Infection Control with Antimicrobial Solid-State ZnO Nanoparticles on Silk Fibroin Gauze
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO-SF Gauze
2.2. Physicochemical Characterization
2.3. In Vitro Assay
2.4. Statistical Methods
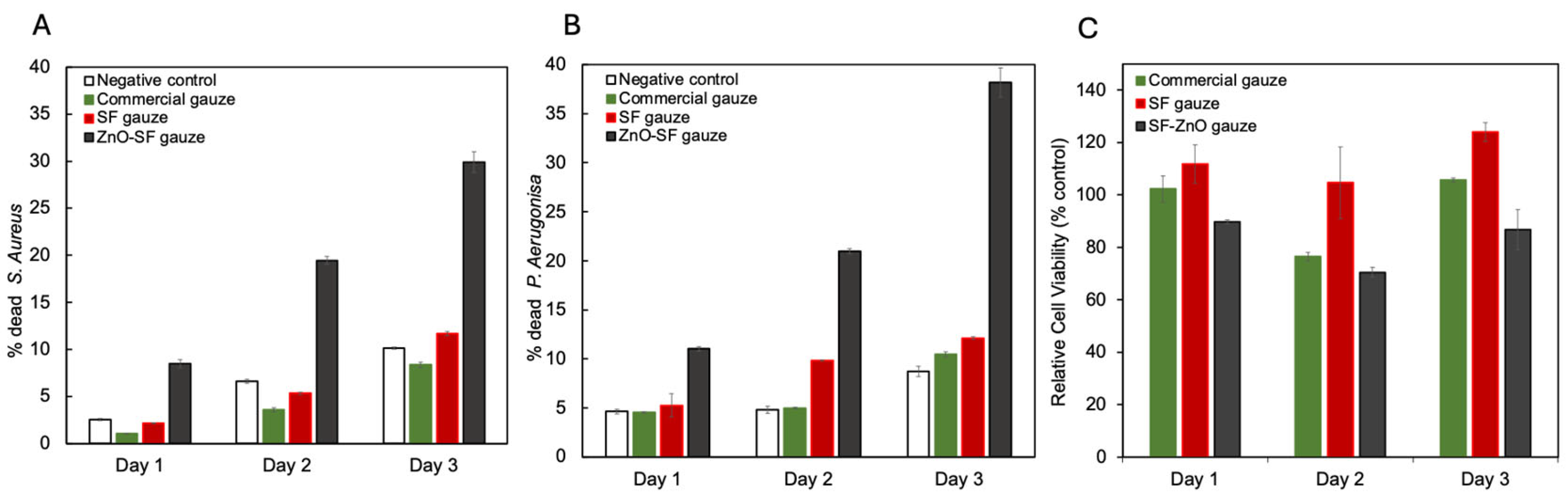
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaynes, R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Ahmed, Z.; Qin, H.; Bhatti, I.A.; Cao, H. Calcium-dependent antimicrobials: Nature-inspired materials and designs. Exploration 2024, 20230099. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Liu, M.; Tang, K.; Li, S.; Fang, Q.; Du, H.; Zhou, X.; Lin, X.; Yang, Y.; et al. Recent design strategies for boosting chemodynamic therapy of bacterial infections. Exploration 2024, 4, 20230087. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Cai, X.; Hou, X.; Zhang, Y.; Liu, J.; Yang, L.; Liu, Y.; Liu, J. A dynamic covalent polymeric antimicrobial for conquering drug-resistant bacterial infection. Exploration 2022, 2, 20210145. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandrasekar, V.; Paudel, N.; Laux, P.; Luch, A.; Gemmati, D.; Tisato, V.; Prabhu, K.S.; Uddin, S.; Dakua, S.P. Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomed. Pharmacother. 2023, 163, 114784. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Angel, S.N.; Honjol, Y.; Masse, M.; Gruenheid, S.; Harvey, E.J.; Merle, G. Engineering surgical stitches to prevent bacterial infection. Sci. Rep. 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Angel, S.; Honjol, Y.; Gruenheid, S.; Gbureck, U.; Harvey, E.; Merle, G. Electroceutical Silk–Silver Gel to Eradicate Bacterial Infection. Adv. Biosyst. 2020, 4, 1900242. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; E Dellinger, P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Anghel, E.L.; DeFazio, M.V.; Barker, J.C.; Janis, J.E.; Attinger, C.E. Current concepts in debridement: Science and strategies. Plast. Reconstr. Surg. 2016, 138, 82S–93S. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-based wound dressing: Recent progress in the detection and therapy of bacterial infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Graves Jr, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Rosenberg, M.; Visnapuu, M.; Vija, H.; Kisand, V.; Kasemets, K.; Kahru, A.; Ivask, A. Selective antibiofilm properties and biocompatibility of nano-ZnO and nano-ZnO/Ag coated surfaces. Sci. Rep. 2020, 10, 13478. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F. Bioinspired materials for wound healing application: The potential of silk fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Preda, N.; Evanghelidis, A.; Enculescu, M.; Florica, C.; Enculescu, I. Zinc oxide electroless deposition on electrospun PMMA fiber mats. Mater. Lett. 2015, 138, 238–242. [Google Scholar] [CrossRef]
- Singh, A.V.; Bansod, G.; Mahajan, M.; Dietrich, P.; Singh, S.P.; Rav, K.; Thissen, A.; Bharde, A.M.; Rothenstein, D.; Kulkarni, S.; et al. Digital Transformation in Toxicology: Improving Communication and Efficiency in Risk Assessment. ACS Omega 2023, 8, 21377–21390. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.M.; Shelar, A.; Kasabe, U.; Nikam, L.K.; Pawar, R.A.; Sangshetti, J.; Kale, B.B.; Singh, A.V.; Patil, R.; Chaskar, M.G. Biofilm inhibition in Candida albicans with biogenic hierarchical zinc-oxide nanoparticles. Biomater. Adv. 2022, 134, 112592. [Google Scholar] [CrossRef] [PubMed]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.J.; Kim, D.W.; Kim, Y.J.; Kweon, H.Y.; Cho, Y.-J.; Choi, S.Y.; Lee, H.R. Toxicity assessment of a novel silk fibroin and poly-methyl-methacrylate composite material. Mol. Cell. Toxicol. 2014, 10, 277–283. [Google Scholar] [CrossRef]
- Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrosp. J. Mater. Chem. B. 2021, 9, 1452–1465. [Google Scholar]
- Seyfi, J.; Goodarzi, V.; Wurm, F.R.; Shojaei, S.; Jafari-Nodoushan, M.; Najmoddin, N.; Khonakdar, H.A.; Baghersad, M.H.; Uzun, L. Developing antibacterial superhydrophobic coatings based on polydimethylsiloxane/silver phosphate nanocomposites: Assessment of surface morphology, roughness and chemistry. Prog. Org. Coat. 2020, 149, 105944. [Google Scholar] [CrossRef]
- Pinto, N.; Silva, A.N.; Rosim-Fachini, E.; Carrión, P.; Furlan, R.; Ramos, I. Electroless deposition of thin metallic films on polymer fibers prepared via electrospinning. Polym. Prepr. 2003, 44, 138–139. [Google Scholar]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, V.P.; Biji, P.; Ashok, A.; Dhara, S.K.; Kamruddin, M.; Tyagi, A.K.; Raj, B. Plasmon-mediated, highly enhanced photocatalytic degradation of industrial textile dyes using hybrid ZnO@Ag core–shell nanorods. RSC Adv. 2014, 4, 58930–58940. [Google Scholar] [CrossRef]
- Xu, J.; Su, H.; Han, J.; Chen, Y.; Song, W.; Gu, Y.; Moon, W.-J.; Zhang, D. In situ deposition of flower-like ZnO on silk fibroin fibers. Appl. Phys. A 2012, 108, 235–238. [Google Scholar] [CrossRef]
- Guo, L.; Ji, Y.L.; Xu, H.; Simon, P.; Wu, Z. Regularly shaped, single-crystalline ZnO nanorods with wurtzite structure. J. Am. Chem. Soc. 2002, 124, 14864–14865. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cai, H.; Li, C.; Liu, X.; Huang, F. Rocksalt-Zincblende-Wurtzite Mixed-Phase ZnO Crystals With High Activity as Photocatalysts for Visible-Light-Driven Water Splitting. Front. Chem. 2020, 8, 351. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Dai, F.; Zhang, H.-H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.-Z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef]
- Ha, S.W.; Tonelli, A.E.; Hudson, S.M. Structural studies of bombyx mori silk fibroin during regeneration from solutions and wet fiber spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Jones, F.; Tran, H.; Lindberg, D.; Zhao, L.; Hupa, M. Thermal stability of zinc compounds. Energy Fuels 2013, 27, 5663–5669. [Google Scholar] [CrossRef]
- Kalra, A.; Lowe, A.; Al-Jumaily, A.M. Mechanical Behaviour of Skin: A Review. J. Mater. Sci. Eng. 2016, 5, 1000254. [Google Scholar]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core-Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, e1900328. [Google Scholar] [CrossRef] [PubMed]
- Britto, E.J.; Nezwek, T.A.; Popowicz, P.; Robins, M. Wound Dressings; StatPearls, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Slayton, R.B.; Toth, D.; Lee, B.Y.; Tanner, W.; Bartsch, S.M.; Khader, K.; Wong, K.; Brown, K.; McKinnell, J.A.; Ray, W.; et al. Vital signs: Estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 826. [Google Scholar] [CrossRef] [PubMed]
- Toon, C.D.; Ramamoorthy, R.; Davidson, B.R.; Gurusamy, K.S. Early versus delayed dressing removal after primary closure of clean and clean-contaminated surgical wounds. Cochrane Database Syst. Rev. 2015, 2015, CD010259. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, C. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surf. B Biointerfaces 2006, 47, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Simunec, D.P.; Kyratzis, I.L.; Sola, A.; Wen, C. Biodegradable PLA-ZnO nanocomposite biomaterials with antibacterial properties, tissue engineering viability, and enhanced biocompatibility. Smart Mater. Manuf. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, W.; Wang, M.; Guan, W.; Yan, X.; Zhang, Q.; Wang, H.; Wang, Z.; Zhang, Y.; Zou, L. Fabrication, characterization and antibacterial properties of ZnO nanoparticles decorated electrospun polyacrylonitrile nanofibers membranes. Mater. Today Commun. 2022, 32, 103958. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, Y.; Yin, S.; Song, B.; Wei, L.; Chen, L.; Shao, L. Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int. J. Nanomed. 2017, 12, 8085–8099. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, N.; Sheng, W.; Ji, X.; Liang, X.; Kong, B.; Yin, P.; Li, Y.; Zhang, X.; Liu, K. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int. 2021, 146, 106179. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef]
- Orazizadeh, M.; Khodadadi, A.; Bayati, V.; Saremy, S.; Farasat, M.; Khorsandi, L. In Vitro Toxic Effects of Zinc Oxide Nanoparticles on Rat Adipose Tissue-Derived Mesenchymal Stem Cells. Cell J. 2015, 17, 412–421. [Google Scholar] [CrossRef]
- Chen, F.-C.; Huang, C.-M.; Yu, X.-W.; Chen, Y.-Y. Effect of nano zinc oxide on proliferation and toxicity of human gingival cells. Human. Exp. Toxicol. 2022, 41, 09603271221080236. [Google Scholar] [CrossRef]
- Badry, A.; Ibrahim, A.A.E.H.; Said, M.I.; Nasr, A.A.E.; Mohamed, M.A.; Hassan, A.K.; Safwat, M.M. In vitro assessment of PEG-6000 coated-ZnO nanoparticles: Modulating action to the resisted antibiotic activity against APEC. BMC Vet. Res. 2023, 19, 1. [Google Scholar] [CrossRef]
- Chong, S.F.; Smith, A.A.; Zelikin, A.N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 2013, 9, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Ribeiro, F.; Wojnarowicz, J.; Łojkowski, W.; Jurkschat, K.; Crossley, A.; Soares, A.M.; Loureiro, S. Zinc oxide nanoparticles toxicity to Daphnia magna: Size-dependent effects and dissolution. Environ. Toxicol. Chem. 2014, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, L.; Dong, J.; Yuan, X.; Ye, J.; Fan, Y.; Liu, B.; Xie, J.; Ji, X. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat. Commun. 2024, 15, 1042. [Google Scholar] [CrossRef]
- Zhang, F.; Kang, Y.; Feng, L.; Xi, G.; Chen, W.; Kong, N.; Tao, W.; Luan, T.; Koo, S.; Ji, X. Infected wound repair with an ultrasound-enhanced nanozyme hydrogel scaffold. Mater. Horiz. 2023, 10, 5474–5483. [Google Scholar] [CrossRef]
- Singh, K.; Yadav, V.B.; Yadav, U.; Nath, G.; Srivastava, A.; Zamboni, P.; Kerkar, P.; Saxena, P.S.; Singh, A.V. Evaluation of biogenic nanosilver-acticoat for wound healing: A tri-modal in silico, in vitro and in vivo study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131575. [Google Scholar] [CrossRef]



| Material | Size | Concentration | Gram + Antimicrobial Activity | Gram − Antimicrobial Activity | Cell Viability |
|---|---|---|---|---|---|
| SF/ZnO | 230 ± 45 nm | 11.5% wt | 29.89 ± 1.10% (Live/dead assay, 72 h, S. aureus) | 38.19 ± 1.46% (Live/dead assay, 72 h, P. aeruginosa) | >80, 90, 70% after 24, 48 and 72 h (Alamar Blue Assay, CHO) |
| PLGA/SF/ZnO [22] | 30–40 nm | 1%, 2%, 3% (v/v) | 45.1%, 87.57%, 100% (turbidity, S. aureus) Found MIC = 39.06 µg/mL (turbidity, 24 h, S. aureus) | 30.54%, 75.42%, 98.63% (turbidity, E. coli) Found MIC = 78.12 µg/mL (turbidity, 24 h, E. coli) | Same as PLGA/SF for day 1, 3, 7; cytotoxic at day 3 and 7; cytotoxic at day 3 and 7 (CCK8 Assay, L929) |
| PVA/CS/ZnO [40] | 30 nm | 0.5%, 1% wt | 75%, 85% (OD, 24 h, S. aureus) | 70%, 80% (OD, 24 h, E. coli) | 74%, >85% (24 h) >80%, >80% (48 h) (MTT assay, L929) |
| Electrospun PVA/ZnO [41] | 50–150 nm | 500 µg/mL | MIC = 250 μg/mL MBC = 250 μg/mL (24 h, S. aureus) | MIC = 62.5 μg/mL MBC = 125 μg/mL (24 h, E. coli) | 82.8%, 48 h (MTT assay, HFF) |
| PVA/starch/ZnO [42] | <100 nm | 2.6%, 3.2%, 3.8%, 4.4% wt | Significant (agar diffusion assay, 48 h, S. aureus) | Significant (agar diffusion assay, 48 h, E. coli) | N/A |
| CS/ZnO [43] | 80 nm | 0.001%, 0.0025%, 0.005%, 0.01% | Significant for all but 0.001% (still decreased number of bacteria) Best with higher concentrations of ZnO NP (colony counting, 24 h, S. aureus) | Significant for all (colony counting, 24 h, E. coli) | 0.01%: >80% viability after 24, 48 and 72 h Others: toxicity after 24 h (<60%) but good viability afterwards (>80%) (cell staining, nHDF) |
| Co-electrospun HA/SF-ZnO [33] | <50 nm | 6.5%, 19.35%, 32.26% wt (A VERIFIER) | 116 ± 3, 42 ± 2, and 6 ± 1 bacteria (CFU, 24 h, S. aureus) Antibacterial effect increases with concentration | 267 ± 7, 79 ± 3, and 28 ± 2 bacteria (CFU, 24 h, E. coli) Antibacterial effect increases with concentration | > 85% for all, but 32.26% wt decreased with time (1, 3, 7 days, MTT assay, HaCat) |
| PLA/ZnO [45] | 48 nm | 0.5, 1, 2% | 86%, 93%, 98% (colony counting, 72 h, S. aureus) | 40%, 57%, 75% (colony counting, 72 h, E. coli) | 79.6% (ZnO 2%wt, MTT Assay, 72 h, HSF) 70.5% (ZnO 2%wt, MTT Assay, 72 h, MSC) |
| PDLA/ZnO [46] | 6.36 ± 2.08 nm | 1, 3, 5% wt | 69.07 ± 1.07%, 69.01 ± 36.92%, 97.07 ± 2.63% (24 h, S. aureus) | 8.12 ± 2.49%, 25.50 ± 12.20%, 99.59 ± 0.14% (24 h, E. coli) | For all samples: 80% day 1 and 3, >100% day 5 and 7 (resazurin, MC3T3-E1) |
| PLA/PCL/TPS (thermoplastic starch)/ZnO [47] | 30–50 nm | 3%, 5% wt | 10 mm, 12 mm (inhibition zone, 24 h, S. aureus) | 8 mm, 9 mm (inhibition zone, 24 h, E. coli) | 25%, 15%: cytotoxic (MTT assay, 24 h, L929) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, D.; Dang, C.-T.; Monk, R.; Angel, S.; Marion, A.; Gbureck, U.; Harvey, E.; Merle, G. Infection Control with Antimicrobial Solid-State ZnO Nanoparticles on Silk Fibroin Gauze. Appl. Sci. 2024, 14, 7103. https://doi.org/10.3390/app14167103
Vieira D, Dang C-T, Monk R, Angel S, Marion A, Gbureck U, Harvey E, Merle G. Infection Control with Antimicrobial Solid-State ZnO Nanoparticles on Silk Fibroin Gauze. Applied Sciences. 2024; 14(16):7103. https://doi.org/10.3390/app14167103
Chicago/Turabian StyleVieira, Daniela, Cat-Thy Dang, Rachel Monk, Samuel Angel, Alexis Marion, Uwe Gbureck, Edward Harvey, and Geraldine Merle. 2024. "Infection Control with Antimicrobial Solid-State ZnO Nanoparticles on Silk Fibroin Gauze" Applied Sciences 14, no. 16: 7103. https://doi.org/10.3390/app14167103






