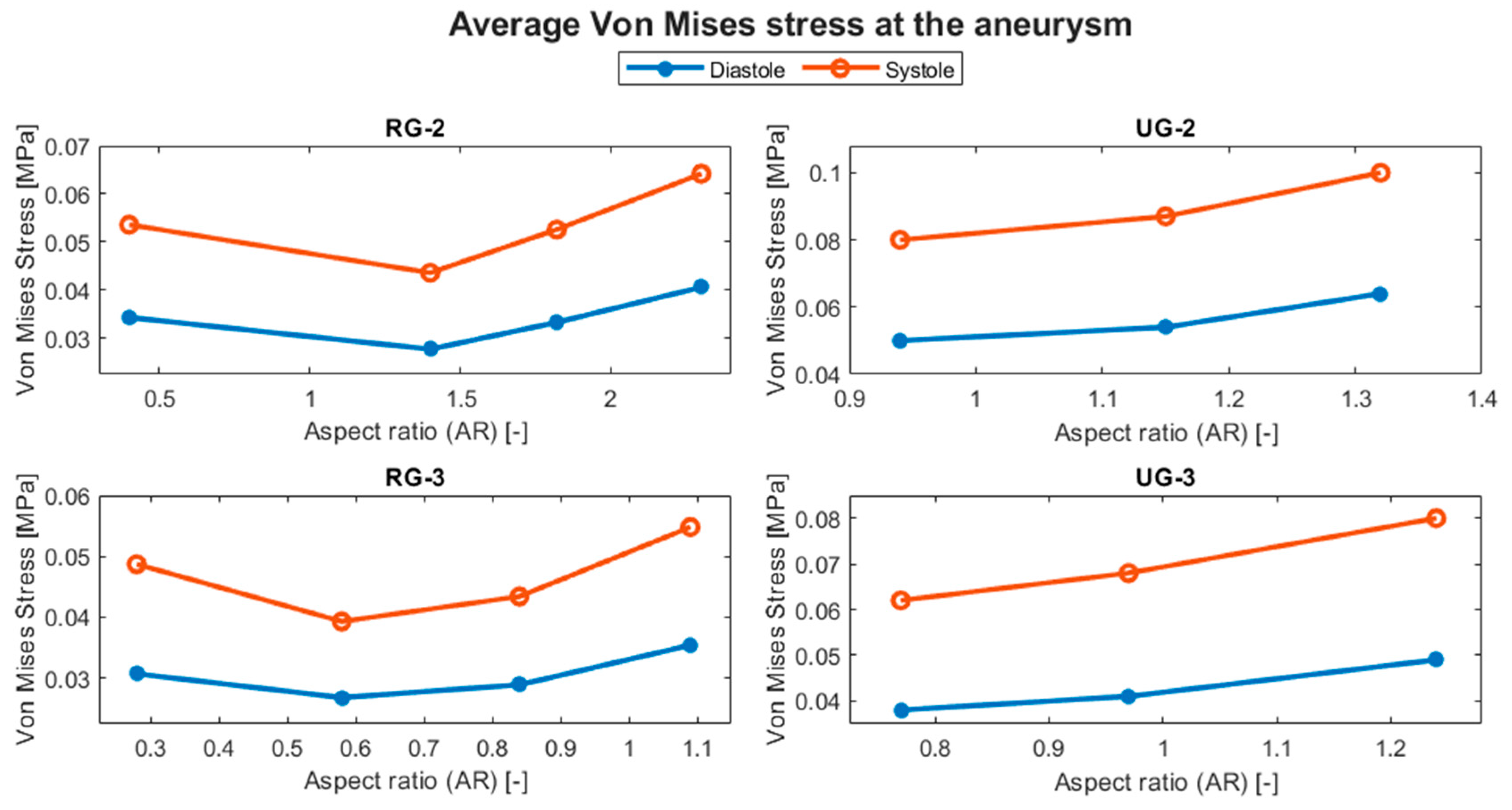
The evolution of the aneurysm size made a large difference to both hemodynamic and structural parameters. The average temporal evolution of WSS for each of the various geometry sizes is shown in Figure 12, and Figure 13 shows the average WSS evolution against the aspect ratio (AR) of each variation and geometry. Except for UG-3, as the AR increased, the WSS reduced.
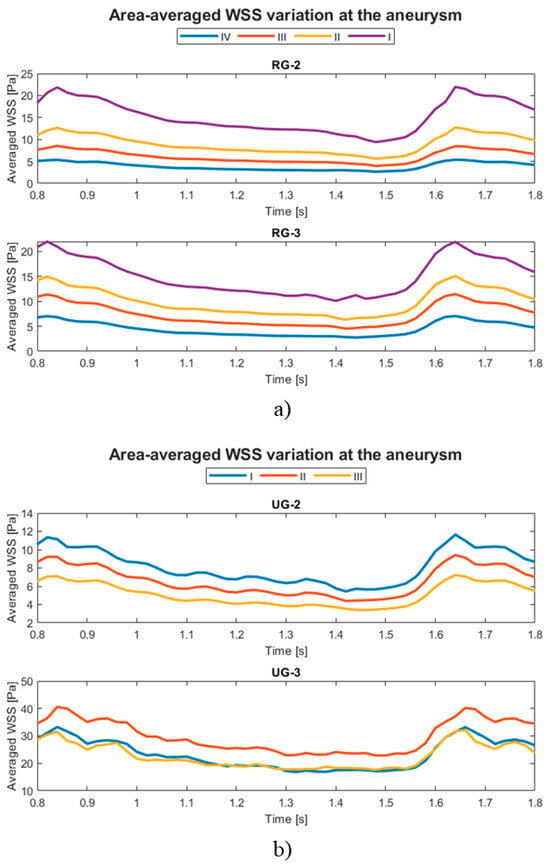
Figure 12.
Temporal evolution of the average WSS at the aneurysm for original aneurysms (a) with rupture and (b) without rupture and their size variations.
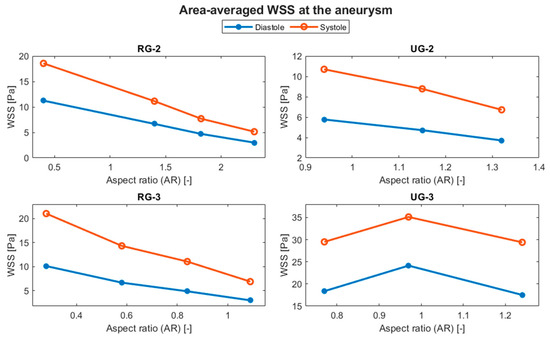
Figure 13.
Evolution of the average WSS at the aneurysm with respect to the AR.
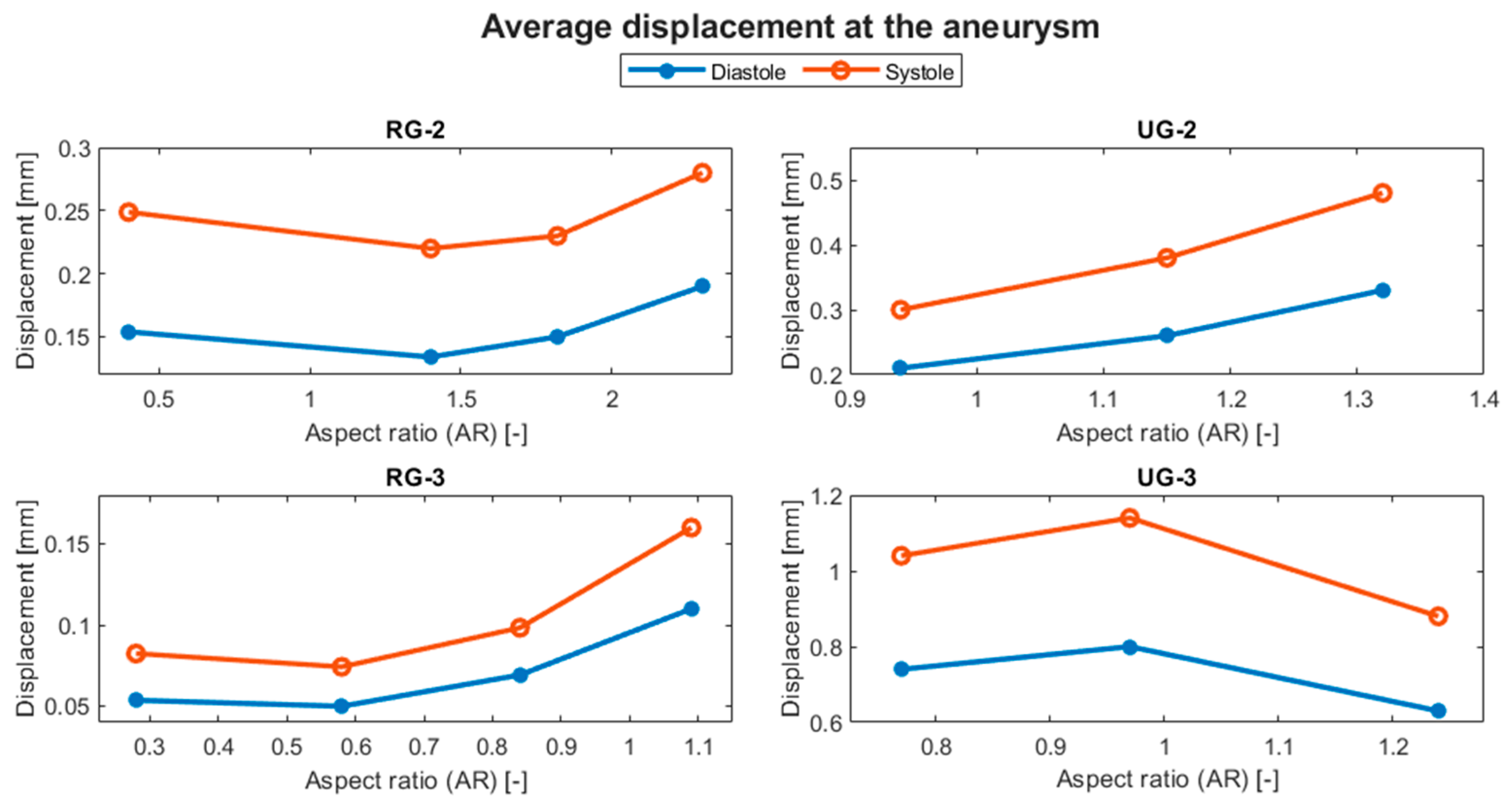
Figure 14 shows the evolution of the average displacement at the aneurysm against the AR for each case. Once again, UG-3 exhibited different behavior to the rest of the geometries. As the AR increased, the displacement also increased, except for in the size I variations of the ruptured geometries. This was repeated for the von Mises stress, as shown in Figure 15. Here, the behavior of UG-3 was similar to that of the other cases, where an aneurysm with a greater AR suffered from greater von Mises stresses. Examples of the obtained von Mises contours can be seen in Figure 16 and Figure 17 for both terminal geometries.
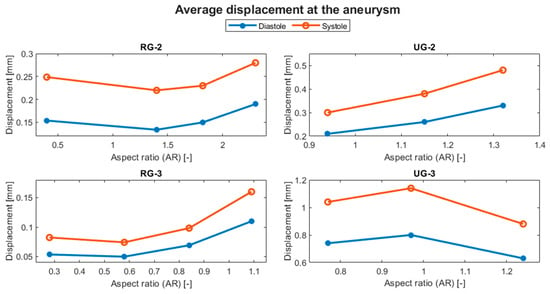
Figure 14.
Evolution of the average displacement at the aneurysm with respect to the AR.
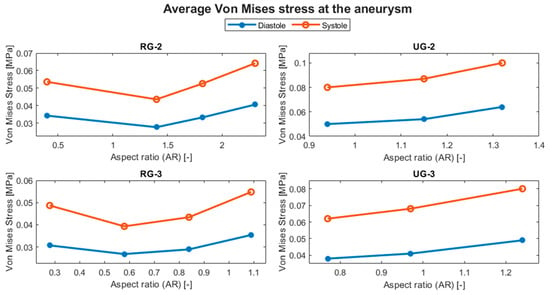
Figure 15.
Evolution of the average von Mises stress at the aneurysm with respect to the AR.
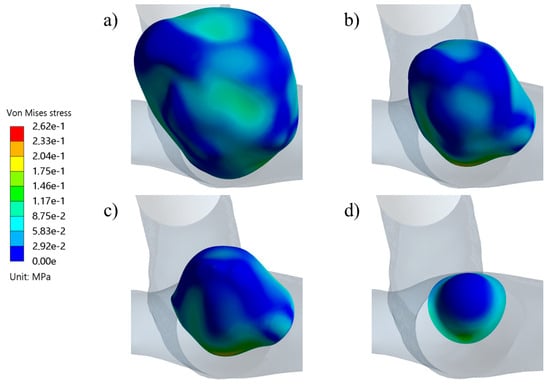
Figure 16.
Von Mises stress distribution contours during systole for the RG-3 geometry at sizes (a) IV, (b) III, (c) II, and (d) I.
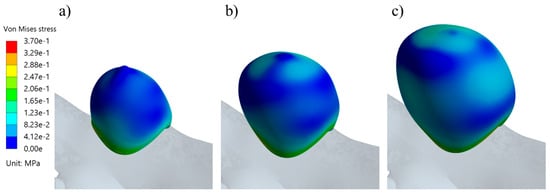
Figure 17.
Von Mises stress distribution contours during systole for the UG-3 geometry at sizes (a) III, (b) II, and (c) I.
3.3. Aneurysm Removal
To compare the pre-aneurysm state with the aneurysm, the time-averaged WSS was calculated using the following equation:
where T is the cardiac cycle time and WSSa is the area-averaged WSS at the aneurysm. Figure 18 shows the TAWSS distribution for the healthy arteries, with an arrow pointing to the future lesion location. In Figure 19, the same can be seen for the pressure distribution. It is important to note that is very likely that the parent vessel will suffer from geometrical changes after aneurysm formation and growth; however, that information was not available, and there is no method to retrieve the previous geometry from the available data. Therefore, for this study, the comparison was performed with the same parent vessel.
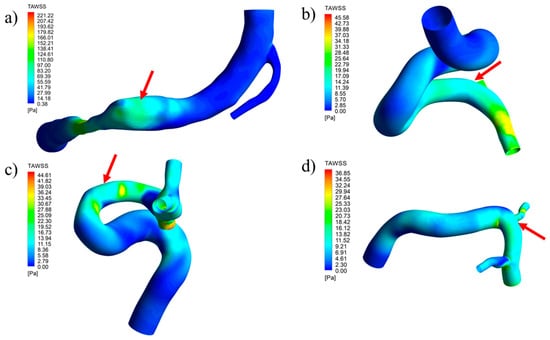
Figure 18.
TAWSS distribution contours for geometries with a removed aneurysm: (a) RG-1, (b) UG-1, (c) RG-2, and (d) UG-2.
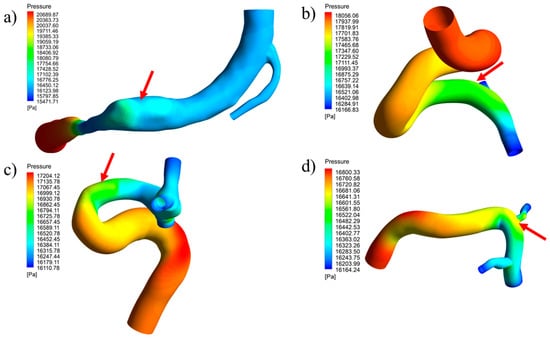
Figure 19.
Pressure distribution contours during systole for geometries with a removed aneurysm: (a) RG-1, (b) UG-1, (c) RG-2, and (d) UG-2.
4. Discussion
4.1. Effect of the Aneurysm Wall Thickness Modification
Although the hemodynamic parameters did not significantly indicate an effect of modifying the aneurysm wall thickness, the structural parameters did. As seen in Table 3, as the aneurysm wall thinned, in this case, changing from 0.35 to 0.10 mm, the average von Mises stress increased by more than 200%, indicating that the aneurysm had a greater rupture risk due to structural failure of its wall.

Table 3.
Percentage differences in the von Mises stress at the aneurysm for a wall thickness of 0.35 mm.
This type of change in the stress magnitude is in accordance with Laplace’s law, a principle that relates the pressure, radius, wall thickness, and tension of a sphere. However, it gives only an approximate idea of how the tension evolves with a change in thickness, whereas a numerical simulation can yield results for the entire dome of an aneurysm taking into consideration other factors, such as the geometry. This could give a better idea of how close the aneurysm is to structural failure. Additionally, it would not be realistic to assume that an aneurysm is a sphere, and such an assumption has previously been shown to give different results [36]. A comparison between anisotropic and isotropic models for cerebral aneurysms were reported in [37].
On the other hand, the displacement exhibited non-intuitive behavior, which contrasted between the geometries. The ruptured geometry, RG-1, displayed the expected trend, where the thinner the wall, the greater was the displacement. Furthermore, this geometry was ten times more sensitive to the change in wall thickness compared with UG-1, according to Table 4.

Table 4.
Percentage differences for the displacement at the aneurysm with respect to a wall thickness of 0.35 mm.
The difference in behaviors and sensitivities can be explained by the parent artery’s geometry and the blood flow path that it imposes. This can be seen for both cases in Figure 20, where the blood flow of RG-1 points to the aneurysm entering that zone more directly and with more velocity than in UG-1. Once the fluid entered the RG-1 aneurysm, it travelled to its upper zone, where it changed direction and decreased in velocity, exchanging momentum with the aneurysm zone that had the highest contour displacement values, as can be seen in Figure 10. In the other case, almost the entire blood flow followed the artery path with very low deviation into the aneurysm, making its displacement very similar to the surrounding zone in the artery, as shown in Figure 11.
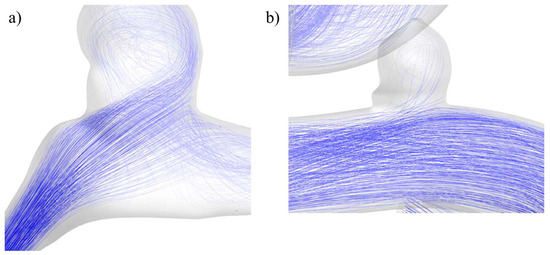
Figure 20.
Blood flow at the aneurysm region for geometries (a) RG-1 and (b) UG-1.
4.2. Effect of the Aneurysm Size Modification
As the WSS time evolution showed, there was a clear distinction in the magnitude when the aneurysm evolved in size, except for UG-3, which showed an increase followed by a decrease. These results were similar to those obtained by Sun, who found that the WSS curves were clearly separated from each other, with the scaled geometry being the one with a higher WSS. The curves obtained by that research group were closer to each other than those found for the present case. This is because, in the present case, the aneurysm size was modified but the wall thickness remained constant for each case. Other differences were that the geometries were different, and a non-Newtonian blood model was used.
Table 5 shows that ruptured geometries demonstrated an increment in the average WSS of between 205 and 276% for the size I case. UG-2 showed a reduction in its WSS of around 36%, considering both systole and diastole, for its largest variation. UG-3 showed an increase of up to 31% at diastole for variation II, but there was a decrease of less than 5% for its largest size, a difference that can be considered negligible.

Table 5.
Percentage variation in the WSS at the aneurysm dome with respect to the original size.
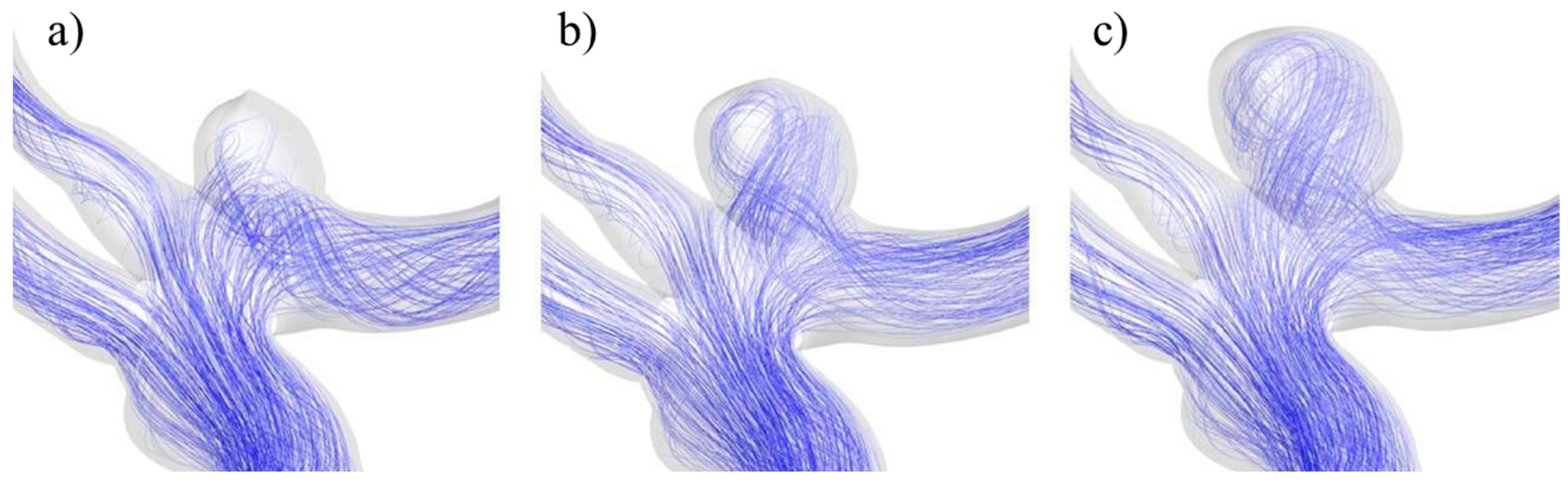
The distinctive behavior of UG-3 can be explained by the changes in the blood flow path as the aneurysm evolved, as seen in Figure 21. In the original geometry, for the smallest size, the blood travelled through a recirculation zone prior to entry into the aneurysm and then the main bifurcation. For the size II aneurysm, the blood flow entered the aneurysm more directly and with more velocity, which explains the WSS increment. The blood flow pattern was similar in the largest variation, but just like for the rest of the cases, there was a decrease in the WSS, because the blood flow reached the dome with a lower velocity.
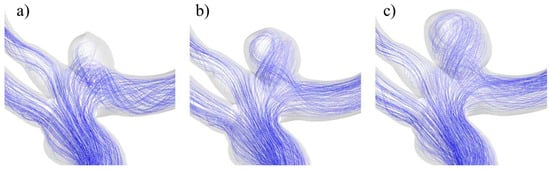
Figure 21.
Blood flow at the aneurysm region for UG-3 for sizes (a) I, (b) II, and (c) III.
In terms of displacement, a larger aneurysm might be expected to have greater displacements because the distance from the anchor point to the artery is longer. This was observed for most cases, and the maximum value was achieved at the top of the aneurysms, where the blood flow changed in magnitude and direction, transferring its momentum to the aneurysm wall. The distinctive behavior of UG-3 can be explained by the complexity of its geometry, which included three bifurcations, and the tendency of its maximum values to be located near the neck.
As can be seen in Table 6, the maximum reduction in displacement for both ruptured geometries was achieved for the size II variation. For RG-2, this reduction was almost 30%, whereas for RG-3, the displacement was reduced at both diastole and systole by about 54%. UG-2 had the maximum displacement increment of about 60% for its largest variation. For UG3, the size II variation showed an increase in displacement of approximately 10%, while its size III variation showed a reduction of about 22% with respect to the original size.

Table 6.
Percentage variation in the average displacement at the aneurysm dome with respect to the original size.
The evolution of von Mises stress with respect to the aneurysm size increment indicated that the larger aneurysms had a higher rupture risk. The percentage differences are presented in Table 7. For both ruptured geometries, the greatest reduction was achieved for size II variations, approximately 30% for RG-2 and between 23 and 29% for RG-3. For unruptured geometries, in both cases, the increase in the von Mises stress at the aneurysm was between 25 and 29%.

Table 7.
Percentage variation in the average von Mises stress at the aneurysm dome with respect to the original size.
Figure 16 and Figure 17 show examples of the von Mises stress during aneurysm evolution for RG-3 and UG-3. It can be noted that the tops of the aneurysms showed higher levels of stress than other zones (except for the neck), even in the latter case, which had a smooth surface. This is in accordance with the zones where ruptures are typically found. For the ruptured geometries, a reduction in stress from size I to size II was observed, followed by an increment. This difference in behavior can be explained by the very different morphologies of the size I variations of the ruptured geometries. In both cases, these were semi-spherical aneurysms, and the change in curvature at their necks led to stress concentrations. Since these aneurysms were smaller than the rest of the cases, this had a greater impact on the average results.
4.3. Pre-Aneurysm State
The contours presented for the TAWSS and pressure show that lesions formed in zones where these parameters had medium magnitudes, never in those with minimum values. As a comparison, the approximate value of the TAWSS in the healthy artery zone where the aneurysm was located (shown in Figure 22) was compared with the average value of the same parameter in the original aneurysm. Table 8 shows that, for all four cases, there was a reduction in the TAWSS when the aneurysm was present. Interestingly, for both ruptured cases, the reduction was approximately 75%, while for the unruptured cases, the reduction was lower, 45% for UG-1 and 33% for UG-2.
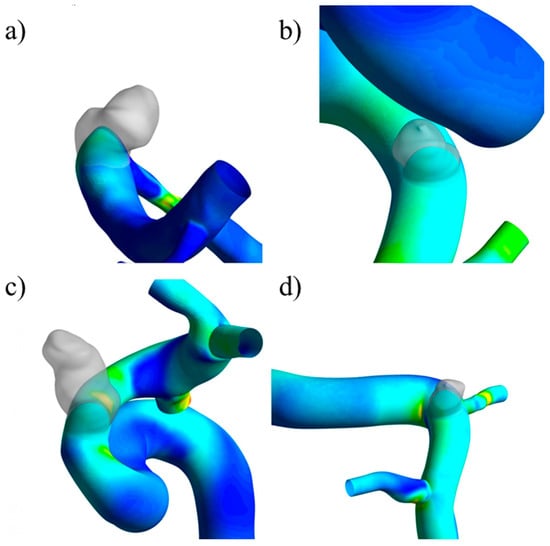
Figure 22.
Location of the imported aneurysm and the TAWSS distribution: (a) RG-1, (b) UG-1, (c) RG-2, (d) UG-2.

Table 8.
TAWSS variation between the pre-aneurysm and aneurysm states for lateral geometries.
The reduction occurred because when the aneurysm was not present, the blood was in contact with the artery at a higher velocity than when the aneurysm occurred, because it needed to travel a longer path into the cavity. It was more difficult for the blood to flow, due to the change in direction and because there was blood with a lower velocity filling the cavity.
Cornelissen et al. [38] clinically studied hemodymamic changes in aneurysms for four years, and the growth that was reported showed qualitatively similar geometric changes as in our investigation. The WSS decreased after growth, and for other hemodynamic characteristics, a large variability between the aneurysms was observed in the study population. Singla et al. [39] reported that the pressure, velocity, and WSS decreased with the aneurysm size, increasing the rupture risk; they varied the aneurysm size considering a spherical aneurysm. Wall thickness heterogeneity affects the FSI results when modelling cerebral aneurysms [40], and the von Misses stress can be overestimated or underestimated using a constant aneurysm wall thickness, depending on the value used. The formation of paraclinoid aneurysms was reported near the location of high WSS and high strain in [41]; our results showed the same tendency in terms of TAWSS. Kim et al. [41] also reported that the presence of the dura mater affected the FSI results. For CFD simulations of cerebral aneurysms, the rheology model is relevant. We used a non-Newtonian Casson model; the differences from the Newtonian blood model were more important in abdominal aortic aneurysms, and the effects of turbulence on WSS were negligible for the flow simulation of cerebral aneurysms [42].
5. Limitations
Real aneurysms exhibit complex mechanical behavior along with a non-uniform distribution of wall thickness and non-predictable size and shape evolution as there are several factors that can affect their evolution and that can change from patient to patient. Nevertheless, assumptions such as a constant wall thickness, no pre-stretching, and an isotropic wall structure are common practices that have been shown to produce realistic structural responses [26,35].
This allows investigators to develop models with a level of complexity that makes it possible to find solutions and predict structural and hemodynamic evolution. Such models can serve as early clinical aids in future applications of the present methodology. In this study, only saccular aneurysms were studied, and fusiform aneurysms were not considered. The parent vessel suffers geometrical changes after aneurysm formation and growth. However, that information was not available, and there is no method to retrieve the previous geometry from the available data. Therefore, for this study, the comparison was performed with the same parent vessel.
6. Conclusions
Using computational simulations, we studied the past evolution of aneurysms and explored potential future developments, a task that would otherwise not have been possible due to ethical concerns. Our results indicated that, for the studied group, as cerebral aneurysms grew, they triggered significant alterations in hemodynamic and structural parameters, reflecting an environment with a greater risk of rupture. Furthermore, the use of FSI simulations made it possible to assess the effects of aneurysm wall thinning while keeping the size unaltered. We revealed that, even if an aneurysm did not grow, it could exist in a high-rupture-risk state because of its thin wall that was prone to structural failure as the average von Mises stress increased. The use of simulations also enabled the study of the lateral arteries following the removal of the aneurysm. We found that for both ruptured and unruptured aneurysms, the TAWSS was reduced.
We conclude that, even for healthy patients, either of the geometrical modifications presented in this study can lead to a significant increment in the rupture risk of the studied cerebral aneurysms and that the rupture point is indicated when a reduction in TAWSS occurs. More samples are needed to obtain more statistically significant conclusions about this and the geometrical factor effects indicated in this pilot study.
Author Contributions
Conceptualization, Á.V.; Methodology, D.D.; Validation, Á.V.; Investigation, D.D.; Writing—original draft, D.D.; Supervision, Á.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keedy, A. An overview of intracranial aneurysms. Mcgill J. Med. 2006, 9, 141–146. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; White, P.M. The detection and management of unruptured intracranial aneurysms. Brain 2000, 123, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Sforza, D.M.; Putman, C.M.; Cebral, J.R. Hemodynamics of Cerebral Aneurysms. Annu. Rev. Fluid Mech. 2009, 41, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Munarriz, P.M.; Gómez, P.A.; Paredes, I.; Castaño-Leon, A.M.; Cepeda, S.; Lagares, A. Basic Principles of Hemodynamics and Cerebral Aneurysms. World Neurosurg. 2016, 88, 311–319. [Google Scholar] [CrossRef]
- Gao, L.; Hoi, Y.; Swartz, D.D.; Kolega, J.; Siddiqui, A.; Meng, H. Nascent Aneurysm Formation at the Basilar Terminus Induced by Hemodynamics. Stroke 2008, 39, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Metaxa, E.; Tremmel, M.; Natarajan, S.K.; Xiang, J.; Paluch, R.A.; Mandelbaum, M.; Siddiqui, A.H.; Kolega, J.; Mocco, J.; Meng, H. Characterization of Critical Hemodynamics Contributing to Aneurysmal Remodeling at the Basilar Terminus in a Rabbit Model. Stroke 2010, 41, 1774–1782. [Google Scholar] [CrossRef]
- Kulcsár, Z.; Ugron, Á.; Marosfoi, M.; Berentei, Z.; Paál, G.; Szikora, I. Hemodynamics of Cerebral Aneurysm Initiation: The Role of Wall Shear Stress and Spatial Wall Shear Stress Gradient. Am. J. Neuroradiol. 2011, 32, 587–594. [Google Scholar] [CrossRef]
- Boussel, L.; Rayz, V.; McCulloch, C.; Martin, A.; Acevedo-Bolton, G.; Lawton, M.; Higashida, R.; Smith, W.S.; Young, W.L.; Saloner, D. Aneurysm Growth Occurs at Region of Low Wall Shear Stress. Stroke 2008, 39, 2997–3002. [Google Scholar] [CrossRef]
- Jung, K.-H. New Pathophysiological Considerations on Cerebral Aneurysms. Neurointervention 2018, 13, 73–83. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or Low WSS? Complex Interactions of Hemodynamics with Intracranial Aneurysm Initiation, Growth, and Rupture: Toward a Unifying Hypothesis. Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef]
- Savastano, L.E.; Bhambri, A.; Wilkinson, D.A.; Pandey, A.S. Biology of Cerebral Aneurysm Formation, Growth, and Rupture. In Intracranial Aneurysms; Elsevier: Amsterdam, The Netherlands, 2018; pp. 17–32. [Google Scholar] [CrossRef]
- Koffijberg, H.; Buskens, E.; Algra, A.; Wermer, M.J.H.; Rinkel, G.J.E. Growth rates of intracranial aneurysms: Exploring constancy. J. Neurosurg. 2008, 109, 176–185. [Google Scholar] [CrossRef]
- Watanabe, Z.; Tomura, N.; Akasu, I.; Munakata, R.; Horiuchi, K.; Watanabe, K. Comparison of Rates of Growth between Unruptured and Ruptured Aneurysms Using Magnetic Resonance Angiography. J. Stroke Cerebrovasc. Dis. 2017, 26, 2849–2854. [Google Scholar] [CrossRef]
- Leemans, E.L.; Cornelissen, B.M.; Said, M.; van den Berg, R.; Slump, C.H.; Marquering, H.A.; Majoie, C.B. Intracranial aneurysm growth: Consistency of morphological changes. Neurosurg. Focus 2019, 47, E5. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.W.; Lee, S.-I.; Noh, S.J.; Jeong, Y.G.; Kim, M.S.; Jeong, Y.T. What Is the Significance of a Large Number of Ruptured Aneurysms Smaller than 7 mm in Diameter? J. Korean Neurosurg. Soc. 2009, 45, 85. [Google Scholar] [CrossRef] [PubMed]
- Sherif, C.; Kleinpeter, G.; Mach, G.; Loyoddin, M.; Haider, T.; Plasenzotti, R.; Bergmeister, H.; Di Ieva, A.; Gibson, D.; Krssak, M. Evaluation of cerebral aneurysm wall thickness in experimental aneurysms: Comparison of 3T-MR imaging with direct microscopic measurements. Acta Neurochir. 2014, 156, 27–34. [Google Scholar] [CrossRef]
- Crompton, M.R. Mechanism of Growth and Rupture in Cerebral Berry Aneurysms. BMJ 1966, 1, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.T.; Sze, K.Y.; Tang, A.Y.S.; Tsang, A.C.O.; Yu, A.C.H.; Chow, K.W. Effects of aspect ratio, wall thickness and hypertension in the patient-specific computational modeling of cerebral aneurysms using fluid-structure interaction analysis. Eng. Appl. Comput. Fluid Mech. 2019, 13, 229–244. [Google Scholar] [CrossRef]
- Nath Yadav, P.; Singh, G.; Chanda, A. Biomechanical modeling of cerebral aneurysm. Mater. Today Proc. 2022, 62, 3295–3300. [Google Scholar] [CrossRef]
- Watton, P.N.; Ventikos, Y.; Holzapfel, G.A. Modelling the growth and stabilization of cerebral aneurysms. Math. Med. Biol. 2009, 26, 133–164. [Google Scholar] [CrossRef][Green Version]
- Tobe, Y.; Yagi, T.; Kawamura, K.; Suto, K.; Sawada, Y.; Hayashi, Y.; Yoshida, H.; Nishitani, K.; Okada, Y.; Kitahara, S.; et al. Three-dimensional wall-thickness distributions of unruptured intracranial aneurysms characterized by micro-computed tomography. Biomech. Model. Mechanobiol. 2024. [Google Scholar] [CrossRef]
- Shang, E.K.; Nathan, D.P.; Woo, E.Y.; Fairman, R.M.; Wang, G.J.; Gorman, R.C.; Gorman, J.H., III; Jackson, B.M. Local wall thickness in finite element models improves prediction of abdominal aortic aneurysm growth. J. Vasc. Surg. 2015, 61, 217–223. [Google Scholar] [CrossRef]
- Voß, S.; Glaßer, S.; Hoffmann, T.; Beuing, O.; Weigand, S.; Jachau, K.; Preim, B.; Thévenin, D.; Janiga, G.; Berg, P. Fluid-Structure Simulations of a Ruptured Intracranial Aneurysm: Constant versus Patient-Specific Wall Thickness. Comput. Math. Methods Med. 2016, 2016, 9854539. [Google Scholar] [CrossRef]
- Torii, R.; Oshima, M.; Kobayashi, T.; Takagi, K.; Tezduyar, T.E. Fluid–structure interaction modeling of blood flow and cerebral aneurysm: Significance of artery and aneurysm shapes. Comput. Methods Appl. Mech. Eng. 2009, 198, 3613–3621. [Google Scholar] [CrossRef]
- Bazilevs, Y.; Hsu, M.C.; Zhang, Y.; Wang, W.; Kvamsdal, T.; Hentschel, S.; Isaksen, J.G. Computational vascular fluid–structure interaction: Methodology and application to cerebral aneurysms. Biomech. Model. Mechanobiol. 2010, 9, 481–498. [Google Scholar] [CrossRef]
- Khe, A.K.; Cherevko, A.A.; Chupakhin, A.P.; Bobkova, M.S.; Krivoshapkin, A.L.; Orlov, K.Y. Haemodynamics of giant cerebral aneurysm: A comparison between the rigid-wall, one-way and two-way FSI models. J. Phys. Conf. Ser. 2016, 722, 012042. [Google Scholar] [CrossRef]
- Valencia, A.; Morales, H.; Rivera, R.; Bravo, E.; Galvez, M. Blood flow dynamics in patient-specific cerebral aneurysm models: The relationship between wall shear stress and aneurysm area index. Med. Eng. Phys. 2008, 30, 329–340. [Google Scholar] [CrossRef]
- Suzuki, J.; Ohara, H. Clinicopathological study of cerebral aneurysms. J. Neurosurg. 1978, 48, 505–514. [Google Scholar] [CrossRef]
- Amigo, N.; Valencia, Á. Determining Significant Morphological and Hemodynamic Parameters to Assess the Rupture Risk of Cerebral Aneurysms. J. Med. Biol. Eng. 2019, 39, 329–335. [Google Scholar] [CrossRef]
- Dunn, L.T. Raised Intracranial Pressure. J. Neurol. Neurosurg. Psychiatry 2002, 73 (Suppl. 1), i23–i27. [Google Scholar] [CrossRef] [PubMed]
- Valencia, Á.; Contente, A.; Ignat, M.; Mura, J.; Bravo, E.; Rivera, R.; Sordo, J. Mechanical test of human cerebral aneurysm specimens obtained from surgical clipping. J. Mech. Med. Biol. 2015, 15, 1550075. [Google Scholar] [CrossRef]
- Oliveira, I.L.; Cardiff, P.; Baccin, C.E.; Gasche, J.L. A numerical investigation of the mechanics of intracranial aneurysms walls: Assessing the influence of tissue hyperelastic laws and heterogeneous properties on the stress and stretch fields. J. Mech. Behav. Biomed. Mater. 2022, 136, 105498. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Burdiles, P.; Ignat, M.; Mura, J.; Bravo, E.; Rivera, R.; Sordo, J. Fluid Structural Analysis of Human Cerebral Aneurysm Using Their Own Wall Mechanical Properties. Comput. Math. Methods Med. 2013, 2013, 293128. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Torrens, P.; Rivera, R.; Galvez, M.; Bravo, E. A mechanical study of patient-specific cerebral aneurysm models: The correlations between stress and displacement with geometrical indices. Mech. Res. Commun. 2009, 36, 642–651. [Google Scholar] [CrossRef]
- Cornejo, S.; Guzmán, A.; Valencia, A.; Rodríguez, J.; Finol, E. Flow-induced wall mechanics of patient-specific aneurysmal cerebral arteries: Nonlinear isotropic versus anisotropic wall stress. Proc. Inst. Mech. Eng. H 2014, 228, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.M.W.; Leemans, E.L.; Slump, C.H.; Berg, R.v.d.; Marquering, H.A.; Majoie, C.B.L.M. Hemodynamic changes after intracranial aneurysm growth. J. Neurosurg. 2022, 136, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Gupta, S.; Chanda, A. A Computational Fluid Dynamics-Based Model for Assessing Rupture Risk in Cerebral Arteries with Varying Aneurysm Sizes. Math. Comput. Appl. 2023, 28, 90. [Google Scholar] [CrossRef]
- Oliveira, I.L.; Gasche, J.L.; Militzer, J.; Baccin, C.E.; Cardiff, P. On the influence of wall thickness heterogeneity in the mechanics of intracranial aneurysms. In Proceedings of the 26th ABCM International Congress of Mechanical Engineering (COBEM 2021), Florianópolis, Brazil, 22–26 November 2021. [Google Scholar] [CrossRef]
- Kim, J.-J.; Yang, H.; Kim, Y.B.; Oh, J.H.; Cho, K.-C. The quantitative comparison between high wall shear stress and high strain in the formation of paraclinoid aneurysms. Sci. Rep. 2021, 11, 7947. [Google Scholar] [CrossRef]
- Brambila-Solórzano, A.; Méndez-Lavielle, F.; Naude, J.L.; Martínez-Sánchez, G.J.; García-Rebolledo, A.; Hernández, B.; Escobar-del Pozo, C. Influence of Blood Rheology and Turbulence Models in the Numerical Simulation of Aneurysms. Bioengineering 2023, 10, 1170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).