3D-Printed Conductive Polymers as Alternative for Bioelectrochemical Systems Electrodes: Abiotic Study and Biotic Start-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Design and Fabrication
2.2. Analytical Techniques
2.3. Biological Activation of Electrodes
3. Results and Discussion
3.1. Electrochemical Characterization of the PLA Electrodes
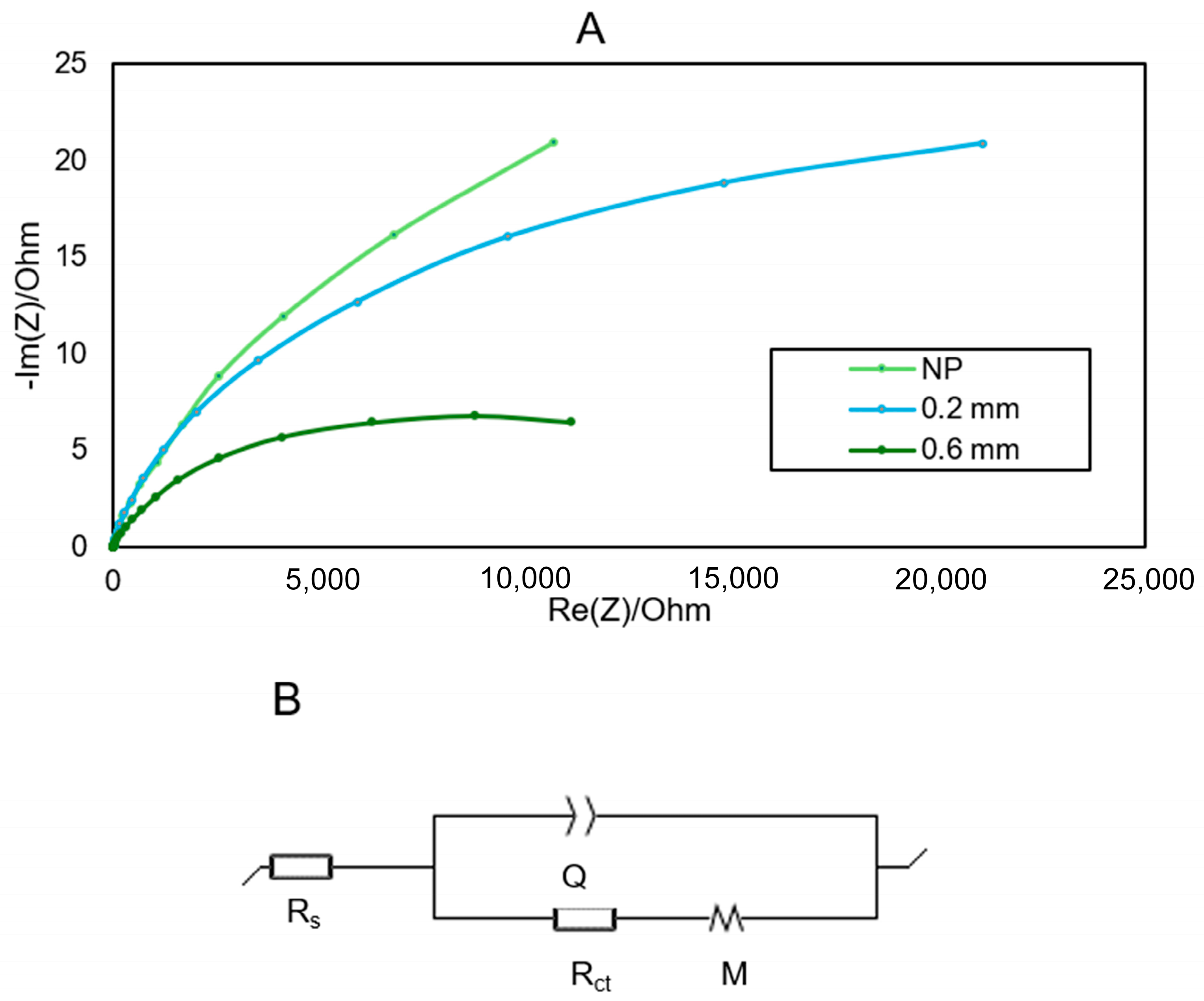
3.2. Electrochemical Characterization of the TPU Electrodes
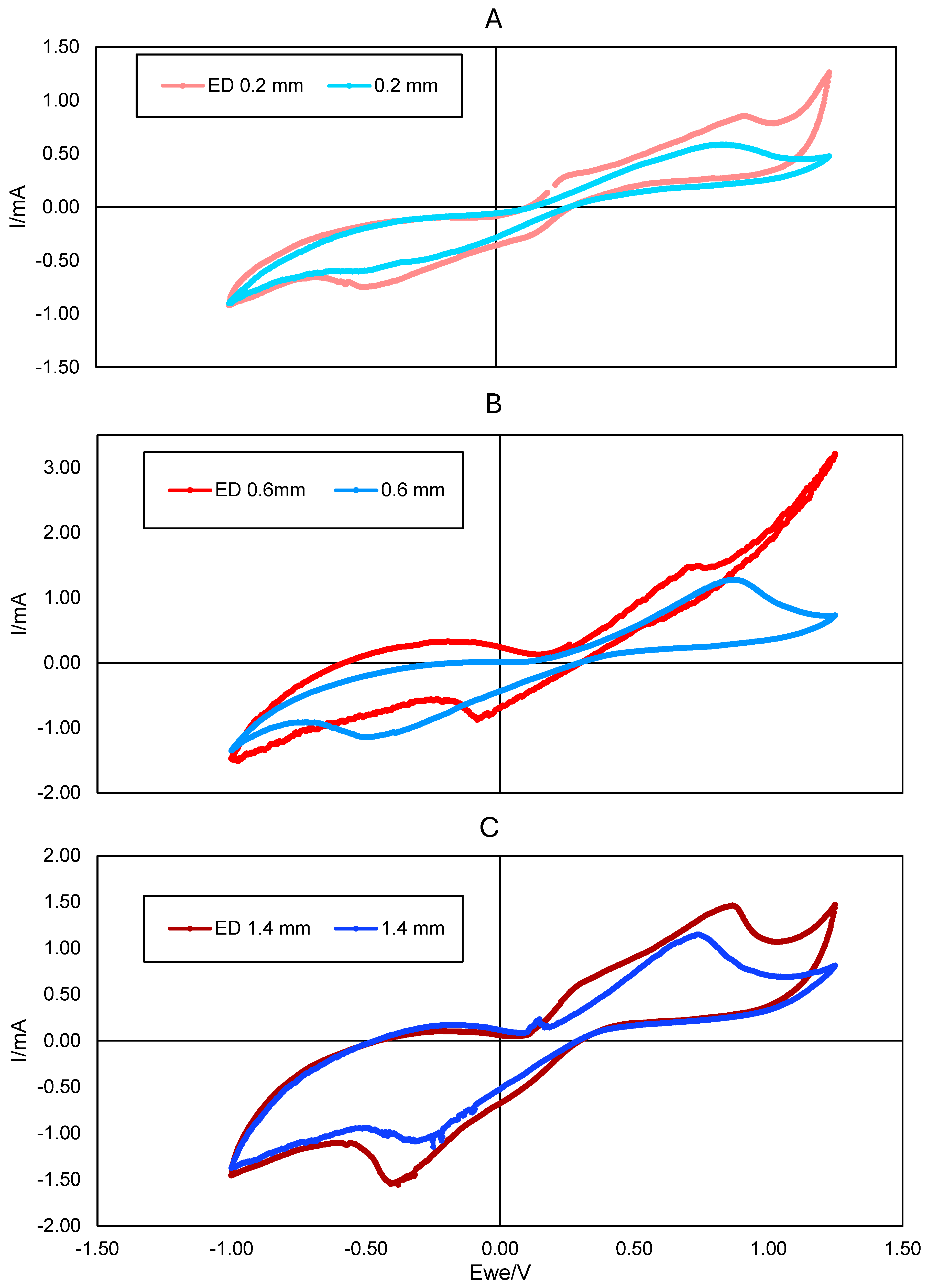
3.3. Electrochemical Characterization of the PLA Electrodes Electrodeposited with Graphene Oxide
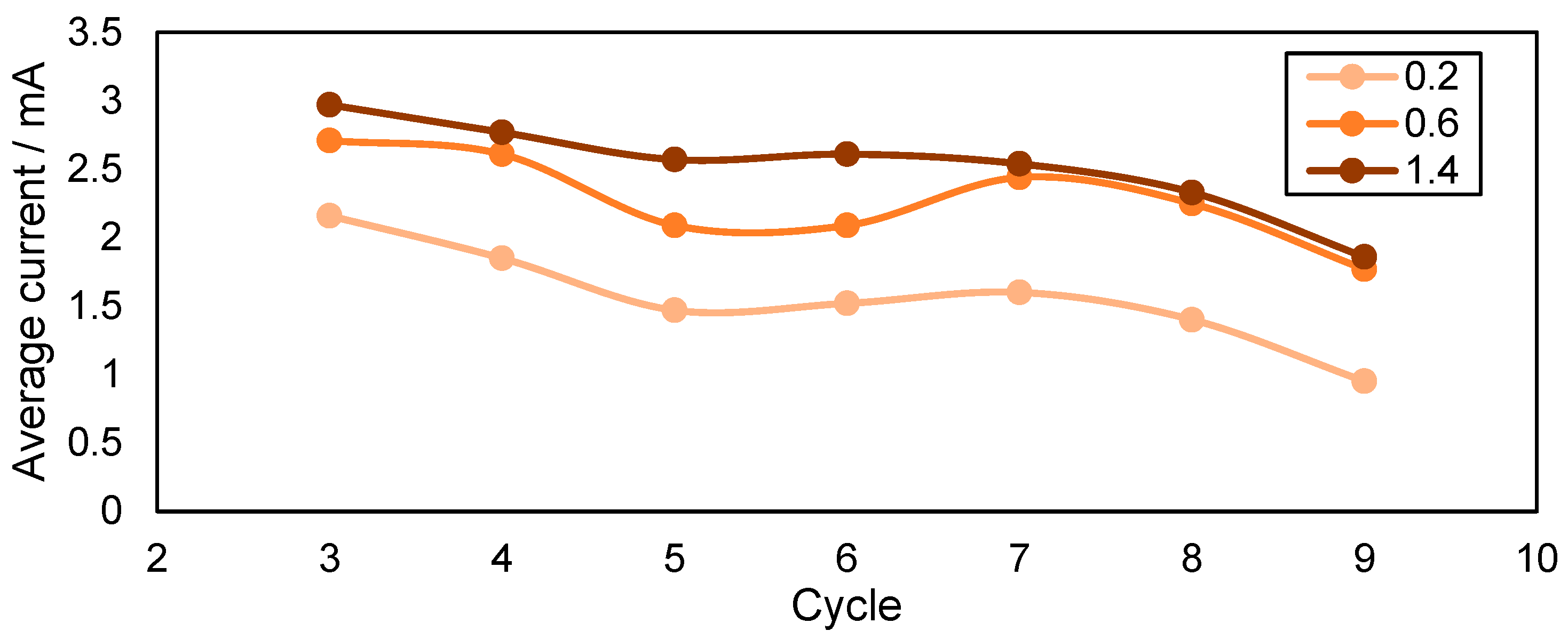
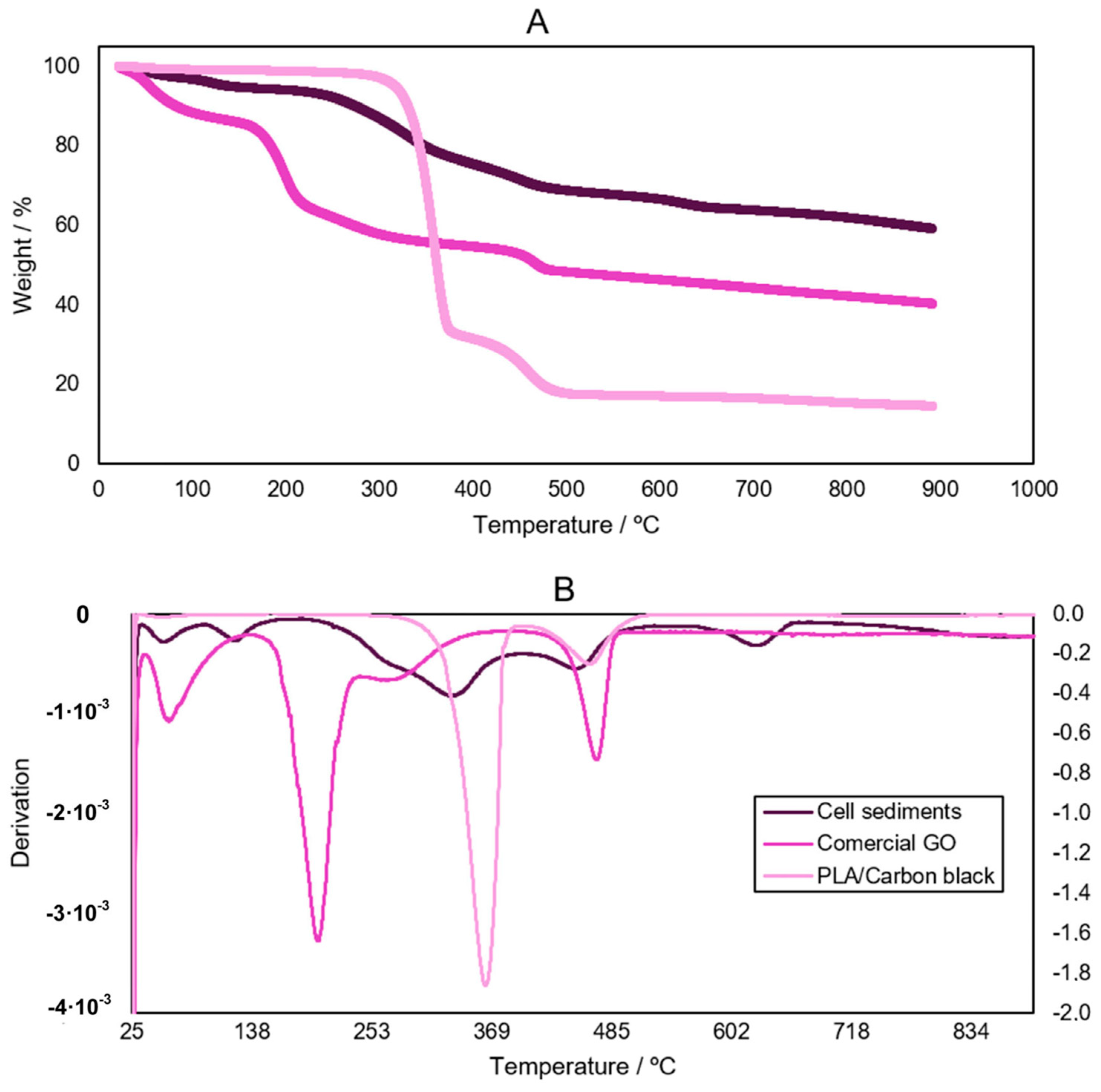
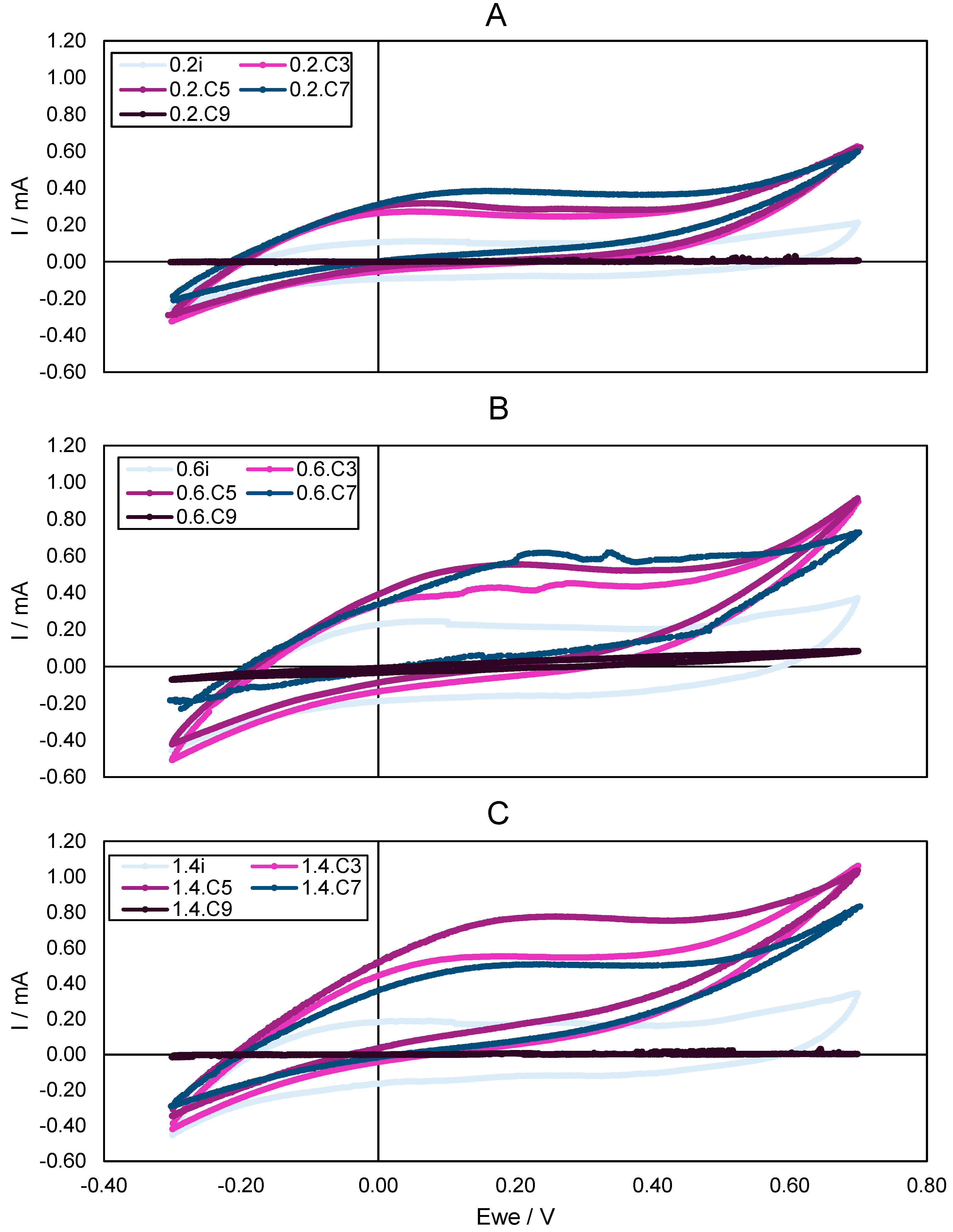
3.4. Characterization of the Biologically Activated Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ieropoulos, I.A.; Singh, A.; Moreno, D.Z.; Greenman, J. Bioelectrochemical Systems and their readiness for commercialisation. Curr. Opin. Electrochem. 2024, 46, 101540. [Google Scholar] [CrossRef]
- Ramanaiah, S.V.; Chandrasekhar, K.; Cordas, C.M.; Potoroko, I. Bioelectrochemical systems (BESs) for agro-food waste and wastewater treatment, and sustainable bioenergy-A review. Environ. Pollut. 2023, 325, 121432. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rezae, A.; Ghosh, S.; Malloum, A.; Banach, A. A review on bioelectrochemical systems for emerging pollutants remediation: A computational approaches. J. Environ. Chem. Eng. 2023, 11, 110021. [Google Scholar] [CrossRef]
- Resitano, M.; Tucci, M.; Mezzi, A.; Kaciulis, S.; Matturro, B.; D’Ugo, E.; Bertuccini, L.; Fazi, S.; Rossetti, S.; Aulenta, F.; et al. Anaerobic treatment of groundwater co-contaminated by toluene and copper in a single chamber bioelectrochemical system. Bioelectrochemistry 2024, 158, 108711. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Srivastava, N.; Pathak, T.S.; Priyanka, P. Advancement in electrode materials and membrane separators for scaling up of MES. In Scaling Up of Microbial Electrochemical Systems: From Reality to Scalability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–172. [Google Scholar] [CrossRef]
- Saratale, G.D.; Banu, J.R.; Nastroc, R.A.; Kadier, A.; Ashokkumar, V.; Lay, C.-H.; Jung, J.-H.; Shin, H.S.; Saratale, R.G.; Chandrasekhar, K. Bioelectrochemical systems in aid of sustainable biorefineries for the production of value-added products and resource recovery from wastewater: A critical review and future perspectives. Bioresour. Technol. 2022, 359, 127435. [Google Scholar] [CrossRef]
- Alonso, R.M.; San-Martín, M.I.; Mateos, R.; Morán, A.; Escapa, A. Scale-up of bioelectrochemical systems for energy valorization of waste streams. In Microbial Electrochemical Technologies, 1st ed.; Tiquia-Arashiro, S., Pant, D., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 447–459. [Google Scholar] [CrossRef]
- Bajracharya, S.; Bian, B.; Jimenez-Sandoval, R.; Matsakas, L.; Katuri, K.P.; Saikaly, P.E. Nature inspired catalysts: A review on electroactive microorganism-based catalysts for electrochemical applications. Electrochim. Acta 2024, 488, 144215. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, J.; Xu, M.Y.; Li, S.W.; Li, C.; Zhou, X.; Wei, J. A biocompatible electrode/exoelectrogens interface augments bidirectional electron transfer and bioelectrochemical reactions. Bioelectrochemistry 2024, 158, 108723. [Google Scholar] [CrossRef]
- Larzillière, V.; de Fouchécour, F.; Bureau, C.; Bouchez, T.; Moscoviz, R. Urban wastewater oxidation by bioelectrochemical systems: To what extent does the inoculum matter? Bioelectrochemistry 2024, 155, 108577. [Google Scholar] [CrossRef]
- Li, X.; Cao, H.; Feng, Q.; Lv, Y.; Zhao, Y.; Pan, H. Effects of applied voltages on electron transfer pathways for bioelectrochemical methane production from maize straw. Energy 2024, 296, 131215. [Google Scholar] [CrossRef]
- Li, C.; Cheng, S. Functional group surface modifications for enhancing the formation and performance of exoelectrogenic biofilms on the anode of a bioelectrochemical system. Crit. Rev. Biotechnol. 2019, 39, 1015–1030. [Google Scholar] [CrossRef]
- Grover, S.; Doyle, L.E. Advanced electrode materials for microbial extracellular electron transfer. Trends Chem. 2024, 6, 144–158. [Google Scholar] [CrossRef]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.J.; Arias, D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: From biofilm-bearing anodes to specialized cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Dhar, B.R. A Mini-Review on Applications of 3D Printing for Microbial Electrochemical Technologies. Front. Energy Res. 2021, 9, 679061. [Google Scholar] [CrossRef]
- Baş, F.; Kaya, M.F. 3D printed anode electrodes for microbial electrolysis cells. Fuel 2022, 317, 123560. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Li, Y.; Wang, Y.; Lu, X.; Wei, Z.; Mo, Q.; Zhang, S.; Sheng, Y.; Huang, C.; et al. Research progress in polylactic acid processing for 3D printing. J. Manuf. Process. 2024, 112, 161–178. [Google Scholar] [CrossRef]
- Mohammed-Sadhakathullah, A.H.M.; Paulo-Mirasol, S.; Molina, B.G.; Torras, J.; Armelin, E. PLA-PEG-Cholesterol biomimetic membrane for electrochemical sensing of antioxidants. Electrochim. Acta 2024, 476, 143716. [Google Scholar] [CrossRef]
- Cruz, R.; Nisar, M.; Palza, H.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Development of bio degradable nanocomposites based on PLA and functionalized graphene oxide. Polym. Test. 2023, 124, 108066. [Google Scholar] [CrossRef]
- Khalil, M.; Dincer, I. An investigation of hydrogen production using 3D-printed cathodes for alkaline water electrolysis. Energy Convers. Manag. 2024, 302, 118169. [Google Scholar] [CrossRef]
- McDonagh, P.; McCole, M.; Skillen, N.; Robertson, P.K.J.; McCrudden, D. 3D printed carbon black-polylactic acid/copper (CB-PLA/Cu) sensor for improved sustainability in monitoring photocatalytic hydrogen peroxide evolution. J. Environ. Chem. Eng. 2023, 11, 111123. [Google Scholar] [CrossRef]
- You, J.; Preen, R.J.; Bull, L.; Greenman, J.; Ieropoulos, I. 3D printed components of microbial fuel cells: Towards monolithic microbial fuel cell fabrication using additive layer manufacturing. Sustain. Energy Technol. Assess. 2017, 19, 94–101. [Google Scholar] [CrossRef]
- Pumera, M. Three-dimensionally printed electrochemical systems for biomedical analytical applications. Curr. Opin. Electrochem. 2019, 14, 133–137. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Mateos, R.; Morán, A.; Escapa, A. Reduced graphene oxide improves the performance of a methanogenic biocathode. Fuel 2022, 321, 123957. [Google Scholar] [CrossRef]
- Alonso, R.M.; Martín, I.S.; Morán, A.; Escapa, A. Comparison of Activation Methods for 3D-Printed Electrodes for Microbial Electrochemical Technologies. Appl. Sci. 2022, 12, 275. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Pelaz, G.; González, R.; Morán, A.; Escapa, A. Elucidating the impact of power interruptions on microbial electromethanogenesis. Appl. Energy 2023, 331, 120382. [Google Scholar] [CrossRef]
- Mateos, R.; Alonso, R.M.; Escapa, A.; Morán, A. Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up. Materials 2017, 10, 79. [Google Scholar] [CrossRef]
- Bonanos, N.; Steele, B.C.H.; Butler, E.P. Applications of Impedance Spectroscopy. In Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 205–537. [Google Scholar] [CrossRef]
- Sayed, E.T.; Alawadhi, H.; Olabi, A.G.; Jamal, A.; Almahdi, M.S.; Khalid, J.; Abdelkareem, M.A. Electrophoretic deposition of graphene oxide on carbon brush as bioanode for microbial fuel cell operated with real wastewater. Int. J. Hydrogen Energy 2021, 46, 5975–5983. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Pelaz, G.; Mateos, R.; Escapa, A. Charge storage capacity of electromethanogenic biocathodes. J. Energy Storage 2024, 76, 109789. [Google Scholar] [CrossRef]

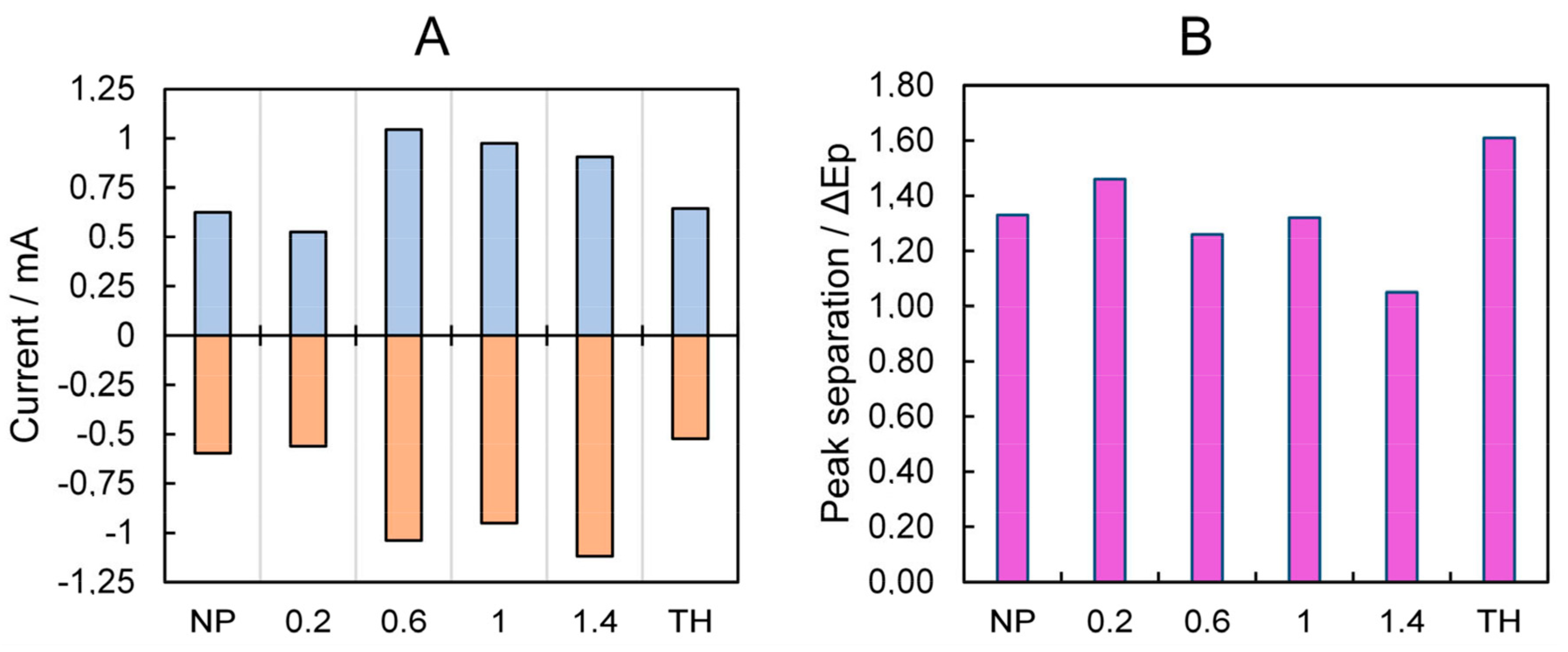

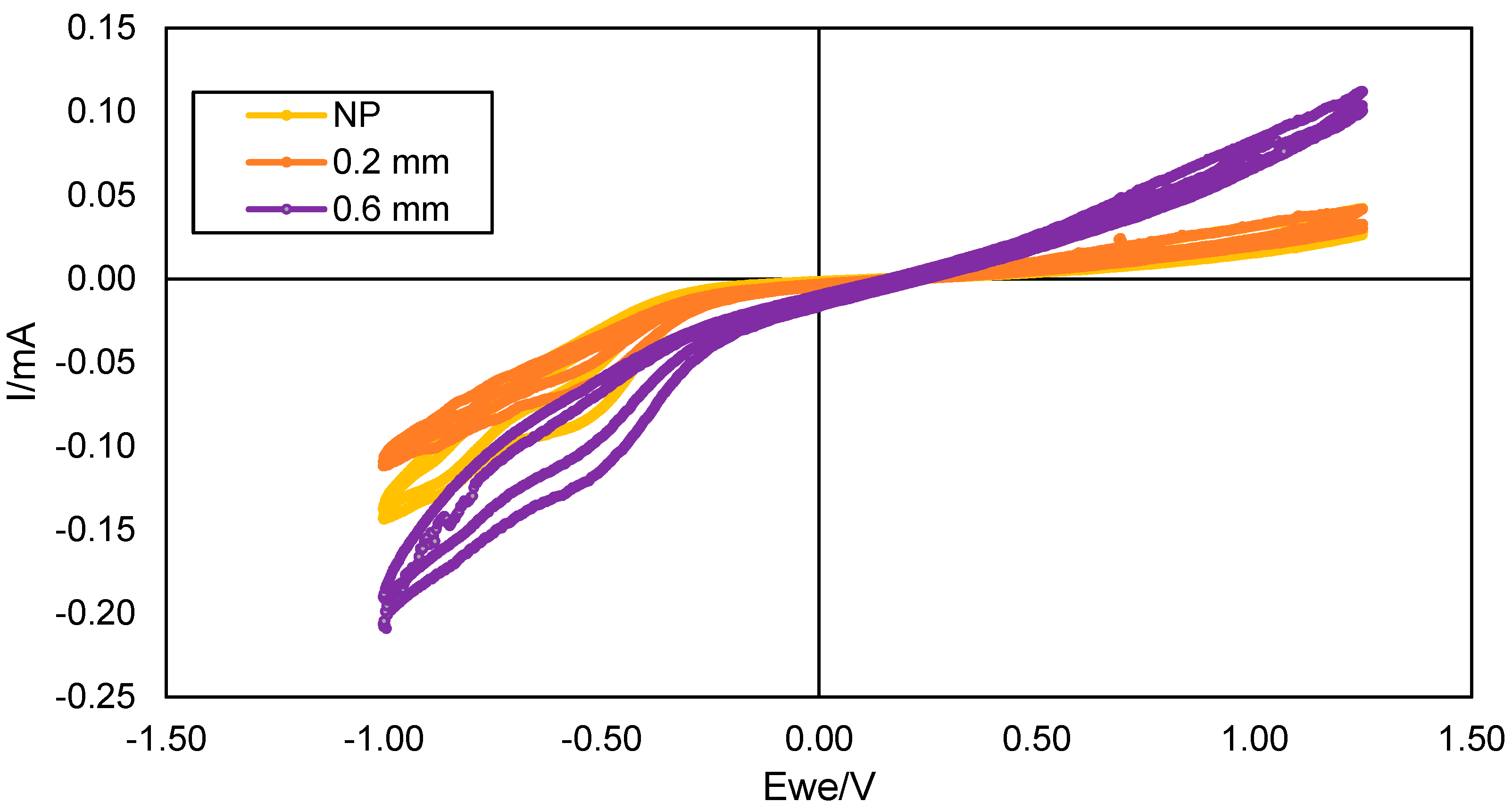
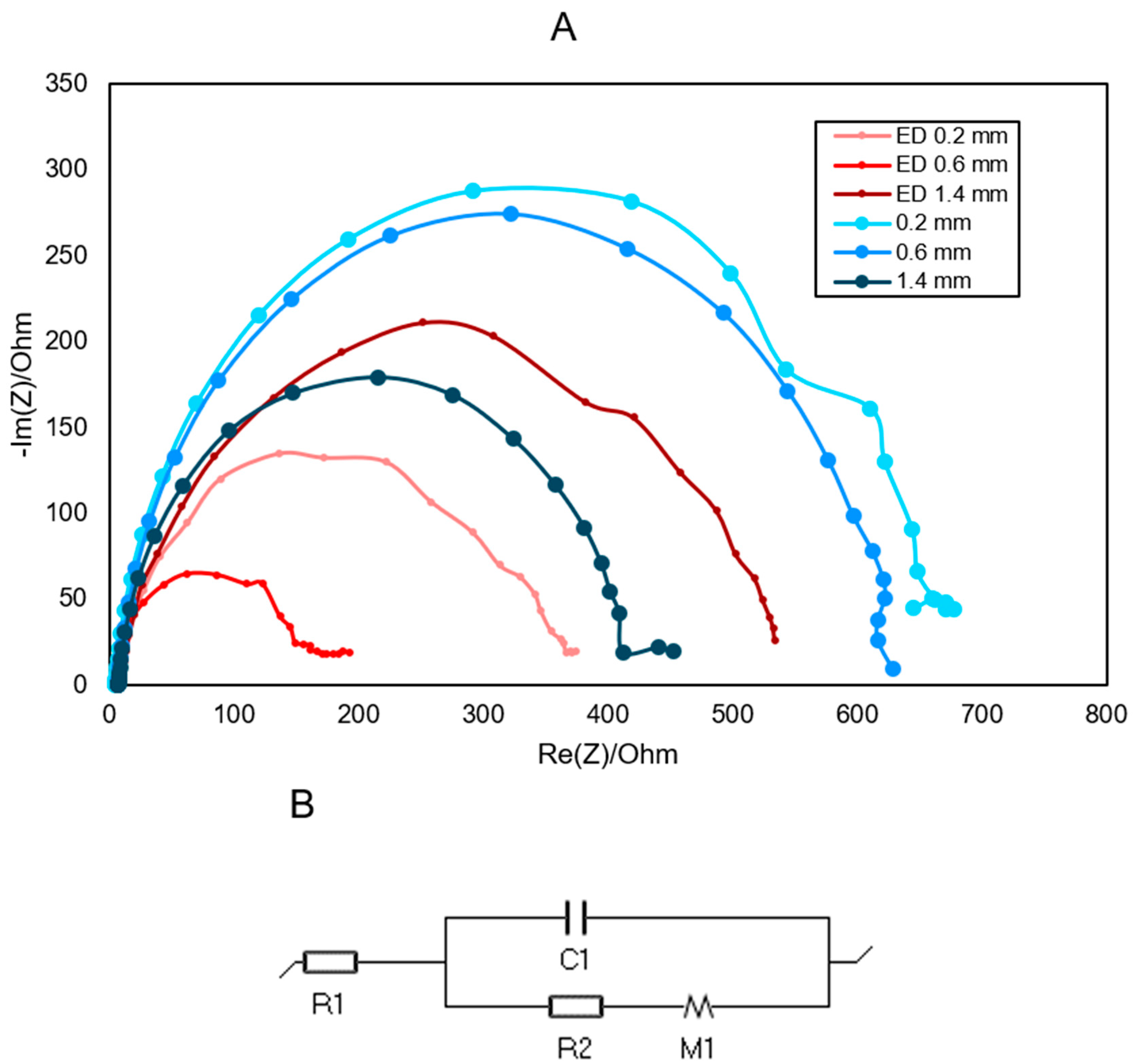
| NP | 0.2 | 0.6 | 1 | 1.4 | TH | |
| Rs (Ω) | 14.22 | 6.009 | 7.291 | 5.821 | 7.637 | 4.169 |
| C (F) | 3.07·10−5 | 1.42·10−5 | 2.88·10−5 | 2.65·10−5 | 6.68·10−5 | 3.5·10−5 |
| Rct (Ω) | 647.9 | 713.7 | 563.3 | 687.4 | 369 | 1450 |
| Rd1 (Ω) | 3.08·102 | 2.81·102 | 1.80·102 | 2.06·102 | 1.77·102 | 5.27·102 |
| td1 (s) | 5.959 | 7.802 | 6.78 | 9.678 | 9.56 | 7.376 |
| NP | 0.2 | 0.6 | |
| Rs (Ω) | 9.467 | 8.553 | 5.631 |
| Q1 (F s(a−1)) | 5.34·10−5 | 3.28·10−5 | 6.19·10−5 |
| a1 | 0.92 | 0.93 | 0.89 |
| Rct (Ω) | 4.65·104 | 4.01·104 | 1.62·104 |
| Rd1 (Ω) | 6.5·104 | 2.98·104 | 0.19 |
| td1 (s) | 14.5 | 24.04 | 9.232 |
| 0.2 | 0.6 | 1.4 | |
| Rs (Ω) | 8.894 | 2.581 | 8.631 |
| C (F) | 1.56·10−5 | 2.16·10−5 | 3.4·10−5 |
| Rct (Ω) | 302.1 | 143.4 | 422.7 |
| Rd1 (Ω) | 1.92·102 | 1.38·102 | 3.18·102 |
| td1 (s) | 7.023 | 7.734 | 7.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mur-Gorgas, A.; Martínez-Pellitero, S.; Joglar, T.; Escapa, A.; Mateos, R. 3D-Printed Conductive Polymers as Alternative for Bioelectrochemical Systems Electrodes: Abiotic Study and Biotic Start-Up. Appl. Sci. 2024, 14, 7199. https://doi.org/10.3390/app14167199
Mur-Gorgas A, Martínez-Pellitero S, Joglar T, Escapa A, Mateos R. 3D-Printed Conductive Polymers as Alternative for Bioelectrochemical Systems Electrodes: Abiotic Study and Biotic Start-Up. Applied Sciences. 2024; 14(16):7199. https://doi.org/10.3390/app14167199
Chicago/Turabian StyleMur-Gorgas, Alberto, Susana Martínez-Pellitero, Tamara Joglar, Adrián Escapa, and Raúl Mateos. 2024. "3D-Printed Conductive Polymers as Alternative for Bioelectrochemical Systems Electrodes: Abiotic Study and Biotic Start-Up" Applied Sciences 14, no. 16: 7199. https://doi.org/10.3390/app14167199






