Response Surface Methodology for Ultrasound-Assisted Oil Extraction Optimization from Blackberry, Chokeberry, and Raspberry Waste Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Ultrasound-Assisted Extraction
2.2.2. Experimental Design
2.2.3. Oil Yield Determination
2.2.4. Conventional Extraction
2.2.5. Oxidation Induction Time
2.2.6. Oxidation Kinetics
2.2.7. Melting and Crystallization Behavior
2.2.8. Gas Chromatography
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
3.2. Oxidation Induction Time
3.3. Optimization of Ultrasound Oil Extraction
3.4. Kinetic Parameters of Oil Oxidation
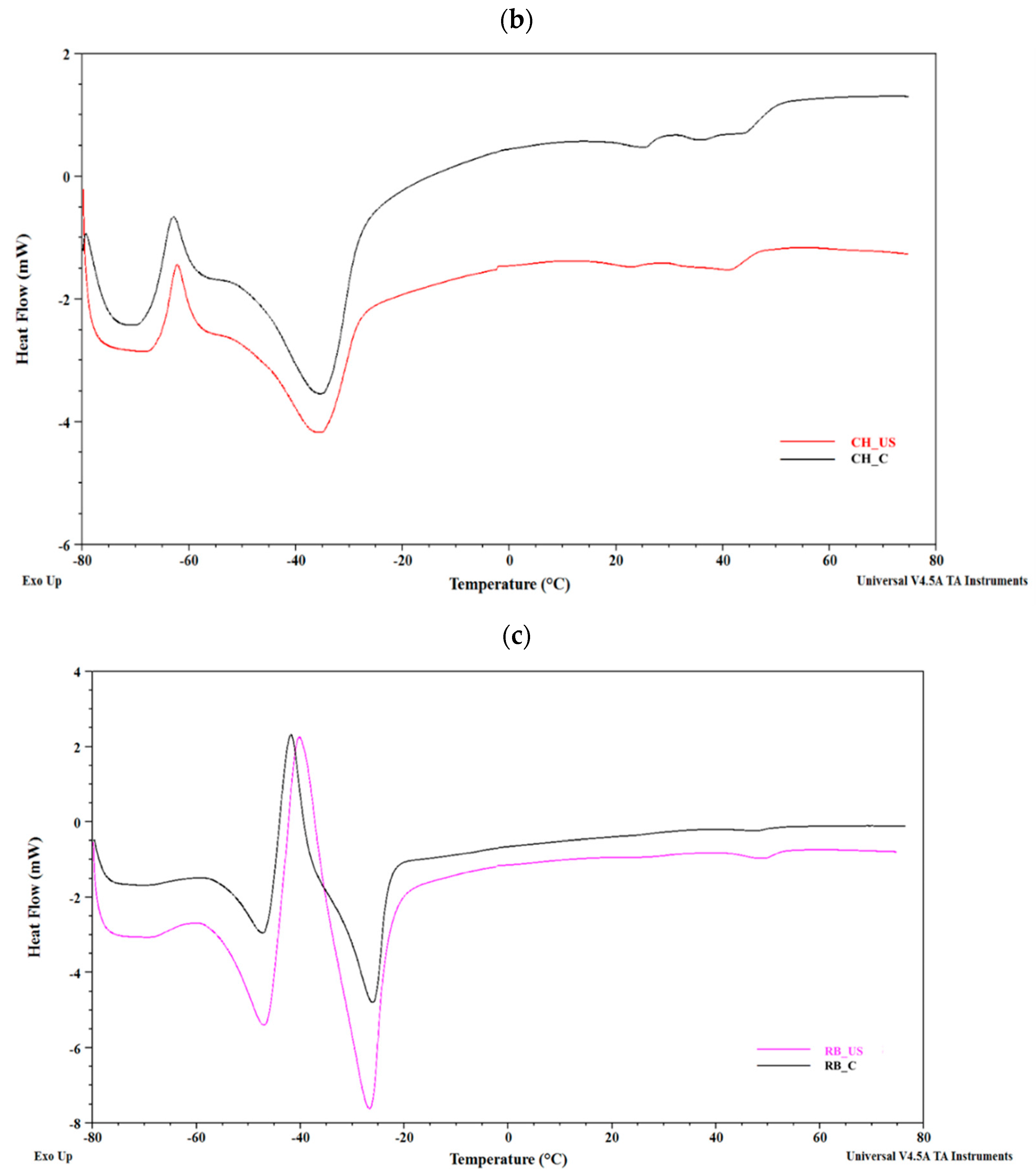
3.5. DSC Assessment
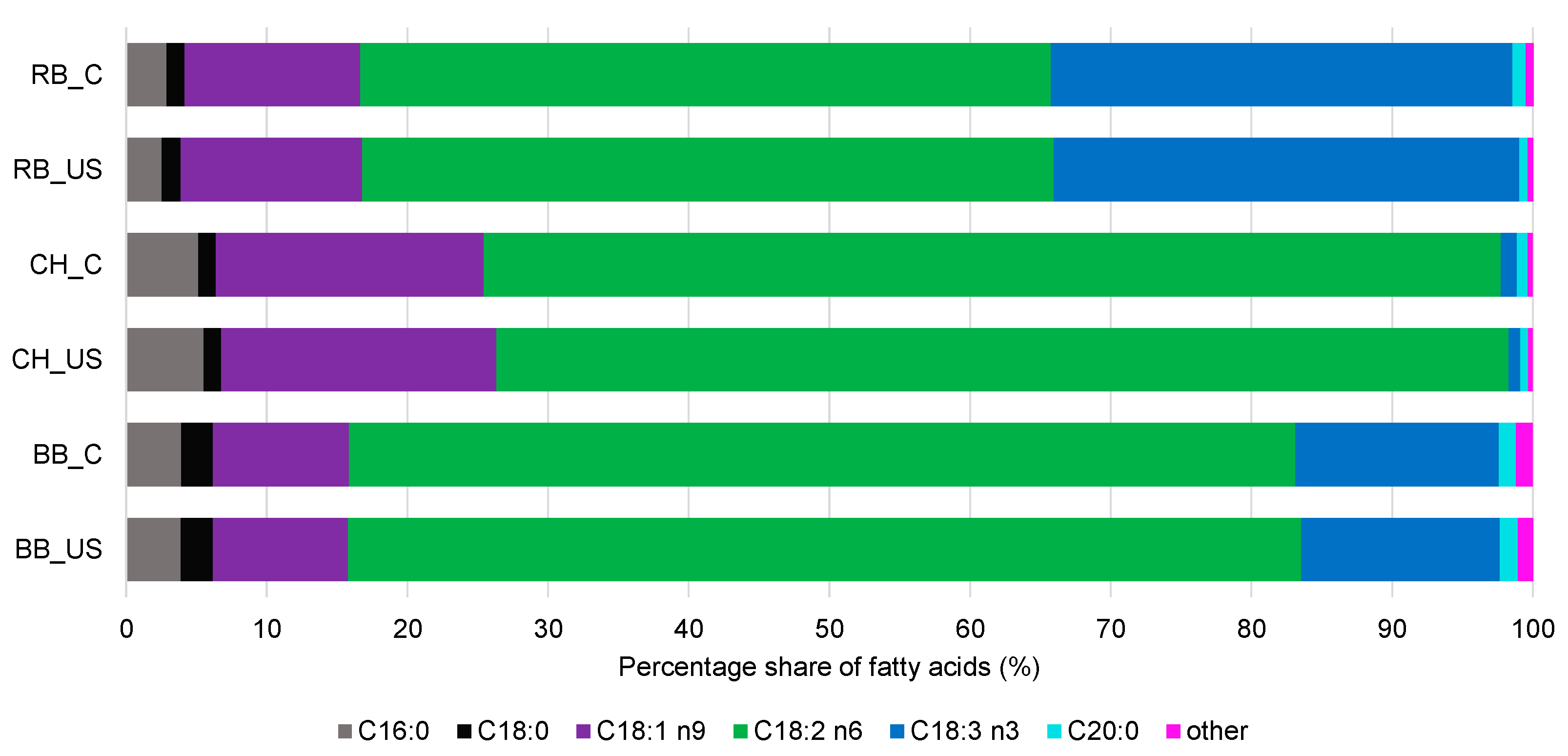
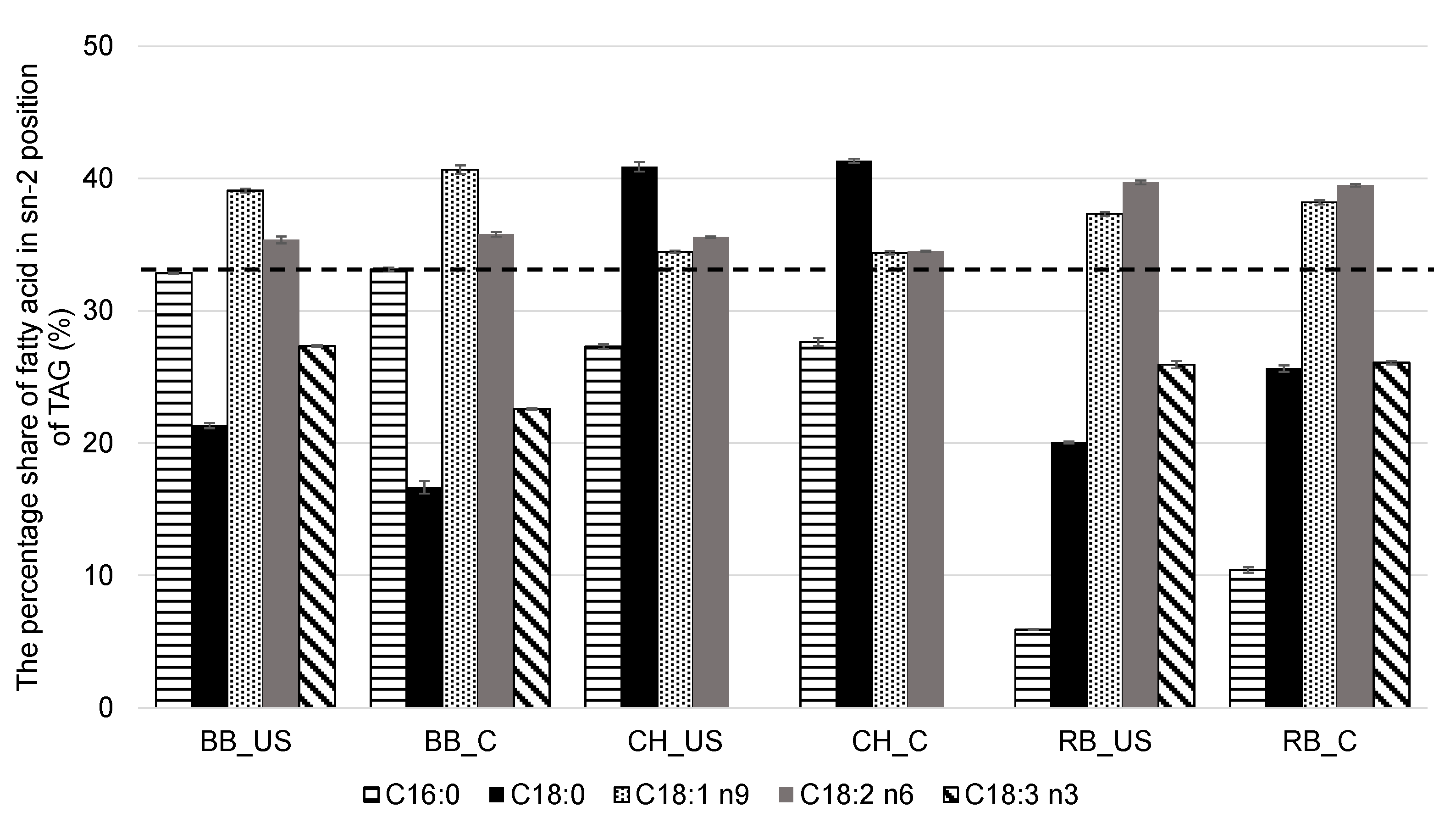
3.6. GC Analysis of Fatty Acid Profile and Distribution in sn-1,3 and sn-2 Positions in Triacylglycerols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of Berry Pomace to Design Functional Foods. Food Rev. Int. 2023, 39, 3204–3224. [Google Scholar] [CrossRef]
- Pecyna, A.; Krzywicka, M.; Blicharz-Kania, A.; Buczaj, A.; Kobus, Z.; Zdybel, B.; Domin, M.; Siłuch, D. Impact of Incorporating Two Types of Dried Raspberry Pomace into Gluten-Free Bread on Its Nutritional and Antioxidant Characteristics. Appl. Sci. 2024, 14, 1561. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Oancea, A.G.; Varzaru, I.; Vlaicu, P.A. Comparative Analysis of Black Chokeberry (Aronia melanocarpa L.) Fruit, Leaves, and Pomace for Their Phytochemical Composition, Antioxidant Potential, and Polyphenol Bioaccessibility. Foods 2024, 13, 1856. [Google Scholar] [CrossRef] [PubMed]
- Čechovičienė, I.; Šlepetienė, A.; Gumbytė, M.; Paulauskienė, A.; Tarasevičienė, Ž. Composition and Physicochemical Properties of Pomace of Various Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2023, 10, 38. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A.; Ferrentino, G.; Angeli, L.; Morozova, K.; Zatelli, D.; Scampicchio, M. Ultrasound Assisted Extraction of Oils from Apple Seeds: A Comparative Study with Supercritical Fluid and Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2023, 86, 103370. [Google Scholar] [CrossRef]
- Milanović, J.; Malićanin, M.; Rakić, V.; Jevremović, N.; Karabegović, I.; Danilović, B. Valorization of Winery Waste: Prokupac Grape Seed as a Source of Nutritionally Valuable Oil. Agronomy 2021, 11, 1864. [Google Scholar] [CrossRef]
- Pérez-Saucedo, M.R.; Jiménez-Ruiz, E.I.; Rodríguez-Carpena, J.G.; Ragazzo-Sánchez, J.A.; Ulloa, J.A.; Ramírez-Ramírez, J.C.; Gastón-Peña, C.R.; Bautista-Rosales, P.U. Properties of the Avocado Oil Extracted Using Centrifugation and Ultrasound-Assisted Methods. Food Sci. Biotechnol. 2021, 30, 1051–1061. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound-Assisted Extraction of Cranberry Seed Oil: Food Waste Valorization Approach. Eur. Food Res. Technol. 2023, 249, 2763–2775. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Kalisz, S.; Wiktor, A.; Górska, A. Recovery of Antioxidants and Oils from Blackcurrant and Redcurrant Wastes by Ultrasound-Assisted Extraction. Food Biosci. 2024, 57, 103511. [Google Scholar] [CrossRef]
- Ni, Q.; Gao, Q.; Yu, W.; Liu, X.; Xu, G.; Zhang, Y. Supercritical Carbon Dioxide Extraction of Oils from Two Torreya Grandis Varieties Seeds and Their Physicochemical and Antioxidant Properties. LWT-Food Sci. Technol. 2015, 60, 1226–1234. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A.; Koczoń, P. The Use of DSC and FT-IR Spectroscopy for Evaluation of Oxidative Stability of Interesterified Fats. J. Therm. Anal. Calorim. 2013, 112, 481–487. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E. Effect of Enzymatic Interesterification on Physiochemical and Thermal Properties of Fat Used in Cookies. LWT 2016, 74, 99–105. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Ostrowska-Ligęza, E.; Kalisz, S. The Study of Thermal Properties of Blackberry, Chokeberry and Raspberry Seeds and Oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Embaby, H.E.; Miyakawa, T.; Hachimura, S.; Muramatsu, T.; Nara, M.; Tanokura, M. Crystallization and Melting Properties Studied by DSC and FTIR Spectroscopy of Goldenberry (Physalis peruviana) Oil. Food Chem 2022, 366, 130645. [Google Scholar] [CrossRef]
- PN-EN ISO: 5509:2001; Animal and Vegetable Fats and Oils. Preparation of Methyl Esters of Fatty Acids. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Bryś, J.; Flores Lnês, F.V.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC Methods to Characterize Human Milk Fat Substitutes Obtained from Lard and Milk Thistle Oil Mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Pina-Rodriguez, A.M.; Akoh, C.C. Enrichment of Amaranth Oil with Ethyl Palmitate at the Sn-2 Position by Chemical and Enzymatic Synthesis. J. Agric. Food Chem. 2009, 57, 4657–4662. [Google Scholar] [CrossRef]
- Dash, D.R.; Pathak, S.S.; Pradhan, R.C. Extraction of Oil from Terminalia Chebula Kernel by Using Ultrasound Technology: Influence of Process Parameters on Extraction Kinetics. Ind. Crops Prod. 2021, 171, 113893. [Google Scholar] [CrossRef]
- Böger, B.R.; Salviato, A.; Valezi, D.F.; Di Mauro, E.; Georgetti, S.R.; Kurozawa, L.E. Optimization of Ultrasound-Assisted Extraction of Grape-Seed Oil to Enhance Process Yield and Minimize Free Radical Formation. J. Sci. Food Agric. 2018, 98, 5019–5026. [Google Scholar] [CrossRef]
- Wong, S.T.; Tan, M.C.; Geow, C.H. Optimization of Ultrasound-Assisted Ethanol Extraction of Hazelnut Oil. J. Food Process. Preserv. 2019, 43, e14138. [Google Scholar] [CrossRef]
- Sanwal, N.; Mishra, S.; Sahu, J.K.; Naik, S.N. Effect of Ultrasound-Assisted Extraction on Efficiency, Antioxidant Activity, and Physicochemical Properties of Sea Buckthorn (Hippophae salicipholia) Seed Oil. LWT 2022, 153, 112386. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. Optimization of Ultrasound-Assisted Solvent Extraction (UASE) Based on Oil Yield, Antioxidant Activity and Evaluation of Fatty Acid Composition and Thermal Stability of Coriandrum sativum L. Seed Oil. Food Sci. Biotechnol. 2019, 28, 377–386. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the Oxidative Stability of Hazelnut Oils by PDSC and Rancimat Methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on Their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.A.; Wali, A.; Aadil, R.M. Advances in Green Processing of Seed Oils Using Ultrasound-Assisted Extraction: A Review. J. Food Process. Preserv. 2020, 44, e14740. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K.; Lee, Y.Y. A Review on Application of Ultrasound and Ultrasound Assisted Technology for Seed Oil Extraction. J. Food Sci. Technol. 2022, 60, 1222–1236. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Antioxidants: Science, Technology, and Applications. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Malićanin, M.; Rac, V.; Antić, V.; Antić, M.; Palade, L.M.; Kefalas, P.; Rakić, V. Content of Antioxidants, Antioxidant Capacity and Oxidative Stability of Grape Seed Oil Obtained by Ultra Sound Assisted Extraction. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 989–999. [Google Scholar] [CrossRef]
- Stevanato, N.; da Silva, C. Radish Seed Oil: Ultrasound-Assisted Extraction Using Ethanol as Solvent and Assessment of Its Potential for Ester Production. Ind. Crops Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules 2023, 28, 2486. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic-Assisted Extraction of Raspberry Seed Oil and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. PLoS ONE 2016, 11, e0153457. [Google Scholar] [CrossRef]
- Isopencu, G.; Stroescu, M.; Brosteanu, A.; Chira, N.; Pârvulescu, O.C.; Busuioc, C.; Stoica-Guzun, A. Optimization of Ultrasound and Microwave Assisted Oil Extraction from Sea Buckthorn Seeds by Response Surface Methodology. J. Food Process. Eng. 2019, 42, e12947. [Google Scholar] [CrossRef]
- Amft, J.; Meissner, P.M.; Steffen-Heins, A.; Hasler, M.; Stöckmann, H.; Meynier, A.; Birault, L.; Velasco, J.; Vermoesen, A.; Perez-Portabella, I.; et al. Interlaboratory Study on Lipid Oxidation during Accelerated Storage Trials with Rapeseed and Sunflower Oil Analyzed by Conjugated Dienes as Primary Oxidation Products. Eur. J. Lipid Sci. Technol. 2023, 125, 2300067. [Google Scholar] [CrossRef]
- Farhoosh, R.; Hoseini-Yazdi, S.Z. Evolution of Oxidative Values during Kinetic Studies on Olive Oil Oxidation in the Rancimat Test. J. Am. Oil Chem. Soc. 2014, 91, 281–293. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Liu, Z.S.; Perez, J.M. Oxidation Kinetic Studies of Oils Derived from Unmodified and Genetically Modified Vegetables Using Pressurized Differential Scanning Calorimetry and Nuclear Magnetic Resonance Spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Islam, M.; Bełkowska, L.; Konieczny, P.; Fornal, E.; Tomaszewska-Gras, J. Differential Scanning Calorimetry for Authentication of Edible Fats and Oils—What Can We Learn from the Past to Face the Current Challenges? J. Food Drug Anal. 2022, 30, 185–201. [Google Scholar] [CrossRef]
- Gila, A.; Sánchez-Ortiz, A.; Jiménez, A.; Beltrán, G. The Ultrasound Application Does Not Affect to the Thermal Properties and Chemical Composition of Virgin Olive Oils. Ultrason. Sonochem. 2021, 70, 105320. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Investigation on the Extraction Yield, Quality, and Thermal Properties of Hempseed Oil during Ultrasound-Assisted Extraction: A Comparative Study. J. Food Process. Preserv. 2018, 42, e13766. [Google Scholar] [CrossRef]
- Micić, D.M.; Ostojić, S.B.; Simonović, M.B.; Pezo, L.L.; Simonović, B.R. Thermal Behavior of Raspberry and Blackberry Seed Flours and Oils. Thermochim. Acta 2015, 617, 21–27. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Islam, M.; Tomaszewska-Gras, J. Influence of Seeds’ Age and Clarification of Cold-Pressed Raspberry (Rubus idaeus L.) Oil on the DSC Oxidative Stability and Phase Transition Profiles. Foods 2023, 12, 358. [Google Scholar] [CrossRef]
- Dahimi, O.; Rahim, A.A.; Abdulkarim, S.M.; Hassan, M.S.; Hashari, S.B.T.Z.; Siti Mashitoh, A.; Saadi, S. Multivariate Statistical Analysis Treatment of DSC Thermal Properties for Animal Fat Adulteration. Food Chem. 2014, 158, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Marikkar, J.M.N.; Ghazali, H.M.; Che Man, Y.B.; Lai, O.M. The Use of Cooling and Heating Thermograms for Monitoring of Tallow, Lard and Chicken Fat Adulterations in Canola Oil. Food Res. Int. 2002, 35, 1007–1014. [Google Scholar] [CrossRef]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Comparison of Subcritical CO2 and Ultrasound-Assisted Aqueous Methods with the Conventional Solvent Method in the Extraction of Avocado Oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of Raspberry (Rubus idaeus L.) Seed Oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Siger, A.; Kowalczewski, P.Ł.; Rudzińska, M. Changes in Chemical Composition and Oxidative Stability of Cold-Pressed Oils Obtained from by-Product Roasted Berry Seeds. LWT 2019, 111, 541–547. [Google Scholar] [CrossRef]
- Ispiryan, A.; Bobinaite, R.; Urbonaviciene, D.; Sermuksnyte-Alesiuniene, K.; Viskelis, P.; Miceikiene, A.; Viskelis, J. Physico-Chemical Properties, Fatty Acids Profile, and Economic Properties of Raspberry (Rubus idaeus L.) Seed Oil, Extracted in Various Ways. Plants 2023, 12, 2706. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K.; Chan, E.S.; Lee, Y.Y. Physicochemical and Antioxidative Properties of Ultrasound-Assisted Extraction of Mahua (Madhuca longifolia) Seed Oil in Comparison with Conventional Soxhlet and Mechanical Extractions. Ultrason. Sonochem. 2023, 92, 106280. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Linderborg, K.M.; Tarvainen, M.; Kalpio, M.; Zhang, Y.; Yang, B. Direct Inlet Negative Ion Chemical Ionization Tandem Mass Spectrometric Analysis of Triacylglycerol Regioisomers in Human Milk and Infant Formulas. Food Chem. 2020, 328, 126991. [Google Scholar] [CrossRef]
- Endo, Y.; Hoshizaki, S.; Fujimoto, K. Oxidation of Synthetic Triacylglycerols Containing Eicosapentaenoic and Docosahexaenoic Acids: Effect of Oxidation System and Triacylglycerol Structure. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 1041–1045. [Google Scholar] [CrossRef]



| Run | Ultrasound Amplitude X1 | Extraction Time X2 | ||
|---|---|---|---|---|
| Coded | Actual [%] | Coded | Actual [min] | |
| 1 | –1 | 30 | –1 | 5 |
| 2 | +1 | 80 | –1 | 5 |
| 3 | –1 | 30 | +1 | 15 |
| 4 | +1 | 80 | +1 | 15 |
| 5 | –1.414 | 19.7 | 0 | 10 |
| 6 | +1.414 | 90.4 | 0 | 10 |
| 7 | 0 | 55 | –1.414 | 2.93 |
| 8 | 0 | 55 | +1.414 | 17.07 |
| 9 | 0 | 55 | 0 | 10 |
| 10 | 0 | 55 | 0 | 10 |
| Oil | Model | R2 | CV (%) | Model p-Value | Model F-Value | Lack of Fit p-Value |
|---|---|---|---|---|---|---|
| BB | Quadratic | 0.9676 | 3.48 | 0.0044 | 23.97 | 0.4097 |
| CH | Quadratic | 0.9030 | 2.47 | 0.0373 | 7.44 | 0.5320 |
| RB | 2FI | 0.7258 | 7.42 | 0.0402 | 5.29 | 0.1294 |
| Oil | Model | R2 | CV (%) | Model p-Value | Model F-Value | Lack of Fit p-Value |
|---|---|---|---|---|---|---|
| BB | Quadratic | 0.9412 | 2.99 | 0.0142 | 12.81 | 0.3282 |
| CH | Quadratic | 0.9067 | 5.02 | 0.0346 | 7.78 | 0.1272 |
| RB | Linear | 0.5815 | 8.04 | 0.0474 | 4.86 | 0.1355 |
| Oil | Optimum Ultrasound Amplitude (%) | Actual Ultrasound Amplitude Applied (%) | Optimum Extraction Time (min) | Predicted Yield (%) | Predicted OIT (min) | Yield (%) | OIT (min) |
|---|---|---|---|---|---|---|---|
| BB | 72.98 | 70 | 8.20 | 8.05 | 75.05 | 8.10 ± 0.41 | 71.15 ± 3.79 |
| CH | 59.18 | 60 | 10.11 | 6.10 | 49.38 | 6.02 ± 0.97 | 48.78 ± 0.69 |
| RB | 95.57 | 95 | 8.43 | 7.17 | 58.29 | 6.96 ± 0.60 | 61.22 ± 6.65 |
| Kinetic Parameter | BB_US | BB_C | CH_US | CH_C | RB_US | RB_C |
|---|---|---|---|---|---|---|
| −a | 5054 | 4741 | 4923 | 4914 | 4976 | 4558 |
| b | 11.961 | 11.230 | 11.776 | 11.746 | 11.659 | 10.817 |
| R2 | 0.9993 | 0.9994 | 0.9954 | 0.9883 | 0.9978 | 0.9952 |
| Ea (kJ/mol) | 92.01 | 86.30 | 89.63 | 89.47 | 90.59 | 82.98 |
| Z (min−1) | 1.71 × 1010 | 3.38 × 109 | 1.14 × 1010 | 1.07 × 1010 | 8.58 × 109 | 1.36 × 109 |
| k at 100 °C (min−1) | 0.0022 | 0.0027 | 0.0032 | 0.0032 | 0.0018 | 0.0033 |
| k at 110 °C (min−1) | 0.0048 | 0.0057 | 0.0068 | 0.0067 | 0.0039 | 0.0066 |
| k at 120 °C (min−1) | 0.010 | 0.011 | 0.014 | 0.014 | 0.0078 | 0.013 |
| k at 130 °C (min−1) | 0.020 | 0.022 | 0.028 | 0.027 | 0.016 | 0.024 |
| k at 140 °C (min−1) | 0.039 | 0.041 | 0.053 | 0.052 | 0.030 | 0.043 |
| Oil | Melting peak Temperature (°C) | Melting Enthalpy (J/g) | Crystallization Peak Temperature (°C) | Crystallization Enthalpy (J/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Tm1 | Tm2 | Tm3 | ΔHm1 | ΔHm2 | ΔHm3 | Tc | ΔHc | |
| BB_US | –42.2 ± 0.1 c | –21.6 ± 0.2 c | – | 38.7 ± 3.0 c | 1.6 ± 1.1 b | – | –71.3 ± 0.8 b | –9.3 ± 2.4 b |
| BB_C | –41.7 ± 0.2 c | –21.0 ± 0.8 c | – | 37.5 ± 1.5 c | 0.4 ± 0.1 b | – | –70.1 ± 0.1 c | –14.0 ± 0.1 ab |
| CH_US | –62.1 ± 0.2 a | –35.0 ± 0.4 b | – | –5.9 ± 0.7 a | 22.9 ± 2.6 c | – | –73.6 ± 1.0 b | –8.6 ± 4.3 b |
| CH_C | –63.9 ± 3.7 a | –35.2 ± 1.4 b | – | –5.2 ± 3.5 a | 21.2 ± 4.8 c | – | –70.8 ± 0.2 c | –11.8 ± 3.8 ab |
| RB_US | –46.5 ± 0.4 b | –39.5 ± 0.7 a | –26.5 ± 0.2 a | 19.2 ± 0.4 b | –23.0 ± 1.8 a | 37.5 ± 1.5 a | –77.2 ± 0.3 a | –17.8 ± 0.6 a |
| RB_C | –47.0 ± 0.2 b | –41.5 ± 0.2 a | –25.8 ± 0.1 b | 17.1 ± 1.2 b | –27.4 ± 1.4 a | 44.3 ± 0.5 b | –75.7 ± 0.1 a | –14.7 ± 0.6 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piasecka, I.; Brzezińska, R.; Wiktor, A.; Górska, A. Response Surface Methodology for Ultrasound-Assisted Oil Extraction Optimization from Blackberry, Chokeberry, and Raspberry Waste Products. Appl. Sci. 2024, 14, 7214. https://doi.org/10.3390/app14167214
Piasecka I, Brzezińska R, Wiktor A, Górska A. Response Surface Methodology for Ultrasound-Assisted Oil Extraction Optimization from Blackberry, Chokeberry, and Raspberry Waste Products. Applied Sciences. 2024; 14(16):7214. https://doi.org/10.3390/app14167214
Chicago/Turabian StylePiasecka, Iga, Rita Brzezińska, Artur Wiktor, and Agata Górska. 2024. "Response Surface Methodology for Ultrasound-Assisted Oil Extraction Optimization from Blackberry, Chokeberry, and Raspberry Waste Products" Applied Sciences 14, no. 16: 7214. https://doi.org/10.3390/app14167214
APA StylePiasecka, I., Brzezińska, R., Wiktor, A., & Górska, A. (2024). Response Surface Methodology for Ultrasound-Assisted Oil Extraction Optimization from Blackberry, Chokeberry, and Raspberry Waste Products. Applied Sciences, 14(16), 7214. https://doi.org/10.3390/app14167214








