Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus spp.
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Harvesting of Fungal Conidia
2.3. Synthesis and Physical Characterization of Functionalized Nanoparticles
2.4. Antifungal Evaluation and Minimal Inhibitory Concentration (MIC) Determination
2.5. Studies on Germination Attenuation of Aspergillus Conidia
2.6. Laser Scanning Confocal Microscopy for the Assessment of Conidial Viability
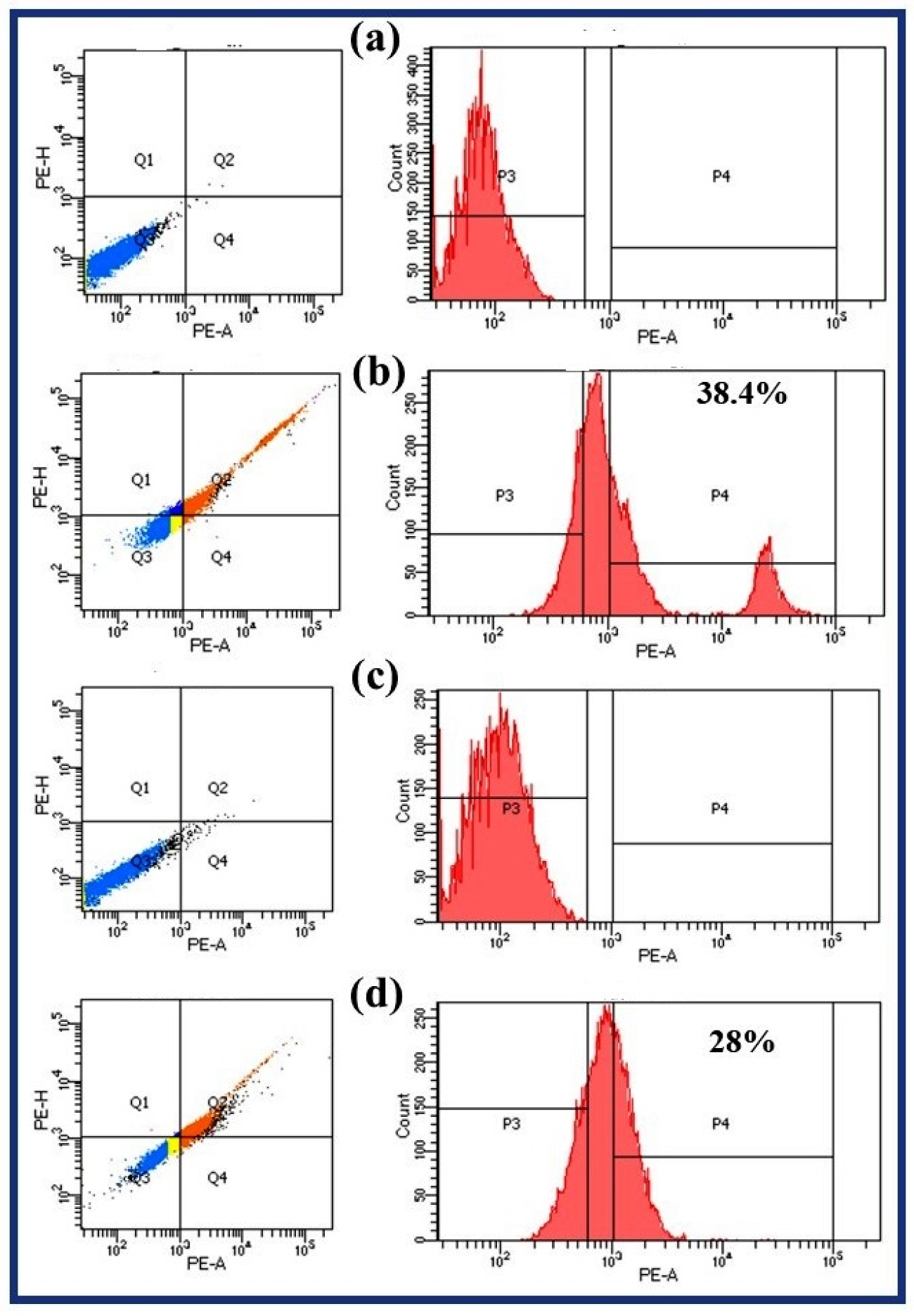
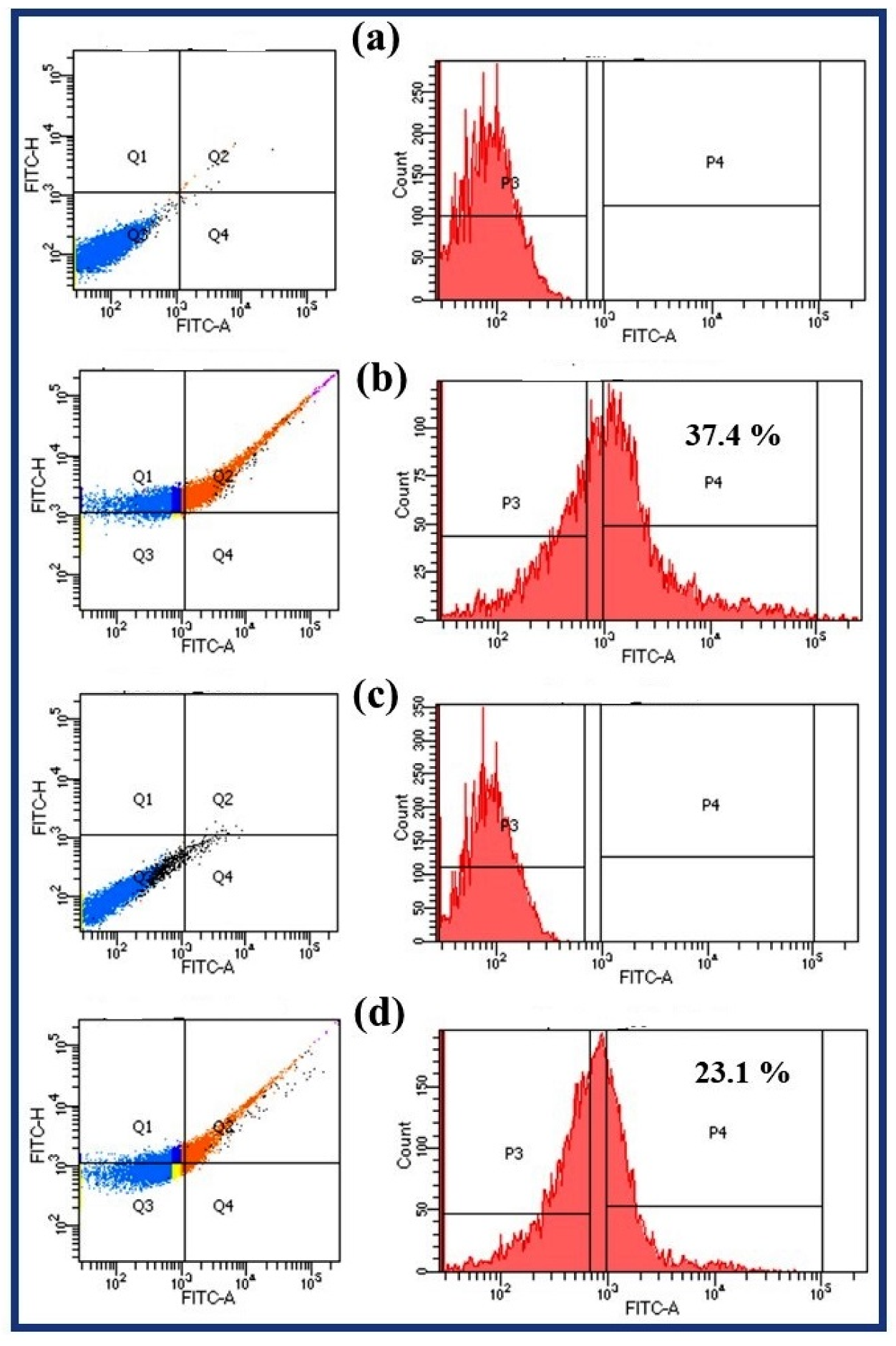
2.7. Fluorescence Activated Cell Sorting (FACS)
2.8. Endogenous Reactive Oxygen Species (ROS) Accumulation Study
2.9. Raman Spectroscopy Studies
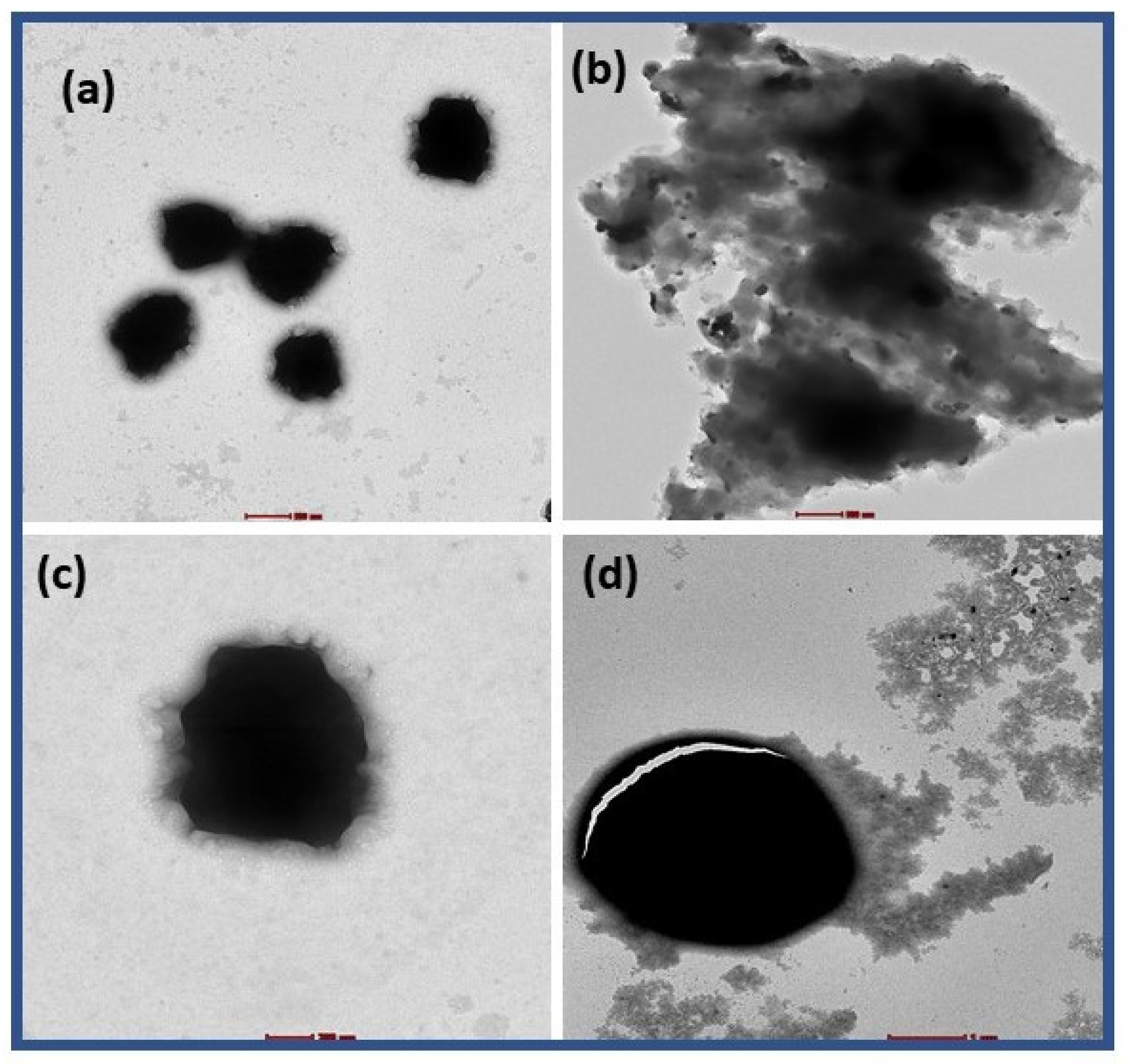
2.10. TEM Characterization of PEI-AuNP@Van Treated Conidia
3. Results
3.1. Synthesis and Characterization of Vancomycin-Functionalized Gold Nanoparticles
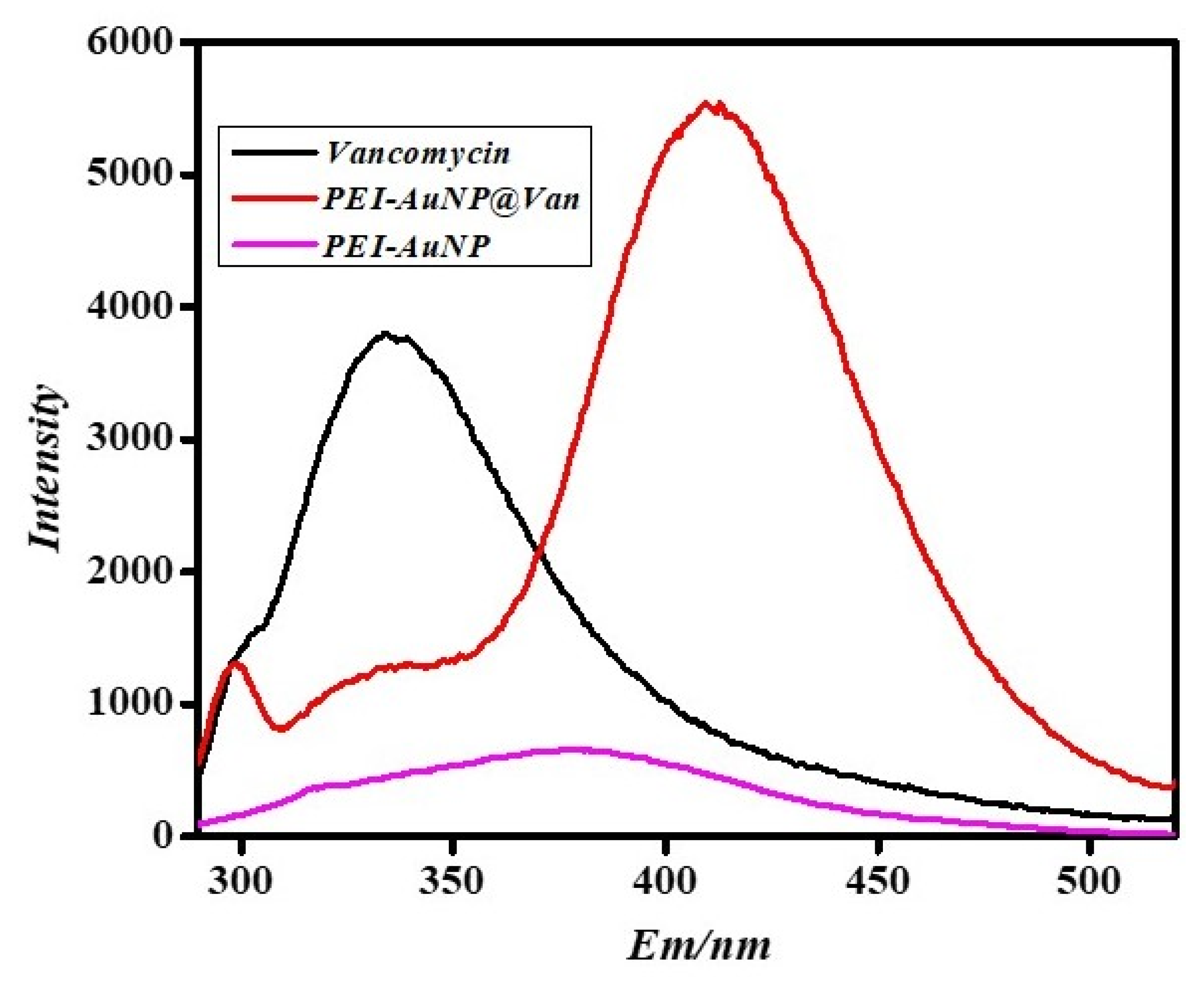
3.2. Confirmation of Vancomycin Functionalization on Gold Nanoparticles by Using Fluorescence Spectroscopy
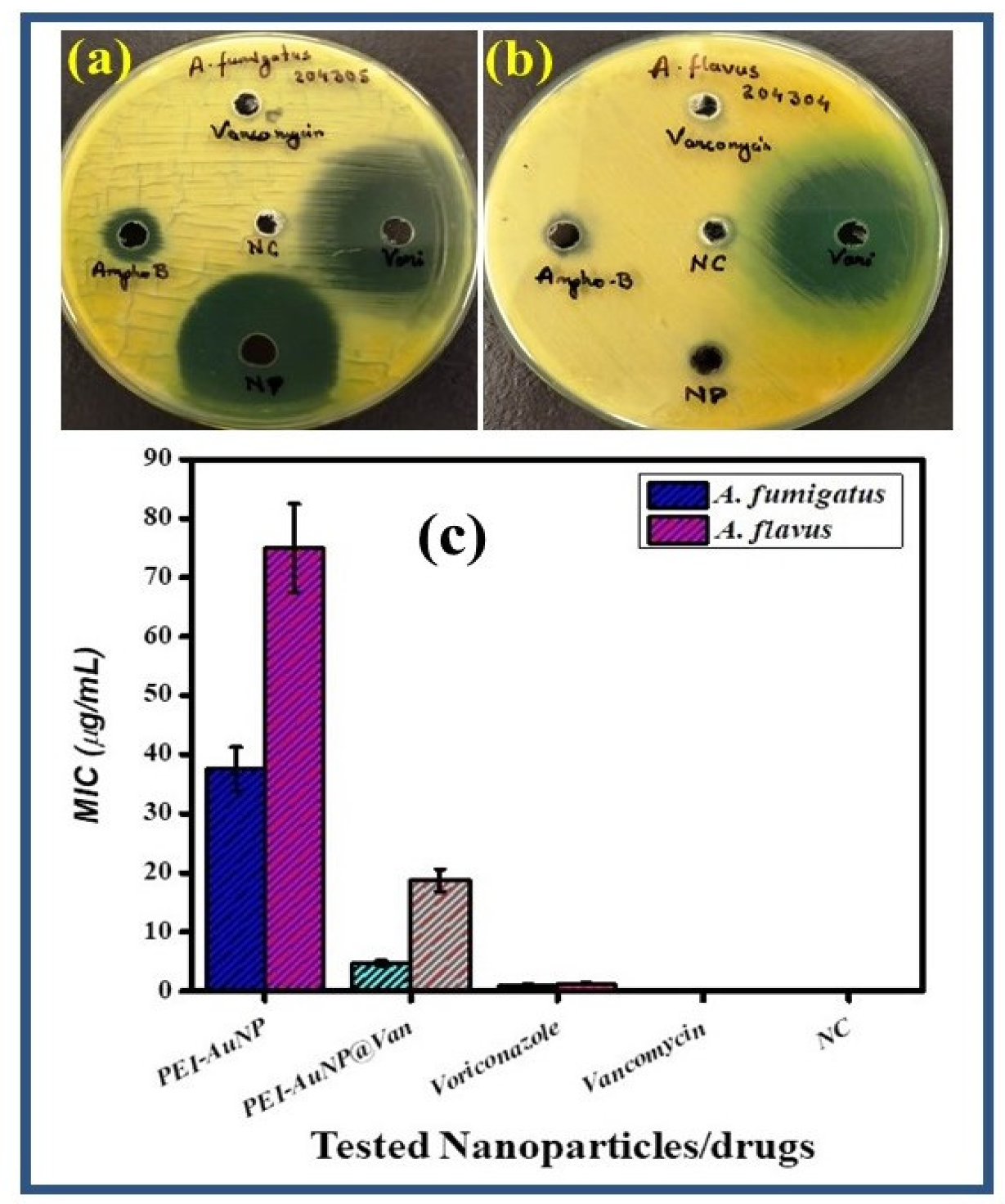
3.3. Antifungal Activity and Minimal Inhibitory Concentration (MIC)
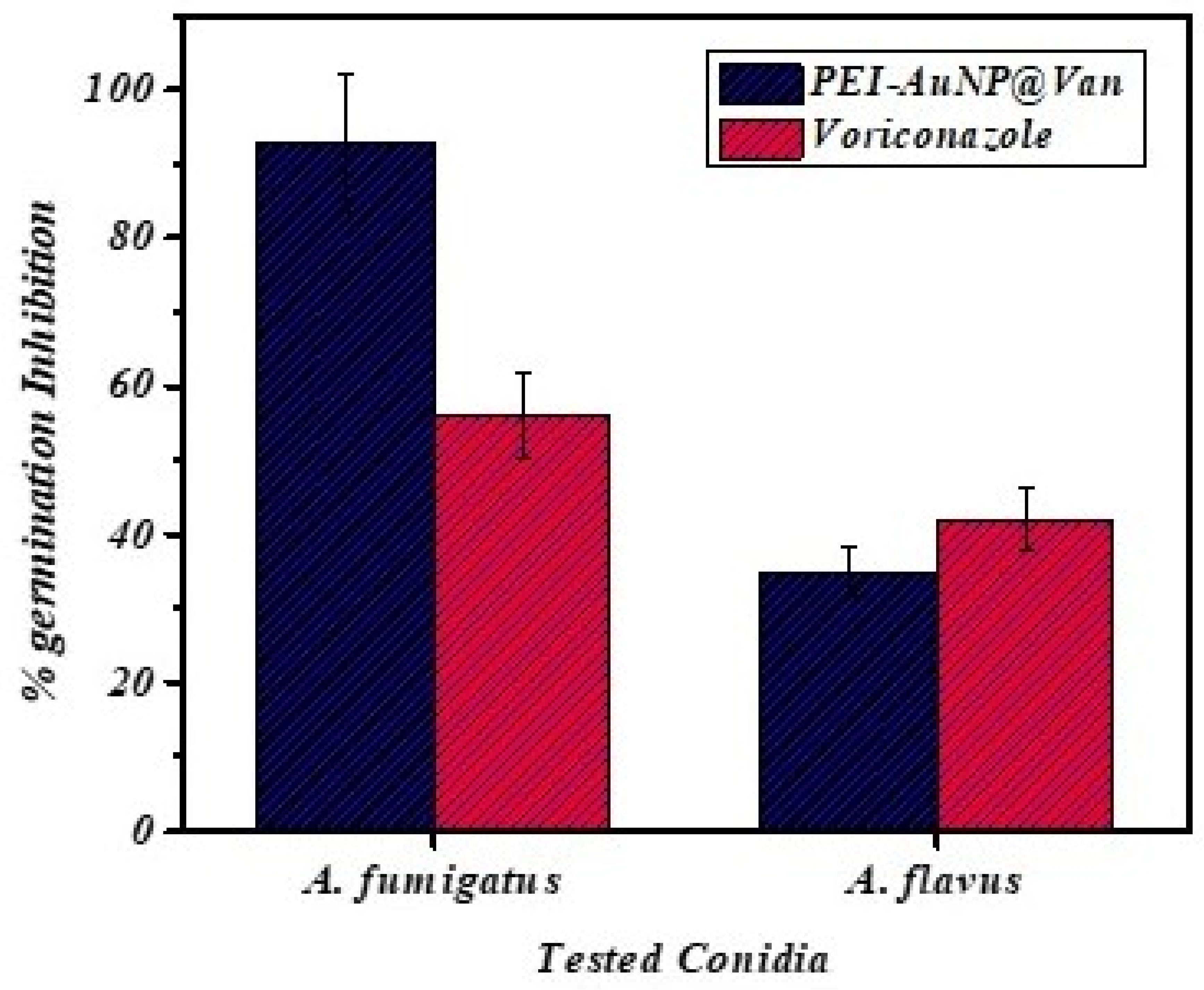
3.4. Germination Inhibition of Conidia by PEI-AuNP@Van
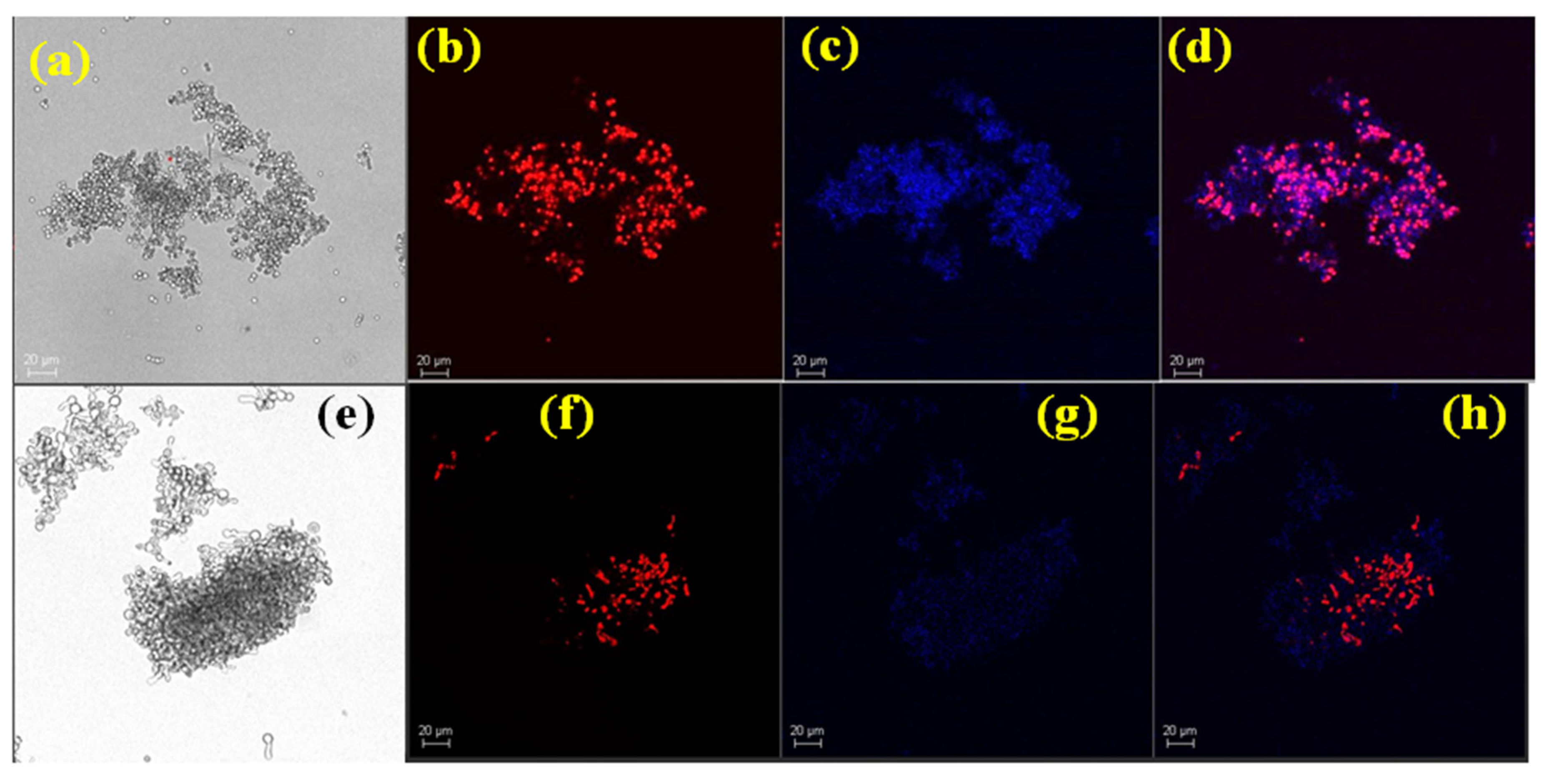
3.5. Conidial Viability Studies
4. Antifungal Mechanism
4.1. Endogenous ROS Generation
4.2. Raman Spectroscopy Analysis
4.2.1. Raman Spectroscopy Analysis of PEI-AuNP@Van and Voriconazole-Treated A. flavus Conidia
4.2.2. Raman Spectroscopy Analysis of PEI-AuNP@Van-Treated Conidia of A. fumigatus
4.3. TEM Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEI-AuNPs | Polyethyleneimine-stabilized gold nanoparticles |
| PEI-AuNP@Van | Vancomycin-functionalized and polyethyleneimine-stabilized gold nanoparticle |
| Van | Vancomycin |
| MIC | Minimum inhibitory concentration |
| PEI | Polyethyleneimine |
| µg | Microgram |
| mL | Microliter |
| AgNPs | Silver nanoparticles |
| AuNPs | Gold nanoparticles |
| TEM | Transmission Electron Microscopy |
| DLS | Dynamic light scattering |
| XRD | X-ray diffraction |
References
- Stevens, D.A.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—State of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 2003, 37 (Suppl. 3), S225–S264. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2013, 41, 621–626. [Google Scholar] [CrossRef]
- Rees, J.R.; Pinner, R.W.; Hajjeh, R.A.; Brandt, M.E.; Reingold, A.L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: Results of population-based laboratory active surveillance. Clin. Infect. Dis. 1998, 27, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Benedict, K.; Derado, G.; Mody, R.K. Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000–2013. Open Forum Infect. Dis. 2017, 4, ofw268. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.; Daneman, N. Bacterial coinfection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Liu, W. Fungal coinfection in COVID-19 patients: Evidence from a systematic review and meta-analysis. Aging 2021, 13, 7745. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control and Prevention. Antimicrobial Resistant—Aspergillus. Available online: https://www.cdc.gov/aspergillosis/php/guidance/index.html (accessed on 1 July 2024).
- Gupta, M.K.; Chandra, A.; Prakash, P.; Tilak, R. Necessity to identify the causative agent for appropriate treatment in fungal corneal ulcer: An in vitro study. J. De Mycol. Médicale 2018, 28, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.; Bentvelsen, R.G.; Schauwvlieghe, A.F.; Schalekamp, S.; van der Velden, W.J.; Kuiper, E.J.; van Paassen, J.; van der Hoven, B.; van der Lee, H.A.; Melchers, W.J.; et al. Voriconazole resistance and mortality in invasive aspergillosis: A multicenter retrospective cohort study. Clin. Infect. Dis. 2019, 68, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate antibiotic systems as antibacterial agents and antibiotic delivery platforms to fight infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold nanoparticles: Can they be the next magic bullet for multidrug-resistant bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.; Arab, H.H.; Hegazy, W.A. Synthesis of gold nanoparticles by using green machinery: Characterization and in vitro toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Whiley, H.; Köper, I. Antibiotic delivery using gold nanoparticles. SN Appl. Sci. 2020, 2, 1022. [Google Scholar] [CrossRef]
- Rahimi, H.; Roudbarmohammadi, S.; Delavari, H.H.; Roudbary, M. Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of Candida albicans isolated from patients with burn infection. Int. J. Nanomed. 2019, 14, 5323–5338. [Google Scholar] [CrossRef] [PubMed]
- Watanakunakorn, C. Mode of action and in-vitro activity of vancomycin. J. Antimicrob. Chemother. 1984, 14 (Suppl. D), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.S.; Palanisamy, S.K.; Lee, S.A. Susceptibility of Candida albicans biofilms to azithromycin, tigecycline and vancomycin and the interaction between tigecycline and antifungals. Int. J. Antimicrob. Agents 2010, 36, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shivaprakash, M.R.; Chakrabarti, A. Biofilm formation by zygomycetes: Quantification, structure and matrix composition. Microbiology 2011, 157, 2611–2618. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. Controlled synthesis of Polyethyleneimine coated gold nanoparticles: Application in glutathione sensing and nucleotide delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1191–1199. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Yadav, H.P.; Gupta, M.K.; Narayan, R.J.; Pandey, P.C. Synthesis of vancomycin functionalized fluorescent gold nanoparticles and selective sensing of mercury (II). Front. Chem. 2023, 11, 1238631. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Non dermatophyte Filamentous Fungi. In Approved Guideline, CLSI Document M51-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gupta, M.K.; Pandey, G.; Tilak, R.; Narayan, R.J.; Pandey, P.C. Size and zeta potential clicked germination attenuation and anti-sporangiospores activity of PEI-functionalized silver nanoparticles against COVID-19 associated Mucorales (Rhizopus arrhizus). Nanomaterials 2022, 12, 2235. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Yadav, S.; Sharma, K.; Firdaus, Z.; Aditi, P.; Neogi, K.; Bansal, M.; Gupta, M.K.; Shanker, A.; Singh, R.K.; et al. Quantum curcumin mediated inhibition of gingipains and mixed-biofilm of Porphyromonas gingivalis causing chronic periodontitis. RSC Adv. 2018, 8, 40426–40445. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. Int. J. Orig. Work All Asp. Raman Spectrosc. Incl. High. Order Process. Also Brillouin Rayleigh Scatt. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Freire, P.T.; Barboza, F.M.; Lima, J.A.; Melo, F.E.; Mendes Filho, J. Raman spectroscopy of amino acid crystals. Raman Spectrosc. Appl. 2017, 201, 171. [Google Scholar]
- Xia, J.; Li, W.; Sun, M.; Wang, H. Application of SERS in the Detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef]
- Raza, A.; Parveen, S.; Majeed, M.I.; Nawaz, H.; Javed, M.R.; Iqbal, M.A.; Rashid, N.; Haider, M.Z.; Ali, M.Z.; Sabir, A.; et al. Surface-enhanced Raman spectral characterization of antifungal activity of selenium and zinc based organometallic compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121903. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, E.; Jagielski, T.; Kamińska, A.; Kowalska, A.; Hryncewicz-Gwóźdź, A.; Waluk, J. Detection and identification of human fungal pathogens using surface-enhanced Raman spectroscopy and principal component analysis. Anal. Methods 2016, 8, 8427–8434. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Yi, X.; Lin, K.; Meng, S.; Zhang, X.; Jiang, C.; Tang, Y.; Wang, M.; He, J.; et al. Stain-free Gram staining classification of pathogens via single-cell Raman spectroscopy combined with machine learning. Anal. Methods 2022, 14, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- Sujith, A.; Itoh, T.; Abe, H.; Yoshida, K.I.; Kiran, M.S.; Biju, V.; Ishikawa, M. Imaging the cell wall of living single yeast cells using surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Assmann, C.; Kirchhoff, J.; Beleites, C.; Hey, J.; Kostudis, S.; Pfister, W.; Schlattmann, P.; Popp, J.; Neugebauer, U. Identification of vancomycin interaction with Enterococcus faecalis within 30 min of interaction time using Raman spectroscopy. Anal. Bioanal. Chem. 2015, 407, 8343–8352. [Google Scholar] [CrossRef]
- Neugebauer, U.; Rösch, P.; Schmitt, M.; Popp, J.; Julien, C.; Rasmussen, A.; Budich, C.; Deckert, V. On the way to nanometer-sized information of the bacterial surface by tip-enhanced Raman spectroscopy. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2006, 7, 1428–1430. [Google Scholar] [CrossRef]
- Rak, M.J.; Saadé, N.K.; Friščić, T.; Moores, A. Mechanosynthesis of ultra-small monodisperse amine-stabilized gold nanoparticles with controllable size. Green Chem. 2014, 16, 86–89. [Google Scholar] [CrossRef]
- Mitra, M.D.; Pandey, P.C. Functional trialkoxysilane mediated controlled synthesis of fluorescent gold nanoparticles and fluoremetric sensing of dopamine. Opt. Mater. 2022, 132, 112810. [Google Scholar] [CrossRef]
- Pandey, P.C.; Tiwari, A.K.; Gupta, M.K.; Pandey, G.; Narayan, R.J. Effect of the organic functionality on the synthesis and antimicrobial activity of silver nanoparticles. Nano Life 2020, 10, 2050002. [Google Scholar] [CrossRef]
- Kim, E.J.; Yeum, J.H.; Choi, J.H. Effects of polymeric stabilizers on the synthesis of gold nanoparticles. J. Mater. Sci. Technol. 2014, 30, 107–111. [Google Scholar] [CrossRef]
- Cho, T.J.; Gorham, J.M.; Pettibone, J.M.; Liu, J.; Tan, J.; Hackley, V.A. Parallel multi-parameter study of PEI-functionalized gold nanoparticle synthesis for bio-medical applications: Part 1—A critical assessment of methodology, properties, and stability. J. Nanopart. Res. 2019, 21, 188. [Google Scholar] [CrossRef]
- Li, A.; Qiu, J.; Zhou, B.; Xu, B.; Xiong, Z.; Hao, X.; Shi, X.; Cao, X. The gene transfection and endocytic uptake pathways mediated by PEGylated PEI-entrapped gold nanoparticles. Arab. J. Chem. 2020, 13, 2558–2567. [Google Scholar] [CrossRef]
- Jin, Y.; Adams, F.; Möller, J.; Isert, L.; Zimmermann, C.M.; Keul, D.; Merkel, O.M. Synthesis and Application of Low Molecular Weight PEI-Based Copolymers for siRNA Delivery with Smart Polymer Blends. Macromol. Biosci. 2023, 23, 2200409. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. Polyethylenimine-mediated synthetic insertion of gold nanoparticles into mesoporous silica nanoparticles for drug loading and biocatalysis. Biointerphases 2017, 12, 011005. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Pandey, G. Synthesis of gold nanoparticles resistant to pH and salt for biomedical applications; functional activity of organic amine. J. Mater. Res. 2016, 31, 3313–3323. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gupta, M.K.; Narayan, R.J.; Pandey, P.C. A whole cell fluorescence quenching-based approach for the investigation of polyethyleneimine functionalized silver nanoparticles interaction with Candida albicans. Front. Microbiol. 2023, 14, 1131122. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, G.; Pellegrino, S.; Bucci, R.; Nava, D.; Gandolfi, R.; Christodoulou, M.S.; Rimoldi, I. Vancomycin-Iridium (III) Interaction: An Unexplored Route for Enantioselective Imine Reduction. Molecules 2019, 24, 2771. [Google Scholar] [CrossRef] [PubMed]
- Świątek, M.; Valensin, D.; Migliorini, C.; Gaggelli, E.; Valensin, G.; Jeżowska-Bojczuk, M. Unusual binding ability of vancomycin towards Cu 2+ ions. Dalton Trans. 2005, 23, 3808–3813. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, T.; Delangle, A.; Simenel, C.; Coddeville, B.; van Vliet, S.J.; Van Kooyk, Y.; Bozza, S.; Moretti, S.; Schwarz, F.; Trichot, C.; et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011, 7, e1002372. [Google Scholar] [CrossRef]
- Wong, S.S.; Venugopalan, L.P.; Beaussart, A.; Karnam, A.; Mohammed, M.R.; Jayapal, J.M.; Bretagne, S.; Bayry, J.; Prajna, L.; Kuppamuthu, D.; et al. Species-specific immunological reactivities depend on the cell-wall organization of the two Aspergillus, Aspergillus fumigatus and A. flavus. Front. Cell. Infect. Microbiol. 2021, 11, 643312. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Adv. Protoc. Oxidative Stress II 2010, 594, 57–72. [Google Scholar]
- Oliveira, M.; Pereira, C.; Bessa, C.; Araujo, R.; Saraiva, L. Hydrogen peroxide-induced secondary necrosis in conidia of Aspergillus fumigatus. Can. J. Microbiol. 2016, 62, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly; CRC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioassays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikhil, A.; Tiwari, A.K.; Tilak, R.; Kumar, S.; Bharti, P.S.; Pandey, P.C.; Narayan, R.J.; Gupta, M.K. Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus spp. Appl. Sci. 2024, 14, 6926. https://doi.org/10.3390/app14166926
Nikhil A, Tiwari AK, Tilak R, Kumar S, Bharti PS, Pandey PC, Narayan RJ, Gupta MK. Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus spp. Applied Sciences. 2024; 14(16):6926. https://doi.org/10.3390/app14166926
Chicago/Turabian StyleNikhil, Aishwarya, Atul Kumar Tiwari, Ragini Tilak, Saroj Kumar, Prahlad Singh Bharti, Prem C. Pandey, Roger J. Narayan, and Munesh Kumar Gupta. 2024. "Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus spp." Applied Sciences 14, no. 16: 6926. https://doi.org/10.3390/app14166926
APA StyleNikhil, A., Tiwari, A. K., Tilak, R., Kumar, S., Bharti, P. S., Pandey, P. C., Narayan, R. J., & Gupta, M. K. (2024). Vancomycin-Conjugated Polyethyleneimine-Stabilized Gold Nanoparticles Attenuate Germination and Show Potent Antifungal Activity against Aspergillus spp. Applied Sciences, 14(16), 6926. https://doi.org/10.3390/app14166926







