Combined Process for Caffeine Treatment in Aqueous Solution by Adsorption/Regeneration and Fenton Oxidation
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Characterization of the Adsorbent Solid
2.3. Adsorption Experiments
2.4. Fenton Experiments
2.5. Combined Process: Adsorption and Fenton Reaction
Regeneration of Granular Activated Carbon
3. Results and Discussion
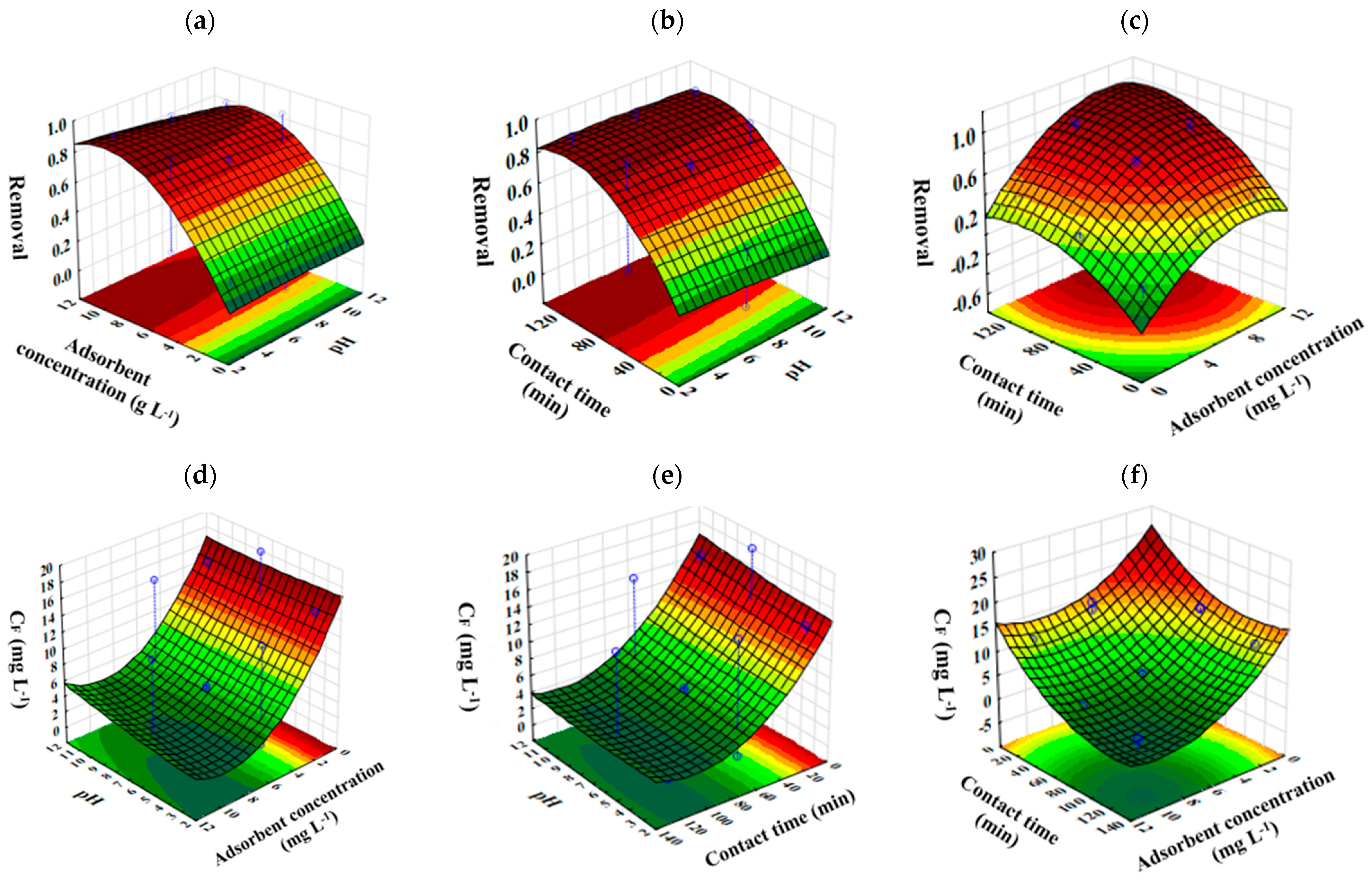
3.1. CAF Adsorption onto GAC
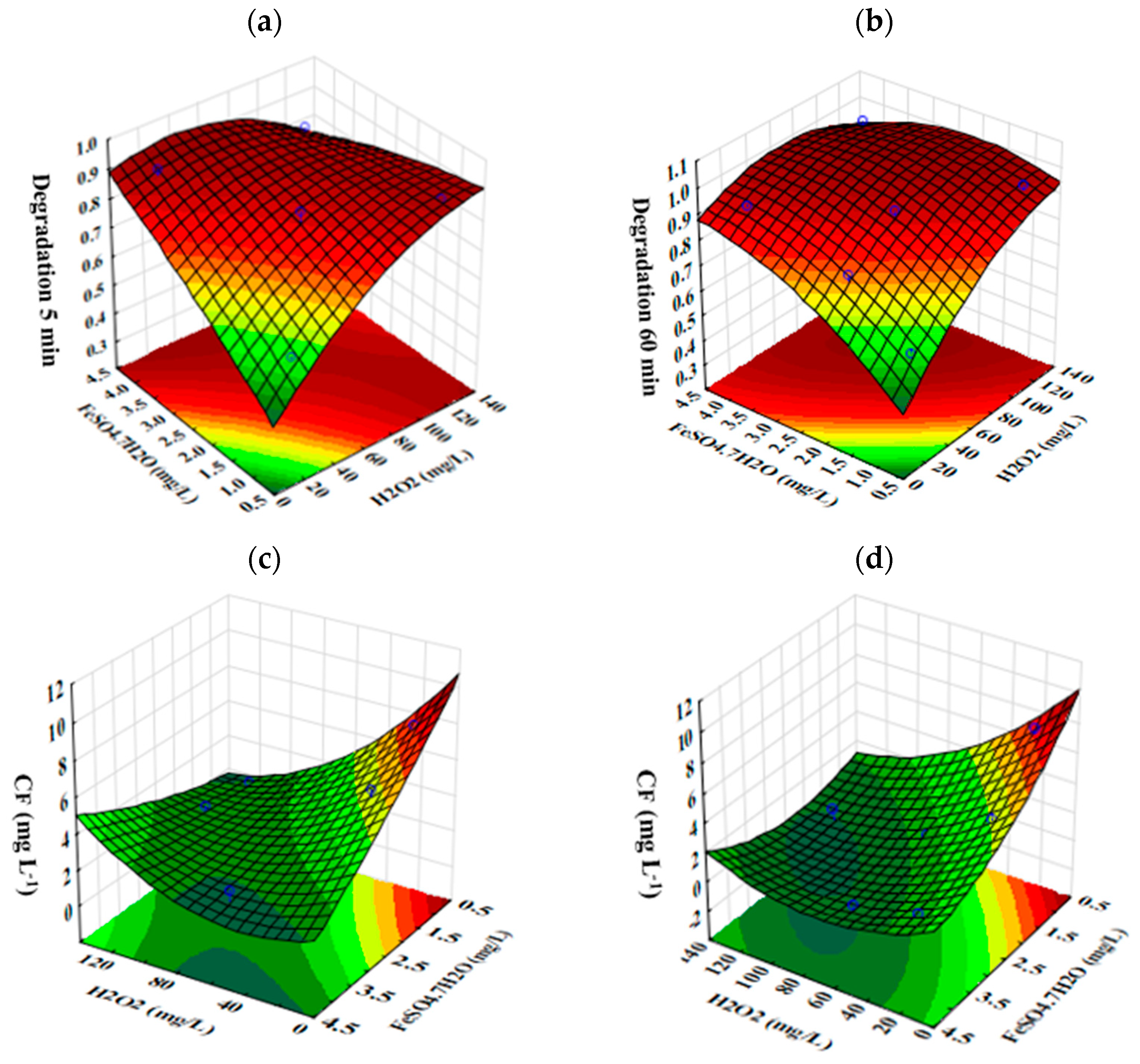
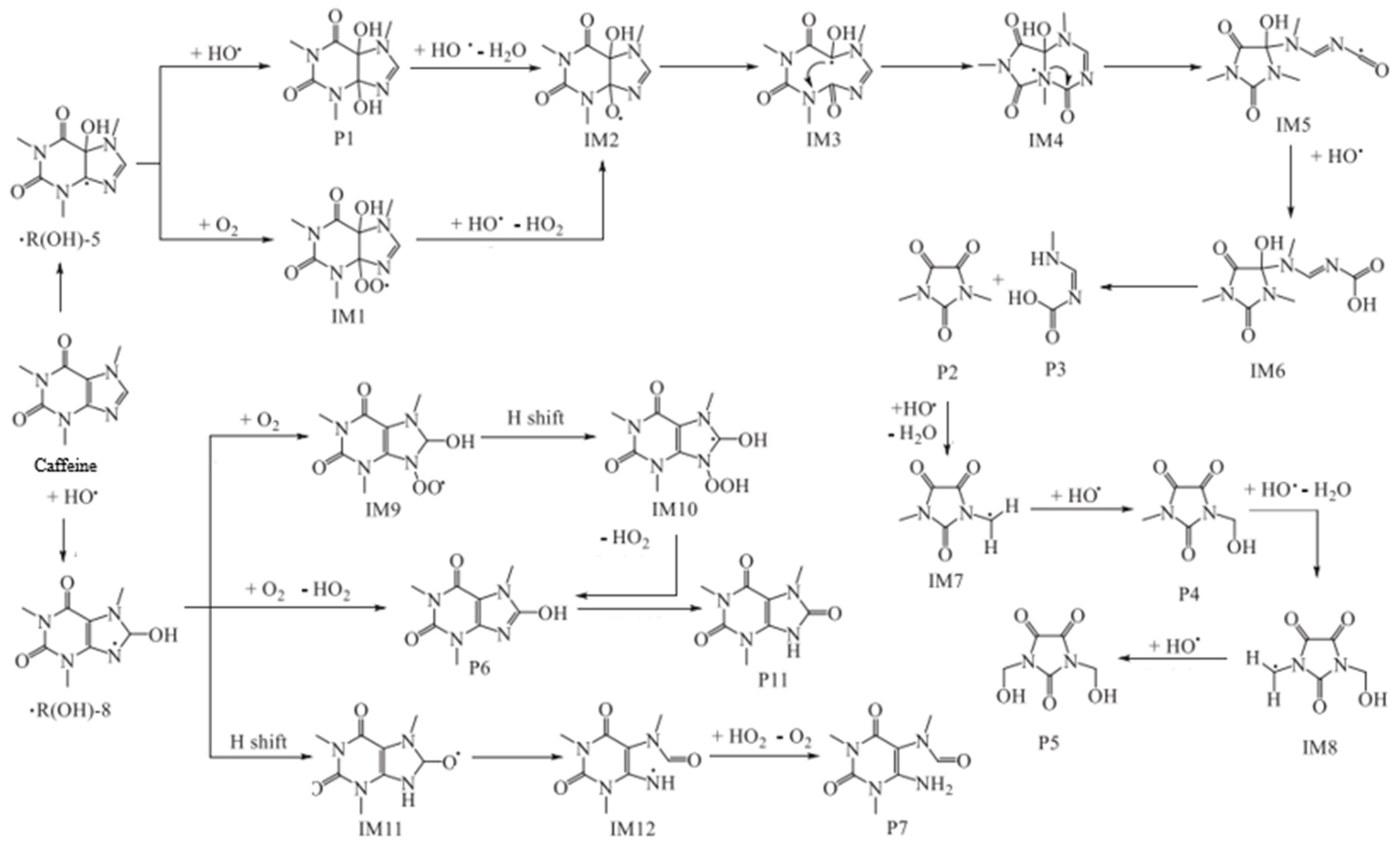
3.2. CAF Degradation by Fenton Process
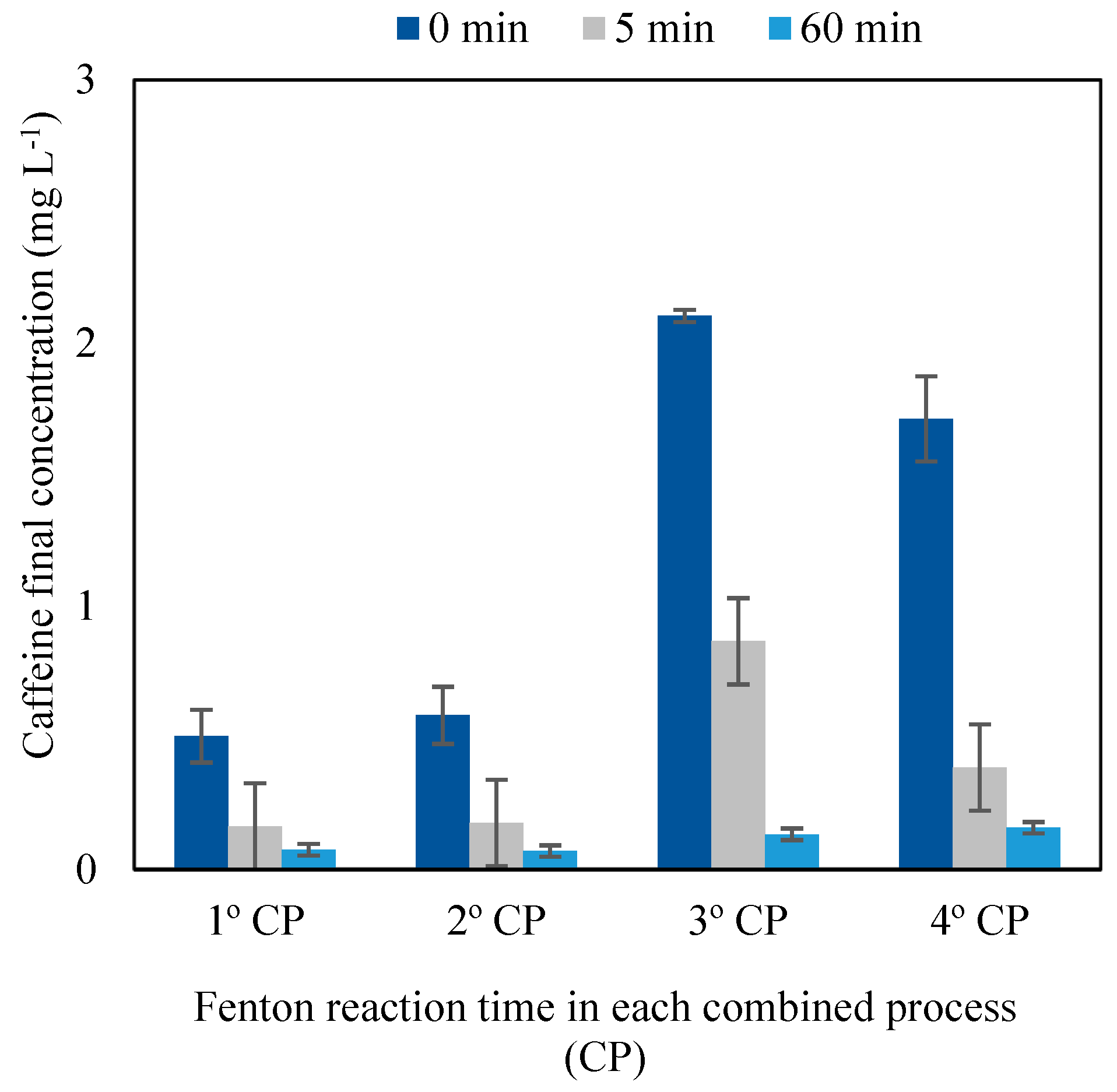
3.3. Combined Process
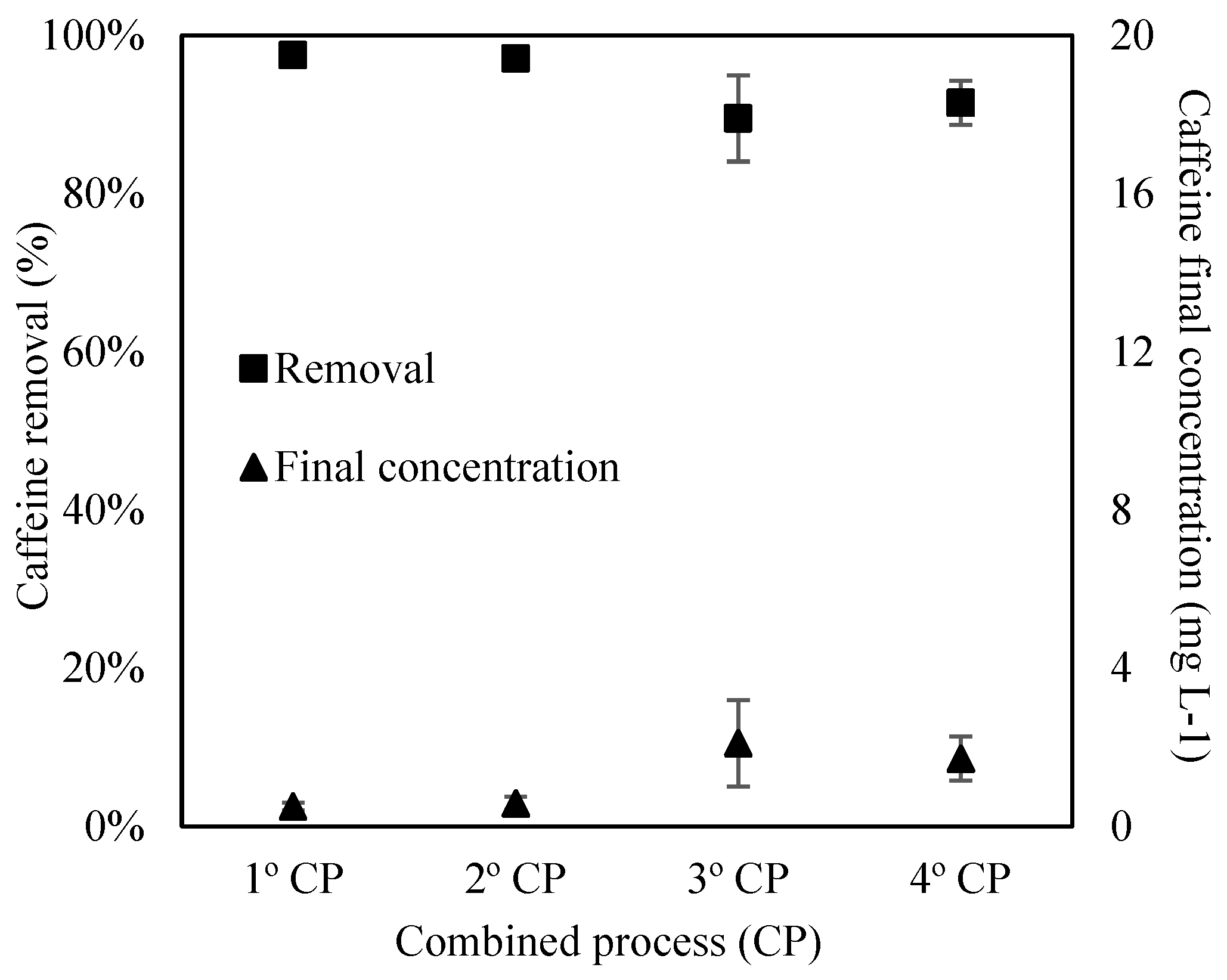
3.3.1. Caffeine Removal and Degradation Efficiency
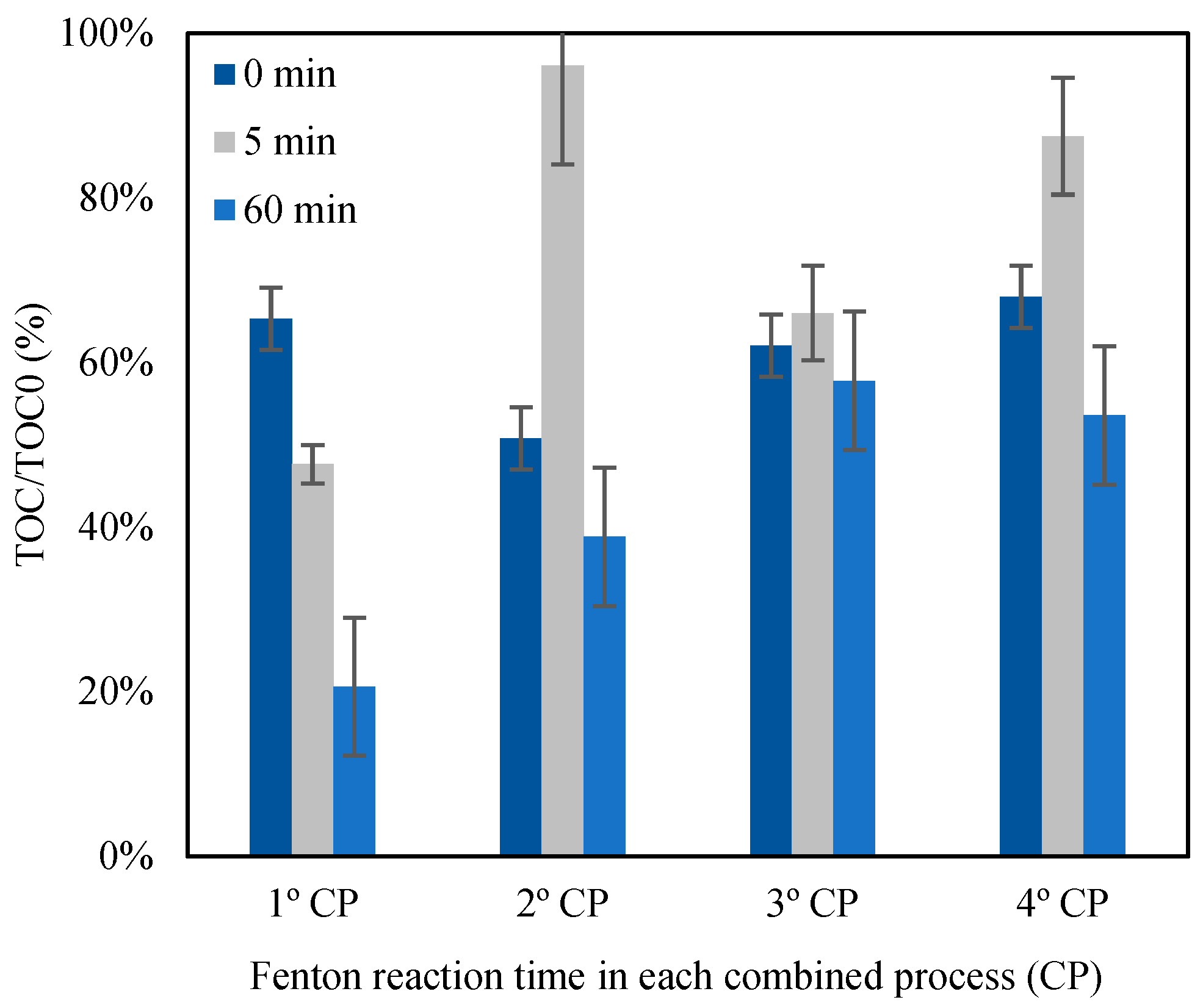
3.3.2. Total Organic Carbon Monitoring
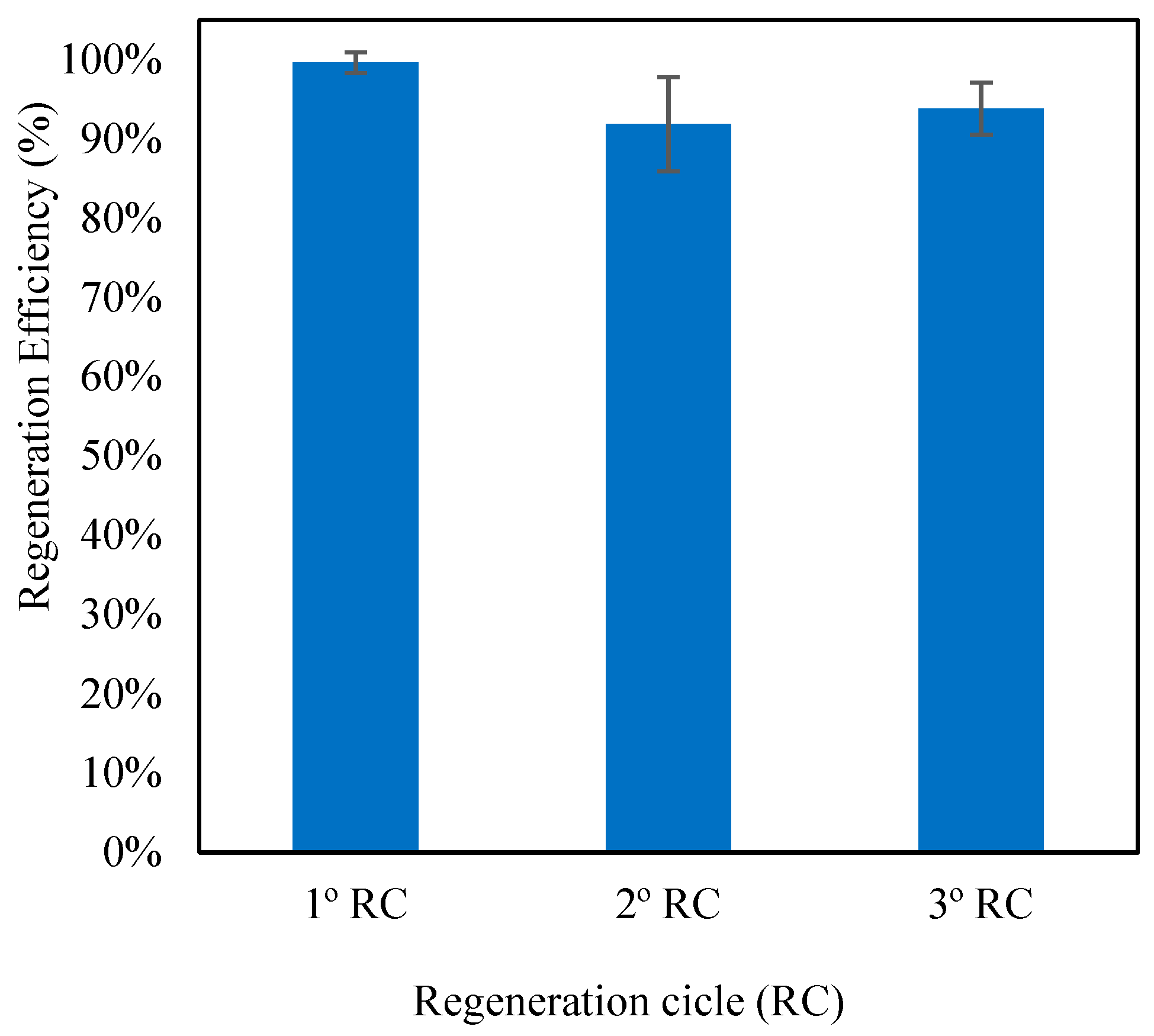
3.3.3. Regeneration Efficiency of Granular Activated Carbon
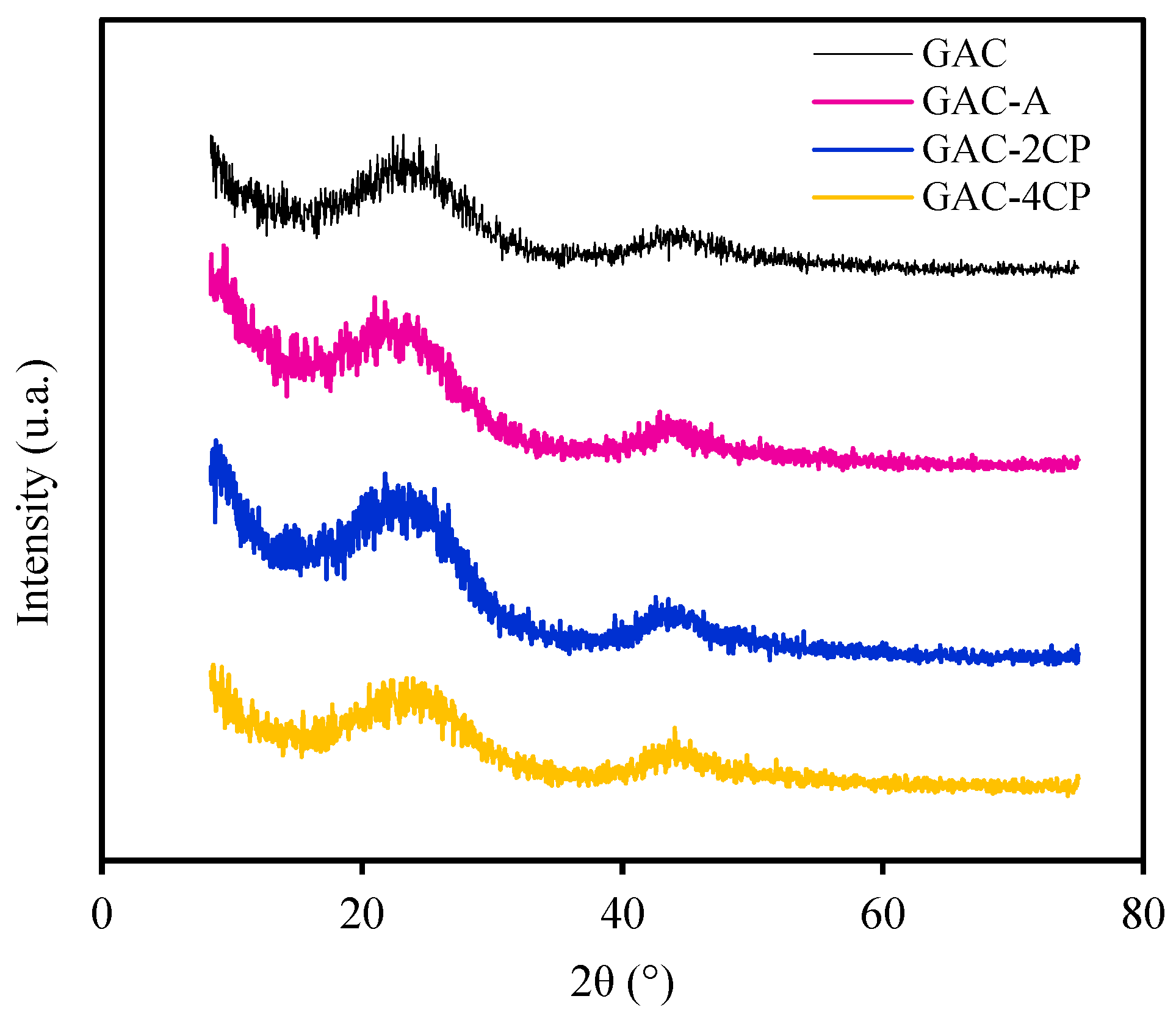
3.4. Characterization of Solid Adsorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alhowail, A. Candidate mechanisms of caffeine improving memory dysfunction. Die Pharmazie 2019, 74, 705–710. [Google Scholar]
- Fiani, B.; Zhu, L.; Musch, B.L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A.Z. The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus J. Med. Sci. 2021, 13, e15032. [Google Scholar] [CrossRef]
- Lima-Silva, A.E.; Cristina-Souza, G.; Silva-Cavalcante, M.D.; Bertuzzi, R.; Bishop, D.J. Caffeine during high-intensity whole-body exercise: An integrative approach beyond the central nervous system. Nutrients 2021, 13, 2503. [Google Scholar] [CrossRef]
- Malviya, A.K.; Saranlal, A.M.; Mulchandani, M.; Gupta, A. Caffeine–Essentials for anesthesiologists: A narrative review. J. Anesthesiol. Clin. Pharmacol. 2023, 39, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.A.F.; Camargo, C.A.C.M. Effects of caffeine on the organism—Literature. Open Access Lib. J. 2019, 6, 5265. [Google Scholar]
- Montagner, C.C.; Umbuzeiro, G.A.; Pasquini, C.; Jardim, W.F. Caffeine as an indicator of estrogenic activity in source water. Environ. Sci. Process. Impacts 2014, 16, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative techniques for caffeine removal from wastewater: An overview of opportunities and challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, control, and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewater and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Cruz, N.; Mierzwa, J.C. Saúde pública e inovações tecnológicas para abastecimento público. Saúde Soc. 2020, 29, e180824. [Google Scholar] [CrossRef]
- Velloso, V. Poluentes Emergentes: Um Report, via Revisão Sistemática, Sobre os Efeitos em Microrganismos Planctônicos de Água Doce; Editora Dialética: São Paulo, SP, Brazil, 2022. [Google Scholar]
- Malaj, E.; Von Der Ohe, P.C.; Grote, M.; Kühne, R.; Mondy, C.P.; Usseglio-Polatera, P.; Brack, W.; Schäfer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef]
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental pollutants and child health—A review of recent concerns. Int. J. Hyg. Environ. Health 2016, 219, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Koumaki, E.; Noutsopoulos, C.; Mamais, D.; Fragkiskatos, G.; Andreadakis, A. Fate of emerging contaminants in high rate activated sludge systems. Int. J. Environ. Res. Public Health 2020, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, G.; De Kreuk, M.K.; Houtman, C.; Van Lier, J.B. Perspectives of coagulation/flocculation for the removal of pharmaceuticals from domestic wastewater: A critical view at experimental procedures. J. Water Process Eng. 2020, 34, 101161. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Thathola, P.; Agnihotri, V.; Pandey, A. Microbial Degradation of Caffeine Using Himalayan Psychrotolerant Pseudomonas sp. GBPI_Hb5 (MCC 3295). Curr. Microbiol. 2021, 78, 3924–3935. [Google Scholar] [CrossRef] [PubMed]
- Nayak, V.; Cuhorka, J.; Mikulášek, P. Separation of drugs by commercial nanofiltration membranes and their modelling. Membranes 2022, 12, 528. [Google Scholar] [CrossRef]
- Dávila, I.V.J.; Hübner, J.V.M.; Nunes, K.G.P.; Féris, L.A. Caffeine removal by adsorption: Kinetics, equilibrium thermodynamic and regeneration studies. J. Solid Waste Technol. Manag. 2021, 47, 95–103. [Google Scholar] [CrossRef]
- Sönmez, G.; Işik, T.B.M. Removal of selected pharmaceuticals from tap water by the Fenton process. Int. J. Environ. Anal. Chem. 2022, 102, 3855–3867. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Baltrėnaitė, E. Sustainable Environmental Protection Technologies; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ghaedi, M. Adsorption: Fundamental Processes and Applications; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Chemical Engineers’ Handbook, 9th ed.; McGraw-Hill: New York, NY, USA, 2019. [Google Scholar]
- Singh, J.K.; Verma, N. Aqueous Phase Adsorption: Theory, Simulations and Experiments; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101 (Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption- and decomposition-based techniques for the regeneration of activated carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Yang, Z.; Tan, D. Regeneration performance of spent granular activated carbon for tertiary treatment of dyeing wastewater by Fenton reagent and hydrogen peroxide. J. Mater. Cycles Waste Manag. 2015, 19, 256–264. [Google Scholar] [CrossRef]
- De Las Casas, C.L.; Bishop, K.G.; Bercik, L.M.; Johnson, M.; Potzler, M.; Ela, W.P.; Sáez, A.E.; Huling, S.G.; Arnold, R.G. In-place regeneration of granular activated carbon using Fenton’s reagents. Remediation Hazard. Waste Subsurface 2006, 4, 43–65. [Google Scholar]
- Muranaka, C.T.; Julcour, C.; Wilhelm, A.M.; Delmas, H.; Nascimento, C.A. Regeneration of activated carbon by (photo)-Fenton oxidation. Ind. Eng. Chem. Res. 2010, 49, 989–995. [Google Scholar]
- Santos, D.H.; Duarte, J.L.; Tonholo, J.; Meili, L.; Zanta, C.L. Saturated activated carbon regeneration by UV-light, H2O2 and Fenton reaction. Sep. Purif. Technol. 2020, 250, 117112. [Google Scholar] [CrossRef]
- Dalmázio, I.; Santos, L.S.; Lopes, R.P.; Eberlin, M.N.; Augusti, R. Advanced oxidation of caffeine in water: On-line and real-time monitoring by electrospray ionization mass spectrometry. Environ. Sci. Technol. 2005, 39, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mei, Q.; Han, D.; Wei, B.; An, Z.; Cao, H.; Xie, J.; He, M. The roles of HO•, ClO•, and BrO• radicals in caffeine degradation: A theoretical study. Sci. Total Environ. 2021, 768, 144733. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.B.; Rosa, I.R.; Del Vecchio, P.; Dávila, I.V.J.; Nunes, K.G.P.; Marcilio, N.R.M.; Féris, L.A. Degradation of ampicillin by combined process: Adsorption and Fenton reaction. Environ. Technol. Innov. 2022, 26, 102365. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and eTOCoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Gil, A.; Galeano, L.A.; Vicente, M.Á. Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 67. [Google Scholar]
- Kabuba, J.; Lukusa, T. Process Optimization Using Response Surface Methodology for the Removal of Cu(II) and Co(II) from Aqueous Solution Using Gelatin-Cellulose Nanocrystals Hydrogel Membrane. Int. J. Environ. Sci. Dev. 2023, 14, 252–258. [Google Scholar] [CrossRef]
- Regalbuto, J.R.; Robles, J.O. The Engineering of Pt/Carbon Catalyst Preparation for Application on Proton Exchange Fuel Cell Membrane; Catalysis Laboratory, University of Illinois at Chicago: Chicago, IL, USA, 2004. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- De Oliveira, J.T. Estimativa de Parâmetros e Seleção de Modelos Através da Aplicação de Técnicas Bayesianas ao Processo de Adsorção de cafeína: Cinética, Isoterma e Curva de Ruptura; Postgraduate Program in Chemical Engineering; Federal University of Rio Grande do Sul: Porto Alegre, Brazil, 2022. [Google Scholar]
- Michael, S.G.; Michael-Kordatou, I.; Beretsou, V.G.; Jäger, T.; Michael, C.; Schwartz, T.; Fatta-Kassinos, D. Solar photo-Fenton oxidation followed by adsorption on activated carbon for the minimization of antibiotic resistance determinants and toxicity present in urban wastewater. Appl. Catal. B Environ. 2019, 244, 871–880. [Google Scholar] [CrossRef]
- Posser, Y.M. Degradação da Cafeína Através dos Processos Oxidativos Avançados Fenton e Foto-Fenton; Postgraduate Program in Chemical Engineering; Federal University of Rio Grande do Sul: Porto Alegre, Brazil, 2016. [Google Scholar]
- Trovó, A.G.; Silva, T.F.; Gomes, O., Jr.; Machado, A.E.; Neto, W.B.; Muller, P.S., Jr.; Daniel, D. Degradation of caffeine by photo-Fenton process: Optimization of treatment conditions using experimental design. Chemosphere 2013, 90, 170–175. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.D.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- François, L.L.; Haro, N.K.; Souza, F.S.; Féris, L.A. Remoção de cafeína por adsorção em carvão ativado. Scientia Cum Industria 2016, 4, 64–68. [Google Scholar] [CrossRef][Green Version]
- Kaur, H.; Bansiwal, A.; Hippargi, G.; Pophali, G.R. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 20473–20485. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.D.D.; Martini, W.S.; Santos, M.D.; Matos, M.A.C.; Rocha, L.L.D. Caffeine oxidation in water by Fenton and Fenton-like processes: Effects of inorganic anions and ecotoxicological evaluation on aquatic organisms. J. Braz. Chem. Soc. 2015, 26, 178–184. [Google Scholar] [CrossRef]
- García-Negueroles, P.; García-Ballesteros, S.; Amat, A.M.; Laurenti, E.; Arques, A.; Santos-Juanes, L. Unveiling the dependence between hydroxyl radical generation and performance of Fenton systems with complexed iron. ACS Omega 2019, 4, 21698–21703. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, K.S.; Antonelli, R.; Gaydeczka, B.; Granato, A.C.; Malpass, G.R.P. Advanced oxidation processes: A review of fundamentals and applications in the treatment of urban and industrial wastewaters. Ambiente Água 2016, 11, 387–401. [Google Scholar] [CrossRef]
- Ubillus, M.A.R. Óxido de Grafeno Magnético Para Degradação de Cafeína por Processo Fenton Heterogêneo; Institute of Chemistry, São Paulo State University: Araraquara, Brazil, 2020. [Google Scholar]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Degradation of caffeine and identification of the transformation products generated by ozonation. Chemosphere 2009, 74, 825–831. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Ince, N.H.; Ozon, E.; Arslan, E.; Aviyente, V.; Savun-Hekimoğlu, B.; Erdincler, A. Oxidative decomposition and mineralization of caffeine by advanced oxidation processes: The effect of hybridization. Ultrason. Sonochem. 2021, 76, 105635. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Reyes-Vidal, Y.; Irigoyen-Campuzano, J.R.; Reynoso-Cuevas, L. Simultaneous oxidation of emerging pollutants in real wastewater by the advanced Fenton oxidation process. Catalysts 2023, 13, 748. [Google Scholar] [CrossRef]
- De Almeida, L.N.B.; Josué, T.G.; Fidelis, M.Z.; Abreu, E.; Bechlin, M.A.; Dos Santos, O.A.A.; Lenzi, G.G. Process comparison for caffeine degradation: Fenton, photo-Fenton, UV/H2O2, and UV/Fe3⁺. Water Air Soil Pollut. 2021, 232, 147. [Google Scholar] [CrossRef]
- Costa, L.R.C.; Ribeiro, L.M.; Hidalgo, G.E.N.; Féris, L.A. Determination of optimal operating parameters for tetracycline removal by adsorption from synthetic and real aqueous solutions. J. Environ. Sci. Health Part A 2020, 55, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.; Pereira, M.F.; Órfão, J.J.; Figueiredo, J.L. Surface chemistry of activated carbons. Chem. Phys. Res. J. 2011, 4, 291. [Google Scholar]
- Nunes, K.G.P.; Dávila, I.V.J.; Arnold, D.; Moura, C.H.R.; Estumano, D.C.; Féris, L.A. Kinetics and thermodynamic study of laponite application in caffeine removal by adsorption. Environ. Process. 2022, 9, 47. [Google Scholar] [CrossRef]
- Hübner, J.V.M. Avaliação Experimental da Habilidade do Carvão Ativado em Adsorver Cafeína Múltiplas Vezes; Postgraduate Program in Chemical Engineering; Federal University of Rio Grande do Sul: Porto Alegre, Brazil, 2020. [Google Scholar]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Yuan, Z.; Zhang, D.; Sun, Z.; Huang, Y.X.; Chen, W.; Tian, D.; Deng, H.; Zhou, Y. Fabrication of TOCton textile waste-based magnetic activated carbon using FeCl3 activation by the Box-Behnken design: Optimization and characteristics. RSC Adv. 2018, 8, 38081–38090. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.M.O.; Aguiar, M.B.; Parolo, M.E.; Avena, M.J. Insights of competitive adsorption on activated carbon of binary caffeine and diclofenac solutions. J. Environ. Manag. 2021, 278, 111523. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, K.K.; Cazetta, A.L.; De Souza, P.S.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Thiravetyan, P.; Suksabye, P. Using “activated carbon from bagasse” for color removal. In Activated Carbon. Chemical Engineering Methods and Technology; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; Gómez, J.M.; García, J. Removal of caffeine from pharmaceutical wastewater by adsorption: Influence of NOM, textural and chemical properties of the adsorbent. Environ. Technol. 2016, 37, 1618–1630. [Google Scholar] [CrossRef]





| Level | pH | Solid Adsorbent Concentration (mg L−1) | Contac Time (min) |
|---|---|---|---|
| (+1) | 11.0 | 10.0 | 120.0 |
| 0 | 7.0 | 5.5 | 65.0 |
| (−1) | 3.0 | 1.0 | 10.0 |
| Level | Fe+2 Concentration (mg L−1) | H2O2 Concentration (mg L−1) |
|---|---|---|
| (+1) | 4.0 | 150.0 |
| 0 | 2.0 | 75.0 |
| (−1) | 0.8 | 25.0 |
| Test Runs | pH | Cads (g L−1) | Contact Time (min) | CF (mg L−1) | Removal (%) |
|---|---|---|---|---|---|
| 1 | 7 | 10.0 | 120 | 1.91 | 90.27 |
| 2 | 3 | 5.5 | 120 | 1.53 | 92.18 |
| 3 | 3 | 1.0 | 65 | 14.80 | 24.56 |
| 4 | 3 | 10.0 | 65 | 1.29 | 93.40 |
| 5 | 11 | 10.0 | 65 | 2.97 | 84.86 |
| 6 | 7 | 5.5 | 65 | 5.36 | 72.65 |
| 7 | 7 | 5.5 | 65 | 5.04 | 74.32 |
| 8 | 7 | 5.5 | 65 | 4.64 | 76.36 |
| 9 | 7 | 1.0 | 10 | 19.12 | 2.48 |
| 10 | 11 | 1.0 | 65 | 14.83 | 24.39 |
| 11 | 11 | 5.5 | 120 | 2.01 | 89.73 |
| 12 | 11 | 5.5 | 10 | 15.23 | 22.35 |
| 13 | 3 | 5.5 | 10 | 13.70 | 30.14 |
| 14 | 7 | 10.0 | 10 | 11.58 | 40.95 |
| 15 | 7 | 1.0 | 120 | 12.45 | 36.53 |
| Factor | SS RCAF | SS CF | DF | F-Value | p-Value |
|---|---|---|---|---|---|
| (1) pH (L) | 0.0044 | 1.7257 | 1 | 0.5048 | 0.5092 |
| (1) pH (Q) | 0.0002 | 0.0914 | 1 | 0.0267 | 0.8765 |
| (2) mads (L) | 0.6134 | 235.9451 | 1 | 69.0123 | 0.0004 |
| (2) mads (Q) | 0.1047 | 40.2711 | 1 | 11.7790 | 0.0186 |
| (3) t (L) | 0.5660 | 217.6919 | 1 | 63.6733 | 0.0005 |
| (3) t (Q) | 0.0836 | 32.1467 | 1 | 9.4027 | 0.0279 |
| 1 by 2 | 0.0018 | 0.6732 | 1 | 0.1969 | 0.6758 |
| 1 by 3 | 0.0007 | 0.2742 | 1 | 0.0802 | 0.7884 |
| 2 by 3 | 0.0058 | 2.2427 | 1 | 0.6560 | 0.4548 |
| Error | 0.0444 | 17.0944 | 5 | ||
| Total SS | 1.4124 | 543.2402 | 14 |
| Factor | Observed Minimum | Critical Values | Observed Maximum |
|---|---|---|---|
| pH | 3 | Natural (pH ~6–8) | 11 |
| Cads (g L−1) | 1 | 10 | 10 |
| Contac time (min) | 10 | 116 | 120 |
| H2O2 (mg L−1) | FeSO4·7H2O (mg L−1) | Surface Response | Reaction Time (min) | |||
|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | |||
| 60.00 | 4.00 | DCAF (%) CF (mg L−1) | 87.48% 2.37 | 97.61% 0.45 | 98.39% 0.31 | 96.98% 0.57 |
| 20.00 | 2.00 | DCAF (%) CF (mg L−1) | 68.65% 5.94 | 75.88% 4.57 | 81.02% 3.59 | 80.46% 3.70 |
| 20.00 | 0.80 | DCAF (%) CF (mg L−1) | 58.72% 7.82 | 64.92% 6.64 | 63.01% 7.00 | 60.17% 7.54 |
| 20.00 | 4.00 | DCAF (%) CF (mg L−1) | 90.10% 1.87 | 86.18% 2.62 | 90.50% 1.80 | 91.58% 1.59 |
| 120 | 4.00 | DCAF (%) CF (mg L−1) | 83.74% 3.08 | 87.70% 2.33 | 96.13% 0.73 | 98.92% 0.21 |
| 60 | 2.00 | DCAF (%) CF (mg L−1) | 86.01% 2.65 | 90.85% 1.73 | 87.59% 2.35 | 93.57% 1.22 |
| 120 | 0.80 | DCAF (%) CF (mg L−1) | 88.66% 2.15 | 98.35% 0.31 | 86.46% 2.56 | 96.46% 0.67 |
| 120 | 2.00 | DCAF (%) CF (mg L−1) | 86.89% 2.48 | 79.83% 3.82 | 81.64% 3.48 | 96.12% 0.73 |
| Factor | SS RCAF | DF | F-Value | p-Value |
|---|---|---|---|---|
| (1) H2O2 (L) | 0.023 | 1 | 13.491 | 0.067 |
| (1) H2O2 (Q) | 0.003 | 1 | 1.907 | 0.301 |
| (2) FeSO4·7H2O (L) | 0.015 | 1 | 8.577 | 0.100 |
| (2) FeSO4·7H2O (Q) | 0.0003 | 1 | 0.187 | 0.708 |
| 1 by 2 | 0.033 | 1 | 19.279 | 0.048 |
| Error | 0.003 | 2 | ||
| Total SS | 0.090 | 7 |
| Factor | SS RCAF | DF | F-Value | p-Value |
|---|---|---|---|---|
| (1) H2O2 (L) | 0.0516 | 1 | 25.587 | 0.037 |
| (1) H2O2 (Q) | 0.003 | 1 | 1.548 | 0.340 |
| (2) FeSO4·7H2O (L) | 0.028 | 1 | 13.95 | 0.065 |
| (2) FeSO4·7H2O (Q) | 0.004 | 1 | 1.896 | 0.302 |
| 1 by 2 | 0.019 | 1 | 9.227 | 0.093 |
| Error | 0.004 | 2 | ||
| Total SS | 0.120 | 7 |
| Factor | Observed Minimum | Critical Values | Observed Maximum |
|---|---|---|---|
| H2O2 (mg L−1) | 25.0 | 129.7 | 150.0 |
| Fe+2 (mg L−1) | 0.8 | 2.3 | 4.0 |
| Stage | ABET (m2/g) | Vpore (cm3/g) | Dpore (Å) |
|---|---|---|---|
| GAC | 534.415 | 0.120 | 4.818 |
| GAC-A | 483.715 | 0.105 | 4.816 |
| GAC-2CP | 531.059 | 0.108 | 4.837 |
| GAC-4CP | 576.459 | 0.139 | 4.836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanatta, N.P.; Jurado Davila, V.; Hugue, K.; Féris, L.A. Combined Process for Caffeine Treatment in Aqueous Solution by Adsorption/Regeneration and Fenton Oxidation. Appl. Sci. 2024, 14, 6993. https://doi.org/10.3390/app14166993
Zanatta NP, Jurado Davila V, Hugue K, Féris LA. Combined Process for Caffeine Treatment in Aqueous Solution by Adsorption/Regeneration and Fenton Oxidation. Applied Sciences. 2024; 14(16):6993. https://doi.org/10.3390/app14166993
Chicago/Turabian StyleZanatta, Natalia Pollon, Vanessa Jurado Davila, Katianna Hugue, and Liliana Amaral Féris. 2024. "Combined Process for Caffeine Treatment in Aqueous Solution by Adsorption/Regeneration and Fenton Oxidation" Applied Sciences 14, no. 16: 6993. https://doi.org/10.3390/app14166993
APA StyleZanatta, N. P., Jurado Davila, V., Hugue, K., & Féris, L. A. (2024). Combined Process for Caffeine Treatment in Aqueous Solution by Adsorption/Regeneration and Fenton Oxidation. Applied Sciences, 14(16), 6993. https://doi.org/10.3390/app14166993






