A Feature Selection-Incorporated Simulation Study to Reveal the Effect of Calcium Ions on Cardiac Repolarization Alternans during Myocardial Ischemia
Abstract
:1. Introduction
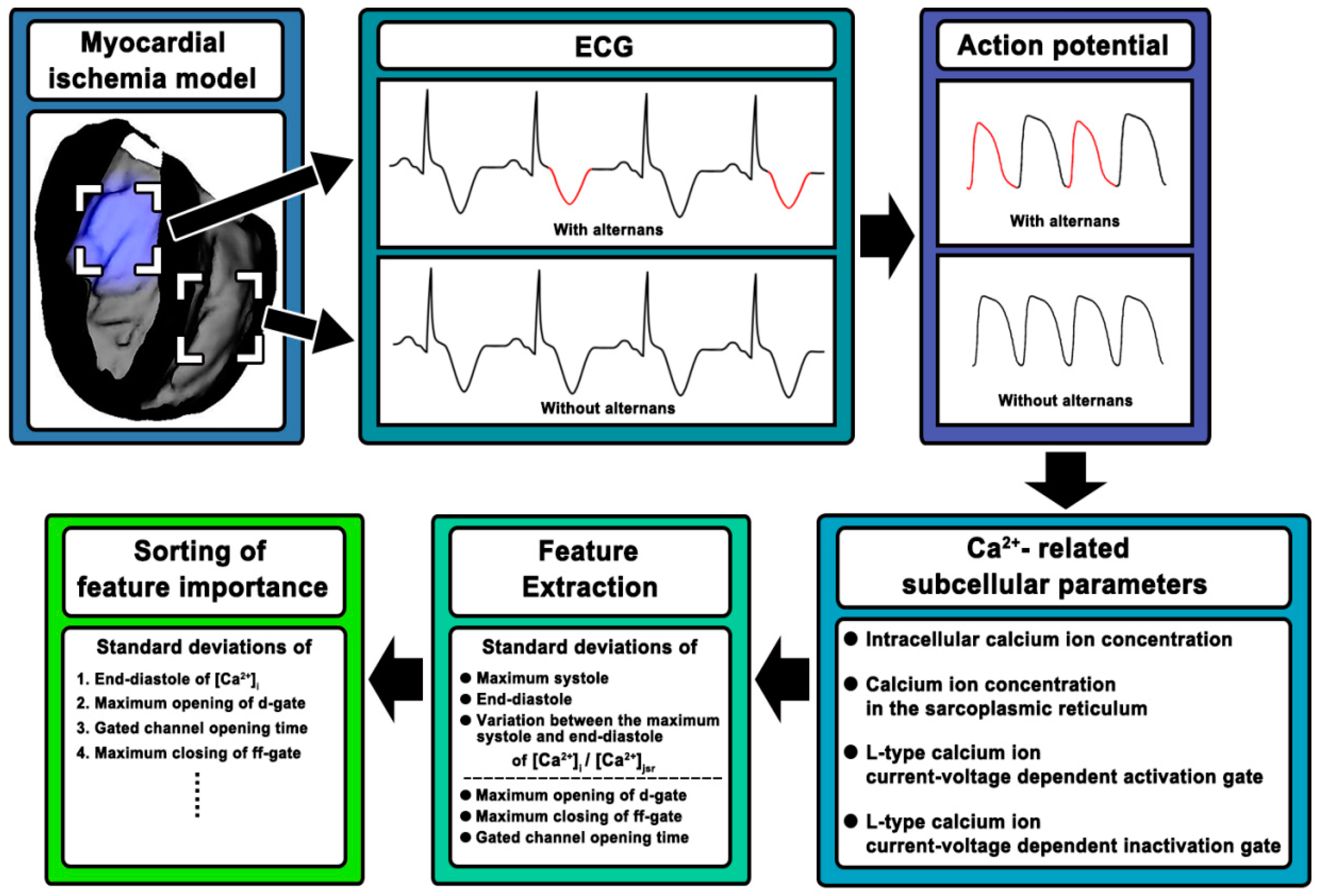
2. Materials and Methods
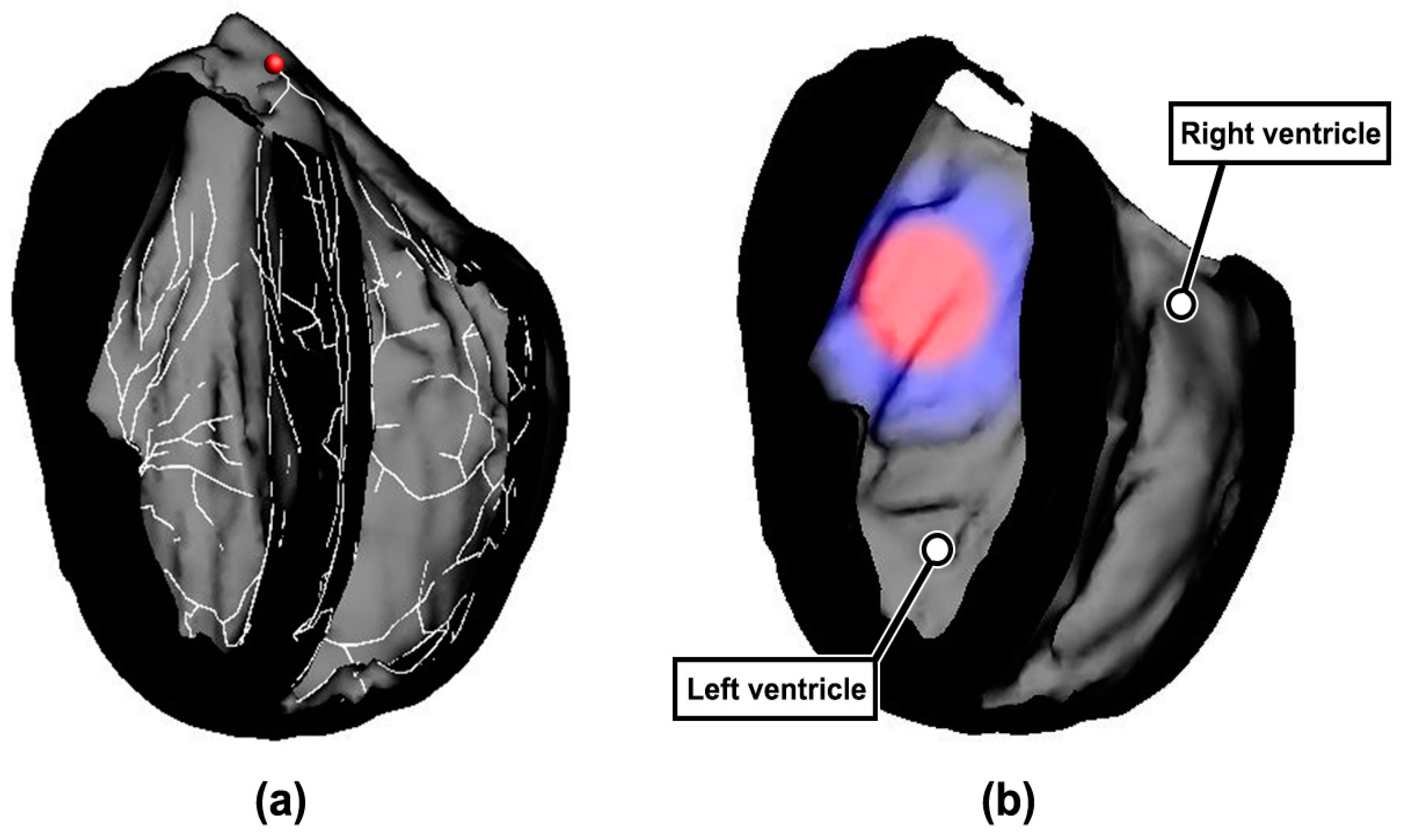
2.1. Construction of Rabbit Ventricular Model with MI
2.2. Simulation Strategy
2.3. Ca2+-Related Subcellular Parameters
2.4. Feature Extraction and Sorting
3. Results
3.1. Feature Extraction
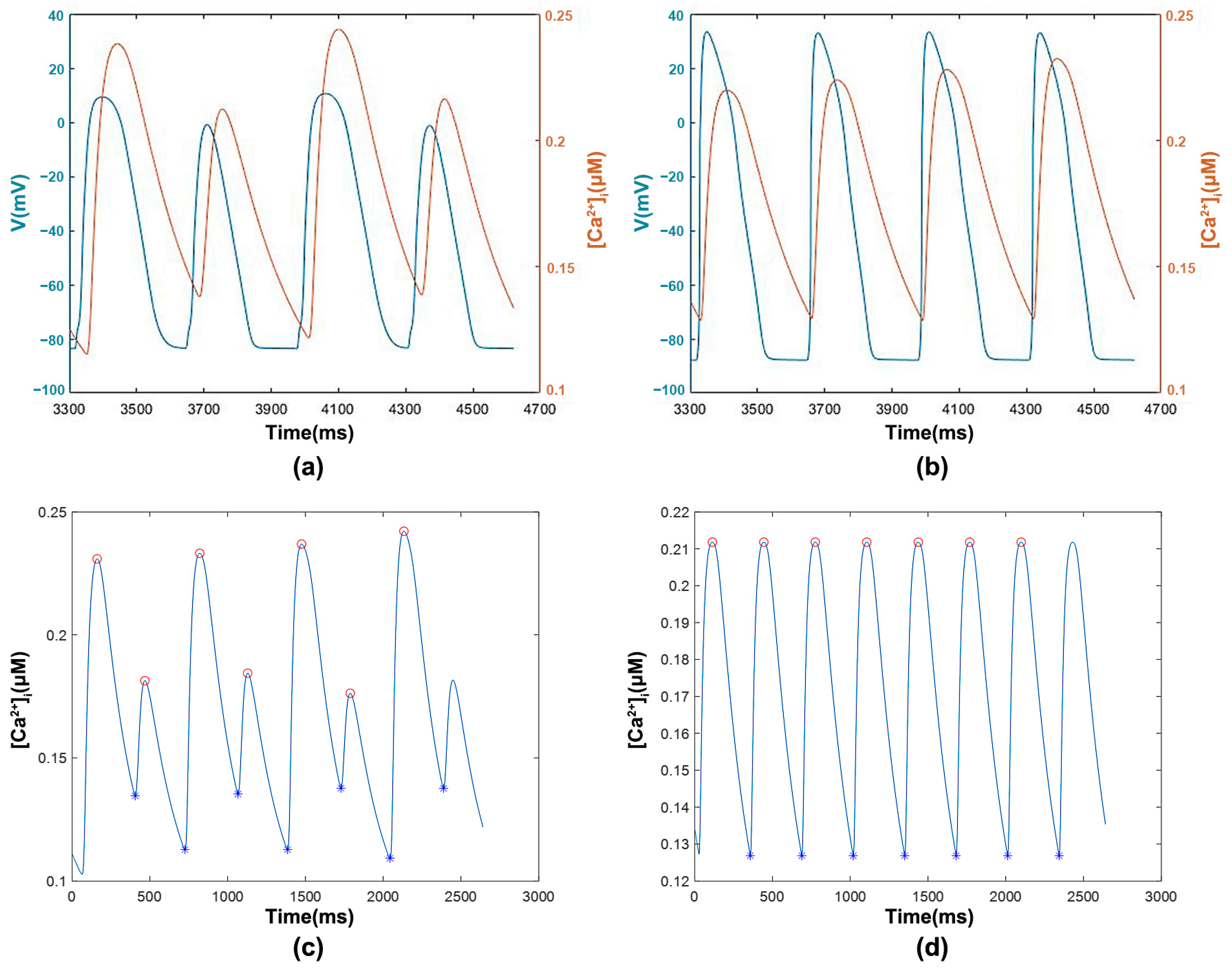
3.1.1. [Ca2+]i with Transmembrane Potential and Features
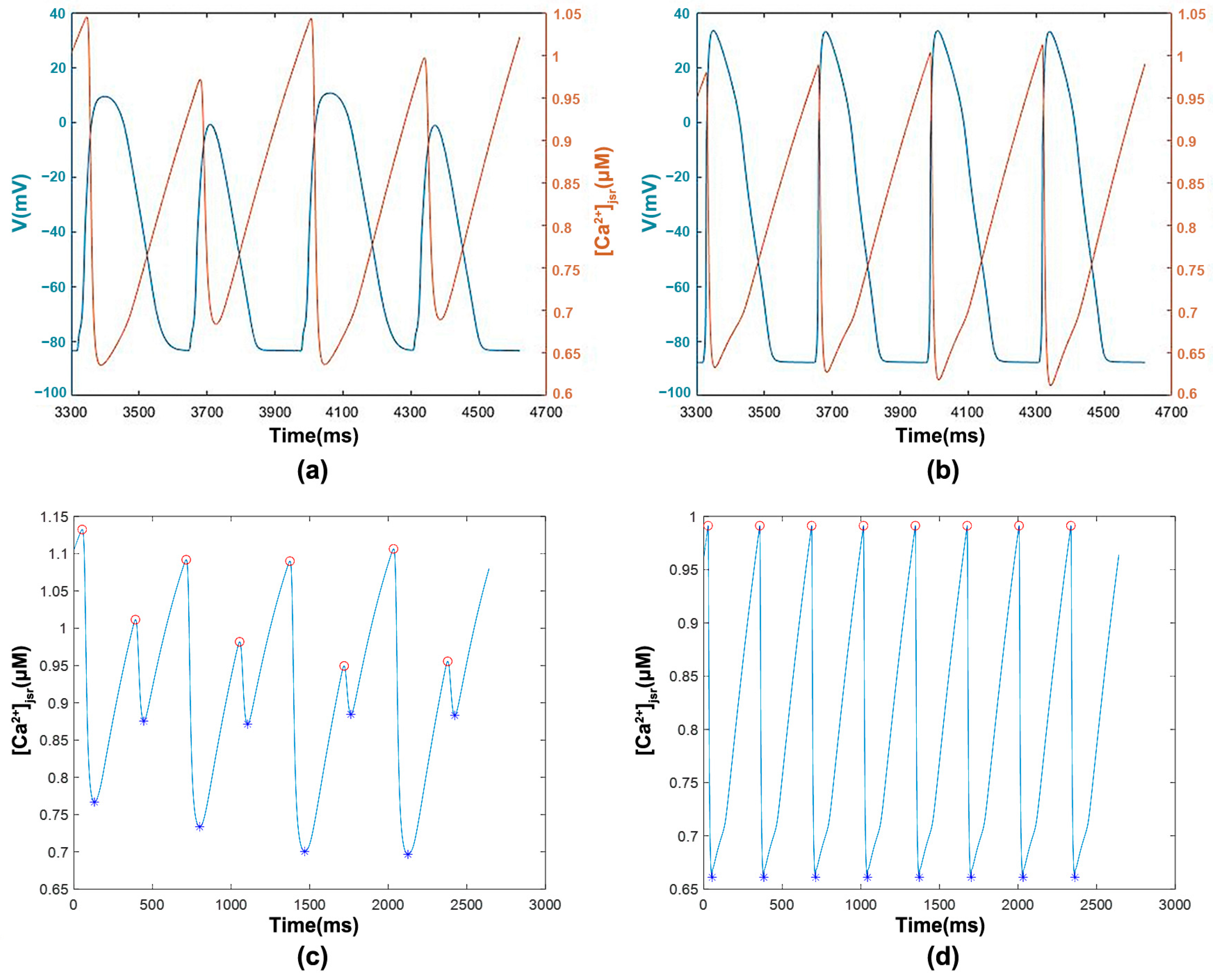
3.1.2. [Ca2+]jsr with Transmembrane Potential and Features
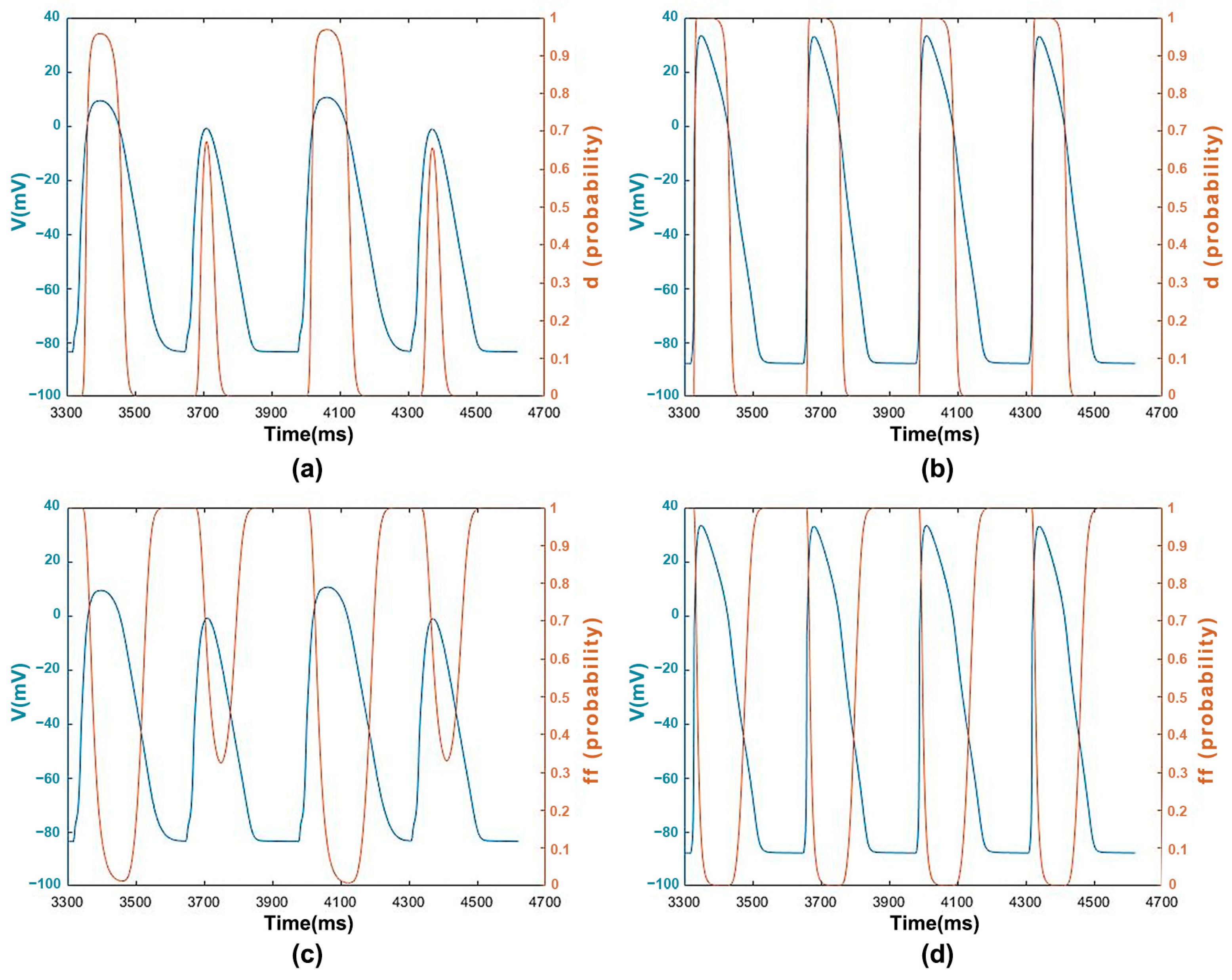
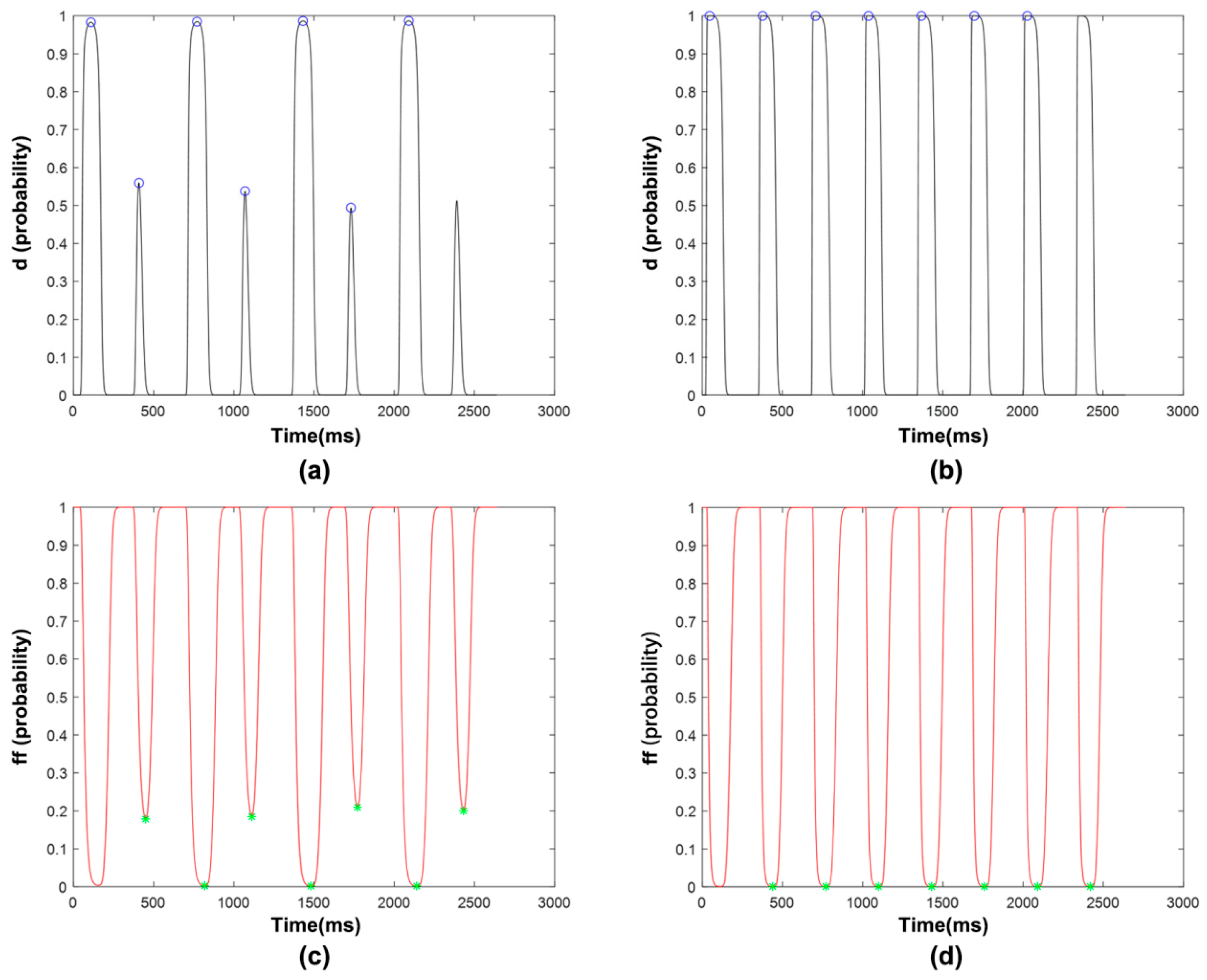
3.1.3. ICaL Voltage-Dependent Gated Channels and Features
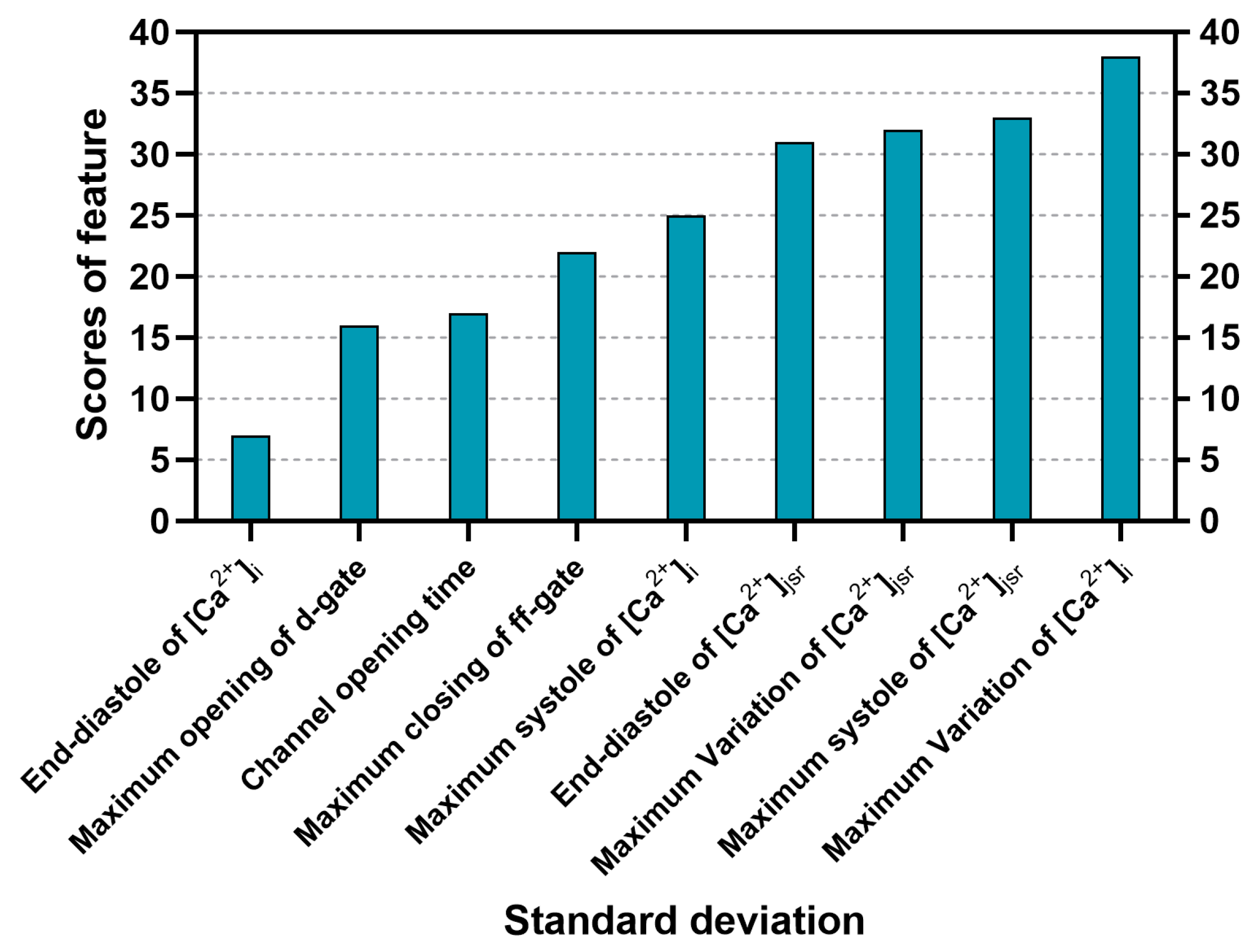
3.2. Sorting of Feature Importance
4. Discussion
4.1. The Influences of [Ca2+]i and ICaL
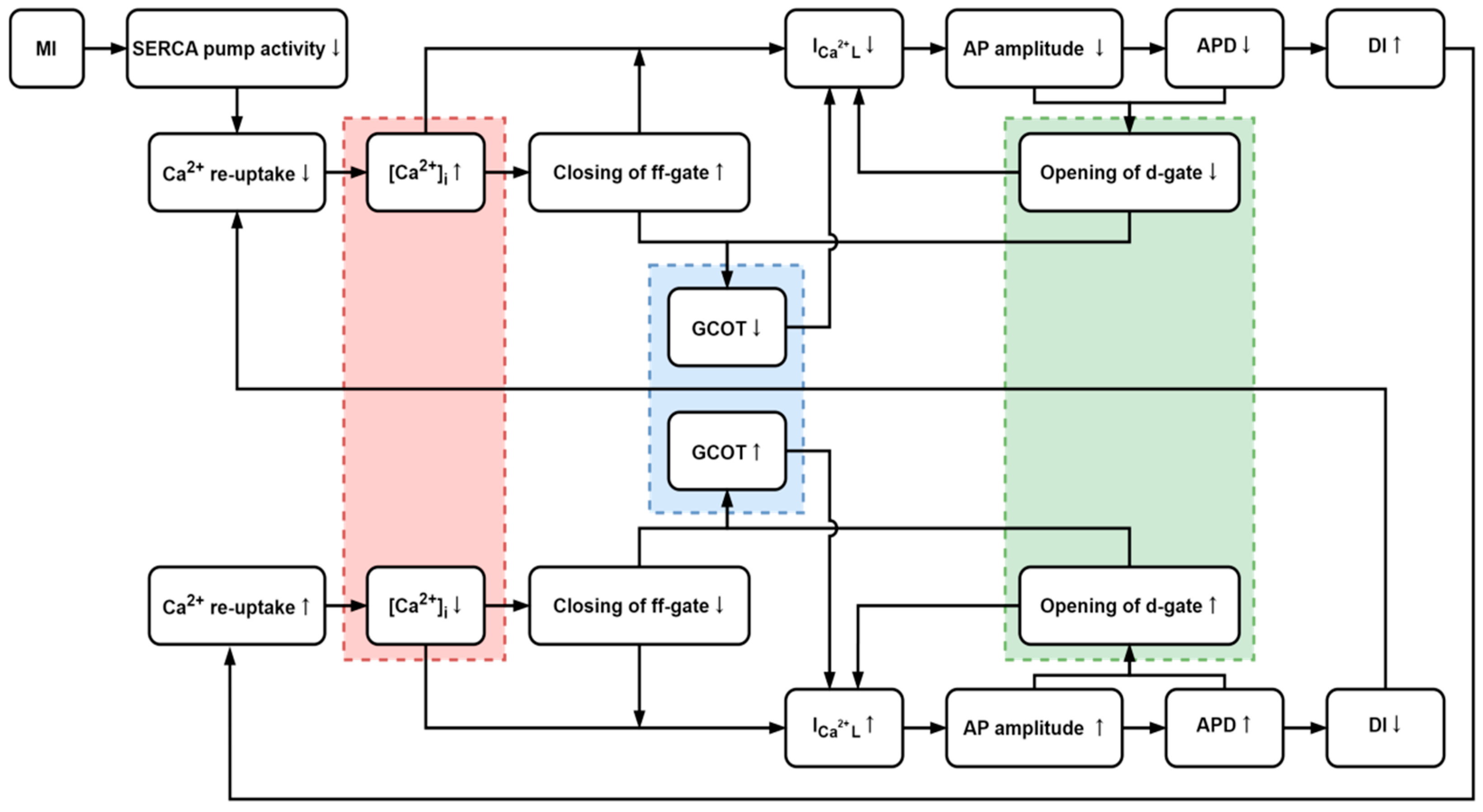
4.2. Generation of CRA
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Expression | Function | |
|---|---|---|
| Variance threshold | n = Number of elements for a given dataset = The average of elements | Calculate the variance of each feature and remove features with variance less than a preset threshold, which are incapable of discriminating samples. |
| Chi-square test | = Observed value = Theoretical value k = Number of observed value | Calculate the difference between observed value and theoretical value, larger result implies stronger correlation between features and classification. |
| MIB | X, Y = Random variables = Joint probability distribution , = Marginal distributions | Assess correlations between random variables, larger mutual information value referring to feature and label implies stronger correlation, accordingly more important features. |
| Fisher score | = Between-class variance of the k-th feature = Intra-class variance of the k-th feature = Number of samples for a given dataset , samples for the k-th feature for the k-th feature = The average value of all classes of sample for the k-th feature | Assess correlations between a feature and sample from the same and the different class. Larger Fisher score proves the feature to be beneficial for classification and more important, in which the variance is small between the feature and sample from the same class, and large between it and sample from the different class. |
| CFS | = Number of features = The average correlation between fea- ture and class = The average correlation between features | Assess correlations between feature subsets and classes to screen the subset that is highly related to a class but not related to each other. The feature combination with the largest is the optimal feature subset. |
| Relif | (near-miss) belonging to the different class for sample (near-hit) belonging to the same class for sample | Appley correlation statistics to weigh the ability of feature to discriminate between nearest neighbor samples. If a random sample is closer to its near-hit than near-miss on a particular feature, then the feature weight is increased as it benefits the classification, and vice versa. |
| MRMR | = The maximum relevance = The minimum redundancy h = Target class | Filter out features with high relevance to the class and low feature redundancy. The MRMR value of a feature is obtained by integrating the maximum relevance and the minimum redundancy through addition or multiplication. Larger implies more important feature. |
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Trew, M.L.; Zgierski-Johnston, C.M. Cardiac Conduction Velocity, Remodeling and Arrhythmogenesis. Cells 2021, 10, 2923. [Google Scholar] [CrossRef]
- Gräni, C.; Benz, D.C.; Gupta, S.; Windecker, S.; Kwong, R.Y. Sudden Cardiac Death in Ischemic Heart Disease: From Imaging Arrhythmogenic Substrate to Guiding Therapies. JACC Cardiovasc. Imaging 2020, 13, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Merchant, F.M.; Kassab, M.B.; Sana, F.; Moazzami, K.; Sayadi, O.; Singh, J.P.; Heist, E.K.; Armoundas, A.A. Cardiac Alternans: Mechanisms and Clinical Utility in Arrhythmia Prevention. J. Am. Hear. Assoc. 2019, 8, e013750. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Karma, A.; Shiferaw, Y.; Chen, P.S.; Garfinkel, A.; Qu, Z. From pulsus to pulseless: The saga of cardiac alternans. Circ. Res. 2006, 98, 1244–1253. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, T.; Li, S.; Zhang, P. The Relationship between T-Wave Alternans and Adverse Cardiac Events in Patients with Congenital Long QT Syndrome: A Systematic Review and Meta-Analysis. Congenit. Heart Dis. 2022, 17, 557–567. [Google Scholar] [CrossRef]
- Carmeliet, E. Cardiac Ionic Currents and Acute Ischemia: From Channels to Arrhythmias. Physiol. Rev. 1999, 79, 917–1017. [Google Scholar] [CrossRef]
- Rakisheva, A.; Marketou, M.; Klimenko, A.; Troyanova-Shchutskaia, T.; Vardas, P. Hyperkalemia in heart failure: Foe or friend? Clin. Cardiol. 2020, 43, 666–675. [Google Scholar] [CrossRef]
- Kormos, A.; Nagy, N.; Acsai, K.; Váczi, K.; Ágoston, S.; Pollesello, P.; Levijoki, J.; Szentandrássy, N.; Papp, J.G.; Varró, A.; et al. Efficacy of selective NCX inhibition by ORM-10103 during simulated ischemia/reperfusion. Eur. J. Pharmacol. 2014, 740, 539–551. [Google Scholar] [CrossRef]
- Szlovák, J.; Tomek, J.; Zhou, X.; Tóth, N.; Veress, R.; Horváth, B.; Szentandrássy, N.; Levijoki, J.; Papp, J.G.; Herring, N.; et al. Blockade of sodium-calcium exchanger via ORM-10962 attenuates cardiac alternans. J. Mol. Cell. Cardiol. 2021, 153, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.E.; O’neill, S.C.; Eisner, D.A. Sarcoplasmic Reticulum Calcium Content Fluctuation Is the Key to Cardiac Alternans. Circ. Res. 2004, 94, 650–656. [Google Scholar] [CrossRef]
- Picht, E.; DeSantiago, J.; Blatter, L.A.; Bers, D.M. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ. Res. 2006, 99, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Myles, R.C.; De Jesus, N.M.; Ohlendorf, A.K.; Bers, D.M.; Ripplinger, C.M. Optical mapping of sarcoplasmic reticulum Ca 2+ in the intact heart. Circ. Res. 2014, 114, 1410–1421. [Google Scholar] [CrossRef]
- Wang, L.; Myles, R.C.; Lee, I.-J.; Bers, D.M.; Ripplinger, C.M. Role of Reduced Sarco-Endoplasmic Reticulum Ca2+-ATPase Function on Sarcoplasmic Reticulum Ca2+ Alternans in the Intact Rabbit Heart. Front. Physiol. 2021, 12, 656516. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Duker, G.; Carlsson, L. Model systems for the discovery and development of antiarrhythmic drugs. Prog. Biophys. Mol. Biol. 2008, 98, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 2000, 525, 285–298. [Google Scholar] [CrossRef]
- Geng, Z.; Jin, L.; Huang, Y.; Wu, X. Rate dependence of early afterdepolarizations in the His-Purkinje system: A simulation study. Comput. Methods Programs Biomed. 2022, 217, 106665. [Google Scholar] [CrossRef]
- Geng, Z.; Jin, L.; Zhu, H.; Wang, J.; Wu, X. Effects of increased extracellular potassium concentration induced by ischemia on the vulnerability of ventricular arrhythmias and the regularity of related ventricular tachycardia. Appl. Sci. 2021, 11, 2189. [Google Scholar] [CrossRef]
- Costabal, F.S.; Hurtado, D.E.; Kuhl, E. Generating Purkinje networks in the human heart. J. Biomech. 2016, 49, 2455–2465. [Google Scholar] [CrossRef]
- O’Hara, T.; Virág, L.; Varró, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Ten Tusscher, K.H.W.J.; Noble, D.; Noble, P.J.; Panfilov, A. A model for human ventricular tissue. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Keener, J.P.; Bogar, K. A numerical method for the solution of the bidomain equations in cardiac tissue. Chaos Interdiscip. J. Nonlinear Sci. 1998, 8, 234–241. [Google Scholar] [CrossRef]
- Deng, D.; Arevalo, H.J.; Prakosa, A.; Callans, D.J.; Trayanova, N.A. A feasibility study of arrhythmia risk prediction in patients with myocardial infarction and preserved ejection fraction. Europace 2016, 18, iv60–iv66. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.A.; Aksnes, G. Myocardial potassium loss and cell depolarisation in ischaemia and hypoxia. Cardiovasc. Res. 1995, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Michailova, A.; Lorentz, W.; McCulloch, A. Modeling transmural heterogeneity of K-ATP current in rabbit ventricular myo-cytes. Am. J. Physiol.-Cell Physiol. 2007, 293, C542–C557. [Google Scholar] [CrossRef]
- Qu, Z.; Xie, Y.; Garfinkel, A.; Weiss, J.N. T-wave alternans and arrhythmogenesis in cardiac diseases. Front. Physiol. 2010, 1, 154. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A. Correlation-based Feature Selection for Machine Learning. Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, April 1999. [Google Scholar]
- Gu, Q.; Li, Z.; Han, J. Generalized fisher score for feature selection. In Proceedings of the 27th Conference on Uncertainty in Artificial Intelli-gence, Barcelona, Spain, 14–17 July 2011. [Google Scholar] [CrossRef]
- Urbanowicz, R.J.; Meeker, M.; La Cava, W.; Olson, R.S.; Moore, J.H. Relief-based feature selection: Introduction and review. J. Biomed. Inform. 2018, 85, 189–203. [Google Scholar] [CrossRef]
- Ding, C.; Peng, H. Minimum redundancy feature selection from microarray gene expression data. J. Bioinform. Comput. Biol. 2005, 3, 185–205. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.J.; Lai, S.; Schär, M.; Wu, K.C.; Steinberg, A.M.; Wei, A.-C.; Anderson, M.E.; Tomaselli, G.F.; Gerstenblith, G.; Bottomley, P.A.; et al. Myocardial ATP depletion detected noninvasively predicts sudden cardiac death risk in patients with heart failure. JCI Investig. 2022, 7, e157557. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Bers, D.M. Effect of intracellular Ca2+ and action potential duration on L-type Ca2+ channel inactivation and recovery from inactivation in rabbit cardiac myocytes. Am. J. Physiol. Circ. Physiol. 2007, 293, H563–H573. [Google Scholar] [CrossRef]
- Fletcher, A. Action potential: Generation and propagation. Anaesth. Intensiv. Care Med. 2019, 20, 243–247. [Google Scholar] [CrossRef]
- Alpert, N.M.; Guehl, N.; Ptaszek, L.; Pelletier-Galarneau, M.; Ruskin, J.; Mansour, M.C.; Wooten, D.; Ma, C.; Takahashi, K.; Zhou, Y.; et al. Quantitative in vivo mapping of myocardial mitochondrial membrane potential. PLoS ONE 2018, 13, e0190968. [Google Scholar] [CrossRef] [PubMed]
- Gauvrit, S.; Bossaer, J.; Lee, J.; Collins, M.M. Modeling Human Cardiac Arrhythmias: Insights from Zebrafish. J. Cardiovasc. Dev. Dis. 2022, 9, 13. [Google Scholar] [CrossRef]
- Sutanto, H.; Heijman, J. Integrative Computational Modeling of Cardiomyocyte Calcium Handling and Cardiac Arrhythmias: Current Status and Future Challenges. Cells 2022, 11, 1090. [Google Scholar] [CrossRef]
- Agrawal, A.; Wang, K.; Polonchuk, L.; Cooper, J.; Hendrix, M.; Gavaghan, D.J.; Mirams, G.R.; Clerx, M. Models of the cardiac L-type calcium current: A quantitative review. WIREs Mech. Dis. 2023, 15, e1581. [Google Scholar] [CrossRef]
- Krishna, A.; Sun, L.; Valderrábano, M.; Palade, P.T.; Clark, J.W. Modeling CICR in rat ventricular myocytes: Voltage clamp studies. Theor. Biol. Med. Model. 2010, 7, 1–66. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, K.; Geng, Z.; Yang, Y.; Yan, S.; Hu, B.; Wu, X. A Feature Selection-Incorporated Simulation Study to Reveal the Effect of Calcium Ions on Cardiac Repolarization Alternans during Myocardial Ischemia. Appl. Sci. 2024, 14, 6789. https://doi.org/10.3390/app14156789
Gu K, Geng Z, Yang Y, Yan S, Hu B, Wu X. A Feature Selection-Incorporated Simulation Study to Reveal the Effect of Calcium Ions on Cardiac Repolarization Alternans during Myocardial Ischemia. Applied Sciences. 2024; 14(15):6789. https://doi.org/10.3390/app14156789
Chicago/Turabian StyleGu, Kaihao, Zihui Geng, Yuwei Yang, Shengjie Yan, Bo Hu, and Xiaomei Wu. 2024. "A Feature Selection-Incorporated Simulation Study to Reveal the Effect of Calcium Ions on Cardiac Repolarization Alternans during Myocardial Ischemia" Applied Sciences 14, no. 15: 6789. https://doi.org/10.3390/app14156789





