Electrocatalytic Nanomaterials Improve Microbial Extracellular Electron Transfer: A Review
Abstract
:1. Introduction
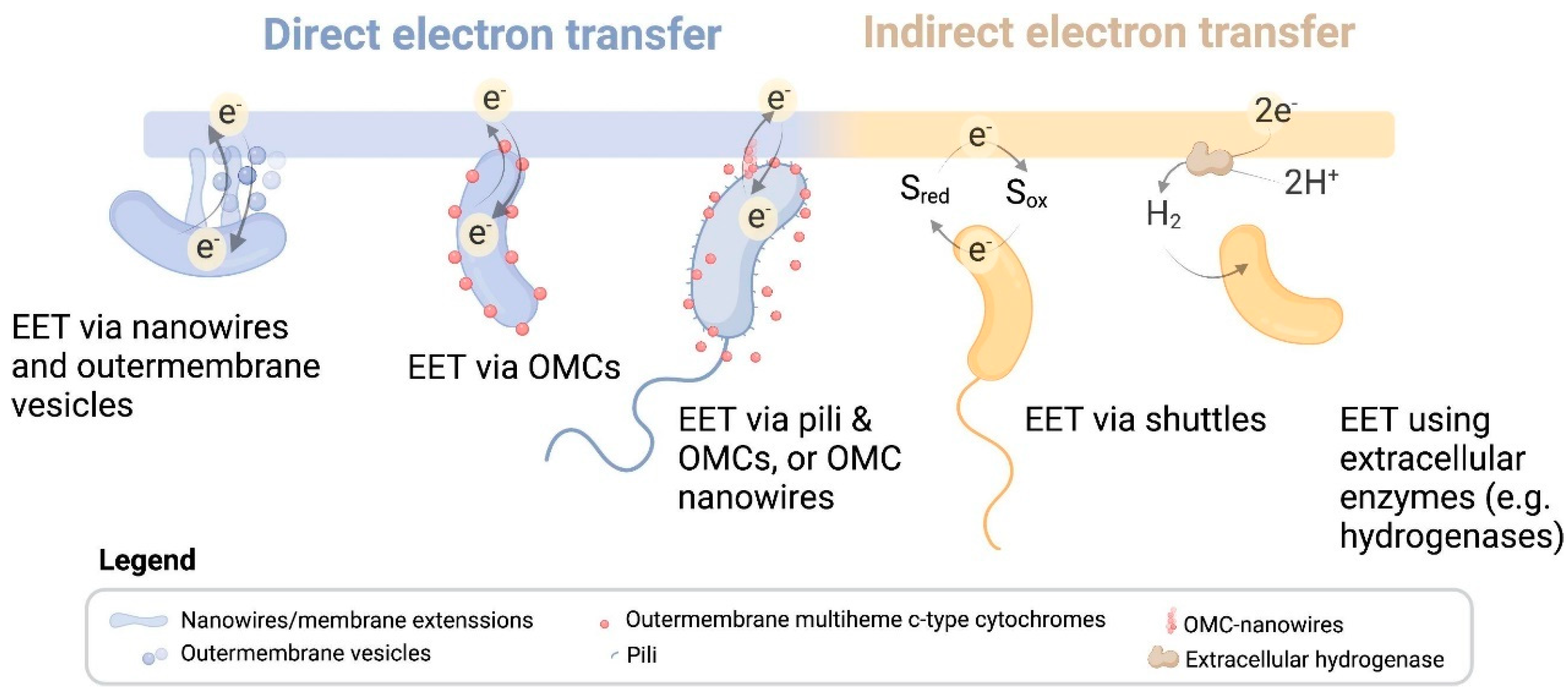
2. EET Mechanisms
2.1. Outward EET from EMAs to Anodes
2.2. Inward EET from Cathodes to EAMs
3. Electrocatalytic Nanomaterials for Promoting EET
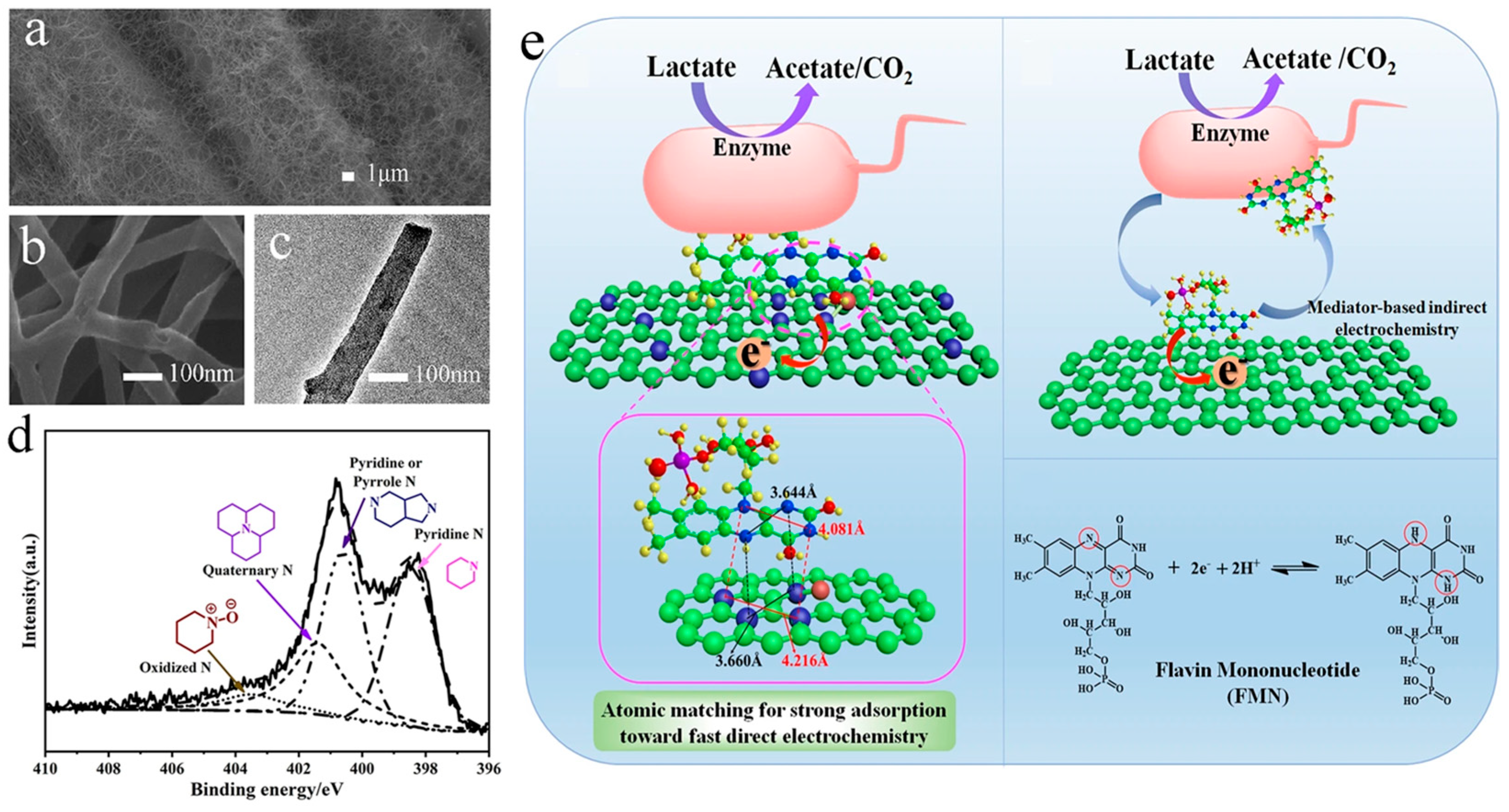
3.1. Heteroatom-Doped Carbons
3.2. Precious Metals
3.3. Metal Oxides
| Nanomaterials | Applications | Performances | Reference |
|---|---|---|---|
| Transition metal oxides (TMOs) | |||
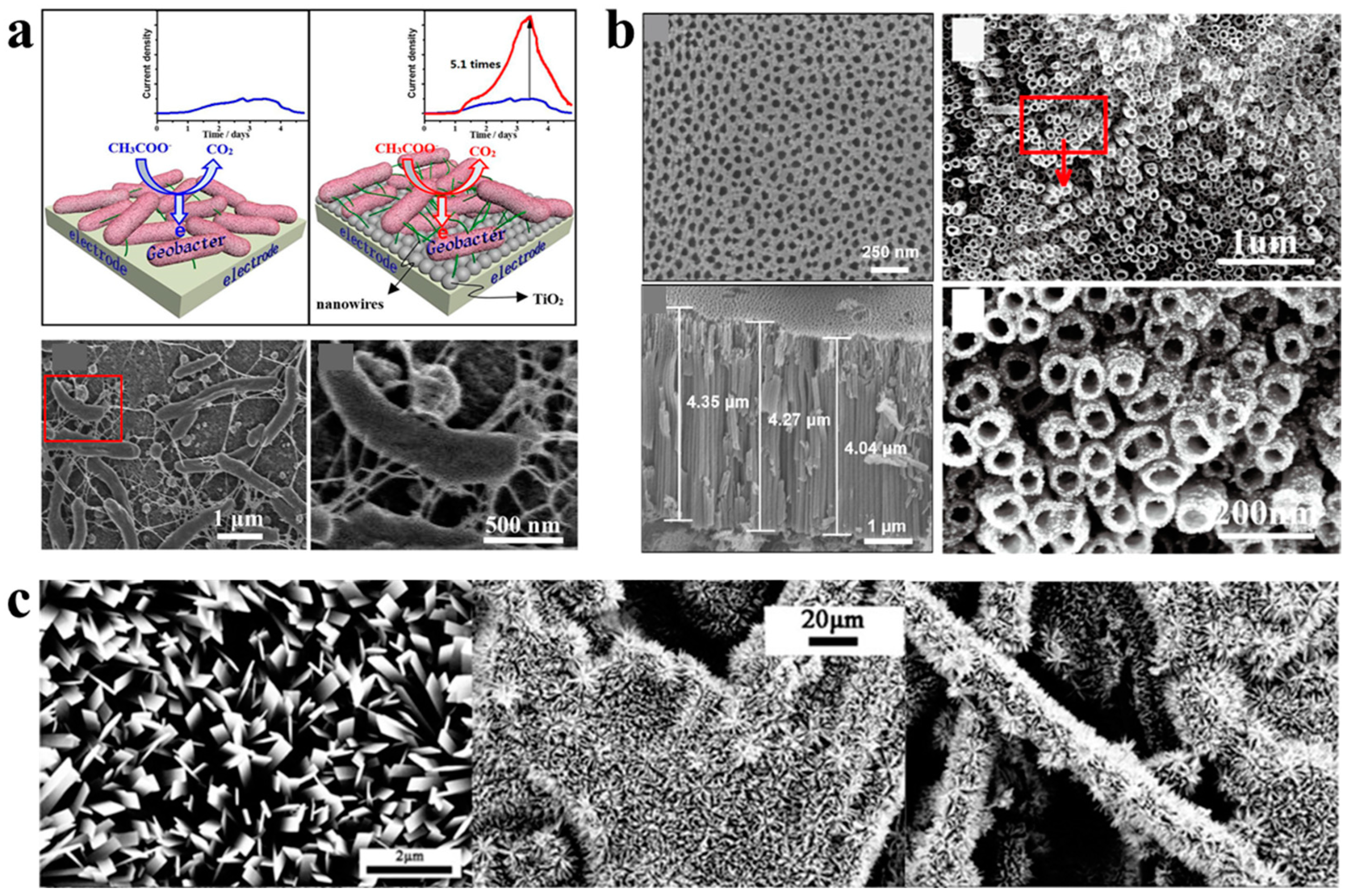
| TiO2 nanoparticles | Microbial anode | Approximate 5.1-fold increase in microbial current generation | [86] |
| TiO2 nanoparticles | Biophotoelectrode | Four-fold increase in photocurrent generation of Synechocystis sp. PCC 6803 | [85] |
| TiO2 nanotubes | MFC anode | A maximal current density of 12.7 A m−2, 190-fold higher than that of bare Ti electrode | [95] |
| TiO2 nanorods | MFC anode | A maximum power density of 2576.3 ± 33 mW m−2, 2.6-fold higher than that of carbon paper electrode | [89] |
| TiO2 nanosheets | MFC anode | A maximum power density of 690 mW m−3, 63% higher than that of bare carbon paper electrode | [90] |
| TiO2 nanosheets | MFC anode | A maximum power density of 497 mW m−2, 97% higher than that of carbon paper electrode | [91] |
| N-doped TiO2 nanosheets | MFC anode | A maximum power density of 747 mW m−2, 196% higher than that of carbon paper electrode | [94] |
| TiO2 nanoclusters | MFC anode | A maximum power density of 25.9 ± 6.2 mW m−2, a record-breaking value | [92] |
| TiO2/polyaniline | MFC anode | A maximum power density of 813 mW m−2, 63.6% higher than that of TiO2 electrode | [113] |
| α-FeOOH nanowhiskers | Microbial anode | A maximal current density of 0.48 A m−2, 60% higher than that of carbon paper electrode | [96] |
| Fe3O4 nanospheres/rGO | MFC anode | A maximum power density of 1837.4 mW m−2, 2.66-fold higher than that of carbon paper electrode | [97] |
| Magnetite nanoparticles | MES modifier | An 8.5-fold increase in acetate production and 52% decrease in methane production | [99] |
| GO/Fe3O4 | MES cathode | A CH4-producing rate of 605 ± 119 mmol m−2 d−1, 24.5-fold higher than that of carbon cloth electrode | [100] |
| N-doped Fe3O4@CD | MEC additive | A 6.37-fold increase in maximum current | [101] |
| α-Fe2O3 nanoarray | MFC anode | A maximum power density of 816 mW m−2, 8-fold higher than that of carbon cloth electrode | [102] |
| rGO/MnO2 | MFC anode | A maximum power density of 2065 mW m−2, 154% higher than that of carbon felt electrode | [103] |
| MnO2 | MEC cathode | A maximum current density of 3.70 ± 0.5 mA m−2, more than double the non-coated carbon felt cathode, and an acetate production rate of 37.9 mmol L−1, 43.0% higher than that of the bare carbon felt | [105] |
| MnO2/rGO | MES cathode | Production of isobutyric acid (15.9 mM) and acetate (3.5 mM), 2.09 and 2.91 folds higher, respectively, than bare carbon cloth | [106] |
| NiO nanoflakes | MFC anode | A 3-fold increase in maximum power density compared to carbon cloth electrode | [108] |
| NiO@N-doped carbon nanowires | MFC anode | An 8.5-fold increase in maximum power density compared to carbon cloth electrode | [109] |
| CeO2 nanoparticles | MFC anode/cathode | A 4.28 and 3.07-fold increase in maximum power density for cathode and anode, respectively | [110] |
| CNTs/m-WO3 | MFC anode | A maximum power density of 1.11 ± 0.022 W m−2, comparable to that of Pt anode | [111] |
| CNTs/SnO2 | MFC anode | A maximum power density of 1421 mW m−2, 3-fold higher than that of bare glassy carbon electrode | [112] |
| Transition metal sulfides (TMSs) | |||
| FeS | Microbial anode | A 3-fold increase in current density | [114] |
| FeS2/graphene | MFC anode | An unprecedented power density of 3220 mW m−2 and a remarkable current density of 3.06 A m−2 | [115] |
| FeS@S. oneidensis | Biohybrid | An increased interfacial electron transfer rate for ferrihydrite reduction | [116] |
| FeS@S. oneidensis | Biohybrid | A record-high interfacial electron transfer efficiency and BES performance | [117] |
| FeS@sulfate-reducing bacteria | Biohybrid | An increased extracellular electron transfer rate for Cr(VI) reduction | [118] |
| FeS@Dehalococcoides mccartyi | Biohybrid | Enhanced dechlorination of trichloroethene | [119] |
| CdS@Moorella thermoacetica | Biohybrid | Continuous production of acetate over several days of light/dark cycles | [120] |
| CdS@Rhodopseudomonas palustris | Biohybrid | A 148%, 122%, and 147% increase in production of solid biomass, carotenoids, and poly-β-hydroxybutyrate (PHB), respectively | [121] |
| CdS@Clostridium autoethanogenum | Biohybrid | Production acetate from CO2 by using only light | [122] |
| CdS@Cupriavidus necator | Biohybrid | Production of 1.41 g PHB from fructose over 120 h and 28 mg from CO2 over 48 h | [123] |
| CdS@Sporomusa ovata | Biohybrid | Production of acetate from CO2 with a quantum yield of 16.8 ± 9% over 5 days | [124] |
| Ni:CdS@Methanosarcina barkeri | Biohybrid | A 250% higher CH4 yield than the CdS@Methanosarcina barkeri biohybrid | [125] |
| Transition metal carbides (TMCs) | |||
| Mo2C/CNTs | MFC anode | A maximum power density of 1.05 ± 0.0264 W m−2, 13-fold higher than that of carbon felt electrode | [126] |
| Nanoporous Mo2C | MFC anode | A maximum power density of 1025 mW m−2, 5-fold higher than that of carbon felt electrode | [19] |
| Mo2C/rGO | MFC anode | A maximum power density of 1697 W m−2, 2-fold and 13-fold higher than that of graphene and carbon cloth electrode, respectively | [127] |
| WC | MFC anode | A maximum power density of 3.26 W m−2, chemical oxygen demand removal rate of 95.5%, coulombic efficiency of 83.2%, 2.14-fold, 1.22-fold, and 1.71-fold of that obtained by naked carbon cloth | [128] |
| Ti3C2 MXene | MFC anode | A maximum power density of 3.74 W m−2, 82.4% higher than that of carbon cloth | [129] |
| Transition metal nitrides (TMNs) | |||
| W2N-MXene | MFC anode | A 523% increase in power density (548 mW m−2), an 83% decrease in chemical oxygen demand, and a 161% increase in electron transfer efficiency compared to those of plain carbon cloth | [130] |
| Fe3N-Fe3C | MFC anode | A maximum power density increased from 28.57 mW m−2 to 58.86 mW m−2 | [131] |
3.4. Metal Sulfides
3.5. Metal Carbides
3.6. Metal Nitrides
4. Conclusion and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E.; Rossi, R.; Ragab, A.A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Kong, C.H. Enhanced removal of p-nitrophenol in a microbial fuel cell after long-term operation and the catabolic versatility of its microbial community. Chem. Eng. J. 2018, 339, 424–431. [Google Scholar] [CrossRef]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sust. Energ. Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Chen, H.; Dong, F.Y.; Minteer, S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat. Catal. 2020, 3, 225–244. [Google Scholar] [CrossRef]
- Qiao, Y.; Bao, S.J.; Li, C.M. Electrocatalysis in microbial fuel cells-from electrode material to direct electrochemistry. Energy Environ. Sci. 2010, 3, 544–553. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis-revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schrorder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Bajracharya, S.; Xu, J.J.; Pant, D.; Saikaly, P.E. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef]
- Chen, G.X.; Wang, R.C.; Sun, M.X.; Chen, J.; Iyobosa, E.; Zhao, J.F. Carbon dioxide reduction to high-value chemicals in microbial electrosynthesis system: Biological conversion and regulation strategies. Chemosphere 2023, 344, 140251. [Google Scholar] [CrossRef]
- Zhou, M.H.; Chi, M.L.; Luo, J.M.; He, H.H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, L.L.; Wang, X.Y.; Liu, T.X. Graphene Oxide-Assisted Dispersion of Pristine Multiwalled Carbon Nanotubes in Aqueous Media. J. Phys. Chem. C 2010, 114, 11435–11440. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Qu, Y.P.; Li, D.; He, W.H.; Feng, Y.J. Nanomaterials for facilitating microbial extracellular electron transfer: Recent progress and challenges. Bioelectrochemistry 2018, 123, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Rezk, H.; Abdelkareem, M.A. Recent progress of graphene based nanomaterials in bioelectrochemical systems. Sci. Total Environ. 2020, 749, 141225. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ayyaru, S.; Ahn, Y.H. Facile one-pot microwave assisted synthesis of rGO-CuS-ZnS hybrid nanocomposite cathode catalysts for microbial fuel cell application. Chemosphere 2021, 278, 130426. [Google Scholar] [CrossRef]
- Li, W.; Yue, H.Y.; Zhang, C.Y.; Hu, J.Y.; Wang, Q.L.; Li, Y.M.; Zhang, S.H.; Chen, J.M.; Zhao, J.K. Engineering multiscale polypyrrole/carbon nanotubes interface to boost electron utilization in a bioelectrochemical system coupled with chemical absorption for NO removal. Chemosphere 2022, 303, 134943. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Qiao, Y.; Wu, X.S.; Ma, C.X.; Li, X.; Li, C.M. Synergistic effect of titanium dioxide nanocrystal/reduced graphene oxide hybrid on enhancement of microbial electrocatalysis. J. Power Sources 2015, 276, 208–214. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, J.X.; Huang, Y.H.; Ni, H.Y.; Long, Z.-E.; Zou, L. Effect of Mo2C-functionalized electrode interface on enhancing microbial cathode electrocatalysis: Beyond electrochemical hydrogen evolution. Electrochim. Acta 2023, 443, 141924. [Google Scholar] [CrossRef]
- Zou, L.; Lu, Z.S.; Huang, Y.H.; Long, Z.-E.; Qiao, Y. Nanoporous Mo2C functionalized 3D carbon architecture anode for boosting flavins mediated interfacial bioelectrocatalysis in microbial fuel cells. J. Power Sources 2017, 359, 549–555. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, X.F.; Liu, X.; Zeng, R.J.; Zhang, F.; Ye, J.; Zhou, S. Facilitated extracellular electron transfer of Geobacter sulfurreducens biofilm with in situ formed gold nanoparticles. Biosens. Bioelectron. 2018, 108, 20–26. [Google Scholar] [CrossRef]
- Fu, X.Z.; Wu, J.; Li, J.; Ding, J.; Cui, S.; Wang, X.M.; Wang, Y.J.; Liu, H.Q.; Deng, X.; Liu, D.F.; et al. Heavy-metal resistant bio-hybrid with biogenic ferrous sulfide nanoparticles: pH-regulated self-assembly and wastewater treatment application. J. Hazard. Mater. 2023, 446, 130667. [Google Scholar] [CrossRef]
- Ma, J.C.; Zhang, J.; Zhang, Y.Z.; Guo, Q.L.; Hu, T.J.; Xiao, H.; Lu, W.B.; Jia, J.F. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Li, J.Y.; Han, H.X.; Chang, Y.H.; Wang, B. The material-microorganism interface in microbial hybrid electrocatalysis systems. Nanoscale 2023, 15, 6009–6024. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Qiao, Y.; Li, C.M. Boosting Microbial Electrocatalytic Kinetics for High Power Density: Insights into Synthetic Biology and Advanced Nanoscience. Electrochem. Energy Rev. 2018, 1, 567–598. [Google Scholar] [CrossRef]
- Paquete, C.M.; Rosenbaum, M.A.; Baneras, L.; Rotaru, A.-E.; Puig, S. Let’s chat: Communication between electroactive microorganisms. Bioresour. Technol. 2022, 347, 126705. [Google Scholar] [CrossRef]
- Breuer, M.; Rosso, K.M.; Blumberger, J.; Butt, J.N. Multi-haem cytochromes in Shewanella oneidensis MR-1: Structures, functions and opportunities. J. R. Soc. Interface 2015, 12, 20141117. [Google Scholar] [CrossRef]
- Marritt, S.J.; Lowe, T.G.; Bye, J.; McMillan, D.G.G.; Shi, L.; Fredrickson, J.; Zachara, J.; Richardson, D.J.; Cheesman, M.R.; Jeuken, L.J.C.; et al. A functional description of CymA, an electron-transfer hub supporting anaerobic respiratory flexibility in Shewanella. Biochem. J. 2012, 444, 465–474. [Google Scholar] [CrossRef]
- McMillan, D.G.G.; Marritt, S.J.; Firer-Sherwood, M.A.; Shi, L.; Richardson, D.J.; Evans, S.D.; Elliott, S.J.; Butt, J.N.; Jeuken, L.J.C. Protein-Protein Interaction Regulates the Direction of Catalysis and Electron Transfer in a Redox Enzyme Complex. J. Am. Chem. Soc. 2013, 135, 10550–10556. [Google Scholar] [CrossRef]
- White, G.F.; Shi, Z.; Shi, L.; Wang, Z.M.; Dohnalkova, A.C.; Marshall, M.J.; Fredrickson, J.K.; Zachara, J.M.; Butt, J.N.; Richardson, D.J.; et al. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals. Proc. Natl. Acad. Sci. USA 2013, 110, 6346–6351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Wang, Z.M.; Liu, J.; Levar, C.; Edwards, M.J.; Babauta, J.T.; Kennedy, D.W.; Shi, Z.; Beyenal, H.; Bond, D.R.; et al. A trans-outer membrane porin-cytochrome protein complex for extracellular electron transfer by Geobacter sulfurreducens PCA. Environ. Microbiol. Rep. 2014, 6, 776–785. [Google Scholar] [CrossRef]
- Shi, L.; Fredrickson, J.K.; Zachara, J.M. Genomic analyses of bacterial porin-cytochrome gene clusters. Front. Microbiol. 2014, 5, 657. [Google Scholar] [CrossRef] [PubMed]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 111, 12883–12888. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gu, Y.; O’Brien, J.P.; Yi, S.M.; Yalcin, S.E.; Srikanth, V.; Shen, C.; Vu, D.; Ing, N.L.; Hochbaum, A.I.; et al. Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers. Cell 2019, 177, 361–369.e10. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Q.; Guberman-Pfeffer, M.J.; Srikanth, V.; Shen, C.; Giska, F.; Gupta, K.; Londer, Y.; Samatey, F.A.; Batista, V.S.; Malvankar, N.S. Structure of Geobacter cytochrome OmcZ identifies mechanism of nanowire assembly and conductivity. Nat. Microbiol. 2023, 8, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella Secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Kluepfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Carlson, H.K.; Iavarone, A.T.; Gorur, A.; Yeo, B.S.; Tran, R.; Melnyk, R.A.; Mathies, R.A.; Auer, M.; Coates, J.D. Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram- positive bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Light, S.H.; Su, L.; Rivera-Lugo, R.; Cornejo, J.A.; Louie, A.; Iavarone, A.T.; Ajo-Franklin, C.M.; Portnoy, D.A. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 2018, 562, 140–144. [Google Scholar] [CrossRef]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef]
- Ross, D.E.; Flynn, J.M.; Baron, D.B.; Gralnick, J.A.; Bond, D.R. Towards Electrosynthesis in Shewanella: Energetics of Reversing the Mtr Pathway for Reductive Metabolism. PLoS ONE 2011, 6, e16649. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Carmona-Martinez, A.A.; Vilajeliu-Pons, A.; Fiset, E.; Baneras, L.; Trably, E.; Dolors Balaguer, M.; Colprim, J.; Bernet, N.; Puig, S. Bidirectional microbial electron transfer: Switching an acetate oxidizing biofilm to nitrate reducing conditions. Biosens. Bioelectron. 2016, 75, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Strycharz, S.M.; Glaven, R.H.; Coppi, M.V.; Gannon, S.M.; Perpetua, L.A.; Liu, A.; Nevin, K.P.; Lovley, D.R. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 2011, 80, 142–150. [Google Scholar] [CrossRef]
- Dantas, J.M.; Tomaz, D.M.; Morgado, L.; Salgueiro, C.A. Functional characterization of PccH, a key cytochrome for electron transfer from electrodes to the bacterium Geobacter sulfurreducens. FEBS Lett. 2013, 587, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.D.; Tran, V.N.; Mohamed, A.; Renslow, R.; Biria, S.; Orfe, L.; Call, D.R.; Beyenal, H. The mechanism of neutral red-mediated microbial electrosynthesis in Escherichia coli: Menaquinone reduction. Bioresour. Technol. 2015, 192, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.Z.; Hu, Y.D.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing Bidirectional Electron Transfer of Shewanella oneidensis by a Synthetic Flavin Pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. Mbio 2010, 1, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Doud, D.F.R.; Angenent, L.T. Toward Electrosynthesis with Uncoupled Extracellular Electron Uptake and Metabolic Growth: Enhancing Current Uptake with Rhodopseudomonas palustris. Environ. Sci. Technol. Lett. 2014, 1, 351–355. [Google Scholar] [CrossRef]
- Karbelkar, A.A.; Rowe, A.R.; El-Naggar, M.Y. An electrochemical investigation of interfacial electron uptake by the sulfur oxidizing bacterium Thioclava electrotropha ElOx9. Electrochim. Acta 2019, 324, 134838. [Google Scholar] [CrossRef]
- Sen Thapa, B.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The ecophysiology of phylogenetically diverse electroactive microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef]
- Zhao, J.T.; Li, F.; Cao, Y.X.; Zhang, X.B.; Chen, T.; Song, H.; Wang, Z.W. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2021, 53, 107682. [Google Scholar] [CrossRef]
- Wu, X.S.; Qjao, Y.; Shi, Z.Z.; Tang, W.; Li, C.M. Hierarchically Porous N-Doped Carbon Nanotubes/Reduced Graphene Oxide Composite for Promoting Flavin-Based Interfacial Electron Transfer in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 11671–11677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Wang, S.P.; Liu, T.B.; Jia, B.Y.; Liu, W.Z.; Dong, P. Metal-organic framework-derived iron oxide modified carbon cloth as a high-power density microbial fuel cell anode. J. Clean Prod. 2022, 341, 130725. [Google Scholar] [CrossRef]
- Chen, M.Q.; Guo, W.X.; Zhang, Y.; Xiao, H.F.; Lin, J.J.; Rao, Y.; Zhang, M.; Cheng, F.L.; Lu, X.H. Activated nitrogen-doped ordered porous carbon as advanced anode for high-performance microbial fuel cells. Electrochim. Acta 2021, 391, 138920. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.Y.; Zhu, X.; Zhang, F.; Ye, D.D.; Liao, Q.; Li, Y. Boosting Power Density of Microbial Fuel Cells with 3D Nitrogen-Doped Graphene Aerogel Electrode. Adv. Sci. 2016, 3, 1600097. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Guo, C.X.; Yong, Y.C.; Li, C.M.; Song, H. Nitrogen doped carbon nanoparticles enhanced extracellular electron transfer for high-performance microbial fuel cells anode. Chemosphere 2015, 140, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Qian, Y.J.; Yang, C.C.; Huang, X.; Li, H.; Xie, X.J.; Huang, L.; Huang, W. Nitrogen-enriched pseudographitic anode derived from silk cocoon with tunable flexibility for microbial fuel cells. Nano Energy 2017, 32, 382–388. [Google Scholar] [CrossRef]
- Huang, H.F.; Wang, H.Q.; Huang, Q.; Song, T.S.; Xie, J.J. Mo2C/N-doped 3D loofah sponge cathode promotes microbial electrosynthesis from carbon dioxide. Int. J. Hydrogen Energy 2021, 46, 20325–20337. [Google Scholar] [CrossRef]
- Hu, J.P.; Zeng, C.P.; Liu, G.L.; Ren, Z.J.; Luo, H.P.; Teng, M. Carbon dots internalization enhances electroactive biofilm formation and microbial acetate synthesis. J. Clean Prod. 2023, 411, 137333. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, W.Q.; He, C.S.; Zhao, H.Q.; Han, J.C.; Liu, X.C.; Mu, Y. Active N dopant states of electrodes regulate extracellular electron transfer of Shewanella oneidensis MR-1 for bioelectricity generation: Experimental and theoretical investigations. Biosens. Bioelectron. 2020, 160, 112231. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.X.; Sun, X.Q.; Zheng, S.Y.; Wang, T.D.; Zhang, X.W.; Li, G.Z. Graphitic N-dominated nitrogen-doped carbon nanotubes as efficient metal-free catalysts for hydrogenation of nitroarenes. Carbon 2019, 146, 60–69. [Google Scholar] [CrossRef]
- Haider, M.R.; Jiang, W.L.; Han, J.L.; Sharif, H.M.A.; Ding, Y.C.; Cheng, H.Y.; Wang, A.J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B-Environ. 2019, 256, 117774. [Google Scholar] [CrossRef]
- Yuan, H.R.; Deng, L.F.; Qian, X.; Wang, L.F.; Li, D.N.; Chen, Y.; Yuan, Y. Significant enhancement of electron transfer from Shewanella oneidensis using a porous N-doped carbon cloth in a bioelectrochemical system. Sci. Total Environ. 2019, 665, 882–889. [Google Scholar] [CrossRef]
- Wu, X.S.; Qiao, Y.; Guo, C.X.; Shi, Z.Z.; Li, C.M. Nitrogen doping to atomically match reaction sites in microbial fuel cells. Comm. Chem. 2020, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; He, W.H.; Yang, J.C.; Sun, J.Q.; Li, H.D.; Han, B.; Zhao, S.L.; Shi, Y.A.; Feng, Y.J.; Tang, Z.Y.; et al. Bread-derived 3D macroporous carbon foams as high performance free-standing anode in microbial fuel cells. Biosens. Bioelectron. 2018, 122, 217–223. [Google Scholar] [CrossRef]
- Du, Y.X.; Sheng, H.T.; Astruc, D.; Zhu, M.Z. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef]
- Zhao, S.L.; Li, Y.C.; Yin, H.J.; Liu, Z.Z.; Luan, E.X.; Zhao, F.; Tang, Z.Y.; Liu, S.Q. Three-dimensional graphene/Pt nanoparticle composites as freestanding anode for enhancing performance of microbial fuel cells. Sci. Adv. 2015, 1, e1500372. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.C.; Sun, B.; Xu, H.D. Anode decoration with biogenic Pd nanoparticles improved power generation in microbial fuel cells. Electrochim. Acta 2015, 182, 815–820. [Google Scholar] [CrossRef]
- Zou, L.; Zhu, F.; Long, Z.-E.; Huang, Y.H. Bacterial extracellular electron transfer: A powerful route to the green biosynthesis of inorganic nanomaterials for multifunctional applications. J. Nanobiotechnol. 2021, 19, 120. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kimber, R.L.; Singh, V.K.; Shende, S.; Behal, A.; Sushkova, S.; Mandzhieva, S.; Lloyd, J.R. Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella. Appl. Environ. Microbiol. 2021, 87, e01390-21. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhao, Z.P.; Peng, L.L.; Shiu, H.Y.; Ding, M.N.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Egan-Morriss, C.; Kimber, R.L.; Powell, N.A.; Lloyd, J.R. Biotechnological synthesis of Pd-based nanoparticle catalysts. Nanoscale Adv. 2022, 4, 654–679. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, F.; Rahunen, N.; Varcoe, J.R.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C.T. A Role for Microbial Palladium Nanoparticles in Extracellular Electron Transfer. Angew. Chem.-Int. Edit. 2011, 50, 427–430. [Google Scholar] [CrossRef] [PubMed]
- You, L.X.; Pan, D.M.; Chen, N.J.; Lin, W.F.; Chen, Q.S.; Rensing, C.; Zhou, S.G. Extracellular electron transfer of Enterobacter cloacae SgZ-5T via bi-mediators for the biorecovery of palladium as nanorods. Environ. Int. 2019, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lin, W.M.; Chen, Y.C.; Hu, Y.Y.; Luo, Q.J. Prompting the FDH/Hases-based electron transfers during Pt(IV) reduction mediated by bio-Pd(0). J. Hazard. Mater. 2021, 417, 126090. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.T.; Qian, D.S.; Chen, Y.C.; Hu, Y.Y. Intra/extracellular electron transfer for aerobic denitrification mediated by in-situ biosynthesis palladium nanoparticles. Water Res. 2021, 189, 116612. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sandoval, R.; Pedireddy, S.; Katuri, K.P.; Saikaly, P.E. Facile Biological-Based Synthesis of Size-Controlled Palladium Nanoclusters Anchored on the Surface of Geobacter sulfurreducens and Their Application in Electrocatalysis. ACS Sustain. Chem. Eng. 2023, 11, 1100–1109. [Google Scholar] [CrossRef]
- Ahmed, E.; Kalathil, S.; Shi, L.; Alharbi, O.; Wang, P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018, 22, 919–929. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, C.; Gu, S.J.; Wei, Y.H.; Li, L.; Qu, Q. Upcycling spent palladium-based catalysts into high value-added catalysts via electronic regulation of Escherichia coli to high-efficiently reduce hexavalent chromium. Environ. Pollut. 2023, 337, 122660. [Google Scholar] [CrossRef]
- Dundas, C.M.; Graham, A.J.; Romanovicz, D.K.; Keitz, B.K. Extracellular Electron Transfer by Shewanella oneidensis Controls Palladium Nanoparticle Phenotype. ACS Synth. Biol. 2018, 7, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Xiao, Y.; Cao, H.L.; Tian, X.C.; Wu, R.R.; Zhang, J.D.; Ulstrup, J.; Zhao, F. Effect of Copper and Phosphate on the Biosynthesis of Palladium Nanoparticles by Shewanella oneidensis MR-1. Chemelectrochem 2020, 7, 4460–4468. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Wang, W.J.; Ding, S.T.; Zhang, M.X.; Tang, A.G.; Zhang, L.; Li, D.B.; Li, B.B.; Deng, G.Z.; Wu, C. Pyruvate Accelerates Palladium Reduction by Regulating Catabolism and the Electron Transfer Pathway in Shewanella oneidensis. Appl. Environ. Microbiol. 2021, 87, e02716-20. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Wickramathilaka, K.Y.; Njeri, E.; Silva, D.; Suib, S.L. A review on transition metal oxides in catalysis. Front. Chem. 2024, 12, 1374878. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Wang, H.W.; Tang, L.F.; Zhu, H.W. Titanium dioxide nanoparticles enhance photocurrent generation of cyanobacteria. Biochem. Biophys. Res. Commun. 2023, 672, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.G.; Tang, J.H.; Yuan, Y.; Yang, G.Q.; Xing, B.S. TiO2 Nanoparticle-Induced Nanowire Formation Facilitates Extracellular Electron Transfer. Environ. Sci. Technol. Lett. 2018, 5, 564–570. [Google Scholar] [CrossRef]
- Santos, J.S.; Tarek, M.; Sikora, M.S.; Praserthdam, S.; Praserthdam, P. Anodized TiO2 nanotubes arrays as microbial fuel cell (MFC) electrodes for wastewater treatment: An overview. J. Power Sources 2023, 564, 232872. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.H.; Kim, H.; Park, Y.H.; In, S.-I. Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies 2018, 11, 3184. [Google Scholar] [CrossRef]
- Chen, M.Q.; Liu, X.Q.; Cheng, F.L.; Lu, X.H.; Tong, Y.X. Oxygen-deficient TiO2 decorated carbon paper as advanced anodes for microbial fuel cells. Electrochim. Acta 2021, 366, 137468. [Google Scholar] [CrossRef]
- Yin, T.; Lin, Z.Y.; Su, L.; Yuan, C.W.; Fu, D.G. Preparation of Vertically Oriented TiO2 Nanosheets Modified Carbon Paper Electrode and Its Enhancement to the Performance of MFCs. ACS Appl. Mater. Interfaces 2015, 7, 400–408. [Google Scholar] [CrossRef]
- Yin, T.; Li, H.; Su, L.; Liu, S.; Yuan, C.; Fu, D. The catalytic effect of TiO2 nanosheets on extracellular electron transfer of Shewanella loihica PV-4. Phys. Chem. Chem. Phys. 2016, 18, 29871–29878. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.D.Z.; Kwon, Y.C. In situ carbon felt anode modification via codeveloping Saccharomyces cerevisiae living-template titanium dioxide nanoclusters in a yeast-based microbial fuel cell. J. Power Sources 2020, 474, 228651. [Google Scholar] [CrossRef]
- Su, L.; Yin, T.; Du, H.X.; Zhang, W.; Fu, D.G. Synergistic improvement of Shewanella loihica PV-4 extracellular electron transfer using TiO2@TiN nanocomposite. Bioelectrochemistry 2020, 134, 107519. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Su, L.; Li, H.; Lin, X.X.; Dong, L.; Du, H.X.; Fu, D.G. Nitrogen doping of TiO2 nanosheets greatly enhances bioelectricity generation of S-loihica PV-4. Electrochim. Acta 2017, 258, 1072–1080. [Google Scholar] [CrossRef]
- Feng, H.J.; Liang, Y.X.; Guo, K.; Chen, W.; Shen, D.S.; Huang, L.J.; Zhou, Y.Y.; Wang, M.Z.; Long, Y.Y. TiO2 Nanotube Arrays Modified Titanium: A Stable, Scalable, and Cost-Effective Bioanode for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424. [Google Scholar] [CrossRef]
- Wang, L.; Su, L.; Chen, H.H.; Yin, T.; Lin, Z.Y.; Lin, X.X.; Yuan, C.W.; Fu, D.G. Carbon paper electrode modified by goethite nanowhiskers promotes bacterial extracellular electron transfer. Mater. Lett. 2015, 141, 311–314. [Google Scholar] [CrossRef]
- Ma, J.C.; Shi, N.; Jia, J.F. Fe3O4 nanospheres decorated reduced graphene oxide as anode to promote extracellular electron transfer efficiency and power density in microbial fuel cells. Electrochim. Acta 2020, 362, 137126. [Google Scholar] [CrossRef]
- Song, R.B.; Zhao, C.-E.; Gai, P.P.; Guo, D.; Jiang, L.P.; Zhang, Q.C.; Zhang, J.R.; Zhu, J.J. Graphene/Fe3O4 Nanocomposites as Efficient Anodes to Boost the Lifetime and Current Output of Microbial Fuel Cells. Chem.-Asian J. 2017, 12, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Viggi, C.C.; Colantoni, S.; Falzetti, F.; Bacaloni, A.; Montecchio, D.; Aulenta, F. Conductive Magnetite Nanoparticles Enhance the Microbial Electrosynthesis of Acetate from CO2 while Diverting Electrons away from Methanogenesis. Fuel Cells 2020, 20, 98–106. [Google Scholar] [CrossRef]
- He, Y.T.; Li, Q.; Li, J.; Zhang, L.; Fu, Q.; Zhu, X.; Liao, Q. Magnetic assembling GO/Fe3O4/microbes as hybridized biofilms for enhanced methane production in microbial electrosynthesis. Renew. Energy 2022, 185, 862–870. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, R.X.; Li, H.; Chen, Z.; Zhou, X.Y.; Ren, X.Y.; Dong, H.Q.; Lin, R.C.; Zhou, J.H. Enhancing Extracellular Electron Transfer of Geobacter sulfurreducens in Bioelectrochemical Systems Using N-Doped Fe3O4@Carbon Dots. ACS Sustain. Chem. Eng. 2022, 10, 3935–3950. [Google Scholar] [CrossRef]
- He, X.; Lu, H.; Fu, J.J.; Zhou, H.; Qian, X.C.; Qiao, Y. Promotion of direct electron transfer between Shewanella putrefaciens CN32 and carbon fiber electrodes via in situ growth of α-Fe2O3 nanoarray. Front. Microbiol. 2024, 15, 1407800. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liang, P.; Yang, X.F.; Jiang, Y.; Bian, Y.H.; Chen, C.M.; Zhang, X.Y.; Huang, X. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens. Bioelectron. 2016, 81, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Tian, T.; Guo, M.; Liu, X.; Liu, X. Cauliflower-like polypyrrole@MnO2 modified carbon cloth as a capacitive anode for high-performance microbial fuel cells. J. Chem. Technol. Biotechnol. 2020, 95, 163–172. [Google Scholar] [CrossRef]
- Anwer, A.H.; Khan, M.D.; Khan, N.; Nizami, A.S.; Rehan, M.; Khan, M.Z. Development of novel MnO2 coated carbon felt cathode for microbial electroreduction of CO2 to biofuels. J. Environ. Manag. 2019, 249, 109376. [Google Scholar] [CrossRef] [PubMed]
- Thatikayala, D.; Pant, D.; Min, B. MnO2/reduced graphene oxide nanohybrids as a cathode catalyst for the microbial reduction of CO2 to acetate and isobutyric acid. Sustain. Energy Technol. Assess. 2021, 45, 101114. [Google Scholar] [CrossRef]
- Kalathil, S.; Katuri, K.P.; Saikaly, P.E. Synthesis of an amorphous Geobacter-manganese oxide biohybrid as an efficient water oxidation catalyst. Green Chem. 2020, 22, 5610–5618. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, X.S.; Li, C.M. Interfacial electron transfer of Shewanella putrefaciens enhanced by nanoflaky nickel oxide array in microbial fuel cells. J. Power Sources 2014, 266, 226–231. [Google Scholar] [CrossRef]
- Wu, X.S.; Shi, Z.Z.; Qiao, Y.; Zou, Z.; Guo, C.X.; Li, C.M. Macroporous spider net-like NiO nanowire on carbon nanowire to grow a biofilm with multi-layered bacterium cells toward high-power microbial fuel cells. J. Power Sources 2021, 506, 230133. [Google Scholar] [CrossRef]
- Pushkar, P.; Prakash, O.; Imran, M.; Mungray, A.A.; Kailasa, S.K.; Mungray, A.K. Effect of cerium oxide nanoparticles coating on the electrodes of benthic microbial fuel cell. Sep. Sci. Technol. 2019, 54, 213–223. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, B.; Xiang, X.D.; Guo, C.L.; Li, W.S. Carbon Nanotubes Conjugated Mesoporous Tungsten Trioxide as Anode Electrocatalyst for Microbial Fuel Cells. ECS J. Solid State Sci. Technol. 2020, 9, 115010. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-walled carbon nanotube/SnO2 nanocomposite: A novel anode material for microbial fuel cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, H.; Yang, G.Q.; Wang, L. Polyaniline composite TiO2 nanosheets modified carbon paper electrode as a high performance bioanode for microbial fuel cells. Synth. Met. 2019, 252, 8–14. [Google Scholar] [CrossRef]
- Jiang, X.C.; Hu, J.S.; Lieber, A.M.; Jackan, C.S.; Biffinger, J.C.; Fitzgerald, L.A.; Ringeisen, B.R.; Lieber, C.M. Nanoparticle Facilitated Extracellular Electron Transfer in Microbial Fuel Cells. Nano Lett. 2014, 14, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.W.; Yan, M.; Li, H.D.; Zhang, L.; Peng, B.Q.; Sun, J.Z.; Liu, D.; Liu, S.Q. FeS2 Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, e1800618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Huang, Y.H.; Ni, H.Y.; Tang, J.; Zhu, Q.; Long, Z.-E.; Zou, L. Biogenic iron sulfide functioning as electron-mediating interface to accelerate dissimilatory ferrihydrite reduction by Shewanella oneidensis MR-1. Chemosphere 2022, 288, 132661. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Wang, Y.Z.; Fang, Z.; Shi, Y.T.; Cheng, Q.W.; Chen, Y.X.; Shi, W.D.; Yong, Y.C. Single cell electron collectors for highly efficient wiring-up electronic abiotic/biotic interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef]
- Qian, D.S.; Liu, H.M.; Hu, F.; Song, S.; Chen, Y.C. Extracellular electron transfer-dependent Cr(VI)/sulfate reduction mediated by iron sulfide nanoparticles. J. Biosci. Bioeng. 2022, 134, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhao, H.P.; Zhu, L.Z. Iron Sulfide Enhanced the Dechlorination of Trichloroethene by Dehalococcoides mccartyi Strain 195. Front. Microbiol. 2021, 12, 665281. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Wong, A.B.; Yang, P.D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Z.; Yu, J.C.; Wang, J.F.; Wong, P.K. Enhanced CO2 reduction and valuable C2+ chemical production by a CdS-photosynthetic hybrid system. Nanoscale 2019, 11, 9296–9301. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Jeon, Y.; Jeon, M.S.; Shin, J.; Song, Y.; Kang, S.; Bae, J.; Cho, S.; Lee, J.-K.; Kim, D.R.; et al. Acetogenic bacteria utilize light-driven electrons as an energy source for autotrophic growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2020552118. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Tremblay, P.-L.; Ding, R.; Xiao, J.X.; Wang, J.T.; Kang, Y.; Zhang, T. Photo-augmented PHB production from CO2 or fructose by Cupriavidus necator and shape-optimized CdS nanorods. Sci. Total Environ. 2021, 753, 106504. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, S.R.; Han, X.Y.; Shen, J.Y.; Lu, Y.W.; Zhao, J.Z.; Shen, C.P.; Qiao, L. Photosynthesis of Acetate by Sporomusa ovata-CdS Biohybrid System. ACS Appl. Mater. Interfaces 2022, 14, 23364–23374. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ren, G.P.; Kang, L.; Zhang, Y.Y.; Liu, X.; Zhou, S.G.; He, Z. Efficient Photoelectron Capture by Ni Decoration in Methanosarcina barkeri-CdS Biohybrids for Enhanced Photocatalytic CO2-to-CH4 Conversion. Iscience 2020, 23, 101287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, B.; Cui, D.; Xiang, X.D.; Li, W.S. Nano-molybdenum carbide/carbon nanotubes composite as bifunctional anode catalyst for high-performance Escherichia coli-based microbial fuel cell. Biosens. Bioelectron. 2014, 51, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Huang, Y.H.; Wu, X.; Long, Z.-E. Synergistically promoting microbial biofilm growth and interfacial bioelectrocatalysis by molybdenum carbide nanoparticles functionalized graphene anode for bioelectricity production. J. Power Sources 2019, 413, 174–181. [Google Scholar] [CrossRef]
- Liu, D.; Chang, Q.H.; Gao, Y.; Huang, W.C.; Sun, Z.Y.; Yan, M.; Guo, C.S. High performance of microbial fuel cell afforded by metallic tungsten carbide decorated carbon cloth anode. Electrochim. Acta 2020, 330, 135243. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.W.; Chang, W.; Zhang, L.; Peng, B.Q.; Li, H.D.; Liu, S.Q.; Yan, M.; Guo, C.S. Ti3C2 MXene as an excellent anode material for high-performance microbial fuel cells. J. Mater. Chem. A 2018, 6, 20887–20895. [Google Scholar] [CrossRef]
- Kolubah, P.D.; Mohamed, H.O.; Ayach, M.; Hari, A.R.; Alshareef, H.N.; Saikaly, P.; Chae, K.-J.; Castano, P. W2N-MXene composite anode catalyst for efficient microbial fuel cells using domestic wastewater. Chem. Eng. J. 2023, 461, 141821. [Google Scholar] [CrossRef]
- Cheng, G.S.; Yang, Y.H.; Luo, E.C.; Suthar, D.K.; Zhao, Y.; Luan, X.; Wang, X.Q.; Dong, C.Q. Degradation and power generation performance of modified-anode microbial fuel cells for kitchen wastewater. J. Power Sources 2024, 591, 233841. [Google Scholar] [CrossRef]
- Nakamura, R.; Okamoto, A.; Tajima, N.; Newton, G.J.; Kai, F.; Takashima, T.; Hashimoto, K. Biological Iron-Monosulfide Production for Efficient Electricity Harvesting from a Deep-Sea Metal-Reducing Bacterium. Chembiochem 2010, 11, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Okamoto, A.; Hashimoto, K.; Nakamura, R. Sulfur-Mediated Electron Shuttling Sustains Microbial Long-Distance Extracellular Electron Transfer with the Aid of Metallic Iron Sulfides. Langmuir 2015, 31, 7427–7434. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Zhang, J.Z.; Sakimoto, K.K.; Yang, P.; Reisner, E. Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat. Nanotechnol. 2018, 13, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.H.; Xu, Q.; Du, M.M.; Zeng, X.F.; Zhong, G.F.; Qiu, B.C.; Zhang, J.L. Recent Progress of Metal Sulfide Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, e2202929. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.Z.; Zhang, L.X.; Li, W.S.; Zhao, S.F.; Lei, J.F.; Zhou, Z.H. Molybdenum carbide as anodic catalyst for microbial fuel cell based on Klebsiella pneumoniae. Biosens. Bioelectron. 2010, 25, 2696–2700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.Z.; Chen, X.F.; Li, H.Y.; Xiong, J.; Hu, M.H.; Li, X.; Li, W.S. Highly dispersed polydopamine-modified Mo2C/MoO2 nanoparticles as anode electrocatalyst for microbial fuel cells. Electrochim. Acta 2018, 283, 528–537. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Zhao, F.; Schroeder, U.; Scholz, F. Interfacing electrocatalysis and biocatalysis with tungsten carbide: A high-performance, noble-metal-free microbial fuel cell. Angew. Chem.-Int. Edit. 2006, 45, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.X.; Ding, K.; Huang, C.; Li, Q.W.; Chu, P.K.; Huo, K.F. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Zhu, Q. Electrocatalytic Nanomaterials Improve Microbial Extracellular Electron Transfer: A Review. Appl. Sci. 2024, 14, 6733. https://doi.org/10.3390/app14156733
Wang X, Li X, Zhu Q. Electrocatalytic Nanomaterials Improve Microbial Extracellular Electron Transfer: A Review. Applied Sciences. 2024; 14(15):6733. https://doi.org/10.3390/app14156733
Chicago/Turabian StyleWang, Xiaopin, Xu Li, and Qisu Zhu. 2024. "Electrocatalytic Nanomaterials Improve Microbial Extracellular Electron Transfer: A Review" Applied Sciences 14, no. 15: 6733. https://doi.org/10.3390/app14156733




