Abstract
The increase in emissions of toxic gasses such as hydrogen sulfide (H2S) and carbon dioxide (CO2), resulting from growing urbanization and industrialization, has caused environmental and public health problems, making the implementation of air purification techniques through adsorption important. Thus, modeling the gas adsorption process is fundamental for good agreement with experimental data, employing mathematical models that enable the prediction of adsorption capacity. In this way, the present work aimed to compare different analytical breakthrough curve models (Thomas, Yoon–Nelson, Adams–Bohart, and Yan) for the adsorption of H2S and CO2 in fixed-bed columns, using experimental data from the literature, estimating the curve parameters through the Markov Chain Monte Carlo (MCMC) method with the Metropolis–Hastings algorithm, and ranking using the determination coefficients (R2 and R2Adjusted) and the Bayesian Information Criterion (BIC). The models showed better agreement using the estimation of maximum adsorption capacity (qs, N0) and the constants of each model (kth, kyn, and kba). In the adsorption of H2S, the Yan model stood out for its precision in estimating qs. For the adsorption of CO2, the Adams–Bohart model achieved better results with the estimation of N0, along with the Yoon–Nelson model. Furthermore, the use of this method allows for a reduction in computational effort compared to models based on complex differential equations.
1. Introduction
Increasing urbanization and industrialization worldwide have heightened concerns about air quality due to rising emissions of atmospheric pollutants. With technological advancements and the expansion of industrial activities, the release of substances such as hydrogen sulfide or hydrogen sulfide gas (H2S), nitric oxide (NO), nitrogen dioxide (NO2), carbon dioxide (CO2), and sulfur dioxide (SO2) has become more frequent. These pollutants are known for their adverse impact on both the environment and human health [1].
Hydrogen sulfide is released primarily during industrial processes such as petroleum refineries and water and sewage treatment plants, and it occurs naturally in biological processes such as organic matter decomposition in waste deposits and wetlands. Furthermore, it is a substance known for its toxicity and can pose significant risks to human health when present in high concentrations [2].
On the other hand, carbon dioxide (CO2), a colorless, odorless, and non-flammable gas, is produced on a large scale by the industry, mainly as a byproduct in the production of ammonia and hydrogen, as well as in the burning of fossil fuels and petroleum refining. Although it can be used in some chemical reactions, CO2 is known as the primary greenhouse gas, contributing significantly to climate change and global warming, becoming a globally recognized environmental concern [3,4,5].
In this context, gas adsorption has therefore become an area of great interest due to the need to develop more effective air purification systems. This process involves the adherence of atoms, molecules, or ions of substances (adsorbates) to a solid surface (adsorbent), resulting in the formation of a molecular or atomic layer on this surface. Adsorption has various applications, including water purification, separation of chemical compounds, and especially the capture of polluting gasses [6,7,8,9].
The use of fixed-bed columns for adsorption is preferred in the industry due to their ability to treat large quantities of pollutants in a continuous flow system, enabling their implementation on a large scale. These columns provide breakthrough curves that describe the variation in pollutant concentration over time. Therefore, mathematical modeling of this system is important for understanding, analyzing, and optimizing separation and purification processes [10,11,12].
However, there are challenges in modeling continuous adsorption systems due to their non-linearity. Parameter estimation of the models often relies on linear regression techniques, including the method of least squares; however, such methods can distort parameter interpretation and compromise model assumptions [13].
Bayesian inference emerges as a promising alternative to least squares fitting, surpassing its limitations. This technique provides a method to combine available experimental data with mathematical modeling and the prior probability distribution of parameters. In general, it involves a statistical analysis of the posterior probability density, which represents the conditional probability of the parameters based on observations. The posterior probability density integrates the prior density of parameters—the information available before measurements—with the likelihood function, which expresses the probability of observations given the parameters in question [14,15].
The posterior probability can be approximated through the Markov Chain Monte Carlo (MCMC) method, a computational tool for approximating integrals and generating samples from a posterior probability. The Metropolis–Hastings algorithm, widely used in MCMC, is based on the acceptance–rejection method and is effective in handling complex probability distributions, enabling random sampling from any probability distribution [16,17].
Therefore, the main objective of the study was to compare different breakthrough curve models for the adsorption of H2S and CO2 in a fixed-bed column system using data obtained from the literature, estimating the parameters of the breakthrough curves through the Markov Chain Monte Carlo method (MCMC) with the application of the Metropolis–Hastings algorithm.
2. Analytical Models of Breakthrough Curves
When conducting dynamic adsorption experiments in fixed-bed columns, the breakthrough curve is generated as a response. This curve demonstrates the interaction between kinetic and thermodynamic effects of the solute with the adsorbent along the column as the gas or liquid flows. Throughout the process, factors such as adsorption capacity, selectivity, adsorption rate, initial concentration, and fluid velocity can significantly influence the position and shape of the breakthrough curve [11].
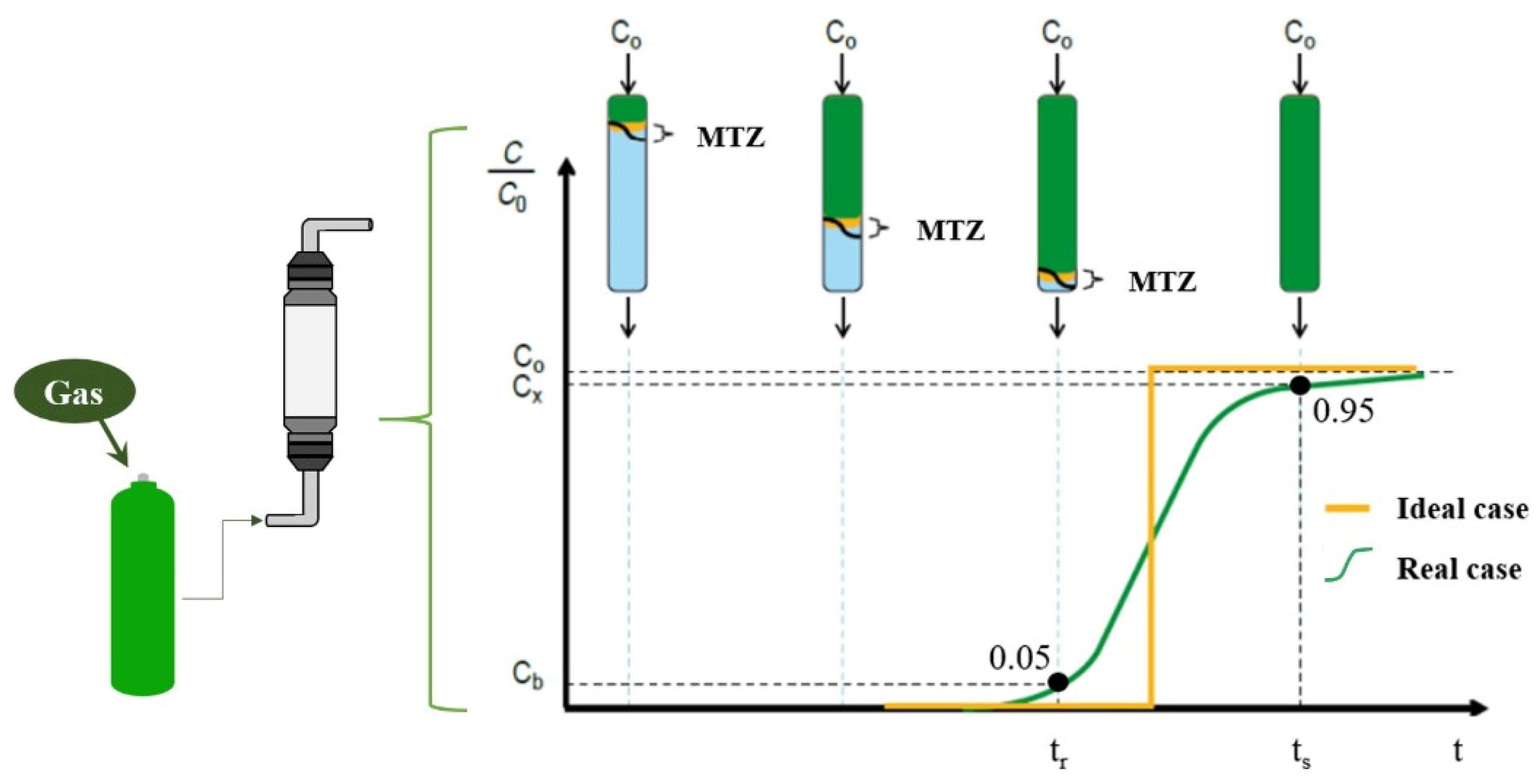
Figure 1 shows a schematic of the fixed-bed adsorption process, where the adsorbate (gas), with initial concentration C0, transfers from the solution to the adsorbent surface. Initially, the concentration at the outlet is zero, but it gradually increases as the sites become saturated. The breakthrough curve, which describes this process, is obtained by relating the concentration of the component at the inlet and outlet of the column (C/C0) over time. The region of highest component removal, known as the mass transfer zone (MTZ), can vary with temperature, component concentration, flow rate, and mass transfer rates [18,19].
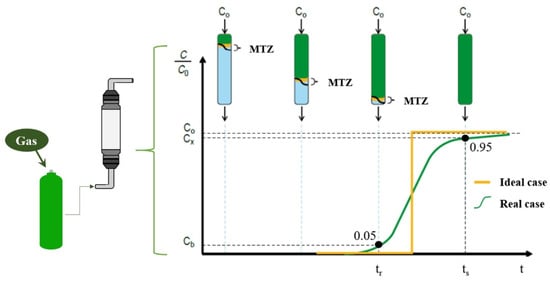
Figure 1.
Schematic representation of the adsorption process in a fixed bed.
The mass transfer region is typically limited by values between 0.95 and 0.05 of the relative concentration (C/C0). Breakthrough (tr) occurs when the relative concentration reaches 0.05. If adsorption continues beyond this point, the outlet concentration increases until it reaches the inlet concentration (C/C0 = 1.0), indicating bed saturation (ts) [18].
Modeling these curves is essential for predicting variables and determining optimal conditions in fixed-bed columns, whether through a theoretical model using mass transfer coefficients and diffusivity or an analytical approach involving experiments with columns of varying lengths and parameters [20,21]. Thus, for fitting to experimental data, the analytical breakthrough curve models of Thomas, Yoon–Nelson, Adams–Bohart, and Yan were used.
2.1. Thomas Model
The Thomas model, initially proposed in 1944 and later refined in 1948, is an approach used to describe adsorption in fixed-beds. In the initial formulation, the capture rate is considered an ion exchange reaction between ionic species “A” in the fluid phase and ionic species “B” in the adsorbent phase, governed by reaction rate constants for ion exchange and regeneration [22,23].
The model is based on Langmuir kinetics for adsorption–desorption and does not consider axial dispersion within the column. The adsorption rate is governed by reversible second-order reaction kinetics, and the model assumes a constant separation factor, applicable to both favorable and unfavorable isotherms [23].
2.2. Yoon–Nelson Model
The study by Yoon and Nelson published in 1984 was developed with the aim of applying gas adsorption kinetics principles to develop a new theoretical model related to the adsorption and breakthrough of vapors or contaminant gasses on solid adsorbents, specifically activated carbon [24].
The model proposed by Yoon and Nelson is based on the idea that the rate at which the probability of adsorption for each adsorbate molecule decreases over time is proportional to both the initial probability of adsorption and the probability of the adsorbate advancing into the interior of the adsorbent. A distinctive feature of this model is its simplification compared to more complex formulations involving mass balance along the adsorption bed, eliminating the need for detailed information on contaminant characteristics, adsorbent type, or physical bed properties [24,25].
2.3. Adams–Bohart Model
In 1920, Adams and Bohart conducted the first investigation of fixed-bed dynamics by analyzing the adsorption of chlorine gas in columns filled with activated carbon. Within this context, the proposed model considers that the adsorption rate is directly linked to the remaining capacity of the adsorbent and the concentrations of the adsorbed species. Additionally, it is recognized that adsorption equilibrium is not instantaneous and there is no axial dispersion in the adsorption bed [26].
The model also posits that the adsorbate is adsorbed irreversibly, with a local removal rate proportional to both the residual capacity of the adsorbent and the adsorbate concentration in the gas phase. This implies that the adsorption rate varies with the availability of adsorption sites on the adsorbent and the concentration of the adsorbate in the gas phase [26,27].
2.4. Yan Model
The model developed by Yan, known as the “dose-response” model, aimed to enhance the accuracy of mathematical fitting compared to the Thomas model, with a special focus on scenarios characterized by very long or very short operating times. The model considers the absence of axial dispersion, highly reduced internal and external diffusion resistances, second-order reversible reaction kinetics (pseudo-second order), and Langmuir isotherm [28].
3. Methodology
The estimation of parameters for the analytical models (Thomas, Yoon–Nelson, Adams–Bohart, and Yan) of fixed-bed adsorption for hydrogen sulfide (H2S) and carbon dioxide (CO2) gasses was conducted using experimental data from [29], who analyzed the efficiency of babassu biochar as an adsorbent for H2S removal from air under low-pressure conditions in a fixed-bed column, and [30], who conducted an experimental and simulation study using a fixed-bed column for CO2 adsorption using activated carbon. The authors employed the Linear Driving Force (LDF) model for parameter estimation, which is based on a system of partial differential equations that requires greater complexity for equation solving and subsequent estimation. The development of the model used by the authors is detailed in the Supplementary Material [31].
In this perspective, analytical breakthrough curve models of Thomas, Yoon–Nelson, Adams–Bohart, and Yan (dose–response) were used, aimed at assessing the models’ ability to achieve good agreement with experimental data, reducing computational costs, enabling faster analysis, and avoiding the complexity of partial differential equations. This approach is advantageous when working with large datasets or when multiple simulations are required [32].
Each model involves unknown parameters that cannot be directly determined from the observed or available data. Thus, these parameters were inferred through Bayesian inference using the Markov Chain Monte Carlo method (MCMC) with the Metropolis–Hastings algorithm.
In Table 1 are the operating conditions used in the adsorption process; Table 2 presents a summary of the models used with their respective equations, parameters, and assumptions; and Table 3 presents the models with the parameters to be estimated.

Table 1.
Operating conditions established in the works of [29,30].

Table 2.
Summary of the models employed in the study.

Table 3.
Parameters to be estimated.
Markov Chain Monte Carlo (MCMC)
In parameter estimation using the MCMC method with the Metropolis–Hastings algorithm, a uniform prior probability distribution was employed with a lower limit of zero (since all parameters are physically non-negative) and an upper limit chosen to be high (10 times the literature value), as described in Equation (1). This choice is justified by the lack of prior knowledge of such parameters; therefore, a uniform prior probability distribution is chosen because it is equiprobable.
Regarding likelihood, it is assumed that the experimental data follow a Gaussian probability distribution and that the experimental information is uncorrelated (Equation (2)):
where is the variance of the measurement uncertainty; is the solution of the direct model calculated with the parameters to be estimated; is the measured value; and is the value of the parameters of interest.
The sample acceptance/rejection algorithm used was Metropolis–Hastings. In this way, the number of states (n) is defined, and then the interaction counter i = 0 is initiated, and an initial value is defined. A candidate value is generated from the distribution P, according to Equation (3), with w being the search step [15,16,33,34].
Subsequently, the acceptance probability of the candidate value is calculated, according to Equation (4). An auxiliary random sample is generated from a uniform distribution u~U (0,1), so that if , the new value is accepted and set as ; otherwise, . Finally, the counter is incremented to and returns to the third step, where a candidate value is generated from the distribution P.
For the selection of models with the best agreement to the experimental data, classical statistical metrics were applied, such as the coefficient of determination (R2) and the adjusted coefficient of determination (R2Adjusted), presented in Equations (5) and (6), as well as the Bayesian Information Criterion (BIC), represented by Equation (7). Accordingly, a good fit is indicated by R2 values close to 1, reflecting a high capacity of the model to represent the experimental data, while lower BIC values suggest a better model fit.
In the equations for the coefficients of determination (5–6), SSE represents the sum of squared errors, SST the total sum of squares, Y the observed data from i = 1 to the number of observations in the sample (n), the estimated data, the mean of the observed data, and NP the number of parameters to be estimated by the model. In the BIC equation, represents the likelihood, NP the number of parameters, and J the number of observations in the sample.
4. Results and Discussion
In the development of the parameter estimation method, experimental data from [29] for H2S adsorption and from [30] for CO2 adsorption were utilized. The Markov Chain Monte Carlo method with the Metropolis–Hastings algorithm was applied to obtain the parameters. The results obtained for the estimation of maximum adsorption capacity (qs, N0) were compared to those obtained deterministically in the literature. Subsequently, based on selection metrics, it was determined which models showed better agreement with the experimental data.
Thus, the number of states of the Markov chain (n) was set in a range from 2000 to 10,000, and the search step (w) used ranged from 0.003 to 0.03, ensuring the stabilization of the chains. For uncertainties, a variance of 1% of the maximum experimental measurement was applied, which is related to the ratio between concentrations (C/C0) in the breakthrough curves.
The elements used for parameter estimation such as the number of states (n), search step (w), and heating states (aq) for each model, with different initial concentrations, are included in the Supplementary Material. Table 4 presents the parameter estimates of the analytical models for H2S gas adsorption with and without estimation of the maximum adsorption capacity.

Table 4.
Parameter estimation of analytical models for H2S gas.
In the fitting analysis of the Thomas, Yoon–Nelson, Adams–Bohart, and Yan models for H2S gas adsorption, it was observed that all models, except Yoon–Nelson, showed good agreement in estimating the maximum adsorption capacity (qs, N0) for the lowest initial concentration (C0) of H2S (1.327 mg/L). Although the Yoon–Nelson model does not include qs in its equation for this estimation, it also demonstrated a good fit for the respective concentration.
Among the analyzed models, the Yan model showed the best fit using the estimation of qs, with an R2 of 0.9982, R2Adjusted of 0.9981, and BIC of 11.2732. On the other hand, the Adams–Bohart model was the worst fit without the estimation of N0, with an R2 e R2Adjusted of 0.9331 and 0.9284, respectively, and a BIC of 3.6145×103.
The Thomas model, commonly used to describe adsorption in fixed-bed systems, showed a higher estimated qs for the lowest concentration (1.327 mg/L), suggesting that the model is more effective in describing the H2S adsorption process at lower concentrations. The kinetic constant of the model (kth) decreased with increasing concentration, indicating that the adsorption rate decreases as the initial concentration increases. With the qs, estimation, the model exhibited high R2 and R2Adjusted values, suggesting a good fit to the experimental data. The BIC values were relatively low, and the coefficients of determination remained high even without qs estimation, but they were lower compared to when qs was estimated.
The Yoon–Nelson model also showed a good fit with high R2 values for both concentrations, although the model does not include the parameter qs. It demonstrated a good representation of the adsorption data. The kinetic constant kyn was higher for the higher concentration (2.577 mg/L), indicating a higher probability of adsorption per unit time, while τ was lower at higher concentrations, suggesting faster saturation of the adsorbent bed.
For the Adams–Bohart model, it was observed that the constant kba decreased with increasing C0 (whether estimating N0 or not). This behavior suggests that the adsorption rate tends to be higher at lower concentrations, which can be explained by the saturation of adsorption sites. At lower initial concentrations, available adsorption sites fill up more quickly, thereby increasing the adsorption rate represented by kba [21,22,35]. Furthermore, when estimating N0, the kba constant adjusted to reflect the change in C0, reinforcing the inverse relationship between the kinetic constant and the initial concentration of the adsorbate.
In the Yan model, it was observed that ay decreased with increasing initial adsorbate concentration. This behavior is associated with the possibility that, for H2S adsorption, the maximum adsorption capacity decreases as the initial concentration increases. Additionally, a decrease in (qs) with increasing (C0) was observed, which may occur due to competition among adsorbate molecules for adsorption sites [36].
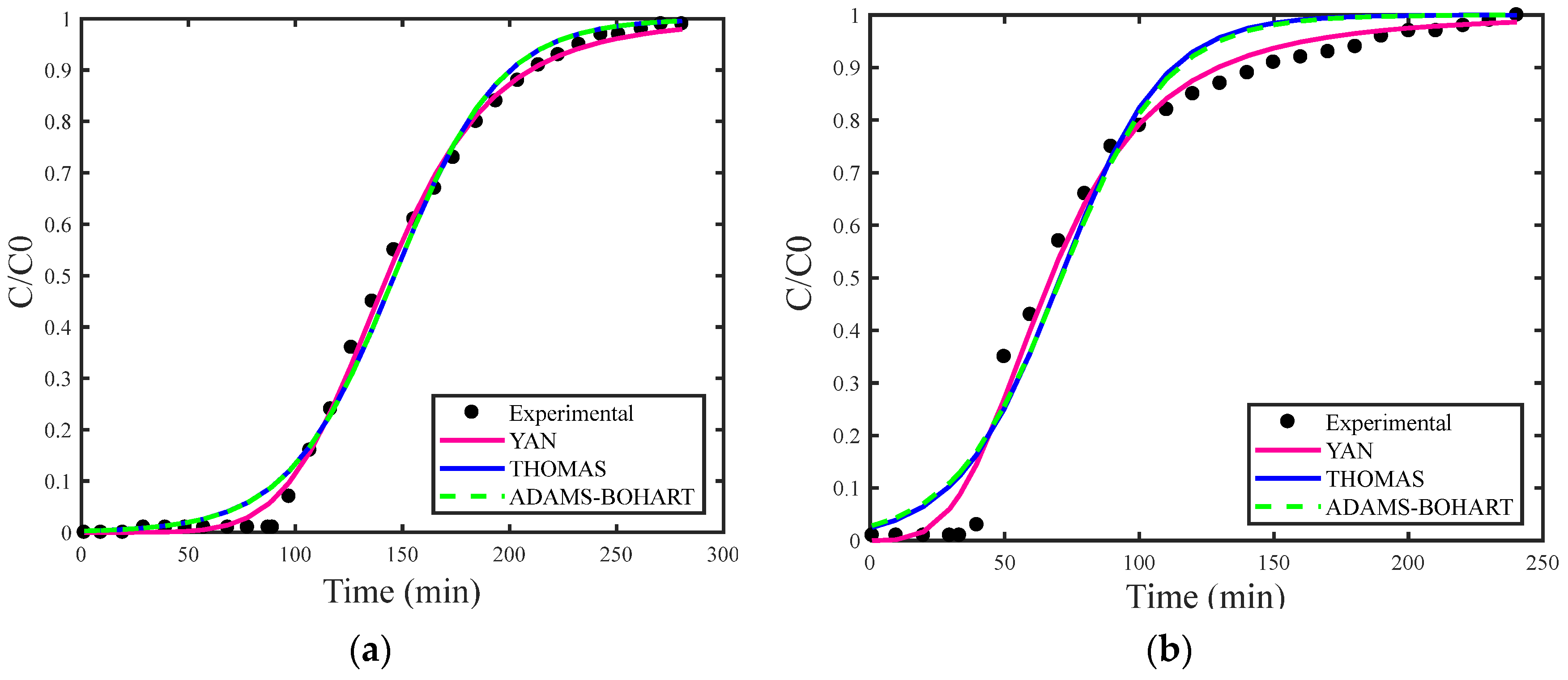
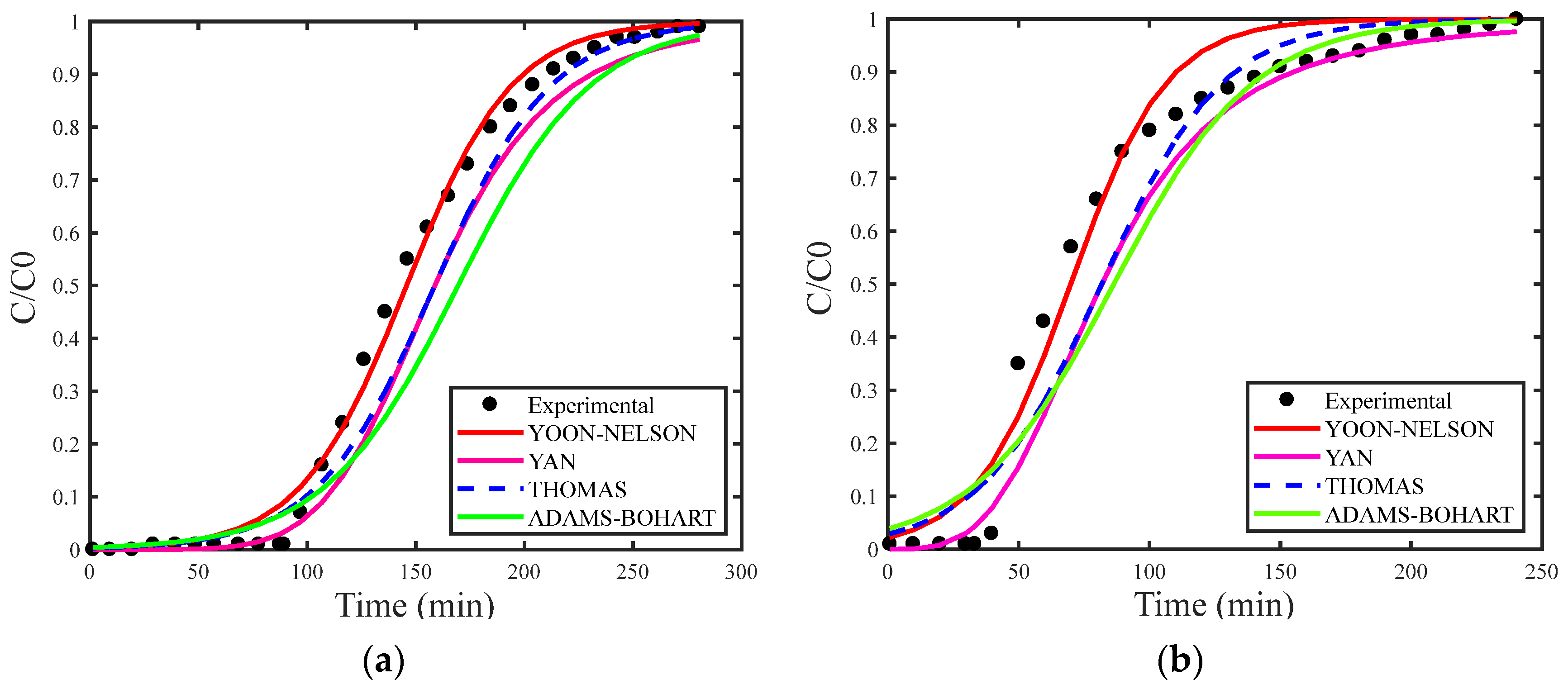
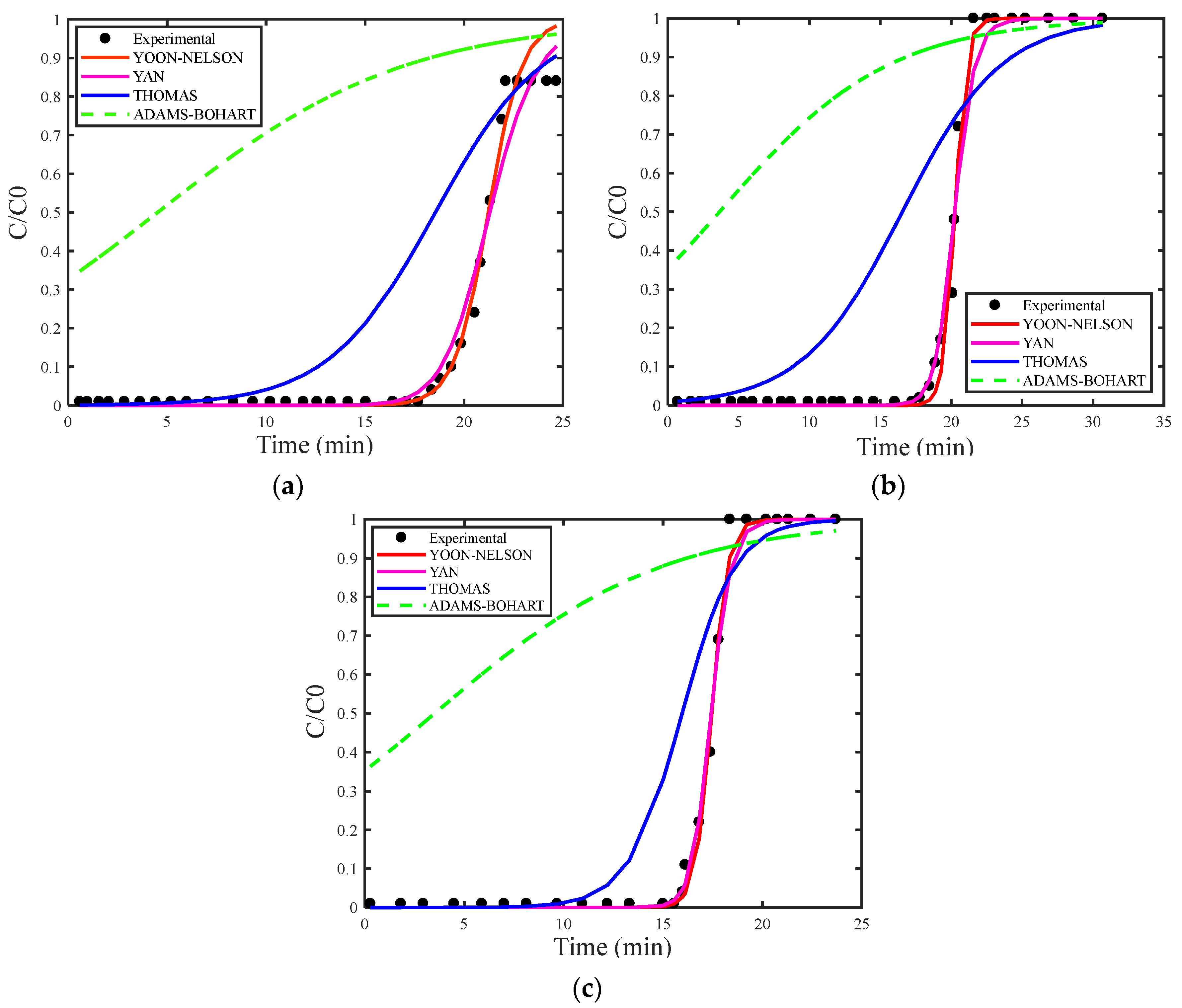
Figure 2a,b presents breakthrough curves simulated using the estimation of maximum adsorption capacity for the Thomas (qs), Adams–Bohart (N0) and Yan (qs). Figure 3a,b displays the simulated breakthrough curves with deterministic qs and N0 obtained from the study by [29], both including the corresponding experimental data curve for the Thomas, Yoon–Nelson, Adams–Bohart, and Yan models.
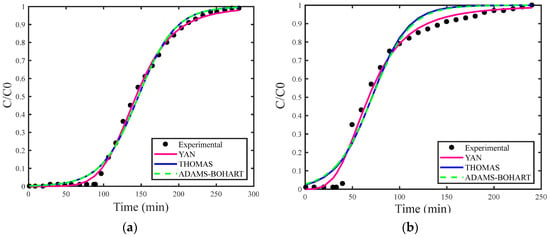
Figure 2.
(a) Breakthrough curves for concentration of 1.327 mg/L and (b) concentration of 2.577 mg/L with estimated qs and N0.
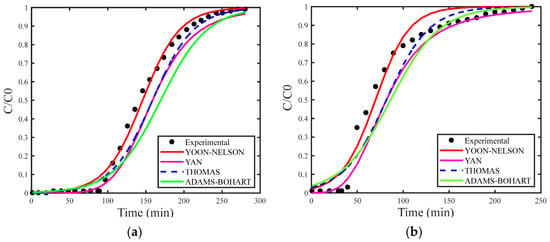
Figure 3.
(a) Breakthrough curves for concentration of 1.327 mg/L and (b) concentration of 2.577 mg/L with deterministic qs and N0.
In general, the models showed better fit when estimating the maximum adsorption capacity, reflecting a more accurate description of the adsorption behavior. Among the models analyzed, the Yan model stood out for providing the best fit. This model was developed to reduce the uncertainty associated with the Thomas model, particularly effective in situations involving extremely long or short operating periods. The Yan model considers the absence of axial dispersion, extremely low internal and external diffusion resistances, reversible second-order reaction kinetics (pseudo-second-order), and Langmuir isotherm [28].
Additionally, Table 5 presents parameter estimates of analytical models for carbon dioxide (CO2) adsorption with and without estimation of maximum adsorption capacity (qs, N0).

Table 5.
Parameter estimation of analytical models for CO2 gas.
For CO2 adsorption, the Thomas, Adams–Bohart, and Yan models showed good fits in estimating the maximum adsorption capacity for the highest concentration (35 mg/L), as indicated by the determination coefficients (R2 e R2Adjusted) and the Bayesian Information Criterion (BIC). The Yoon–Nelson model, although it does not estimate qs, also demonstrated a satisfactory fit for this concentration.
By analyzing the selection metrics (R2 e R2Adjusted e BIC), the Adams–Bohart models (estimating N0) and Yoon–Nelson (without estimating qs) showed the best fits for the 35 mg/L, with R2 and R2Adjusted of 0.9951 and BIC varying between 153.3194 and 153.3209. In contrast, the Adams–Bohart model, without estimating N0, exhibited the worst fit for the lowest concentration (18 mg/L), with an R2 of 0.2020 and R2Adjusted of 0.1522.
Generally, the maximum adsorption capacity for CO2 concentrations increased with the rise of (C0). In the paper [37], the authors state that the concentration gradient between the adsorbate and the solute is higher at elevated concentrations, increasing mass transfer and consequently the adsorption capacity.
The Thomas model provided qs estimates close to the deterministic qs. Moreover, it was observed that, in general, the kinetic constant (kth) decreased with increasing C0, suggesting that the adsorption rate tends to decrease at higher concentrations due to adsorbent saturation, a dynamic also reported by [38].
On the other hand, in the Yoon–Nelson model, the constant kyn increased with the rise of C0. Thus, it was possible to observe a decrease in the parameter τ, indicating a shorter contact time required for adsorption to reach half of its capacity, relating to a more dynamic behavior of the adsorbent at higher concentrations.
In the Adams–Bohart model, when estimating N0, it was noted that the constant kba exhibited non-linear behavior in relation to C0. This behavior may occur due to the adsorption dynamics at different concentrations and the possible interference of interactions between CO2 molecules at high concentrations. Furthermore, the Yan model provided similar maximum adsorption capacity estimates to those of the Thomas model, also indicating higher qs at higher concentrations. It was observed that the constant ay decreased with increasing concentration, both when estimating qs and using deterministic qs.
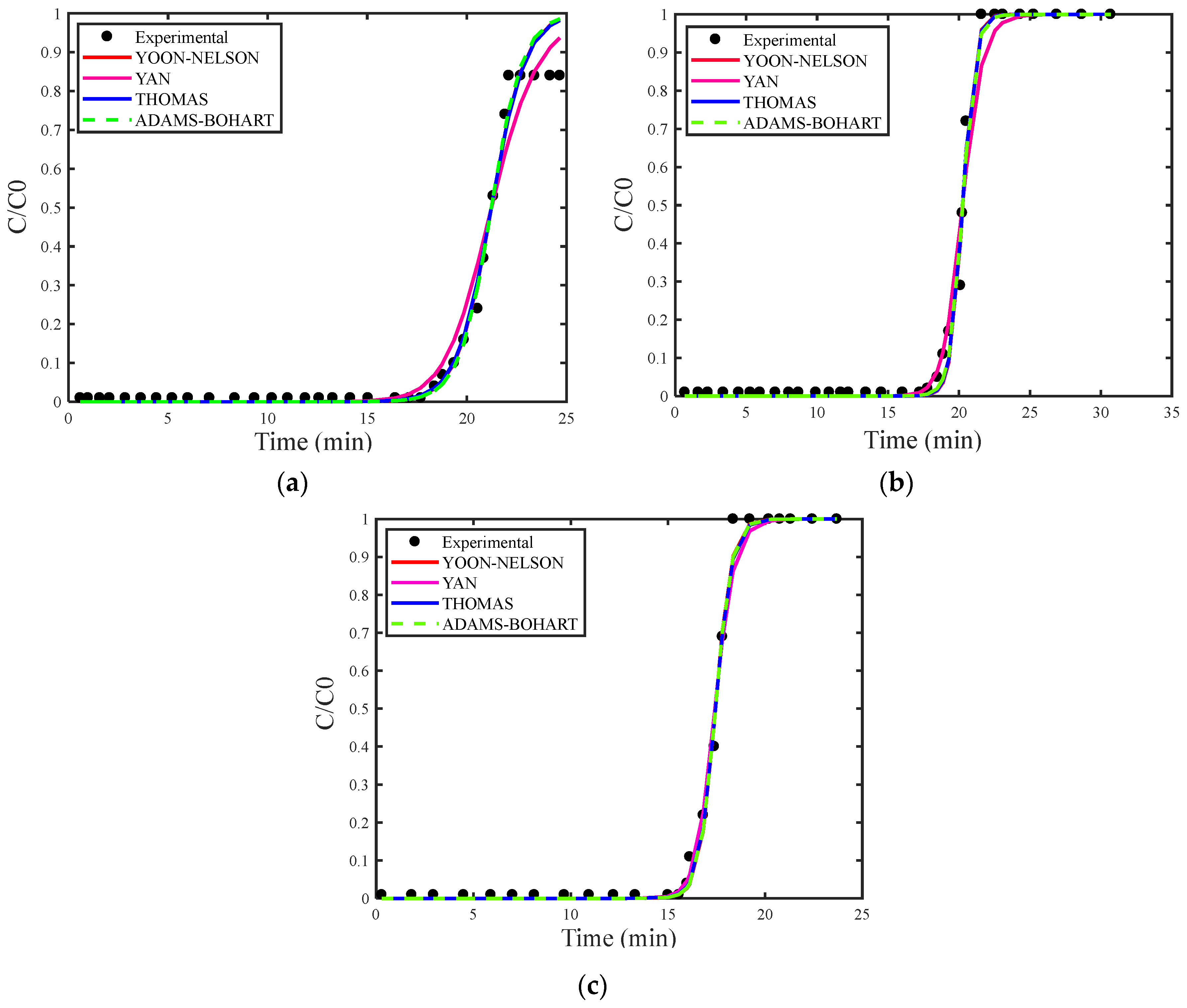
Figure 4a–c show the breakthrough curves simulated using the estimated maximum adsorption capacity for the Thomas (qs), Adams–Bohart (N0) and Yan (qs) models. Figure 5a–c present the breakthrough curves estimated with the deterministic values of qs and N0 obtained from the study by [30], covering the Thomas, Yoon–Nelson, Adams–Bohart, and Yan models, including the curve corresponding to the experimental data for proper comparison.
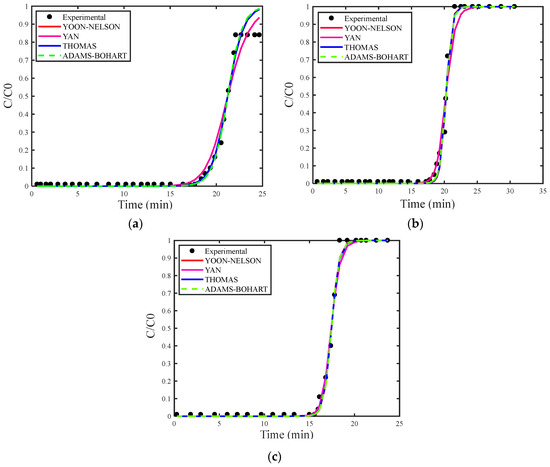
Figure 4.
(a) Concentration of 18 mg/L, (b) concentration of 29 mg/L, and (c) concentration of 35 mg/L with the estimation of qs and N0.
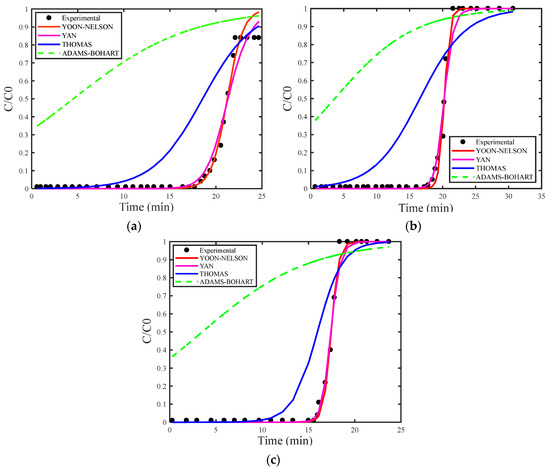
Figure 5.
(a) Concentration of 18 mg/L, (b) concentration of 29 mg/L, and (c) concentration of 35 mg/L with deterministic qs and N0.
As in the analysis for H2S gas, the breakthrough curves for CO2 gas adsorption also showed superior fitting when estimating the maximum adsorption capacity. Among the models examined, the Adams–Bohart model stood out for providing the best fit with the estimate of maximum adsorption capacity at the highest concentration (35 mg/L). However, this model performed the worst in describing the adsorption process without this estimate.
The Adams–Bohart model assumes that the adsorption rate is directly related to the residual capacity of the adsorbent and the concentrations of the adsorbed species, recognizing that adsorption equilibrium does not occur instantly and that there is no axial dispersion in the adsorption bed. Additionally, the model suggests that the adsorbate is irreversibly adsorbed, with a local removal rate proportional to the residual capacity of the adsorbent and the concentration of the adsorbate in the gas phase. Thus, the adsorption rate varies according to the availability of adsorption sites on the adsorbent and the concentration of the adsorbate in the gas phase [26,27].
Although the Adams–Bohart model stood out, the Thomas model also showed good fit for the concentration of 35 mg/g. This model assumes that adsorption kinetics follow a first-order reaction with respect to the adsorbate concentration and that the adsorption isotherm follows the Langmuir model, implying a homogeneous adsorption surface with energetically equivalent adsorption sites. Furthermore, the Thomas model neglects internal and external mass transfer resistances, assuming that the adsorption rate is limited by the adsorption reaction at the active site [21,23].
When comparing the adsorption process of the two gasses, H2S and CO2, models that considered the estimation of maximum adsorption capacity provided a good description of adsorption processes in fixed-bed columns, better reflecting column dynamics. Conversely, treating this parameter as deterministic tends to result in larger differences between estimated and experimental values. This discrepancy arises because estimating it as a random variable allows for a more flexible adjustment, providing an optimized mathematical fit that better represents the reality of the adsorption system [19].
Moreover, it was observed that the accuracy of the models can be influenced by the initial concentrations of the adsorbates. During the adsorption of H2S at low concentrations, the models presented a more precise fit, whereas in the adsorption of CO2 at low concentrations, the models showed a poorer fit to the experimental data. This may occur because analytical models rely on simplified assumptions, such as bed uniformity, the absence of interactions between adsorbed molecules, and the absence of axial dispersion, which may not fully capture the complexity of real systems.
Table 6 presents estimates of the parameters of analytical models of breakthrough curve adsorption of H2S and CO2 gasses in fixed-bed systems found in other reports in the literature. These estimates, although derived from different operational conditions, are roughly consistent with the order of magnitude obtained from the experimental data of [29,30].

Table 6.
Parameter estimates of analytical models obtained from the literature.
5. Conclusions
This study showed that the use of Bayesian inference for model fitting in continuous adsorption systems is promising, enabling better understanding and optimization of gas separation and purification processes.
Overall, models that included the estimation of maximum adsorption capacity provided more accurate descriptions of adsorption system behavior. On the other hand, it was noted that when maximum adsorption capacity is treated as a deterministic parameter, the difference between estimated and experimental values tends to be larger.
The analysis of determination coefficients (R2 and R2Adjusted) and Bayesian Information Criterion (BIC) reinforced the efficiency of the fitted models. For H2S, the Yan model best described the gas adsorption, obtaining R2 values above 0.998 and a lower BIC value of 11.2732, whereas for CO2, the standout models were Adams–Bohart and Yoon–Nelson, with R2 values close to 0.995 and a BIC of 153.3. These results confirm the effectiveness of the analytical models in representing fixed-bed adsorption processes, offering a viable alternative with lower computational effort compared to models based on complex differential equations.
Thus, the study highlights the importance of modeling in fixed-bed column adsorption, contributing to the development of more efficient systems for capturing atmospheric pollutants, such as H2S e CO2, with the potential for large-scale implementation. Additionally, the application of Bayesian inference provides a promising alternative to overcome the limitations of traditional parameter estimation methods, offering improved fits/descriptions and enhanced reliability in analyses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14166956/s1, Table S1: Elements used for parameter estimation of models for hydrogen sulfide (H2S) gas.; Table S2: Elements used for parameter estimation of models for hydrogen sulfide (CO2) gas.
Author Contributions
Conceptualization, methodology, validation, writing—original draft preparation, software H.B.S.L. and A.P.S.d.S.; writing—review and editing, visualization W.B.d.S.; supervision, visualization, writing—review and editing D.S.d.C.; project administration, formal analysis, E.C.R.; software, validation, project administration, supervision, writing—original draft preparation D.C.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pró-Reitoria de Pesquisa e Pós-Graduação (PROPESP) of Federal University of Pará (UFPA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request, the data will be made available from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We thank FAPESPA and PROPESP (UFPA) for financially supporting this research through financing the project entitled “Adsorção De Gases Em Leito Fixo: Uso De adsorventes Produzidos A Partir De Resíduos De Mineração Em Sistema Com Escala Semi Piloto” agreement Nº13/2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baumann, R.; Libanio, G.; Iasco-Pereira, H.; Soares, F.V.; Pereira, K.C.; Neto, W.A.D. Indicadores Quantitativos da OCDE e o Brasil; Instituto de Pesquisa Econômica Aplicada (IPEA): Brasília, Brasil, 2023; Volume 5. [Google Scholar]
- Artaxo, P.E. O Estado da Qualidade do Ar no Brasil; Working Paper; WRI Brasil: São Paulo, Brazil, 2021; p. 32. [Google Scholar]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Majchrzak-Kucęba, I. CO2: A useful reactant. In The Carbon Chain in Carbon Dioxide Industrial Utilization Technologies: A Case Study; CRC Press: Boca Raton, FL, USA, 2022; pp. 17–35. [Google Scholar]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.J. Valorization of orange peel waste to tunable heteroatom-doped hydrochar-derived microporous carbons for selective CO2 adsorption and separation. Sci. Total Environ. 2022, 849, 157805. [Google Scholar] [CrossRef]
- Elgendy, K.; Elmehasseb, I.; Kandil, S. Efficient removal of common organic pollutants from water by Zn-doped TiO2 nanoparticles with different applications. Karbala Int. J. Mod. Sci. 2022, 8, 98–111. [Google Scholar] [CrossRef]
- Engenharia e Tecnologia de Processos Alimentares. In Adsorption and Ion Exchange; Zeki BERK: Istanbul, Turkey, 2009; pp. 279–294.
- Fujiwara, K.; Shibahara, M. Atomic-scale thermal manipulation with adsorbed atoms on a solid surface at a liquid-solid interface. Sci. Rep. 2019, 9, 13202. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Juela, D.; Vera, M.; Cruzat, C.; Alvarez, X.; Vanegas, E. Mathematical modeling and numerical simulation of sulfamethoxazole adsorption onto sugarcane bagasse in a fixed-bed column. Chemosphere 2021, 280, 130687. [Google Scholar] [CrossRef]
- Páscoa, A.P.C. Modelação e Simulação Matemática da Adsorção em Leito Fixo. Aplicação à Adsorção de CO2 em Zeólito 4A. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2018. [Google Scholar]
- Qian, W.; Hu, M.; Su, Y.; Shan, S.; Zhang, Z.; Hu, L.; Lin, X. Insight into mass transfer during adsorption of geniposidic acid onto a fixed-bed column by numerical simulation considering influence of operating conditions on column adsorption performance. Sep. Purif. Technol. 2023, 319, 124021. [Google Scholar] [CrossRef]
- Lima, A.C.A. Adsorção de anions presente em efluente usando pó da casca de coco verde modificado. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2009. [Google Scholar]
- Galagali, N.; Marzouk, Y.M. Bayesian inference of chemical kinetic models from proposed reactions. Chem. Eng. Sci. 2015, 123, 170–190. [Google Scholar] [CrossRef]
- Orlande, H.R.B.; Colaço, M.J.; Cotta, C.P.; Guimarães, G.; Borges, V. Problemas Inversos em Transferencia de Calor; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Gelman, A.; Carlin, J.; Stern, H.; Dunson, D.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 639. [Google Scholar]
- Gilks, W.R.; Roberts, G.O.; Sahu, S.K. Adaptive markov chain monte carlo through regeneration. J. Am. Stat. Assoc. 1998, 93, 1045–1054. [Google Scholar] [CrossRef]
- Mccabe, W.L.; Smith, J.C.; Harriot, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Oliveira, J.T.; Estumano, D.C.; Féris, L.A. Resolution methods for adsorption models, batch and continuous mode, and its impact on process implementation: An experimental and statistical comparison. J. Water Process Eng. 2024, 58, 104888. [Google Scholar] [CrossRef]
- Franco, M.A.E. Adsorção de Fármacos em Carvão Ativado: Processo em Batelada, Leito Fixo e Modelagem das Curvas de Ruptura. 2018. Ph.D. Thesis, Universidade Federal do Rio Grande Do Sul, Porto Alegre, Brazil, 2018. [Google Scholar]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais, 2nd ed.; Imprensa Universitária: Lisbon, Portugal, 2020. [Google Scholar]
- Tien, C. Introduction to Adsorption: Basics, Analysis and Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 53, 493–502. [Google Scholar] [CrossRef]
- Amador, I.C.B.; Nunes, K.G.P.; Franco, M.A.E.; Viegas, B.M.; Macêdo, E.N.; Féris, L.A.; Estumano, D.C. Application of Approximate Bayesian Computational technique to characterize the breakthrough of paracetamol adsorption in fixed-bed column. Int. Commun. Heat Mass Transfer. 2022, 132, 105917. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Chu, K.H. Breakthrough curve analysis by simplistic models of fixed bed adsorption: In defense of the century-old Bohart-Adams model. Chem. Eng. J. 2020, 380, 122513. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A new model for heavy metal removal in a biosorption column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Scheufele, F.B.; Silva, E.S.; Cazula, B.B.; Marins, D.S.; Sequinel, R.; Borba, C.E.; Patuzzo, G.S.; Lopez, T.F.M.; Alves, H.J. Mathematical modeling of low-pressure H2S adsorption by babassu biochar in fixed bed column. J. Environ. Chem. Eng. 2021, 9, 2213–3437. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Amari, A.; Danish, M.; Al Alwan, B.A.; Shah, M. Simulation study of fixed-bed CO2 adsorption from CO2/N2 mixture using using activvated carbono. Chem. Eng. Commun. 2020, 208, 1358–1367. [Google Scholar] [CrossRef]
- Soeiro, W.F.; Moura, C.H.R.; Dias, C.S.; Rodrigues, E.C.; Da Costa, D.S.; Viegas, B.M.; Estumano, D.C. Mathematical Evaluation of Direct and Inverse Problem Applied in Breakthrough Models of Metal Adsorption. Appl. Sci. 2024, 14, 5035. [Google Scholar] [CrossRef]
- Dobbelaere, M.R.; Plehiers, P.P.; Van De Vijver, R.; Stevens, C.V.; Van Geem, K.M. Machine learning in chemical engineering: Strengths, weaknesses, opportunities, and threats. Engineering 2021, 7, 1201–1211. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Dias, C.S.; Moura, C.H.R.D.; Ferreira, J.L.; Rodrigues, E.C.; Macêdo, E.N.; Estumano, D.C.; Viegas, B.M. Use of Bayesian Methods in the Process of Uranium Bioleaching by Acidithiobacillus ferrooxidans. Appl. Sci. 2023, 14, 109. [Google Scholar] [CrossRef]
- Tavares, R.; Santana, C.D.; Rodrigues, C.H.M.; Rodrigues, E.C.; Viegas, B.M.; Estumano, D.C. Parameter Estimation in Mass Balance Model Applied in Fixed Bed Adsorption Using the Markov Chain Monte Carlo Method. J. Heat Mass Transf. Res. 2022, 9, 219–232. [Google Scholar]
- Jurado-Davila, I.V.; Schneider, I.A.H.; Estumano, D.; Féris, L.A. Phosphate removal using dolomite modified with ultrasound: Mathematical and experimental analysis. J. Environ. Sci. Health 2023, 58, 469–482. [Google Scholar] [CrossRef]
- Giles, C.H.; Macewan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption, Part XI. A system of classification of solutions adsorption isotherms, and it’s uses in diagnosis os adsorption mechanisms and in measurement of specific areas of solids. J. Chem. Soc. 1960, 111, 10–1039. [Google Scholar]
- Meng, M.; Feng, Y.; Zhang, M.; Liu, Y.; Ji, Y.; Wang, J.; Yan, Y. Highly efficient adsorption of salicylic acid from aqueous solution by wollastonite-based imprinted adsorbent: A fixed-bed column study. Chem. Eng. J. 2013, 225, 331–339. [Google Scholar] [CrossRef]
- Jurado-Davila, V.; Oshiro, G.P.; Estumano, D.C.; Féris, L.A. Immobilization of Marbofloxacin for Water Treatment by Adsorption in Batch Scale and Fixed-Bed Column: Applying of Monte Carlo Bayesian Modeling. Ind. Eng. Chem. Res. 2024, 63, 9976–9987. [Google Scholar] [CrossRef]
- Aslam, Z.; Hussein, I.A.; Shawabkeh, R.A.; Parvez, M.A.; Ahmad, W.; Ihsanullah. Adsorption Kinetics and modeling of H2S by treated waste oil fly ash. J. Air Waste Manag. Assoc. 2019, 69, 246–257. [Google Scholar] [CrossRef]
- Saleh, A.M.; Mahdi, H.H.; Alias, A.B.; Abd Hadi, N.K.; Qarizada, D.; Jawad, A.H.; Saleh, N.M. Equilibrium and kinetic studies in adsorption of H2S using coconut shell activated carbon xerogel: Effect of mass adsorbent and temperature. Desalination Water Treat. 2024, 317, 100149. [Google Scholar] [CrossRef]
- Ligero, A.; Calero, M.; Martín-Lara, M.Á.; Blázquez, G.; Solís, R.R.; Pérez, A. Fixed-bed CO2 adsorption onto activated char from the pyrolysis of a non-recyclable plastic mixture from real urban residues. J. CO2 Util. 2023, 73, 102517. [Google Scholar] [CrossRef]
- Mrosso, R.; Mecha, A.C.; Kiplagat, J. Performance evaluation of calcined eggshell waste (Sorbent) for biogas upgrading: Adsorption isotherms, adsorption kinetics, and fixed bed studies. Environ. Chall. 2024, 16, 100961. [Google Scholar] [CrossRef]
- Carvalho, G.R. Breakthrough analysis of continuous fixed-bed dehydration of gas streams using 4A zeolite molecular sieve. Acta Sci. 2022, 45, e58764. [Google Scholar] [CrossRef]
- Moraes, S.C.G. Avaliação da Capacidade Adsortiva da Cinza do Bagaço de Cana-de-Açúcar para Remoção de H2S Oriundo da Gaseificação de Biomassas. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2017. [Google Scholar]
- Tourzani, A.A.; Hormozi, F.; Asadollahzadeh, M.; Torkaman, R. Effective CO2 capture by using poly (acrylonitrile) nanofibers based on the radiation grafting procedure in fixed-bed adsorption column. Sci. Rep. 2023, 13, 6173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).