Research Progress in the Construction and Application of In Vitro Vascular Models
Abstract
:1. Introduction
2. Construction of In Vitro Vascular Models
2.1. Construction of In Vitro Vascular Model Based on Microfluidic Technology
2.1.1. Photolithography and Soft Lithography
2.1.2. Self-Assembly
2.2. Construction of In Vitro Vascular Model Based on Non-Microfluidics-Based Methods
2.2.1. Construction of Vascular Models In Vitro Using Templates
2.2.2. Construction of Vascular Model In Vitro by 3D Bioprinting
2.2.3. Construction of Vascular Model In Vitro by Laser Degradation or Laser Cavitation Molding
3. Application
3.1. Endothelial Dysfunction
3.2. Blood Vessels Associated with Cancer
3.3. Blood–Brain Barrier
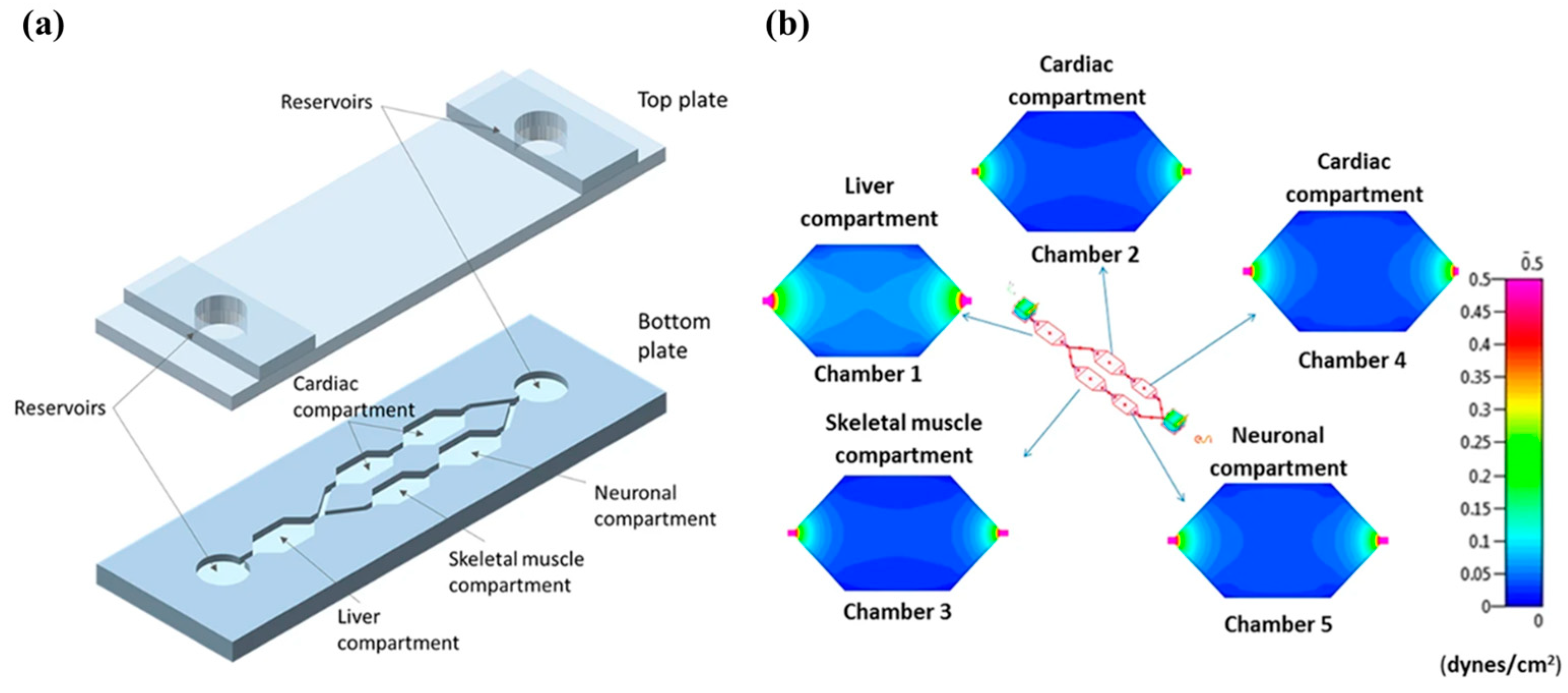
3.4. Vascularized In Vitro Organs-on-Chips
4. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wang, Y.T.; Zhong, L.N.; Pan, F.W.; Wang, J. Advances in Tissue Engineering of Vasculature through Three-Dimensional Bioprinting. Dev. Dyn. 2021, 250, 1717–1738. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Whelan, I.T.; Moeendarbary, E.; Hoey, D.A.; Kelly, D.J. Biofabrication of Vasculature in Microphysiological Models of Bone. Biofabrication 2021, 13, 032004. [Google Scholar] [CrossRef] [PubMed]
- Puryear, J.R.; Yoon, J.K.; Kim, Y. Advanced Fabrication Techniques of Microengineered Physiological Systems. Micromachines 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.X.; Millican, R.; Lynd, T.; Gangasani, M.; Malhotra, S.; Sherwood, J.; Hwang, P.T.; Cho, Y.; Brott, B.C.; et al. Recent Progress in In Vitro Models for Atherosclerosis Studies. Front. Cardiovasc. Med. 2022, 8, 790529. [Google Scholar] [CrossRef] [PubMed]
- Grifno, G.N.; Farrell, A.M.; Linville, R.M.; Arevalo, D.; Kim, J.H.; Gu, L.; Searson, P.C. Tissue-Engineered Blood-Brain Barrier Models via Directed Differentiation of Human Induced Pluripotent Stem Cells. Sci. Rep. 2019, 9, 13957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Zhang, J.T.; Hao, L.Z.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Modeling Endothelialized Hepatic Tumor Microtissues for Drug Screening. Adv. Sci. 2020, 7, 2002002. [Google Scholar] [CrossRef] [PubMed]
- Luque-González, M.A.; Reis, R.L.; Kundu, S.C.; Caballero, D. Human Microcirculation-On-Chip Models in Cancer Research: Key Integration of Lymphatic and Blood Vasculatures. Adv. Biosyst. 2020, 4, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.W.; George, S.C. Engineering Vascularized Organoid-On-A-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.Y.; Guo, F.; Zhang, B.Y. Modeling Organ-Specific Vasculature with Organ-On-A-Chip Devices. Nanotechnology 2018, 30, 024002. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Enrico, A.; Voulgaris, D.; Ostmans, R.; Sundaravadivel, N.; Moutaux, L.; Cordier, A.; Niklaus, F.; Herland, A.; Stemme, G. 3D Microvascularized Tissue Models by Laser-Based Cavitation Molding of Collagen. Adv. Mater. 2022, 34, 2109823. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.; Vogt, F.; Schmitz-Rode, T.; Jockenhoevel, S.; Mela, P. Bioengineered Vascular Constructs as Living Models for in Vitro Cardiovascular Research. Drug Discov. Today 2016, 21, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Balestrini, J.L.; Udelsman, B.V.; Zhou, K.C.; Zhao, L.P.; Ferruzzi, J.; Starcher, B.C.; Levene, M.J.; Humphrey, J.D.; Niklason, L.E. Biaxial Stretch Improves Elastic Fiber Maturation, Collagen Arrangement, and Mechanical Properties in Engineered Arteries. Tissue Eng. Part C Methods 2016, 22, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Schmitz-Rixen, T.; Hamilton, G.; Seifalian, A.M. Achieving the Ideal Properties for Vascular Bypass Grafts Using a Tissue Engineered Approach: A Review. Med. Biol. Eng. Comput. 2007, 45, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Pollet, A.M.A.O.; den Toonder, J.M.J. Recapitulating the Vasculature Using Organ-On-Chip Technology. Bioengineering 2020, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic Vasculature: From Descriptive Heterogeneity to Functional Pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.J.; Coulombe, K.L.K. Integrated Approaches to Spatiotemporally Directing Angiogenesis in Host and Engineered Tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hou, L.Q.; Huang, N.F. Vascularization of Three-Dimensional Engineered Tissues for Regenerative Medicine Applications. Acta Biomater. 2016, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Gerecht, S. Microfluidics and Biomaterials to Study Angiogenesis. Curr. Opin. Chem. Eng. 2016, 11, 114–122. [Google Scholar] [CrossRef]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins and Their Impact on the Blood Vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Pavani, K.; Nikarika, R.; Sumantran, V.N. Intussusceptive Angiogenesis as a Key Therapeutic Target for Cancer Therapy. Life Sci. 2020, 252, 117670. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic Targeting of Angiogenesis Molecular Pathways in Angiogenesis-Dependent Diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Saravanan, S.; Anuradha, D.; Chatterjee, S. Models to Investigate Intussusceptive Angiogenesis: A Special Note on CRISPR/Cas9 Based System in Zebrafish. Int. J. Biol. Macromol. 2018, 123, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, B.; Rong, W.W.; Goede, A.; Hoffmann, B.; Scarpa, F.; Kuebler, W.M.; Secomb, T.W.; Pries, A.R. Coalescent Angiogenesis-Evidence for a Novel Concept of Vascular Network Maturation. Angiogenesis 2022, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Pezzella, F. Sprouting and Nonsprouting Angiogenesis in Tumors. Tumor Vasc. 2020, 25, 2927. [Google Scholar]
- Pasut, A.; Becker, L.M.; Cuypers, A.; Carmeliet, P. Endothelial Cell Plasticity at the Single-Cell Level. Angiogenesis 2021, 24, 311–326. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Muñoz-Félix, J.M.; Pedrosa, A.R.; Hodivala-Dilke, K.M. “Splitting the Matrix”: Intussusceptive Angiogenesis Meets MT1-MMP. EMBO Mol. Med. 2019, 12, e11663. [Google Scholar] [CrossRef] [PubMed]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft Lithography in Biology and Biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Pasman, T.; Grijpma, D.; Stamatialis, D.; Poot, A. Flat and Microstructured Polymeric Membranes in Organs-On-Chips. J. R. Soc. Interface 2018, 15, 20180351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Z.P.; Kamm, R.D. Vascularized Organoids on a Chip: Strategies for Engineering Organoids with Functional Vasculature. Lab Chip 2021, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- He, J.K.; Chen, R.M.; Lu, Y.J.; Zhan, L.; Liu, Y.X.; Li, D.C.; Jin, Z.M. Fabrication of Circular Microfluidic Network in Enzymatically-Crosslinked Gelatin Hydrogel. Mater. Sci. Eng. C 2016, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kacarevic, Z.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of Tissue Engineering Scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.Y.; Gou, M.L.; Mei, D.Q.; Zhang, K.; Chen, S.C. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of Bioprinter Technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef]
- Holzl, K.; Lin, S.M.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.X.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.F.; Ho, V.B.; Gaston, J.D. 3D Bioprinting and Its Application to Military Medicine. Mil. Med. 2020, 185, E1510–E1519. [Google Scholar] [CrossRef] [PubMed]
- Applegate, M.B.; Coburn, J.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-Based Three-Dimensional Multiscale Micropatterning of Biocompatible Hydrogels for Customized Tissue Engineering Scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A Practical Guide to Rapid-Prototyping of PDMS-Based Microfluidic Devices: A Tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Park, K.Y.; Virumbrales-Munoz, M.; Beebe, D.J. Toward Improved In Vitro Models of Human Cancer. APL Bioeng. 2021, 5, 010902. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Mandenius, C.F. Fabrication of a Microfluidic Cell Culture Device Using Photolithographic and Soft Lithographic Techniques. Methods Mol. Biol. 2019, 1994, 227–233. [Google Scholar] [PubMed]
- Wang, Z.; Samanipour, R.; Kim, K. Organ-On-A-Chip Platforms for Drug Screening and Tissue Engineering. In Biomedical Engineering: Frontier Research and Converging Technologies; Springer International Publishing: New York, NY, USA, 2015; pp. 209–233. [Google Scholar]
- Jiang, B.; White, A.; Ou, W.Q.; Belleghem, S.V.; Stewart, S.; Shamul, J.H.; Rahaman, S.O.; Fisher, J.P.; He, X. Noncovalent Reversible Binding-Enabled Facile Fabrication of Leak-Free PDMS Microfluidic Devices without Plasma Treatment for Convenient Cell Loading and Retrieval. Bioact. Mater. 2022, 16, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Borok, A.; Laboda, K.; Bonyar, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Liu, S.C.; Zhang, Y.J. Direct Bonding of Polymer/Glass-Based Microfluidic Chips with Dry Film Photoresist. Microsyst. Technol. 2018, 24, 1659–1665. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Liu, H.H.; Zhang, B.J.; Han, Y.; Shen, C.; Lin, Q.; Chen, H. Development of Antibacterial and High Light Transmittance Bulk Materials: Incorporation and Sustained Release of Hydrophobic or Hydrophilic Antibiotics. Colloids Surf. B Biointerfaces 2016, 141, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, J. Implantable Liquid Metal-Based Flexible Neural Microelectrode Array and Its Application in Recovering Animal Locomotion Functions. J. Micromech. Microeng. 2017, 27, 104002. [Google Scholar] [CrossRef]
- Tavakoli, S.; Nemati, S.; Kharaziha, M.; Akbari-Alavijeh, S. Embedding CuO Nanoparticles in PDMS-SiO2 Coating to Improve Antibacterial Characteristic and Corrosion Resistance. Colloid Interface Sci. Commun. 2019, 28, 20–28. [Google Scholar] [CrossRef]
- Sala, F.; Ficorella, C.; Osellame, R.; Käs, J.A.; Vázquez, R.M. Microfluidic Lab-On-A-Chip for Studies of Cell Migration under Spatial Confinement. Biosensors 2022, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities-Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Tanyeri, M.; Tay, S. Viable Cell Culture in PDMS-Based Microfluidic Devices. Methods Cell Biol. 2018, 148, 3–33. [Google Scholar] [PubMed]
- Kuddannaya, S.; Bao, J.N.; Zhang, Y.L. Enhanced in Vitro Biocompatibility of Chemically Modified Poly(dimethylsiloxane) Surfaces for Stable Adhesion and Long-Term Investigation of Brain Cerebral Cortex Cells. ACS Appl. Mater. Interfaces 2015, 7, 25529–25538. [Google Scholar] [CrossRef] [PubMed]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent Advances in Nonbiofouling PDMS Surface Modification Strategies Applicable to Microfluidic Technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Natyanun, S.; Pussadee, N. Hydrophobicity Recovery of Polydimethylsiloxane Treated with Oxygen Plasma and Ion Implantation. J. Phys. Conf. Ser. 2018, 1144, 012110. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Baassiri, K.; Mahmoudi, Z.; Perumal, A.S.; Rajendran, K.; Rubies, G.M.; Nicolau, D.V. Hydrophobic Recovery of PDMS Surfaces in Contact with Hydrophilic Entities: Relevance to Biomedical Devices. Materials 2022, 15, 2313. [Google Scholar] [CrossRef] [PubMed]
- Deagen, M.E.; Chan, E.P.; Schadler, L.S.; Ullal, C.K. Corona Treatment for Nanotransfer Molding Adhesion. ACS Appl. Polym. Mater. 2019, 1, 997–1005. [Google Scholar]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, M.; Liu, J.C.; Xu, H.Q.; Zhang, Z.Y.; Xu, C.X. Effects of Corona Treatment on Cellular Attachment and Morphology on Polydimethylsiloxane Micropillar Substrates. JOM 2022, 74, 3408–3418. [Google Scholar] [CrossRef]
- Ozcam, A.E.; Efimenko, K.; Genzer, J. Effect of Ultraviolet/Ozone Treatment on the Surface and Bulk Properties of Poly(dimethyl Siloxane) and Poly(vinylmethyl Siloxane) Networks. Polymer 2014, 55, 3107–3119. [Google Scholar] [CrossRef]
- Raveendran, R.; Namboothiry, M.A.G. Surface-Treated Poly(dimethylsiloxane) as a Gate Dielectric in Solution-Processed Organic Field-Effect Transistors. ACS Omega 2018, 3, 11278–11285. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved Cell Adhesion under Shear Stress in PDMS Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sivarapatna, A.; Ghaedi, M.; Xiao, Y.; Han, E.; Aryal, B.; Zhou, J.; Fernandez-Hernando, C.; Qyang, Y.B.; Hirschi, K.K.; Niklason, L.E. Engineered Microvasculature in PDMS Networks Using Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Cell Transp. 2017, 26, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Microfluidic Device Fabrication Mediated by Surface Chemical Bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Chuah, Y.J.; Ang, W.T.; Zheng, N.; Wang, D.A. Optimization of a Polydopamine (PD)-Based Coating Method and Polydimethylsiloxane (PDMS) Substrates for Improved Mouse Embryonic Stem Cell (ESC) Pluripotency Maintenance and Cardiac Differentiation. Biomater. Sci. 2017, 5, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Xu, J.J.; Chen, H.Y. In-Situ Grafting Hydrophilic Polymer on Chitosan Modified Poly(dimethylsiloxane) Microchip for Separation of Biomolecules. J. Chromatogr. A 2007, 1147, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.W.; Ren, X.Q.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N. Surface Modification of Poly(dimethylsiloxane) Microfluidic Devices by Ultraviolet Polymer Grafting. Anal. Chem. 2002, 74, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Oh, J.M.; Kwon, K.W.; Huh, D. Polydopamine-Based Interfacial Engineering of Extracellular Matrix Hydrogels for the Construction and Long-Term Maintenance of Living Three-Dimensional Tissues. ACS Appl. Mater. Interfaces 2019, 11, 23919–23925. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial Flow Regulates the Angiogenic Response and Phenotype of Endothelial Cells in a 3D Culture Model. Lab Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Pauty, J.; Usuba, R.; Cheng, I.G.; Hespel, L.; Takahashi, H.; Kato, K.; Kobayashi, M.; Nakajima, H.; Lee, E.; Yger, F. A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs. EBioMedicine 2018, 27, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kato, K.; Ueyama, K.; Kobayashi, M.; Baik, G.; Yukawa, Y.; Suehiro, J.I.; Matsunaga, Y.Y. Visualizing Dynamics of Angiogenic Sprouting from a Three-Dimensional Microvasculature Model Using Stage-Top Optical Coherence Tomography. Sci. Rep. 2017, 7, 42426. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of Perfused, Functional Microvascular Tubes In Vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin Integrated with Perfusable Vascular Channels on a Chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Seo, T.S. Orthogonal Co-Cultivation of Smooth Muscle Cell and Endothelial Cell Layers to Construct in Vivo-Like Vasculature. Biomicrofluidics 2019, 13, 014115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Chen, M.J.; Fan, X.Q.; Zhou, H.F. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.; Datta, P.; Wu, Y.; Ozbolat, I.T. 3D Bioprinting for Modelling Vasculature. Microphysiol. Syst. 2018, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.J.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.T.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.J.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Sugioka, K.; Vazquez, R.M.; Osellame, R.; Kelemen, L.; Ormos, P. Three-Dimensional Femtosecond Laser Processing for Lab-On-A-Chip Applications. Nanophotonics 2018, 7, 613–634. [Google Scholar] [CrossRef]
- Rayner, S.G.; Howard, C.C.; Mandrycky, C.J.; Stamenkovic, S.; Himmelfarb, J.; Shih, A.Y.; Zheng, Y. Multiphoton-Guided Creation of Complex Organ-Specific Microvasculature. Adv. Healthc. Mater. 2021, 10, 2100031. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft Lithography for Microfluidics: A Review. BioChip J. 2008, 2, 1–11. [Google Scholar]
- Nguyen, D.H.T.; Stapleton, S.C.; Yang, M.T.; Cha, S.S.; Choi, C.K.; Galie, P.A.; Chen, C.S. Biomimetic Model to Reconstitute Angiogenic Sprouting Morphogenesis In Vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Hsu, Y.H.; Lee, A.P.; Hughes, C.C.W.; George, S.C. In Vitro Perfused Human Capillary Networks. Tissue Eng. Part C Methods 2013, 19, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Mayerich, D.; Slater, J.H. Image-Guided, Laser-Based Fabrication of Vascular-Derived Microfluidic Networks. J. Vis. Exp. 2017, 119, e55101. [Google Scholar] [PubMed]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.I. Blood Flow and Arterial Endothelial Dysfunction: Mechanisms and Implications. Comptes Rendus Phys. 2013, 14, 479–496. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In Vitro Modeling of the Microvascular Occlusion and Thrombosis That Occur in Hematologic Diseases Using Microfluidic Technology. J. Clin. Investig. 2012, 122, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chang, S.F.; Lee, P.L.; Chang, K.; Chen, L.J.; Usami, S.; Chien, S.; Chiu, J.J. Neutrophils, Lymphocytes, and Monocytes Exhibit Diverse Behaviors in Transendothelial and Subendothelial Migrations under Coculture with Smooth Muscle Cells in Disturbed Flow. Blood 2006, 107, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Dorweiler, B.; Torzewski, M.; Dahm, M.; Ochsenhirt, V.; Lehr, H.A.; Lackner, K.J.; Vahl, C.F. A Novel in Vitro Model for the Study of Plaque Development in Atherosclerosis. Thromb. Haemost. 2006, 95, 182–189. [Google Scholar] [PubMed]
- Dereli-Korkut, Z.; Akaydin, H.D.; Ahmed, A.H.R.; Jiang, X.J.; Wang, S.H. Three Dimensional Microfluidic Cell Arrays for ex Vivo Drug Screening with Mimicked Vascular Flow. Anal. Chem. 2014, 86, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion Bioprinting of Hydroxyethylcellulose-Based Bioink for Cervical Tumor Model. Carbohydr. Polym. 2021, 260, 117793. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal Tumor-On-A-Chip System: A 3D Tool for Precision Onco-Nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, Y.F.; Xu, L.T.; Li, W.L.; Chen, Y.L.; Zheng, S.N.; Dai, R.; Liu, J. Recapitulation of Dynamic Nanoparticle Transport around Tumors Using a Triangular Multi-Chamber Tumor-On-A-Chip. Lab Chip 2022, 22, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.F.; Voigt, E.E.; Szot, C.S.; Freeman, J.W.; Vlachos, P.P.; Rylander, M.N. Three-Dimensional Microfluidic Collagen Hydrogels for Investigating Flow-Mediated Tumor-Endothelial Signaling and Vascular Organization. Tissue Eng. Part C Methods 2014, 20, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Prabhu, A. In Vitro Blood Brain Barrier Models: Molecular Aspects and Therapeutic Strategies in Glioma Management. Curr. Res. Transl. Med. 2022, 71, 103376. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; de Menezes, L.R.; do Nascimento, D.F.; Souza, D.H.S.; Reynaud, F.; Marques, M.F.V.; Tavares, M.I.B. Polymeric Nanoparticles Assembled with Microfluidics for Drug Delivery across the Blood-Brain Barrier. Eur. Phys. J. Spec. Top. 2016, 225, 779–795. [Google Scholar] [CrossRef]
- Yeon, J.H.; Na, D.; Choi, K.; Ryu, S.W.; Choi, C.; Park, J.K. Reliable Permeability Assay System in a Microfluidic Device Mimicking Cerebral Vasculatures. Biomed. Microdevices 2012, 14, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Donnes, P.; Sanchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-On-Chips at the Frontiers of Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Sun, L.Y.; Wang, Y.; Cai, L.J.; Zhang, Z.H.; Shang, Y.X.; Zhao, Y.J. A Biomimetic Human Lung-On-A-Chip with Colorful Display of Microphysiological Breath. Adv. Mater. 2022, 34, 2108972. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Bernabini, C.; Smith, A.S.T.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.Q.; Santhanam, N.; et al. Multi-Organ Toxicity Demonstration in a Functional Human in Vitro System Composed of Four Organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mehrotra, S.; Zare-Eelanjegh, E.; Rodrigues, R.O.; Akbarinejad, A.; Ge, D.; Amato, L.; Kiaee, K.; Fang, Y.C.; Rosenkranz, A.; et al. A Heart-Breast Cancer-On-A-Chip Platform for Disease Modeling and Monitoring of Cardiotoxicity Induced by Cancer Chemotherapy. Small 2021, 17, e2004258. [Google Scholar] [CrossRef] [PubMed]
- Fritschen, A.; Blaeser, A. Biosynthetic, Biomimetic, and Self-Assembled Vascularized Organ-On-A-Chip Systems. Biomaterials 2021, 268, 120556. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-On-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [PubMed]
- Bogorad, M.I.; Destefano, J.; Wong, A.D.; Searson, P.C. Tissue-Engineered 3D Microvessel and Capillary Network Models for the Study of Vascular Phenomena. Microcirculation 2017, 24, e12360. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Albers, H.J.; Passier, R.; Mummery, C.L.; van den Berg, A.; Orlova, V.V.; van der Meer, A.D. Advanced in Vitro Models of Vascular Biology: Human Induced Pluripotent Stem Cells and Organ-On-Chip Technology. Adv. Drug Deliv. Rev. 2019, 140, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Meshkin, H.; Cornelius, V.A.; Eleftheriadou, M.; Potel, K.N.; Setyaningsih, W.A.W.; Margariti, A. Vascular Organoids: Unveiling Advantages, Applications, Challenges, and Disease Modelling Strategies. Stem Cell Res. Ther. 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Ou, C.W.; Chen, A.Z.; Yang, Z.L. Advances in Hydrogel-Based Vascularized Tissues for Tissue Repair and Drug Screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
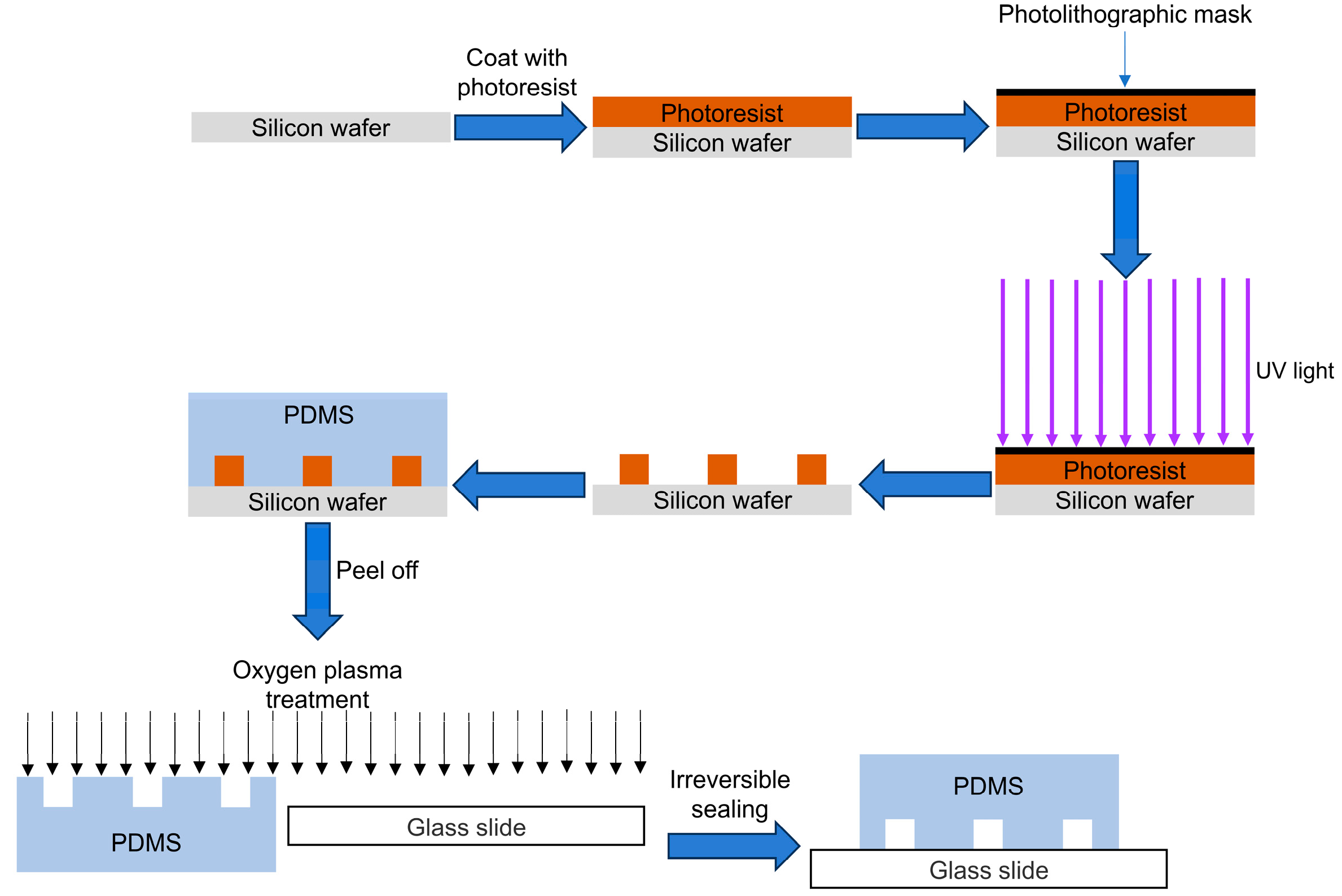
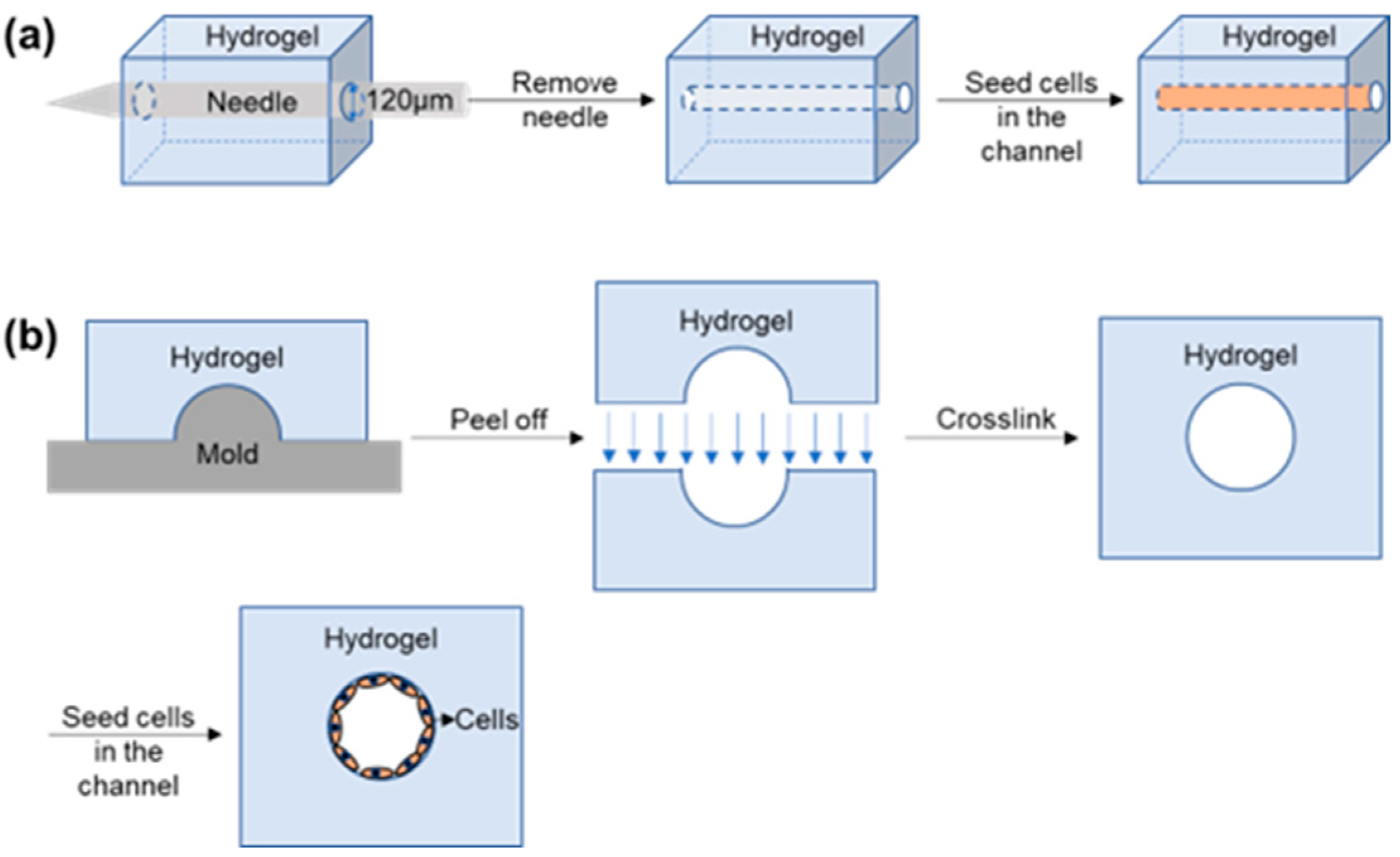


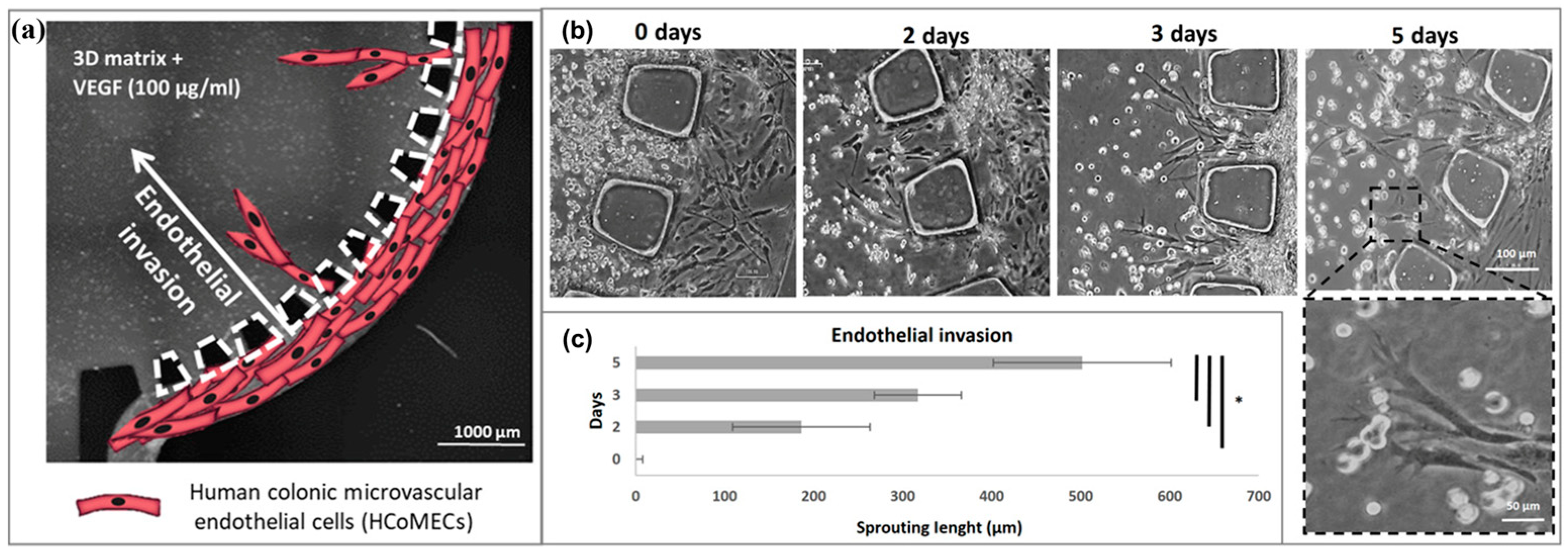
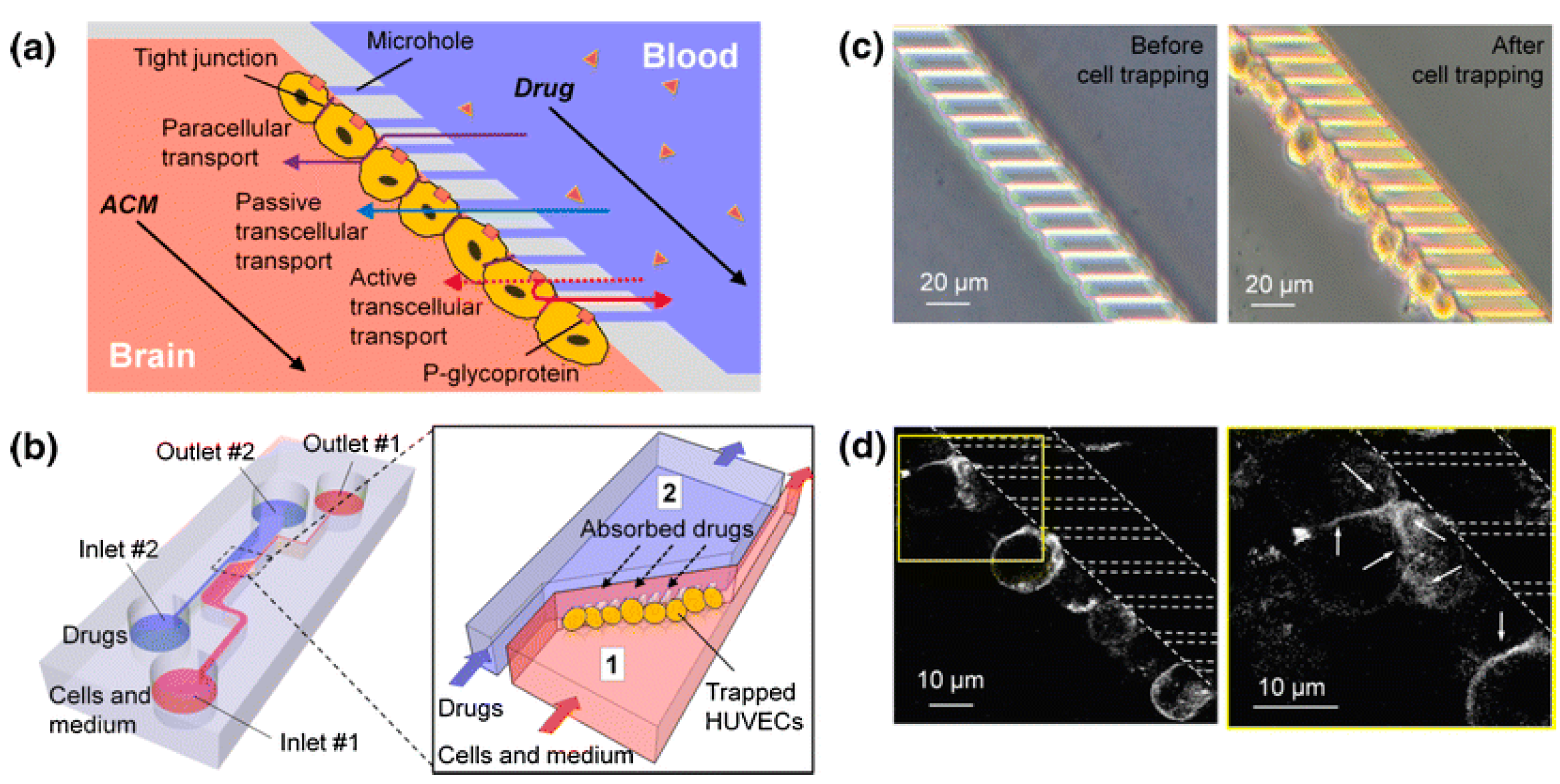
| Traditional In Vitro Vascular Models | Advantages | Disadvantages |
|---|---|---|
| Animal models | Significant contribution to drug development and safety. | Differences in physiological environments between different species; one-sidedness of animal model research reports; not feasible to accurately model complex vascular structures; time-consuming, cumbersome, and raises ethical concerns. |
| In vitro two- dimensional models | Low price, easy operation, and good compatibility with high-resolution microscopes. | Usually static and difficult to reproduce the microenvironment of fluid flow in vivo. |
| In Vitro Vascular Model Construction Methods | Advantages | Disadvantages | |
|---|---|---|---|
| Based on microfluidic technology | Photolithography and soft lithography | Mature technology (lithography). Low cost, convenient, effective, and short in duration (soft lithography). | Time-consuming and costly, difficult to control surface chemical properties, and limited to photosensitive materials (photolithography); vascular channels have a rectangular cross-section. |
| Self-assembly | Similar in function and morphology to blood vessels in the body; precise control of key parameters to regulate the morphological characteristics of blood vessels. | Creates random and unpredictable vascular networks; complexity and low throughput; more time-consuming vascular formation. | |
| Non- microfluidics-based methods | Template | Low cost and easy to operate, the channels produced have a similar cross-section to physiological structures. | High requirements for the mechanical properties of biomaterials; prone to deformation; only used to construct simple vascular structures. |
| 3D bioprinting | Inexpensive and convenient, suitable for most high-viscosity bioinks; widely used (extrusion bioprinting). High cell viability, fast printing speed, high resolution, low cost, and compatibility with various bioinks (inkjet bioprinting). Ability to print high-viscosity bioinks, high cell viability, high resolution, and fast printing speed (light-assisted bioprinting). | Low resolution and cell viability (extrusion bioprinting). Nozzle clogging is prone to occur, requiring bioinks with low viscosity and low cell density (inkjet bioprinting). Operated in a sterile environment; complex, with high costs; not yet widely used (light-assisted bioprinting). | |
| Laser degradation and laser cavitation molding | High resolution, easy to operate, and not limited by complex 3D shapes; guarantees a sterile environment; low impact on cell viability. | High cost, with limitations on certain biomaterials. | |
| PDMS Surface Modification Strategy | Principle | Advantages | Disadvantages | |
|---|---|---|---|---|
| Surface activation modification | Gas plasma | Introduce polar functional groups on the surface of PDMS to increase its surface hydrophilicity. | Short processing time and simple operation. | Longer processing time, and the surface hydrophilicity may decrease or even disappear over time. |
| Corona discharge | Simple and economical. | |||
| UV/ozone treatment | Deeper surface modification; mild process; lower cost. | |||
| Physical adsorption | Protein coating technology | Coating the PDMS surface with extracellular matrix proteins to increase hydrophilicity. | Simple, fast, and effective. | Poor stability makes it difficult to construct a uniform protein layer. |
| Surface chemical modification | Silanization | Utilizing the reaction between alkoxy groups and PDMS substrate to generate Si-O-Si covalent bonds, in order to introduce hydroxyl groups, amino groups, thiol groups, or carboxyl groups. | Higher stability and reproducibility, with the ability to control the parameters of the polymerization process. | Low modification efficiency and uneven surface modification. |
| Polymer surface grafting | Grafting polymer monomers onto the PDMS surface to create a hydrophilic surface. | |||
| Model Construction Methods | First Author/Year of Publication | Main Conclusion | Applications | Advantages and Disadvantages |
|---|---|---|---|---|
| Template | Nguyen/2013 | This model was used to study the morphological mechanisms of a vasculogenesis and the effects of vasculogenesis inhibitors on this process. | Endothelial structure and barrier function; mechanisms and related factors of angiogenesis and vasculogenesis; complex vascular structures such as cancer and blood–brain barrier; drug screening and evaluation. | Simple operation and structure; easy to construct circular channels. |
| Soft lithography and self-assembly | Moya/2013 | Exposure to higher interstitial flow promotes rapid formation of a vascular network, demonstrating perfusion of dynamic human capillary networks in a microphysical system. This system has potential wide-ranging applications in diagnosis and treatment. | Endothelial structure and barrier function; mechanisms and related factors of angiogenesis and vasculogenesis; complex vascular structures such as cancer and blood–brain barrier; organ-on-a-chip; drug screening and evaluation. | Physiological functions are similar to those of blood vessels in the human body; difficult to control the direction of vascular network growth. |
| Laser degradation | Heintz/2017 | By utilizing image- guided laser ablation techniques, a more complex human vascular network can be directly generated through selective photothermal degradation in cell-loaded hydrogels. | Endothelial structure and barrier function; complex vascular structures such as cancer and blood–brain barrier; drug screening and evaluation. | High resolution; expensive; time-consuming. |
| Three-dimensional bioprinting | Gao/2018 | The constructed vessels possess normal vascular functions such as selective permeability, antiplatelet/leukocyte adhesion, and response to physiological shear forces. | Endothelial structure and barrier function; complex vascular structures such as cancer and blood–brain barrier; drug screening and evaluation. | High precision and biological activity; complex printing process. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Cheng, P.; Ying, G.; Ou, Z. Research Progress in the Construction and Application of In Vitro Vascular Models. Appl. Sci. 2024, 14, 6780. https://doi.org/10.3390/app14156780
He Z, Cheng P, Ying G, Ou Z. Research Progress in the Construction and Application of In Vitro Vascular Models. Applied Sciences. 2024; 14(15):6780. https://doi.org/10.3390/app14156780
Chicago/Turabian StyleHe, Zhenyu, Pengpeng Cheng, Guoqing Ying, and Zhimin Ou. 2024. "Research Progress in the Construction and Application of In Vitro Vascular Models" Applied Sciences 14, no. 15: 6780. https://doi.org/10.3390/app14156780





