The Effects of Internal Curing and Shrinkage Cracking Avoidance on the Corrosion of Reinforced Concrete Walls with Superabsorbent Polymers
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
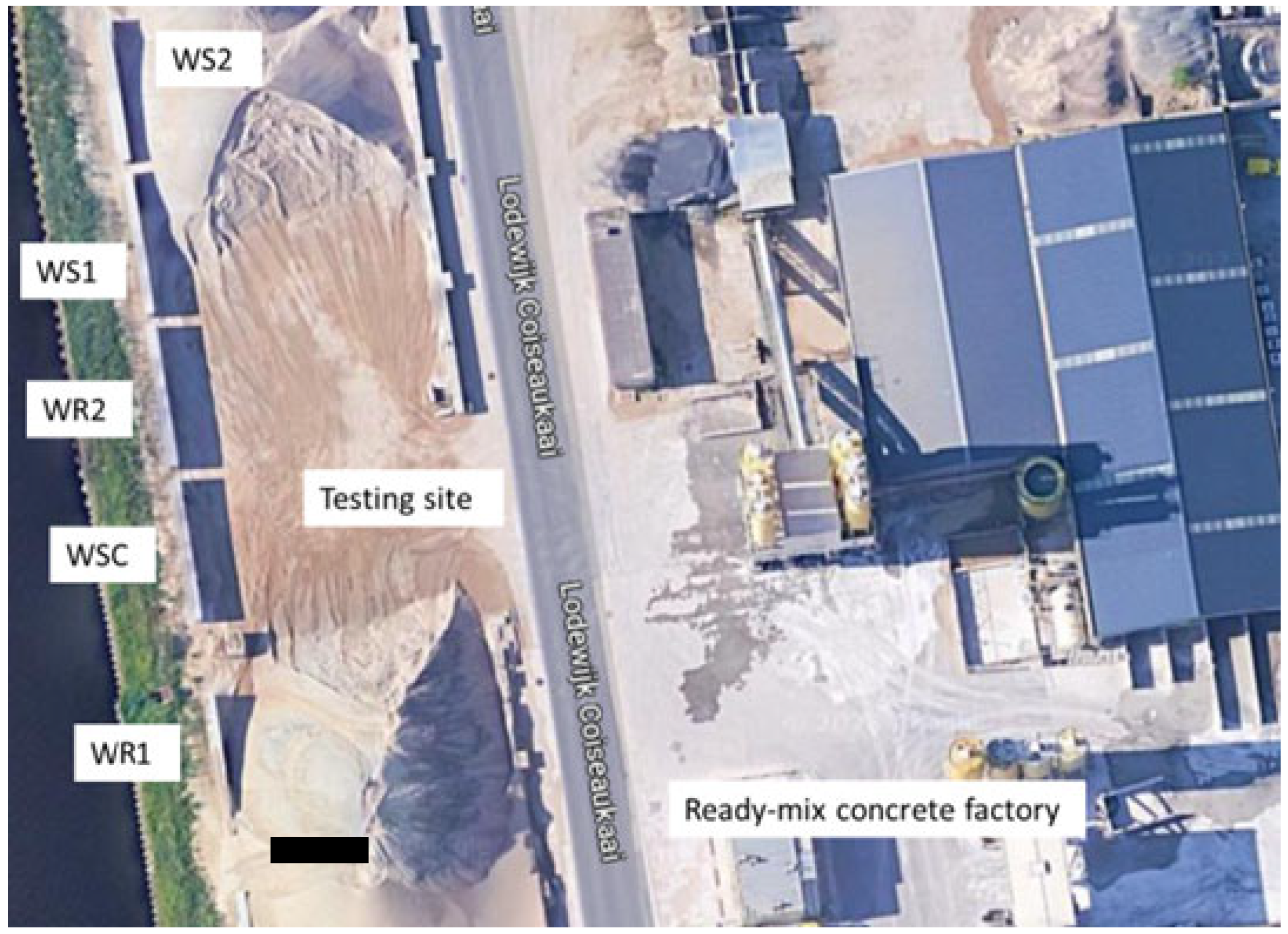
2.1. Overview of the Testing Campaign
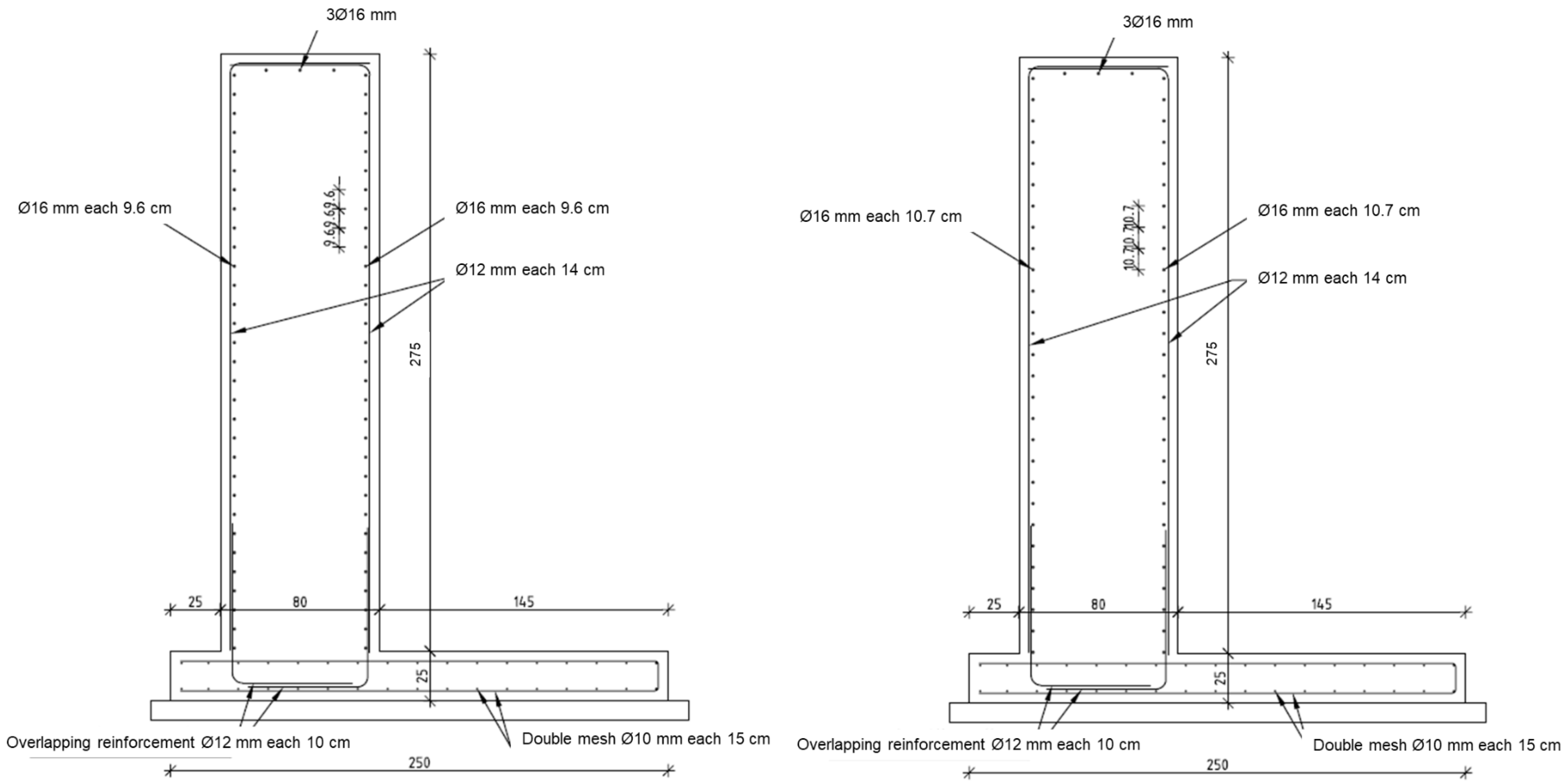
2.2. Description of the Walls and Concrete Mix Design
2.3. Details about the Superabsorbent Polymers Used
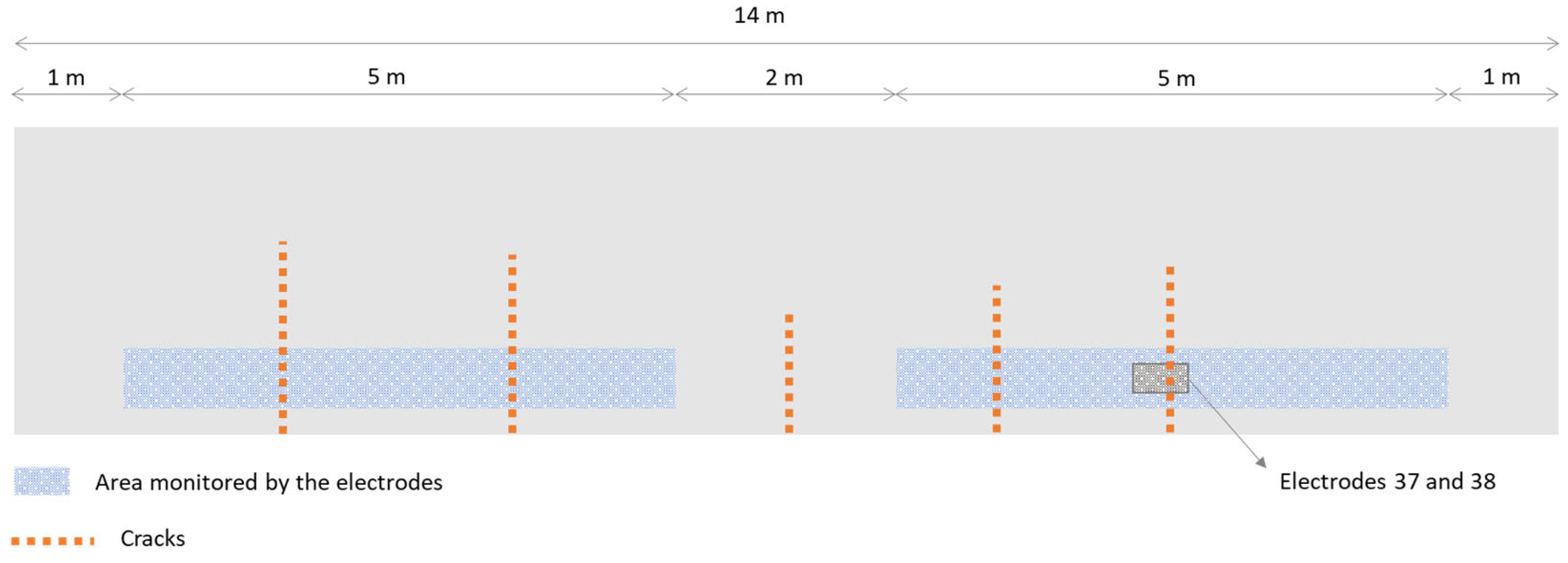
2.4. Sensors and Monitoring System Used in the Walls
2.5. Durability Testing
2.5.1. Accelerated Carbonation
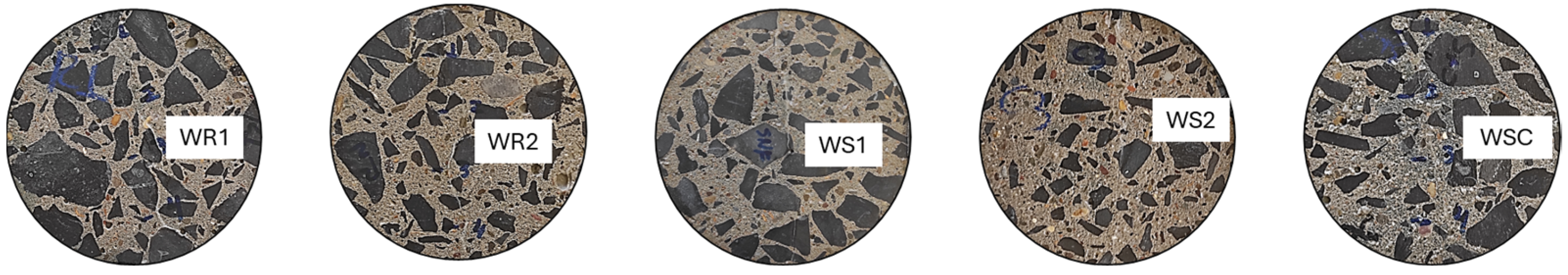
2.5.2. Chloride Penetration Profile
- Uncracked specimens, constantly submerged in a NaCl solution (35 g/L);
- Cracked specimens, constantly submerged in a NaCl solution (35 g/L);
- Cracked specimens subjected to wet/dry cycles (6 h wet/6 h dry) in a NaCl solution (35 g/L).
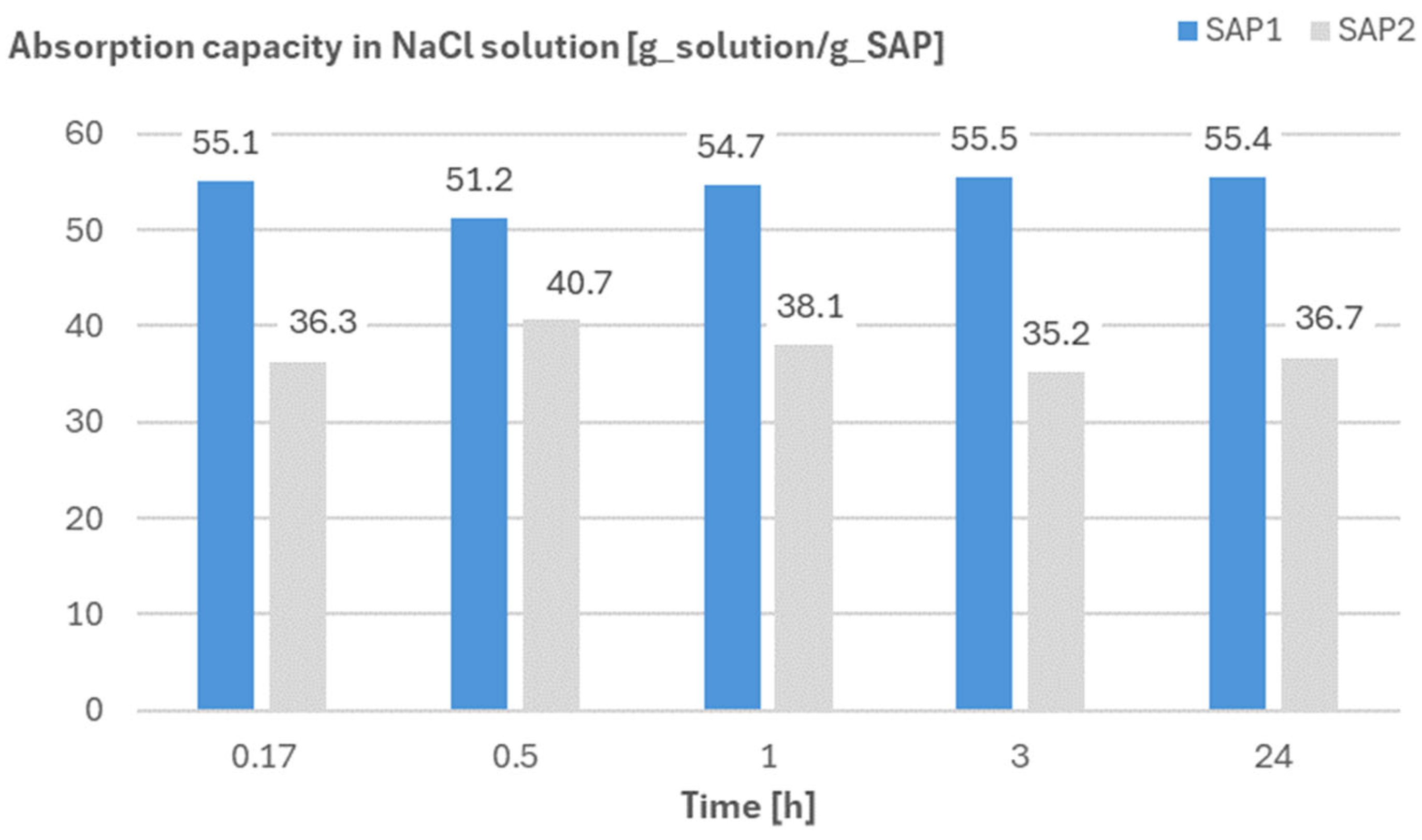
2.5.3. Absorption Capacity of SAPs in NaCl Solution by Means of the Filtration Test
3. Results and Discussion
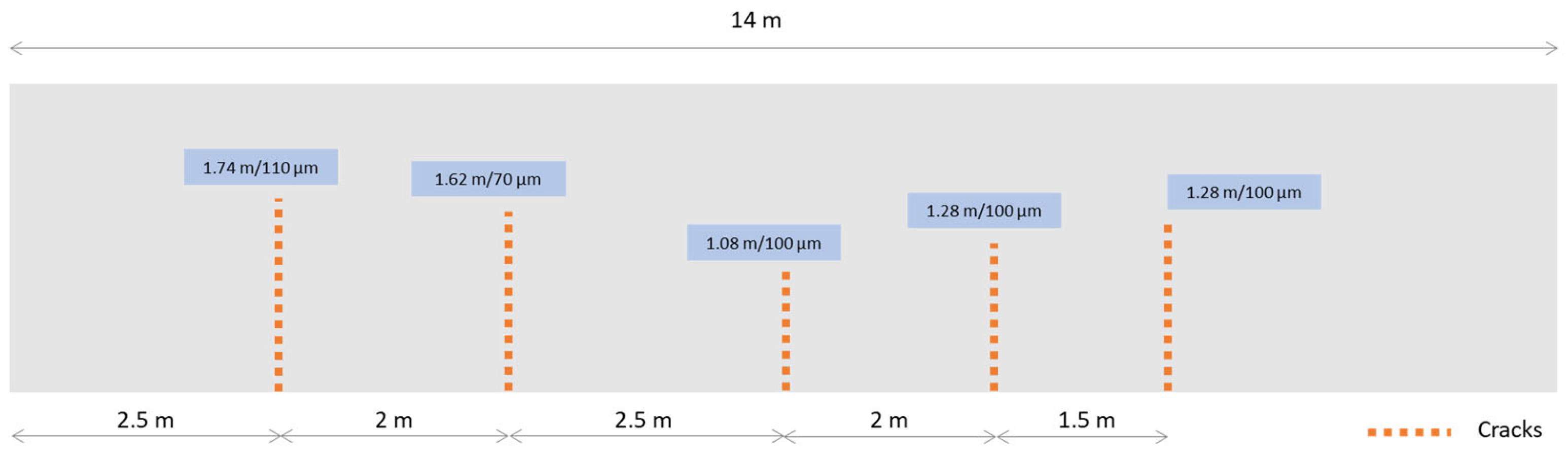
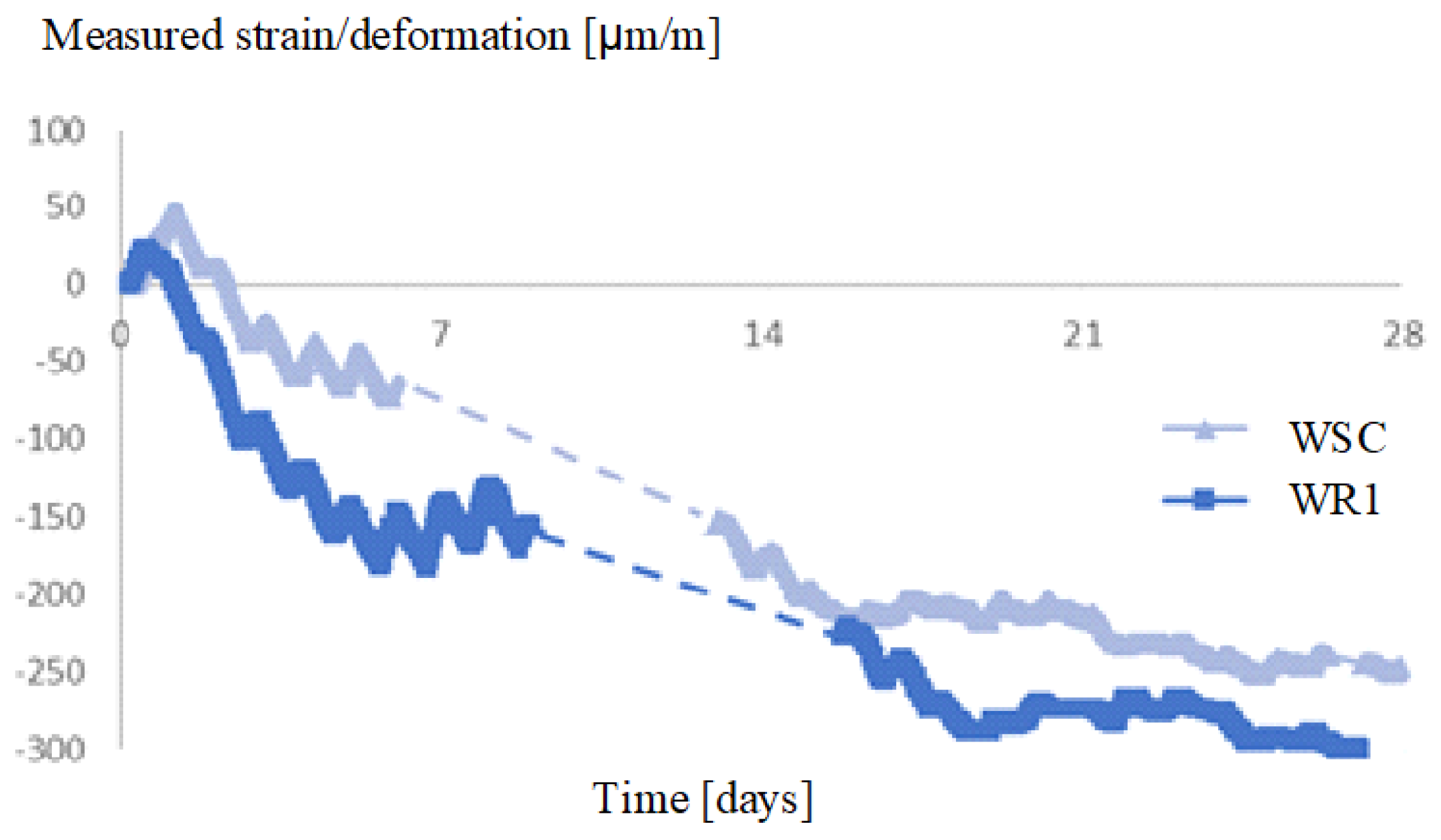
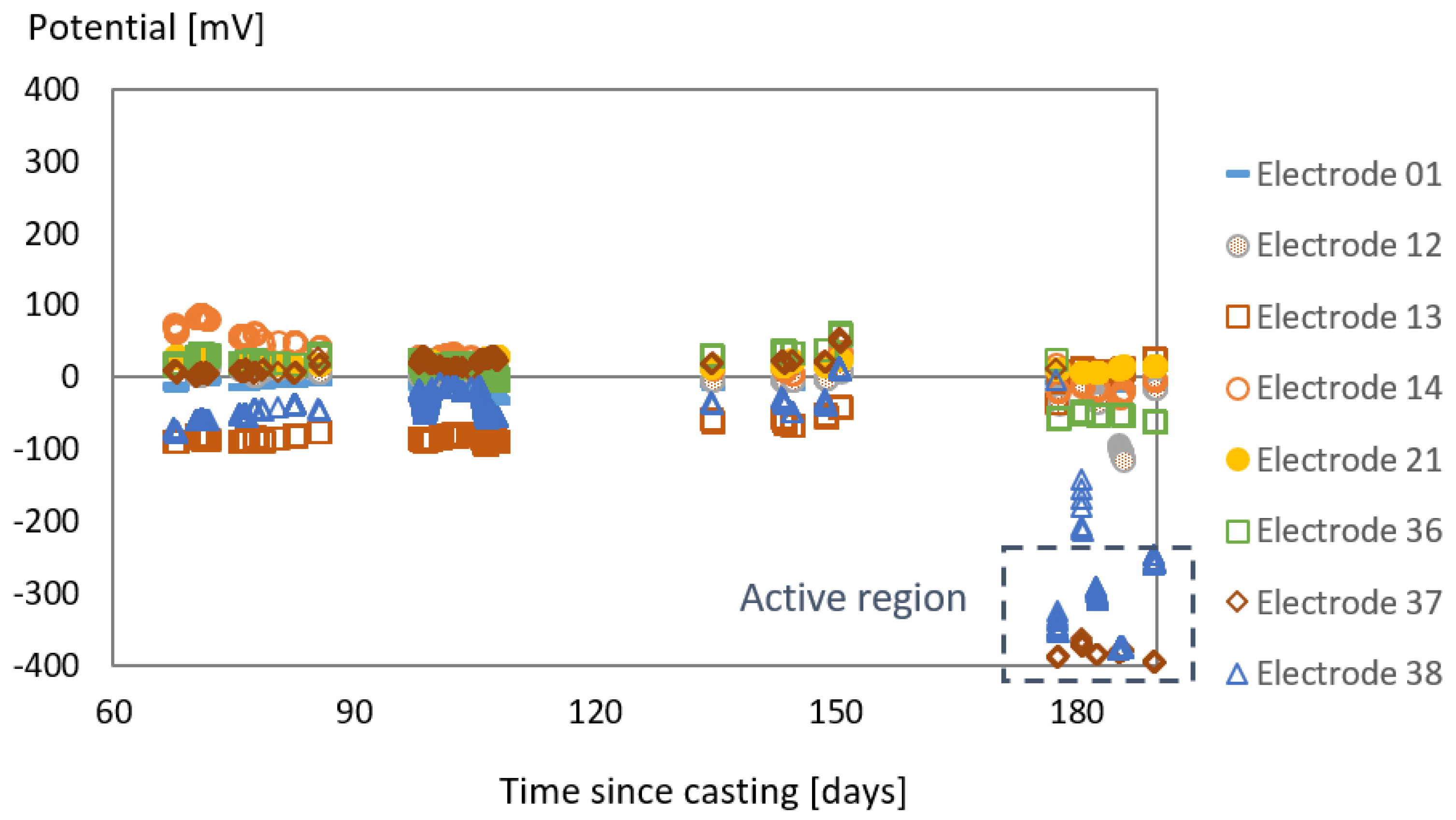
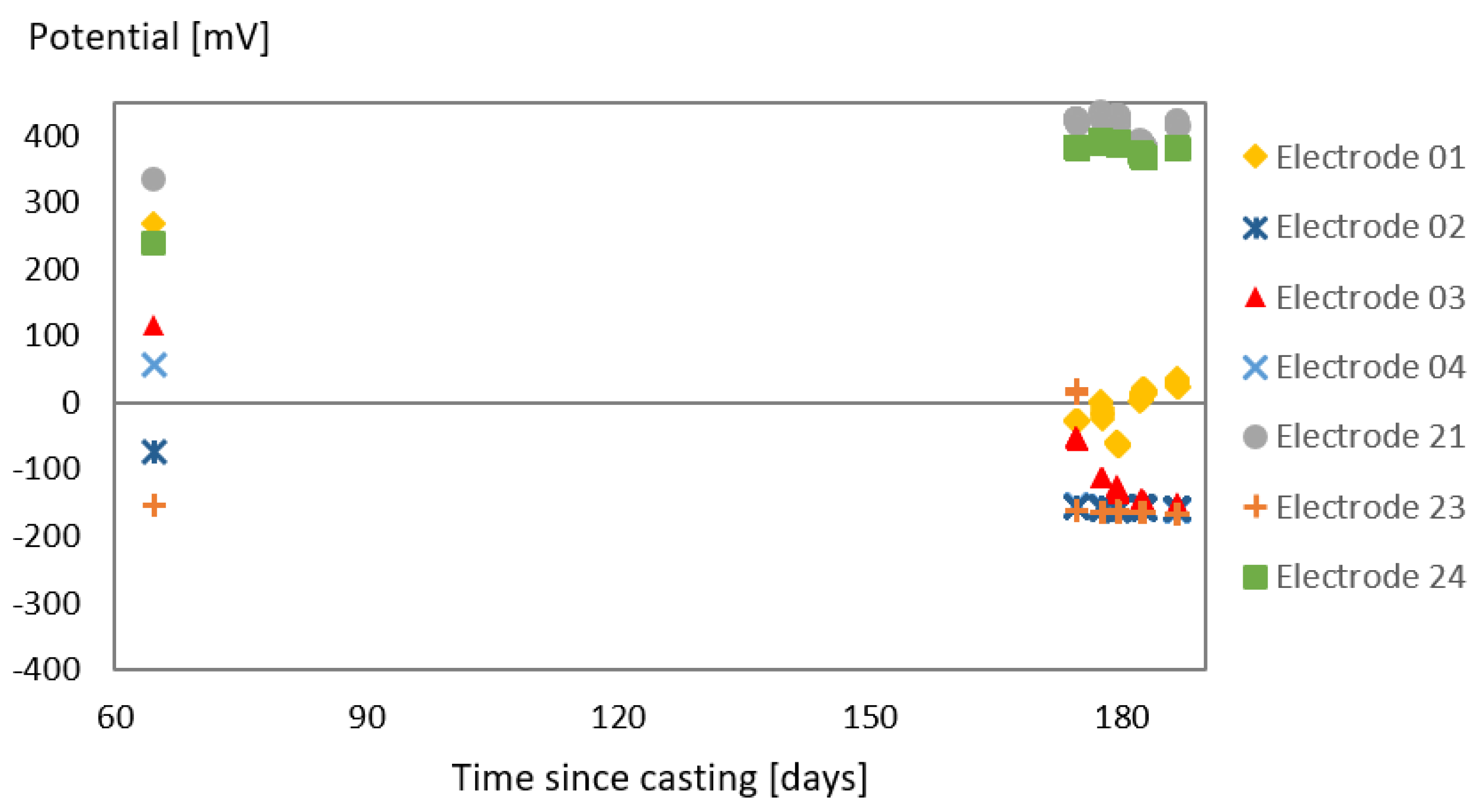
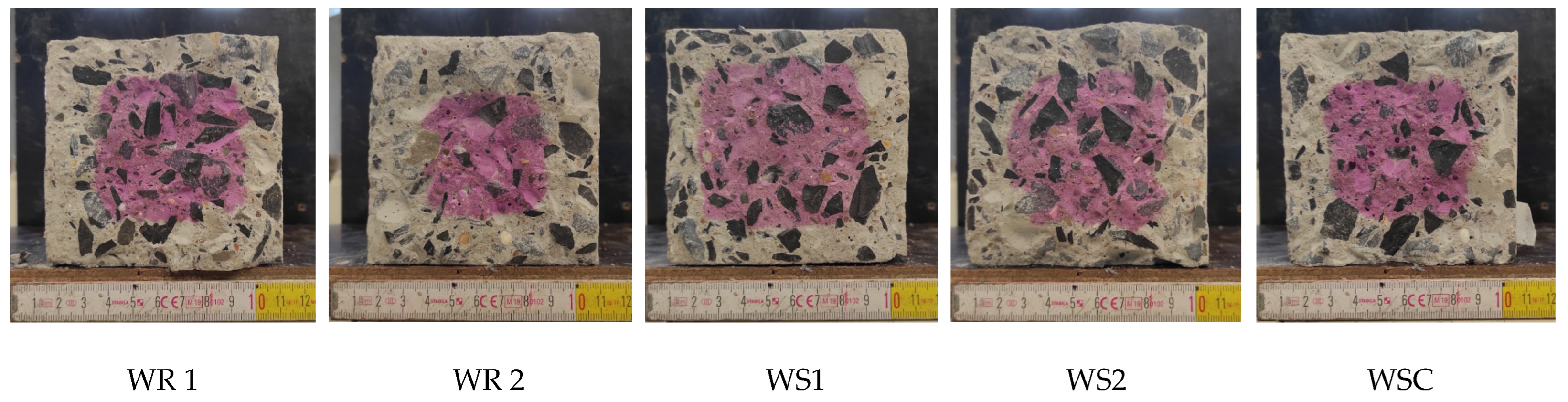
3.1. Effect of SAP Internal Curing on the Cracking and Corrosion Initiation
3.2. Tests with the Laboratory Specimens
3.2.1. Accelerated Carbonation
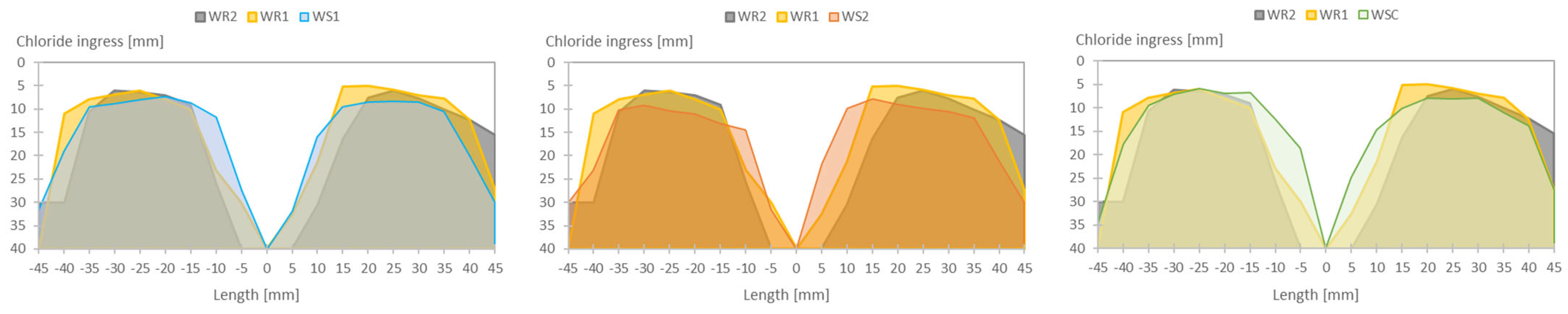
3.2.2. Uncracked Specimens Constantly Submerged in a Solution of NaCl (35 g/L)
3.2.3. Cracked Specimens Constantly Submerged and Subjected to wet/dry Cycles in a NaCl Solution
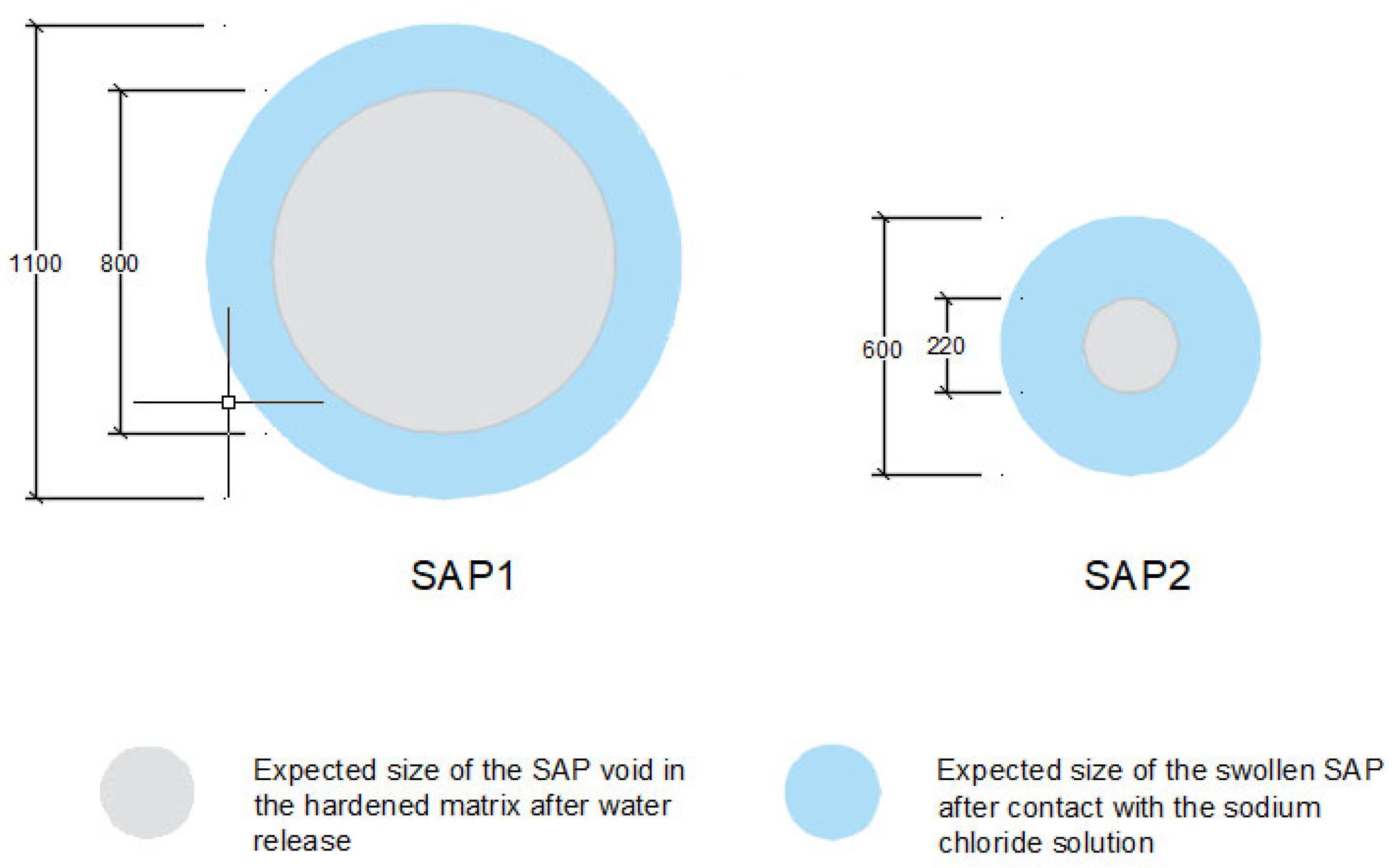
3.3. Absorption Capacity and Swelling and Sealing during Chloride Ingress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechtcherine, V.; Reinhardt, H.W. Application of Super Absorbent Polymers (SAP) in Concrete Construction, State-of-the-Art Report Prepared by Technical Committee 225-SAP; RILEM, Springer: Dordrecht, The Netherlands, 2012; p. 166. [Google Scholar]
- Wong, H.S. Concrete with superabsorbent polymer, In Eco-Efficient Repair and Rehabilitation of Concrete Infrastructures; Pacheco-Torgal, R.E.M.F., Shi, X., De Belie, N., Van Tittelboom, K., Sáez, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 467–499. [Google Scholar]
- Schröfl, C.; Erk, K.A.; Siriwatwechakul, W.; Wyrzykowski, M.; Snoeck, D. Recent progress in superabsorbent polymers for concrete. Cem. Concr. Res. 2022, 151, 106648. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Wyrzykowski, M.; Schröfl, C.; Snoeck, D.; Lura, P.; De Belie, N.; Mignon, A.; Van Vlierberghe, S.; Klemm, A.J.; Almeida, F.C.R.; et al. Application of super absorbent polymers (SAP) in concrete construction—Update of RILEM state-of-the-art report. Mater. Struct. 2021, 54, 80. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; Vermoesen, E.; Mannekens, E.; Van Tittelboom, K.; Van Vlierberghe, S.; De Belie, N.; Snoeck, D. Enhanced durability performance of cracked and uncracked concrete by means of smart in-house developed superabsorbent polymers with alkali-stable and -unstable crosslinkers. Constr. Build. Mater. 2021, 297, 123812. [Google Scholar] [CrossRef]
- Araujo, M.; Van Vlierberghe, S.; Feiteira, J.; Graulus, G.J.; Van Tittelboom, K.; Martins, J.C.; Dubruel, P.; De Belie, N. Cross-linkable polyethers as healing/sealing agents for self-healing of cementitious materials. Mater. Des. 2016, 98, 215–222. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success? Materials 2017, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Monnig, S.; Lura, P. Superabsorbent polymers—An additive to increase the freeze-thaw resistance of high strength concrete. In Advances in Construction Materials; Grosse, C.U., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 351–358. [Google Scholar]
- Laustsen, S.; Hasholt, M.T.; Jensen, O.M. Void structure of concrete with superabsorbent polymers and its relation to frost resistance of concrete. Mater. Struct. 2015, 48, 357–368. [Google Scholar] [CrossRef]
- Bentz, D.P. Influence of internal curing using lightweight aggregates on interfacial transition zone percolation and chloride ingress in mortars. Cem. Concr. Compos. 2009, 31, 285–289. [Google Scholar] [CrossRef]
- Zhutovsky, S.; Kovler, K. Effect of internal curing on durability-related properties of high performance concrete. Cem. Concr. Res. 2012, 42, 20–26. [Google Scholar] [CrossRef]
- Carmelo Di Bella, C.V.E.H.; Jason, W. Chloride Transport Measurements for a Plain and Internally Cured Concrete Mixture; ACI Symposium Publication: Farmington Hills, MI, USA, 2012; Volume 290. [Google Scholar]
- Hasholt, M.T.; Jensen, O.M. Chloride migration in concrete with superabsorbent polymers. Cem. Concr. Compos. 2015, 55, 290–297. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Wu, Z.; Shi, C. Effects of SAP on the properties and pore structure of high performance cement-based materials. Constr. Build. Mater. 2017, 131, 476–484. [Google Scholar] [CrossRef]
- Lee, C.K.; Kim, I.S.; Choi, S.S.Y.; Yang, E.I. Evaluation of Fundamental Properties and Chloride Penetration Resistance of Concrete using Superabsorbent Polymers. J. Korea Inst. Struct. Maint. Insp. 2020, 24, 50–59. [Google Scholar]
- Dang, J.; Zhao, J.; Du, Z.H. Effect of Superabsorbent Polymer on the Properties of Concrete. Polymers 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, M.; Woyciechowski, P. Chloride Diffusion in Concrete Modified with Polyacrylic Superabsorbent Polymer (SAP) Hydrogel—The Influence of the Water-to-Cement Ratio and SAP-Entrained Water. Materials 2021, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Van Mullem, T.; De Brabandere, L.; Van de Voorde, E.; Snoeck, D.; De Belie, N. Influence of superabsorbent polymers on the chloride ingress of mortar measured by chloride diffusion and a quasi-steady-state migration test. Cem. Concr. Compos. 2024, 150, 105563. [Google Scholar] [CrossRef]
- Tenorio Filho, J.R.; Mannekens, E.; Van Tittelboom, K.; Van Vlierberghe, S.; De Belie, N.; Snoeck, D. Innovative SuperAbsorbent Polymers (iSAPs) to construct crack-free reinforced concrete walls: An in-field large-scale testing campaign. J. Build. Eng. 2021, 43, 102639. [Google Scholar] [CrossRef]
- Tenorio Filho, J.R.; Snoeck, D.; De Belie, N. Mixing protocols for plant-scale production of concrete with superabsorbent polymers. Struct. Concr. 2020, 21, 983–991. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; De Belie, N.; Snoeck, D. Reaching Beyond Internal Curing: The Effects of Superabsorbent Polymers on the Durability of Reinforced Concrete Structures. In International RILEM Conference on Synergising Expertise towards Sustainability and Robustness of Cement-based Materials and Concrete Structures; Jędrzejewska, A., Kanavaris, F., Azenha, M., Benboudjema, F., Schlicke, D., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 933–941. [Google Scholar]
- EN 12390-12:2020; Testing Hardened Concrete Determination of the Carbonation Resistance of Concrete. Accelerated Carbonation Method. European Standard: Plzen, Czech Republic, 2020. Available online: https://www.en-standard.eu/bs-en-12390-12-2020-testing-hardened-concrete-determination-of-the-carbonation-resistance-of-concrete-accelerated-carbonation-method/ (accessed on 27 June 2024).
- Snoeck, D.; Schröfl, C.; Mechtcherine, V. Recommendation of RILEM TC 260-RSC: Testing sorption by superabsorbent polymers (SAP) prior to implementation in cement-based materials. Mater. Struct. 2018, 51, 116. [Google Scholar] [CrossRef]
- ASTM C1581-04:2010; Standard Test Method for Determining Age at Cracking and Induced Tensile Stress Characteristics of Mortar and Concrete under Restrained Shrinkage. ASTM International: West Conshohocken, PA, USA, 2018.
- Snoeck, D.; Pel, L.; De Belie, N. The water kinetics of superabsorbent polymers during cement hydration and internal curing visualized and studied by NMR. Sci. Rep. 2017, 7, 9514. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, D.; Goethals, W.; Hovind, J.; Trtik, P.; Van Mullem, T.; Van den Heede, P.; De Belie, N. Internal curing of cement pastes by means of superabsorbent polymers visualized by neutron tomography. Cem. Concr. Res. 2021, 147, 106528. [Google Scholar] [CrossRef]
- Grasley, Z.C.; Lange, D.A.; D’Ambrosia, M.D. Internal relative humidity and drying stress gradients in concrete. Mater. Struct. 2006, 39, 901–909. [Google Scholar] [CrossRef]
- Henkensiefken, R.; Bentz, D.; Nantung, T.; Weiss, J. Volume change and cracking in internally cured mixtures made with saturated lightweight aggregate under sealed and unsealed conditions. Cem. Concr. Compos. 2009, 31, 427–437. [Google Scholar] [CrossRef]
- Poursaee, A.; Ross, B. The Role of Cracks in Chloride-Induced Corrosion of Carbon Steel in Concrete—Review. Corros. Mater. Degrad. 2022, 3, 258–269. [Google Scholar] [CrossRef]
- Tenorio Filho, J.R.; Mannekens, E.; Van Tittelboom, K.; Snoeck, D.; De Belie, N. Assessment of the potential of superabsorbent polymers as internal curing agents in concrete by means of optical fiber sensors. Constr. Build. Mater. 2020, 238, 117751. [Google Scholar] [CrossRef]
- Snoeck, D.; Pel, L.; De Belie, N. Superabsorbent polymers to mitigate plastic drying shrinkage in a cement paste as studied by NMR. Cem. Concr. Compos. 2018, 93, 54–62. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Shen, A.; Chen, Z.; Qin, X.; Zhao, M. Research on drying shrinkage deformation and cracking risk of pavement concrete internally cured by SAPs. Constr. Build. Mater. 2019, 227, 116705. [Google Scholar] [CrossRef]
- Yio, M.H.N.; Mac, M.J.; Yeow, Y.X.; Wong, H.S.; Buenfeld, N.R. Effect of autogenous shrinkage on microcracking and mass transport properties of concrete containing supplementary cementitious materials. Cem. Concr. Res. 2021, 150, 106611. [Google Scholar] [CrossRef]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; Van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; De Belie, N. The effects of superabsorbent polymers on the microstructure of cementitious materials studied by means of sorption experiments. Cem. Concr. Res. 2015, 77, 26–35. [Google Scholar] [CrossRef]
- Snoeck, D.; Pel, L.; De Belie, N. Comparison of different techniques to study the nanostructure and the microstructure of cementitious materials with and without superabsorbent polymers. Constr. Build. Mater. 2019, 223, 244–253. [Google Scholar] [CrossRef]
- Xu, J.; Qin, X.; Huang, Z.; Lin, Y.; Li, B.; Xie, Z. Effect of Superabsorbent Polymer (SAP) Internal Curing Agent on Carbonation Resistance and Hydration Performance of Cement Concrete. Adv. Mater. Sci. Eng. 2022, 2022, 3485373. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; de Araújo, M.A.P.G.; Mannekens, E.; De Belie, N.; Snoeck, D. Alginate- and sulfonate-based superabsorbent polymers for application in cementitious materials: Effects of kinetics on internal curing and other properties. Cem. Concr. Res. 2022, 159, 106889. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, M.; Liu, Z.; Guo, Y.; Zhang, Q. Salt-Tolerant Superabsorbent Polymer with High Capacity of Water-Nutrient Retention Derived from Sulfamic Acid-Modified Starch. ACS Omega 2019, 4, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.A.; Khan, M.; Javed, R.; Sayed, M.; Khan, M.S.; Khan, A.; Ullah, M. Superabsorbent polymer hydrogels with good thermal and mechanical properties for removal of selected heavy metal ions. J. Clean. Prod. 2018, 201, 78–87. [Google Scholar] [CrossRef]
- Bentz, D.P.; Snyder, K.A.; Cass, L.C.; Peltz, M.A. Doubling the service life of concrete structures. I: Reducing ion mobility using nanoscale viscosity modifiers. Cem. Concr. Compos. 2008, 30, 674–678. [Google Scholar] [CrossRef]
- Snoeck, D. Superabsorbent polymers to seal and heal cracks in cementitious materials. RILEM Tech. Lett. 2018, 3, 32–38. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef]
- di Summa, D.; Tenório Filho, J.R.; Snoeck, D.; Van den Heede, P.; Van Vlierberghe, S.; Ferrara, L.; De Belie, N. Environmental and economic sustainability of crack mitigation in reinforced concrete with SuperAbsorbent Polymers (SAPs). J. Clean. Prod. 2021, 358, 131998. [Google Scholar] [CrossRef]







| Wall | Cement | Sand | Limestone | Ad.1 | Ad.2 | SAP1 | SAP2 | w/cto | w/cef |
|---|---|---|---|---|---|---|---|---|---|
| WR1 | 360 | 736 | 1116 | 2.42 | 1.56 | 0 | 0 | 0.44 | 0.44 |
| WR2 | 360 | 695 | 1054 | 0.95 | 0 | 0 | 0 | 0.52 | 0.52 |
| WS1 | 360 | 702 | 1078 | 2.42 | 1.56 | 1.37 | 0 | 0.52 | 0.44 |
| WS2 | 360 | 702 | 1078 | 2.42 | 1.56 | 0 | 3.6 | 0.54 | 0.44 |
| WSC | 360 | 702 | 1078 | 2.42 | 1.56 | 1.37 | 3.6 | 0.62 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenório Filho, J.R.; De Belie, N.; Snoeck, D. The Effects of Internal Curing and Shrinkage Cracking Avoidance on the Corrosion of Reinforced Concrete Walls with Superabsorbent Polymers. Appl. Sci. 2024, 14, 6901. https://doi.org/10.3390/app14166901
Tenório Filho JR, De Belie N, Snoeck D. The Effects of Internal Curing and Shrinkage Cracking Avoidance on the Corrosion of Reinforced Concrete Walls with Superabsorbent Polymers. Applied Sciences. 2024; 14(16):6901. https://doi.org/10.3390/app14166901
Chicago/Turabian StyleTenório Filho, José Roberto, Nele De Belie, and Didier Snoeck. 2024. "The Effects of Internal Curing and Shrinkage Cracking Avoidance on the Corrosion of Reinforced Concrete Walls with Superabsorbent Polymers" Applied Sciences 14, no. 16: 6901. https://doi.org/10.3390/app14166901





