Effects of Whole-Body Vibration Training on Improving Physical Function, Cognitive Function, and Sleep Quality for Older People with Dynapenia in Long-Term Care Institutions: A Randomized Controlled Study
Abstract
:1. Introduction
Aim
2. Methods
2.1. Research Design
2.2. Participants
2.3. Research Tools
2.3.1. Body Composition
2.3.2. Walking Speed Measurement
2.3.3. Demographic Characteristics
2.4. Physical Function
2.4.1. HGS
2.4.2. IADLs
2.5. Cognitive Function
2.6. Sleep Quality
2.7. Whole Body Vibration Training
2.8. Statistical Analysis
2.9. Ethical Considerations
3. Results
3.1. Homogeneity Testing of Basic Data, Physical Function, Cognitive Function, and Sleep Quality in the Experimental and Control Groups
3.2. Analysis of Physical Function, Cognitive Function, and Sleep Quality before and after the Intervention in the Experimental and Control Groups
3.3. Analysis of Demographic Characteristics, Physical Performance, Cognitive Function, and Sleep Quality Using the GEE
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 73rd World Health Assembly Decisions. 2020. Available online: https://www.who.int/news/item/07-08-2020-73rd-world-health-assembly-decisions (accessed on 5 May 2020).
- Ministry of the Interior. Population with the Latest Statistical Indicators. Available online: https://www.moi.gov.tw/cp.aspx?n=602&ChartID=S0401 (accessed on 5 May 2021).
- Kobayashi, K.; Imagama, S.; Ando, K.; Nakashima, H.; Machino, M.; Morozumi, M.; Kanbara, S.; Ito, S.; Inoue, T.; Yamaguchi, H.; et al. Dynapenia and physical performance in community dwelling elderly people in Japan. Nagoya J. Med. Sci. 2020, 82, 415–424. [Google Scholar]
- Noh, H.M.; Park, Y.S. Handgrip strength, dynapenia, and mental health in older Koreans. Sci. Rep. 2020, 10, 4004. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.-J.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Physical function-derived cut-points for the diagnosis of sarcopenia and dynapenia from the Canadian longitudinal study on aging. J. Cachexia Sarcopenia Muscle 2019, 10, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, M.; Kanauchi, M. A cross-sectional study of the association between dynapenia and higher-level functional capacity in daily living in community-dwelling older adults in Japan. BMC Geriatr. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.; Kessler, J. A new scoring system for increasing the sensitivity of the MMSE. Z. Gerontol. Geriatr. 2020, 53, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, D.D.; Walters, K.; Rait, G.; Bazo-Alvarez, J.C.; Weerasinghe, M.C. Cross-cultural adaptation and psychometric evaluation of the Sinhala version of Lawton instrumental activities of daily living scale. PLoS ONE 2018, 13, e0199820. [Google Scholar] [CrossRef] [PubMed]
- Skottheim, A.; Lovheim, H.; Isaksson, U.; Sandman, P.O.; Gustafsson, M. Insomnia symptoms among old people in nursing homes. Int. Psychogeriatr. 2018, 30, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Society of Sleep Medicine. Trends in the Prevalence of Common Sleep Problems in Taiwan: A 10-Year Cross-Sectional Repeat Survey; Taiwan Society of Sleep Medicine: Taiwan, China, 2017. [Google Scholar]
- Lin, T.H.; Chang, S.F.; Liao, M.T.; Chen, Y.H.; Tsa, H.C. The relationships between physical function, nutrition, cognitive function, depression, and sleep quality for facility-dwelling older adults with dynapenia. BMC Geriatr. 2023, 23, 278. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tu, Y.K.; Wang, T.G.; Huang, Y.T.; Chien, K.L. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: A systematic review and network meta-analysis. Age Ageing 2018, 47, 367–373. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2017, 39, 175–191. [Google Scholar] [CrossRef]
- Latham, N.K.; Anderson, C.S.; Bennett, D.A.; Stretton, C. Progressive resistance strength training for physical disability in older people. Cochrane Database Syst. Rev. 2003, 2, CD002759. [Google Scholar]
- Lee, L.-C.; Hsu, P.-S.; Hsieh, K.-C.; Chen, Y.-Y.; Chu, L.-P.; Lu, H.-K.; Chiu, Y.-C.; Li, L.; Lai, C.-L. Standing 8-electrode bioelectrical impedance analysis as an alternative method to estimate visceral fat area and body fat mass in athletes. Int. J. Gen. Med. 2021, 14, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2019, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.W.; Liu, H.C.; Wong, P.F.; Liao, K.K.; Yun, S.H.; Lin, K.P.; Chang, C.Y.; Hsu, T.C. Chinese version and norms of the mini-mental state examination. Taiwan J. Phys. Med. Rehabil. 2021, 12, 52–59. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Ma, T.; Yao, S.; Chen, Z.K.; Xu, W.D.; Jiang, X.Y.; Wang, X.F. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Chang, S.F.; Ho, H.Y. Effect of whole-body vibration training on the physical capability, activities of daily living, and sleep quality of older people with sarcopenia. Appl. Sci. 2020, 10, 1695. [Google Scholar] [CrossRef]
- Young, P.K.; Jung, S.S. The effects of multimodal cognitive intervention focused on instrumental activities of daily living (IADL) for the elderly with high-risk of dementia: A pilot study. J. Converg. Inf. Technol. 2019, 9, 210–216. [Google Scholar]
- Smolarek, A.C.; Ferreira, L.H.; Schoenfeld, B.; Cordeiro, C.R.; Alessi, A.; Laat, E.F.; Mascarenhas, L.P.; Perin, S.C.; Zandoná, B.A.; Souza, W.C.; et al. Cognitive performance changes after a 12-week strength training program in overweight older women. J. Exerc. Physiol. 2020, 22, 1. [Google Scholar]
- Santos, P.R.; Cavalcante, B.R.; Vieira, A.K.; Guimarães, M.D.; Silva, A.M.; Armstrong, A.D.; Carvalho, R.G.; Carvalho, F.O.; Souza, M.F. Improving cognitive and physical function through 12-weeks of resistance training in older adults: Randomized controlled trial. J. Sports Sci. 2020, 38, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Cai, M. Optimal frequency of whole body vibration training for improving balance and physical performance in the older people with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2021, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Palop-Montoro, M.V.; Lozano-Aguilera, E.; Arteaga-Checa, M.; Serrano-Huete, V.; Párraga-Montilla, J.A.; Manzano-Sánchez, D. Sleep quality in older women: Effects of a vibration training program. Appl. Sci. 2020, 10, 8391. [Google Scholar] [CrossRef]
- Yang, H.; Xu, L.; Qin, W.; Hu, F.; Li, L.; Chen, C.; Tang, W. Gender differences in the modifying effect of living arrangements on the association of sleep quality with cognitive function among community-dwelling older adults: A cross-sectional study. Front. Public Health 2023, 11, 1142362. [Google Scholar]
- Pothier, K.; Gagnon, C.; Fraser, S.A.; Lussier, M.; Desjardins-Crépeau, L.; Berryman, N.; Kergoat, M.J.; Vu, T.T.; Li, K.Z.; Bosquet, L.; et al. A comparison of the impact of physical exercise, cognitive training and combined intervention on spontaneous walking speed in older adults. Aging Clin. Exp. Res. 2017, 30, 921–925. [Google Scholar] [CrossRef]
- Tseng, S.J.; Tseng, Y.T.; Lin, P.C. A survey of sleep quality among older adults. New Taipei J. Nurs. 2020, 22, 21–32. [Google Scholar]
- Coelho-Oliveira, A.C.; Monteiro-Oliveira, B.B.; de Oliveira, R.G.; Reis-Silva, A.; Ferreira-Souza, L.F.; Rodrigues Lacerda, A.C.; Mendonça, V.A.; Sartorio, A.; Taiar, R.; Bernardo-Filho, M.; et al. Evidence of use of whole-body vibration in individuals with metabolic syndrome: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 3765. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Laplagne, M.; Dupuy, D.; Sosner, P.; Bosquet, L. Effect of simultaneous exercise and cognitive training on executive functions, barorefex sensitivity, and pre-frontal cortex oxygenation in healthy older adults: A pilot study. GeroScience 2023, 45, 119–140. [Google Scholar] [CrossRef]
- Azeredo, C.F.; Paiva, P.C.; Azeredo, L.; Silva, A.R.; Francisca-Santos, A.; Paineiras-Domingos, L.; Silva, A.L. Effects of whole-body vibration exercises on parameters related to the sleep quality in metabolic syndrome individuals: A clinical trial study. Appl. Sci. 2019, 9, 5183. [Google Scholar] [CrossRef]
| Variable | Experimental (n = 29) | Control (n = 29) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | t-Value | p-Value | ||
| Gender | 0.065 | 0.800 | |||||
| male | 12 | 41.14 | 14 | 48.28 | |||
| female | 17 | 58.86 | 15 | 51.72 | |||
| Age | 0.161 | 0.096 | |||||
| 65–74 years old | 21 | 72.41 | 18 | 62.07 | |||
| 75–84 years old | 6 | 20.69 | 9 | 31.03 | |||
| 85 years old and above | 2 | 6.90 | 2 | 6.90 | |||
| Education level | 2.500 | 0.475 | |||||
| uneducated | 9 | 31.04 | 7 | 24.14 | |||
| educated | 20 | 68.96 | 22 | 75.86 | |||
| Religious beliefs | 3.500 | 0.321 | |||||
| None | 8 | 29.03 | 6 | 22.58 | |||
| Yes | 21 | 70.97 | 23 | 77.42 | |||
| Disease categories | 2.500 | 0.475 | |||||
| Respiratory System | 8 | 27.59 | 4 | 13.56 | |||
| Musculoskeletal System | 3 | 10.59 | 5 | 17.13 | |||
| Circulatory System | 8 | 27.59 | 8 | 27.48 | |||
| Endocrine Metabolism | 10 | 34.23 | 12 | 41.83 | |||
| Mean ± SD | Mean ± SD | t-value | p-value | ||||
| Physical function | |||||||
| Grip Strength (Right Hand) | 24.84 ± 4.48 | 24.69 ± 4.49 | 0.439 | 0.115 | |||
| Grip Strength (Left Hand) | 22.42 ± 3.82 | 22.94 ± 3.91 | 0.461 | 0.417 | |||
| IADL | |||||||
| Shopping Outdoors | 1.84 ± 0.69 | 1.84 ± 0.58 | 0.229 | 0.080 | |||
| Going Out for Activities | 1.81 ± 0.83 | 1.65 ± 0.71 | 0.176 | 0.060 | |||
| food preparation | 1.74 ± 0.68 | 1.58 ± 0.67 | 0.194 | 0.200 | |||
| household maintenance | 1.84 ± 0.74 | 1.57 ± 0.69 | 0.190 | 0.070 | |||
| laundry | 1.71 ± 0.59 | 1.55 ± 0.67 | 0.221 | 0.070 | |||
| telephone use | 1.61 ± 0.56 | 1.61 ± 0.56 | 0.229 | 0.300 | |||
| medication administration | 1.65 ± 0.49 | 1.61 ± 0.50 | 0.263 | 0.081 | |||
| financial management | 1.55 ± 0.51 | 1.36 ± 0.49 | 0.228 | 0.600 | |||
| Cognitive function | |||||||
| Sense of orientation | 7.71 ± 0.53 | 8.23 ± 0.56 | 1.046 | 0.406 | |||
| attention and calculation abilities | 3.36 ± 0.88 | 3.03 ± 0.75 | 0.304 | 0.140 | |||
| memory | 1.65 ± 0.55 | 1.97 ± 0.61 | 0.239 | 0.070 | |||
| language | 2.10 ± 0.65 | 2.39 ± 0.67 | 0.264 | 0.500 | |||
| verbal comprehension | 2.32 ± 0.60 | 1.74 ± 0.63 | 0.237 | 0.080 | |||
| constructional ability | 0.97 ± 0.18 | 0.74 ± 0.45 | 0.190 | 0.090 | |||
| Sleep quality | |||||||
| Subjective sleep quality | 1.32 ± 0.65 | 1.39 ± 0.62 | 0.169 | 0.905 | |||
| sleep onset latency (minutes) | 1.32 ± 0.75 | 1.32 ± 0.65 | 0.149 | 0.603 | |||
| sleep duration (hours) | 1.65 ± 0.61 | 1.32 ± 0.65 | 0.181 | 0.800 | |||
| sleep efficiency (%) | 1.39 ± 0.76 | 1.65 ± 0.61 | 0.172 | 0.100 | |||
| sleep disturbances (minutes) | 1.16 ± 0.82 | 1.26 ± 0.58 | 0.135 | 0.060 | |||
| use of sleep medication | 1.45 ± 0.93 | 1.23 ± 0.62 | 0.134 | 0.180 | |||
| daytime dysfunction (minutes) | 0.87 ± 0.67 | 1.16 ± 0.69 | 0.116 | 0.360 | |||
| total score | 9.16 ± 5.19 | 9.84 ± 4.41 | 0.151 | 0.430 | |||
| Variables | Experimental Group (n = 29) | Control Group (n = 29) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Paired t | p-Value | Mean ± SD | Paired t | p-Value | |||
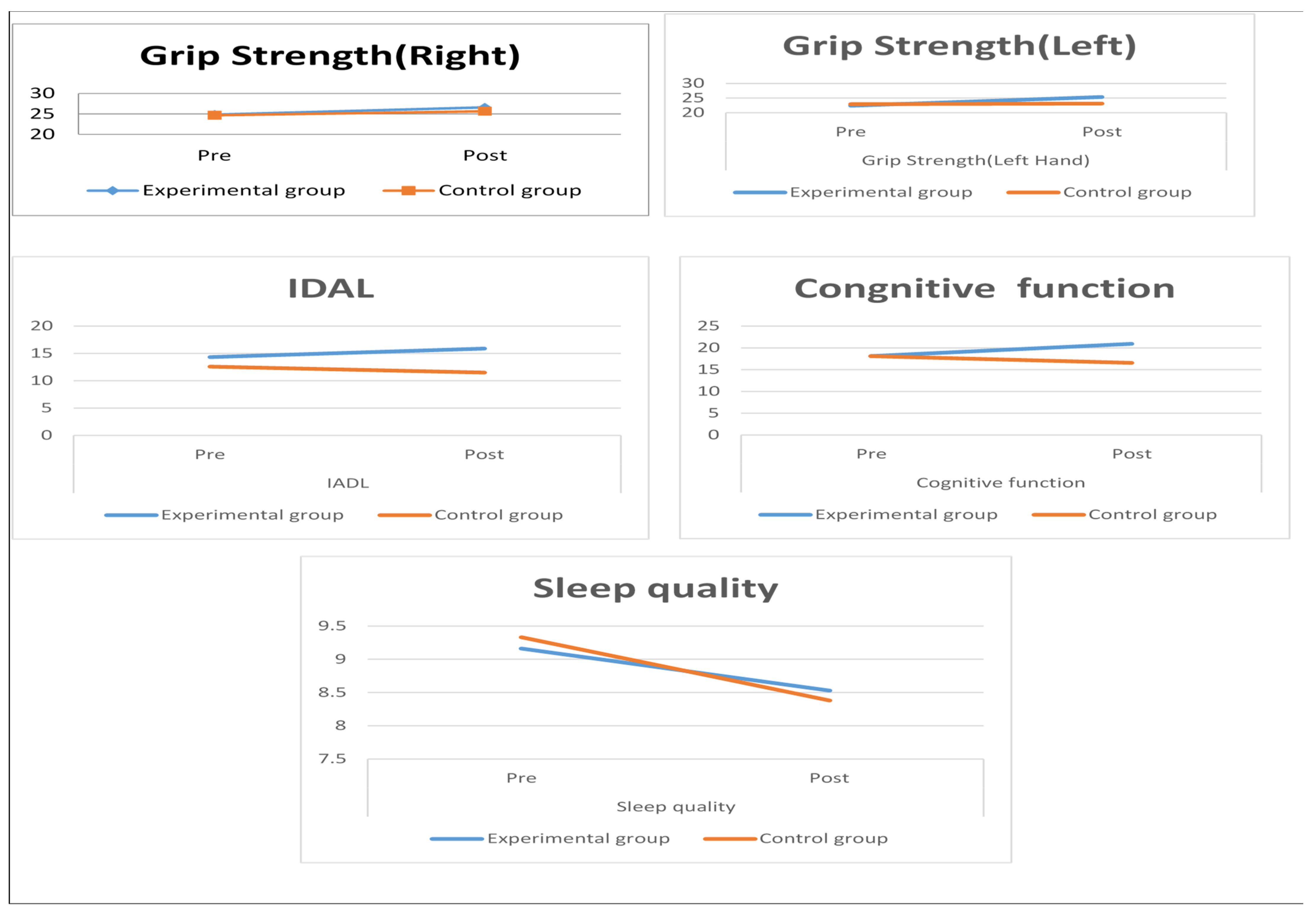
| Physical Function | ||||||||
| Grip strength | ||||||||
| Grip Strength (Right Hand) | Pre-test | 24.84 ± 4.48 | −1.252 | <0.001 *** | 24.70 ± 4.49 | 1.603 | 0.060 | |
| Post-test | 26.62 ± 4.53 | 25.67 ± 4.75 | ||||||
| Grip Strength (Left Hand) | Pre-test | 22.42 ± 3.82 | −1.225 | <0.001 *** | 22.94 ± 3.91 | 0.500 | 0.090 | |
| Post-test | 25.40 ± 4.58 | 23.17 ± 4.77 | ||||||
| IADL | ||||||||
| Shopping Outdoors | Pre-test | 1.84 ± 0.69 | −3.50 | 0.002 *** | 1.64 ± 0.58 | 3.06 | 0.045 | |
| Post-test | 2.13 ± 0.50 | 1.13 ± 0.56 | ||||||
| Going Out for Activities | Pre-test | 1.81 ± 0.83 | −2.68 | 0.012 ** | 1.64 ± 0.71 | 1.72 | 0.096 | |
| Post-test | 2.00 ± 0.82 | 1.61 ± 0.65 | ||||||
| food preparation | Pre-test | 1.74 ± 0.68 | 2.40 | 0.223 | 1.58 ± 0.67 | −2.96 | 0.106 | |
| Post-test | 1.90 ± 0.60 | 1.81 ± 0.60 | ||||||
| household maintenance | Pre-test | 1.74 ± 0.84 | 3.59 | 0.101 | 1.59 ± 0.67 | −2.96 | 0.066 | |
| Post-test | 2.19 ± 0.75 | 1.61 ± 0.70 | ||||||
| laundry | Pre-test | 1.81 ± 0.59 | 2.40 | 0.123 | 1.55 ± 0.57 | −3.23 | 0.073 | |
| Post-test | 1.87 ± 0.50 | 1.51 ± 0.48 | ||||||
| telephone use | Pre-test | 1.81 ± 0.56 | 3.78 | 0.201 | 1.61 ± 0.56 | −3.23 | 0.063 | |
| Post-test | 1.94 ± 0.51 | 1.67 ± 0.50 | ||||||
| medication administration | Pre-test | 1.65 ± 0.49 | −4.35 | 0.001 *** | 1.61 ± 0.50 | −4.65 | 0.100 | |
| Post-test | 2.03 ± 0.61 | 1.03 ± 0.55 | ||||||
| financial management | Pre-test | 1.95 ± 0.51 | 3.50 | 0.092 | 1.36 ± 0.49 | −1.14 | 0.200 | |
| Post-test | 1.84 ± 0.58 | 1.12 ± 0.56 | ||||||
| Cognitive function | ||||||||
| Sense of orientation | Pre-test | 7.71 ± 0.53 | −1.53 | 0.117 | 8.23 ± 0.56 | −2.78 | 0.101 | |
| Post-test | 7.84 ± 0.63 | 8.54 ± 0.72 | ||||||
| attention and calculation abilities | Pre-test | 3.36 ± 0.88 | −7.84 | <0.001 *** | 3.03 ± 0.75 | −1.07 | 0.060 | |
| Post-test | 4.61 ± 0.92 | 2.94 ± 0.85 | ||||||
| memory | Pre-test | 1.65 ± 0.55 | −1.42 | <0.001 *** | 1.97 ± 0.61 | −1.66 | 0.200 | |
| Post-test | 2.52 ± 0.51 | 1.48 ± 0.51 | ||||||
| language | Pre-test | 2.10 ± 0.65 | −5.30 | <0.001 *** | 2.39 ± 0.67 | −1.53 | 0.067 | |
| Post-test | 2.58 ± 0.50 | 1.61 ± 0.50 | ||||||
| verbal comprehension | Pre-test | 2.32 ± 0.60 | −1.00 | 0.326 | 1.74 ± 0.63 | −1.23 | 0.303 | |
| Post-test | 2.36 ± 0.61 | 1.00 ± 0.58 | ||||||
| constructional ability | Pre-test | 0.97 ± 0.18 | −1.00 | 0.325 | 0.74 ± 0.45 | −3.23 | 0.603 | |
| Post-test | 1.00 ± 0.00 | 1.00 ± 0.00 | ||||||
| Sleep quality | ||||||||
| Subjective sleep quality | Pre-test | 1.32 ± 0.66 | 4.35 | 0.060 | 1.39 ± 0.61 | 3.50 | 0.072 | |
| Post-test | 1.94 ± 0.57 | 1.10 ± 0.60 | ||||||
| sleep onset latency (minutes) | Pre-test | 1.32 ± 0.75 | 2.68 | 0.012 * | 1.32 ± 0.65 | 3.23 | 0.103 | |
| Post-test | 1.13 ± 0.72 | 1.37 ± 0.57 | ||||||
| sleep duration (hours) | Pre-test | 1.65 ± 0.61 | 4.06 | 0.003 *** | 1.32 ± 0.65 | 4.06 | 0.300 | |
| Post-test | 1.29 ± 0.53 | 1.97 ± 0.61 | ||||||
| sleep efficiency (%) | Pre-test | 1.39 ± 0.76 | 4.32 | 0.062 | 1.65 ± 0.61 | 5.91 | 0.600 | |
| Post-test | 1.07 ± 0.77 | 1.00 ± 0.58 | ||||||
| sleep disturbances (minutes) | Pre-test | 1.16 ± 0.82 | 3.40 | 0.063 | 1.26 ± 0.58 | 1.79 | 0.083 | |
| Post-test | 1.00 ± 0.68 | 1.16 ± 0.52 | ||||||
| use of sleep medication | Pre-test | 1.45 ± 0.93 | 1.41 | 0.169 | 1.23 ± 0.62 | 2.68 | 0.112 | |
| Post-test | 1.29 ± 0.59 | 1.03 ± 0.71 | ||||||
| daytime dysfunction (minutes) | Pre-test | 0.87 ± 0.67 | 1.44 | 0.161 | 1.16 ± 0.68 | 2.99 | 0.076 | |
| Post-test | 0.81 ± 0.61 | 0.81 ± 0.54 | ||||||
| Variables | Experimental Group (n = 29) | Control Group (n = 29) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical Function | Cognitive Function | Sleep Quality | Physical Function | Cognitive Function | Sleep Quality | |||||||
| Gender | Mean ± SD | t-Value | Mean ± SD | t-Value | Mean ± SD | t-Value | Mean ± SD | t-Value | Mean ± SD | t-Value | Mean ± SD | t-Value |
| Male | 21.56 ± 0.58 | 2.078 * | 4.86 ± 0.56 | 2.104 * | 2.35 ± 0.51 | 2.093 * | 18.36 ± 0.45 | 1.264 | 3.57 ± 0.48 | 1.718 | 2.01 ± 0.11 | 1.546 |
| Female | 20.56 ± 0.67 | 6.86 ± 0.32 | 1.01 ± 0.11 | 19.06 ± 0.61 | 5.52 ± 0.98 | 1.85 ± 0.22 | ||||||
| Group | Post-Test Mean (SE) | Pre-Test Mean (SE) | Mean Difference (SE) | p-Value | Group × Time | p-Value | |
|---|---|---|---|---|---|---|---|
| Physical Function | |||||||
| Grip strength | (Right Hand) | ||||||
| Experimental Group | 26.62 (0.61) | 24.84 (0.45) | 1.78 (0.16) | <0.001 *** | 0.203 (0.057–0.721) | <0.001 *** | |
| Control Group | 22.94 (1.06) | 25.24 (0.37) | −2.30 (0.69) | 0.076 | |||
| (Left Hand) | |||||||
| Experimental Group | 25.58 (0.62) | 23.42 (0.33) | 1.16 (0.29) | 0.022 * | 0.130 (0.037–0.461) | <0.001 *** | |
| Control Group | 22.67 (0.74) | 25.41 (0.52) | −2.74 (0.22) | 0.087 | |||
| IADL | |||||||
| Experimental Group | 14.67 (0.18) | 13.32 (0.17) | 1.35 (0.01) | 0.003 *** | 0.131 (0.035–0.489) | 0.005 ** | |
| Control Group | 12.90 (0.07) | 12.68 (0.08) | 0.22 (0.01) | 0.079 | |||
| Cognitive function | |||||||
| Experimental Group | 20.19 (0.78) | 17.65 (0.04) | 2.54 (0.74) | 0.045 | 0.098 (0.010−0.870) | 0.035 * | |
| Control Group | 18.03 (0.07) | 17.74 (0.14) | 0.29 (0.07) | 0.087 | |||
| Sleep quality | |||||||
| Experimental Group | 7.52 (0.10) | 9.16 (0.15) | −1.64 (0.04) | <0.001 *** | 0.132 (0.03–0.05) | 0.003 ** | |
| Control Group | 7.09 (0.13) | 9.29 (0.12) | −0.21 (0.01) | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-C.; Chang, S.-F. Effects of Whole-Body Vibration Training on Improving Physical Function, Cognitive Function, and Sleep Quality for Older People with Dynapenia in Long-Term Care Institutions: A Randomized Controlled Study. Appl. Sci. 2024, 14, 6830. https://doi.org/10.3390/app14156830
Su Y-C, Chang S-F. Effects of Whole-Body Vibration Training on Improving Physical Function, Cognitive Function, and Sleep Quality for Older People with Dynapenia in Long-Term Care Institutions: A Randomized Controlled Study. Applied Sciences. 2024; 14(15):6830. https://doi.org/10.3390/app14156830
Chicago/Turabian StyleSu, Yu-Chen, and Shu-Fang Chang. 2024. "Effects of Whole-Body Vibration Training on Improving Physical Function, Cognitive Function, and Sleep Quality for Older People with Dynapenia in Long-Term Care Institutions: A Randomized Controlled Study" Applied Sciences 14, no. 15: 6830. https://doi.org/10.3390/app14156830





