Abstract
There are only a few studies about the effects of toothpastes for children on remineralization and surface roughness of primary teeth. The aim of this study was to examine the remineralization capacity of five different toothpastes for children on primary tooth enamels with artificial initial caries, their effects on enamel surface roughness, and the relationship between their abrasive effects and remineralization. Sixty of 74 samples were allocated for microhardness and AFM analyses (after initialization, demineralization, and pH cycling), and 14 samples were evaluated by SEM. Sixty samples were divided into five groups, with each group representing a different toothpaste brand, as follows: Group 1: Splat; Group 2: Logodent; Group 3: Eyup Sabri Tuncer; Group 4: Naturalive; and Group 5: Buccotherm. Fourteen samples were divided into seven groups, each representing a different processing stage, with two samples in each group, for the initial (sound enamel surface) stage, post demineralization, and after applying the five remineralizing toothpastes. Toothpastes were applied to samples in a 7-day pH cycle. Data were analyzed statistically. Each toothpaste showed increased microhardness values; however, this increase was significant only for Group 4 (Naturalive) and Group 5 (Buccotherm). The highest surface roughness values were obtained after demineralization. The toothpastes reduced surface roughness, but these reductions were not statistically significant among the different types of toothpastes. The correlation analysis revealed that the toothpastes affected surface roughness according to their remineralization potential. The results were supported by SEM images. All toothpastes recovered primary tooth enamels with artificial initial caries, but only two had significant values.
1. Introduction
Untreated tooth decay in primary teeth can lead to pain, bacteremia, and high treatment costs. It can also result in disturbances in growth and development, speech and chewing, reduced self-esteem, and damage to permanent teeth [1]. It has been reported that the presence of decay during the primary dentition period may affect the occurrence of decay during the permanent dentition period and could be used as a risk indicator for predicting caries during this stage [2]. Strategies within the minimally invasive dentistry approach, aimed at maintaining the health of permanent teeth throughout life and ensuring that primary teeth remain healthy and functional until their natural exfoliation, include remineralizing demineralized enamel and implementing caries prevention measures [3].
Dental caries, which is defined as a disease that occurs when the balance between the demineralization and remineralization phases that follow each other in the mouth is disturbed in the direction of demineralization, is still the most common childhood disease today, despite advances in preventive and therapeutic practices [4]. Initial caries lesions are white spot lesions without cavitations observed as areas of subsurface demineralization in enamel. They are considered the first stage, in which dental caries become visible [5]. Demineralization is the dissolution of minerals on the tooth surface when the acids released from the metabolism of fermentable carbohydrates taken with nutrition by bacteria come into contact with the tooth [6]. Both in vitro and in vivo studies have reported that initial caries lesions can be treated through remineralization, defined as the re-accumulation of minerals lost by the demineralization of dental tissue on the enamel surface [3,7]. A successful remineralization process for lesions is achieved by providing and maintaining supersaturation on the tooth surface with neutral pH, calcium, and phosphate ions at the local level, enabling mineral ions to reach the lesion body at the lesion level, and by regulating both dietary and oral hygiene habits at the patient level [8].
Oral hygiene measures aim to mechanically remove bacterial biofilms on the enamel, one of the key components in caries development, by regular and sufficient behaviors and also chemically, by using various compounds and active agents such as fluoride-containing agents [9]. The primary mechanism of fluoride’s action is explained by its ability to significantly control the progression of caries and by remineralization through its low and continuous presence in oral fluids, exerting a topical effect. The topical effect mechanism includes inhibiting demineralization, promoting remineralization, and inhibiting bacterial activity in the plaque [10]. Fluoride in plaque fluid through topical sources adsorbs onto demineralized subsurface crystals, forming a fluorapatite-like structure that is less soluble and more resistant to acid attacks than the original tooth mineral. On the other hand, fluoride combines with the hydrogen ions (H+) from acids produced by bacteria’s carbohydrate metabolism, forming hydrofluoric acid (HF). This acid passes through bacterial cell walls and inhibits the glycolytic enzyme enolase, thereby obstructing carbohydrate metabolism and acid production and affecting the production of bacterial adhesive polysaccharides [11].
The caries-preventive effects of fluoride-containing products have been demonstrated in numerous reviews. Among all the active ingredients in toothpaste, the greatest number of evidence-based studies have been carried out on fluoride [12,13]. Reductions in the prevalence of dental caries recorded in many industrialized countries have been attributed to the widespread use of fluoride in various forms, particularly fluoride-containing toothpaste. Using fluoride-containing toothpaste leads to small but sustained increases in fluoride levels in plaque and saliva [14]. Active lesions, which continue to progress under acid attack, can be controlled through dietary adjustments, tooth brushing, and the use of fluoride-containing toothpaste [15]. However, it has been reported that toothpaste can be swallowed by children in the primary dentition period due to their inadequate control of the swallowing reflex and the inability to spit, which causes continuous and high-dose exposure to fluoride, resulting in various side effects [16,17]. Acute fluoride toxicity is characterized by symptoms such as nausea and vomiting occurring shortly after the ingestion of large amounts of fluoride. Due to its affinity for bone and dental tissues, prolonged exposure to high fluoride concentrations can lead to fluoride accumulation in these tissues, resulting in dental fluorosis and skeletal fluorosis [18]. In addition, it has been reported that fluoride can cause damage at both the organelle and organ levels, adversely affect the endocrine system, and that high concentrations of fluoride, particularly with chronic exposure, have neurotoxic effects, as evidenced by animal studies [17,19,20,21].
Although fluoride has been proven effective and is considered the gold standard in remineralization treatments, new agents are being researched as alternatives due to potential side effects. These agents can be added to toothpaste to reduce or eliminate the fluoride content. Promising options are reported to be effective in remineralizing initial carious lesions. While toothpastes containing these agents are available on the market and offer ease of access and use, more information is needed regarding their effectiveness [22,23,24,25].
During tooth brushing with toothpaste, tooth surfaces are cleaned not only by mechanical brushing but also by the toothpaste’s abrasives. Daily oral care needs to differ in adults and children due to physiological and structural differences among primary teeth, young permanent teeth, and adult permanent teeth [26]. Therefore, toothpastes should be evaluated not only in terms of fluoride content but also in terms of abrasiveness, according to the age groups of children.
Since there are only a few studies about the effects of toothpastes for children on the remineralization and surface roughness of primary teeth, further studies are needed on this subject [25,27,28]. The effects of the toothpastes investigated on primary teeth in our study were not compared with those of previous studies and were not evaluated with microhardness, AFM, and SEM analyses. This study aimed to examine the remineralization capacity of five different commercially available toothpastes for children on primary tooth enamel with artificial initial caries formed under in vitro conditions, their effects on enamel surface roughness, and the relationship between their abrasive effects and remineralization.
2. Material and Methods
Approval to conduct the study was obtained from the Clinical Research Ethics Committee of the Faculty of Medicine at Suleyman Demirel University (decision no. 17/242).
2.1. Tooth Collection and Selection
In the power analysis that was performed to determine the ideal sample size and ensure high reliability of the study results, and considering relevant studies on the subject [29,30], the number of observations in the subgroups of this study was determined as at least 10 with 95% power (α = 5% and effect size = 1.43) and in factorial order (repeated measurement). Considering potential data losses, the number of observations was increased to 12.
A total of 37 primary molar teeth were collected from healthy patients aged between 7–9 years who were born and living in the same geographic region (western Mediterranean, Turkey) after their consent was obtained for the study. The inclusion criteria for the teeth, which had been extracted for orthodontic purposes, were as follows: having no caries, discoloration, structural defects, cracks, fractures, restorations, and having root resorption levels of 1/3–1/2 according to the Fanning scale [31]. Calculus, if present on the teeth, was removed using a scaler, and plaque and soft tissue residues were eliminated using a soft brush under running water. The teeth were kept in a 0.5% chloramine-T solution for one week to ensure disinfection. By examining them under a stereomicroscope (Leica S4 E, Wetzlar, Germany), teeth with structural defects, cracks, or fractures were excluded from the study. The teeth included in the study were stored in distilled water to prevent dehydration until the time of the experiment. The solution was replaced weekly, and the teeth were used within six months.
2.2. Formation of the Groups
Toothpastes for children that were available on the market, both with and without fluoride, were examined. These products, which were found to be numerous, were listed and evaluated in as much detail as possible. The toothpastes had basic components and various therapeutic agents, but they were quite different from each other in terms of their contents. Therefore, it was very difficult to detect toothpastes with similar contents.
Toothpastes for children that were easily accessible on the market that did not, according to the manufacturers, contain certain agents but that did contain agents whose remineralization efficiency had been investigated in previous studies, were selected in line with the aim of our study. According to these selection criteria, groups were formed that represented the following different toothpaste brands: Splat (Group 1), Logodent (Group 2), Eyup Sabri Tuncer (Group 3), Naturalive (Group 4), and Buccotherm (Group 5) (Table 1).

Table 1.
Distribution of study groups according to the toothpastes used and their ingredients.
In the study, 60 of 74 enamel samples obtained from 37 lower and upper first and second primary molars were homogeneously distributed into 5 groups by the randomization method, including 12 in each group. The toothpastes were applied to the enamels with artificial initial caries in a 7-day pH cycle. Microhardness and atomic force microscopy (AFM) analyses were performed on 60 samples at the beginning, after demineralization, and after the pH cycle. A total of 14 enamel samples to be used for evaluation by a scanning electron microscope (SEM) were homogeneously distributed into 5 groups by randomization, including 2 enamel samples in each of the beginning, post-demineralization, and post-remineralization stages. Each tooth was designated as the negative control group itself. Therefore, another negative control group was not included.
2.3. Preparation of Enamel Samples
The crowns of the teeth were taken out of distilled water and separated from their roots under the cemento–enamel junction and perpendicularly to the long axis using a water-cooled low-speed diamond disc mounted on a straight handpiece. The crown pulp was removed using an excavator.
The coronal parts of the 37 teeth were embedded in acrylic resin (Meliodent, Kulzer) and placed in the precision cutting machine (Brillant 220, ATM GMBH, Mammelzen, Germany). Then, they were mesiodistally divided into two parts using the precision cutting machine, and a total of 74 enamel samples, including two samples from each tooth’s buccal and lingual surfaces, were obtained.
The enamel samples were removed from acrylic resin and again embedded horizontally in acrylic resin in metal molds with a diameter of 30 mm and a height of 12 mm, as required for the mechanical polishing machine (Saphir 520, ATM GMBH, Mammelzen, Germany), keeping the buccal or lingual surface exposed. To obtain a clear image and a standard enamel surface, they were ground flat and polished in the water-cooled mechanical polishing machine using 320, 600, 800, 1200 grit silicon carbide papers, respectively.
Labels were attached to each enamel sample to prepare standard enamel surfaces with 3 × 2 mm windows to apply the test procedures. The remaining tooth surfaces were covered with a double coat of acid-resistant nail polish (Figure 1).
Figure 1.
(a) Enamel sample with label attached and covered with a double coat of nail polish; and (b) enamel sample after label removal.
The samples were removed from the acrylic blocks for microhardness, AFM, and SEM analyses; their removed surfaces were covered with a double coat of nail polish. One side of the prepared window was marked and used for the microhardness analysis in each sample (Figure 2). The samples were kept in distilled water in 4.7 cm × 2.2 cm numbered and capped glass bottles during the experiments.
Figure 2.
(a) Front side of the enamel sample removed from the acrylic block; and (b) back side of the enamel sample removed from the acrylic block.
2.4. Formation of Artificial Initial Caries in Enamel Samples
To form artificial caries and use the samples in the pH cycle, a demineralization solution which had a pH level of 4.4 was prepared with one liter of distilled water by adding 2.2 mM potassium dihydrogen phosphate (KH2PO4), 0.05 M acetic acid (CH3COOH), 2.2 mM calcium chloride (CaCl2), and 1 M potassium hydroxide (KOH). The samples were kept in an oven (FN 055, Nüve, Ankara, Turkey) at 37 °C for 72 h in glass bottles with enough demineralization solution to cover each sample [32].
2.5. pH Cycle
The demineralization solution used to form artificial caries was used in the pH cycle. A remineralization solution with a pH level of 7.0 was prepared in one liter of distilled water by adding 1.5 mM calcium chloride (CaCl2), 0.9 mM sodium dihydrogen phosphate (NaH2PO4), 0.15 mM potassium chloride (KCl), and 1 M potassium hydroxide (KOH).
Toothpastes were weighed on a precision balance (KD-TBC-300, Tebisan, Beyoglu, Istanbul, Turkey) to apply a pea-sized amount (0.32 g) to each enamel sample and were stirred homogeneously with distilled water at a ratio of 1:3 in a magnetic stirrer (Arec Heating Magnetic Stirrer, VELP Scientifica, Usmate Velate, Italy). The samples were kept in the prepared toothpaste solutions for 1 min before the first demineralization cycle as well as before and after the second demineralization cycle (Figure 3).
Figure 3.
(a) Measuring the amount of toothpaste on a precision balance; (b) preparation of the toothpaste solution in the magnetic stirrer; and (c) application of the prepared toothpaste solution to the samples in numbered glass bottles.
After the artificial caries lesion was formed, a demineralization–remineralization cycle was applied to the samples for 7 days to mimic the pH changes in the intraoral environment throughout the day [28]. According to this method, the samples were kept in an oven at 37 °C for 7 days to imitate body temperature in the demineralization solution twice a day for 3 h; in the remineralization solution for 2 h between demineralization cycles; and in the remineralization solution for the remaining time. The pH values of the solutions were regularly checked using pH measurement papers.
The demineralization and remineralization solutions were renewed before each cycle; the toothpaste solutions were renewed before each application. After the solution and toothpaste applications, the samples were washed using distilled water for the next step.
2.6. Microhardness Analysis
The microhardness values of the samples were measured in 3 stages: at the beginning, after the artificial caries lesion was formed on the samples, and after the pH cycle was completed, by adopting the same conditions and steps and using a microhardness measurement device (Matsuzawa HWMMT-X3, TTS, Okasa, Japan) with a Vickers measuring tip.
Microhardness was measured using the Vickers tip of the device from three different points to eliminate regional differences in each sample and by applying 100 g of force (0.980 Newtons) for 15 s by the square pyramid-shaped diamond tip of the device. The values obtained for each enamel sample were recorded, and the mean microhardness value was determined by averaging the three measurements mentioned above.
For the five toothpastes for children applied in the pH cycles, the percentages of surface microhardness recovery values (%SMHR) were calculated using the following formula:
2.7. AFM Analysis
The roughness (Ra) assessment of the enamel surfaces was performed using AFM (ez-AFM, Nanomagnetics, Oxford, UK).
To measure surface roughness, the enamel sample was fixed using double-sided tape and placed inside the device. By calibrating at each measurement stage and scanning 3 × 3-µm areas, surface roughness (Ra) was measured at 3 stages and in 3 different regions on each enamel sample, as follows: at the beginning; after the artificial caries lesion was formed; and after the pH cycle was completed. The Ra value used in the analyses was recorded by taking the average of the results.
2.8. Examination of Surface Changes with SEM
The intact enamel surface and the structural changes occurring on the enamel surface after the experimental stages were examined and recorded by SEM (Quanta FEG 250, FEI, Hillsboro, OR, USA) at ×10,000 magnification. Before examining the samples, they were not subjected to any preparation (e.g., sputter coating). The “LOW” vacuum mode was selected to ensure conductivity; the chamber pressure was brought to 60 Pa.
2.9. Statistical Analysis
The data were analyzed using the IBM SPSS (IBM Corp., Chicago, IL, USA. Released 2017. IBM SPSS Statistics for Windows, Version 25.0.) package program and evaluated using descriptive statistical methods. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to check whether the data had normal distribution. For comparing the quantitative data, the differences between more than two groups were evaluated by ANOVA for the normally distributed measurements and by Kruskal–Wallis analysis for the non-normally distributed measurements. When a statistically significant difference was found, Bonferroni analysis was performed to determine the source of this significant difference. Friedman’s analysis was used to compare at least three dependent measurements for the non-normally distributed data. ANOVA was used in single-factor repeated measurements for the surface roughness assessments. Pearson’s correlation analysis was used for measurements with a normal distribution. Spearman’s correlation analysis was used for those without a normal distribution to test whether there were statistically significant relationships between the following values: the beginning, post-demineralization, and post-remineralization microhardness values; the beginning, post-demineralization, and post-remineralization surface roughness values; the difference in microhardness values after demineralization and remineralization (R–D)l the difference in surface roughness values after demineralization and remineralization (D–R); and the microhardness surface recovery values. A p-value smaller than 0.05 (p < 0.05) was considered statistically significant.
3. Results
3.1. Microhardness Analysis Findings
There was no statistically significant difference between the median microhardness values of the toothpastes measured at the beginning, post demineralization, and post remineralization (p > 0.05). In the intragroup comparisons, the microhardness values of the toothpastes measured at the beginning, post demineralization, and post remineralization were significantly different from each other (p < 0.05). The Splat, Eyup Sabri Tuncer, and Logodent toothpastes had higher initial microhardness values than those at other stages. For the Naturalive and Buccotherm toothpastes, the difference in microhardness values was observed in all measurement periods, ranking from high to low at the beginning, post-remineralization, and post-demineralization stages. After applying these two toothpastes, the microhardness values that had decreased due to demineralization increased. The remineralization effects of these two toothpastes are shown in Table 2.

Table 2.
Comparison of the microhardness values of the toothpastes measured at the beginning, after demineralization, and after remineralization.
There was no statistically significant difference between the median surface microhardness recovery values of the toothpastes (p > 0.05) (Table 3.)

Table 3.
Comparison of surface microhardness improvement values according to each toothpaste.
3.2. AFM Analysis Findings
There was a statistically significant difference among the mean surface roughness values measured at the beginning, post-remineralization, and post-demineralization in the toothpastes in the intragroup comparisons (p < 0.05) (Table 4). The difference was due to the surface roughness values measured after demineralization, which were higher than those measured at the beginning and after remineralization. According to the pairwise comparisons of toothpastes at each stage, there was no statistically significant difference between the toothpastes (p > 0.05). There was also no statistically significant difference between the toothpastes in terms of their microhardness and surface roughness values measured after demineralization and remineralization (p > 0.05) (Table 5).

Table 4.
Comparison of the surface roughness values of the toothpastes at the beginning, after demineralization and after remineralization.

Table 5.
Comparison of the toothpastes in terms of the difference between microhardness and surface roughness values measured after demineralization and remineralization.
The correlation analysis revealed that the remineralization potential of the toothpastes was associated with their effectiveness on surface roughness. The correlation of numerical measures from the same analysis was an expected result. While only the expected correlations were found in the Eyup Sabri Tuncer and Buccotherm toothpaste groups, the measurements of microhardness analysis and surface roughness analysis in the Splat, Logodent, and Naturalive toothpaste groups were observed to be associated with each other. In the Splat toothpaste group, apart from the expected correlations, a statistically significant moderate positive correlation was found between the beginning surface roughness and microhardness (R-D) values (r = 0.591; p = 0.043) and between the beginning surface roughness and microhardness surface recovery values (r = 0.315; p = 0.043). In the Logodent toothpaste group, apart from the expected correlations, a statistically significant, moderate negative correlation was found between the post-demineralization microhardness and post-remineralization surface roughness values (r = −0.587; p = 0.045) and between the post-remineralization microhardness and post-remineralization surface roughness values (r = −0.615; p = 0.033). In the Naturalive toothpaste group, apart from the expected correlations, a statistically significant, moderate positive correlation was found between the post-demineralization microhardness and post-remineralization surface roughness values (r = 0.584; p = 0.046), and a statistically significant, moderate negative correlation was found between the surface roughness (D-R) and microhardness surface recovery values (r = −0.622; p = 0.031).
3.3. SEM Analysis Findings
Figure 4 presents the SEM images obtained from the samples at ×10,000 magnification.
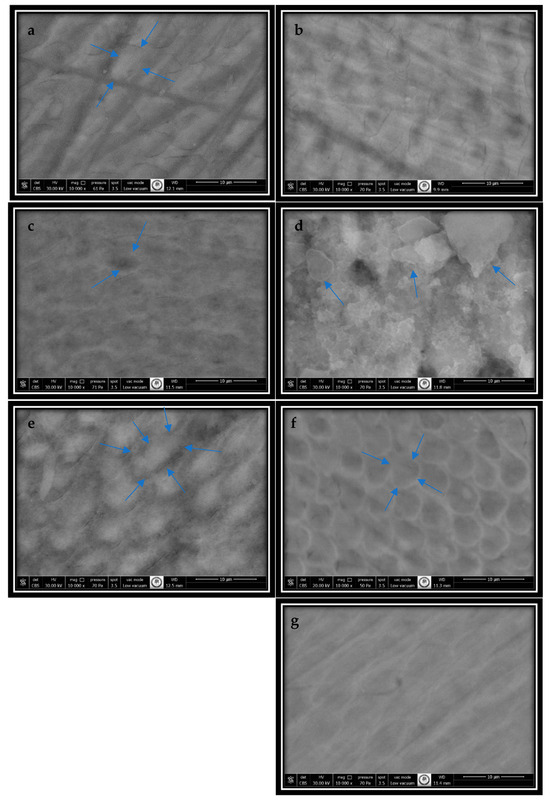
Figure 4.
SEM images obtained at ×10,000 magnification: (a) intact enamel surface. The circular-shaped prisms are distinctly separated from the interprismatic enamel. The blue arrows show circular-shaped prism boundaries; (b) artificial caries formed on the enamel surface (the body material dissolved, resulting in enlarged enamel prisms); (c) Group 1’s (Splat) enamel surface, with pores in the prism body and surface irregularities (the blue arrows point to the pore on the enamel surface); (d) Group 2’s (Logodent) enamel surface (non-homogeneous, irregular accumulations (the blue arrows show the deposits)); (e) Group 3’s (Eyup Sabri Tuncer) enamel surface (the blue arrow points to the filled enamel prism with a honeycomb appearance); (f) Group 4’s (Naturalive) enamel surface (the blue arrows show the enamel prism with a prominent honeycomb appearance.); and (g) Group 5’s (Buccotherm) enamel surface, with the filling showing a diffuse, cloud-like structure.).
In images of the intact enamel surface, circular enamel prisms with distinct boundaries, interprismatic enamel, and their homogeneous distribution were observed. The boundaries of the enamel prisms and the interprismatic enamel resembled the structure of the prism body, and continuity was maintained between both prismatic and interprismatic enamel (Figure 4a).
The enamel surface with artificial caries exhibited a honeycomb appearance. The periphery of the prisms in the enamel with artificial caries remained intact, but the body material had dissolved and emptied. The prisms were enlarged, and the interprismatic enamel had decreased (Figure 4b).
Although the honeycomb appearance of the enamel prisms and a decrease in the interprismatic enamel due to demineralization continued to be observed on the enamel surface on which the toothpastes were applied, the prism bodies were fuller compared to their demineralized condition.
In Figure 4c, although there are fillings on the surface, the pores in the prism body and surface irregularities are noticeable.
In Figure 4d, non-homogeneous, irregular accumulations are observed.
In Figure 4e, the prisms with a honeycomb pattern are filled inside.
In Figure 4f, although small filling areas are observed, the pores in the enamel prisms and the honeycomb appearance are noticeable.
In Figure 4g, the enamel surface is regular, with fillings showing a diffuse, cloud-like structure.
4. Discussion
In our study, a 7-day pH cycle, as used by Thaveesangpanich et al. (2005), was implemented to evaluate the remineralization effects of five different toothpastes for children on artificially carious primary teeth [28]. The samples in our study were treated with a demineralization solution prepared using 2.2 mM CaCl2, 2.2 mM KH2PO4, 0.05 M CH3COOH, and 1.0 M KOH, and were kept in an incubator at 37 °C for 72 h, taking into account the study by El Habashy and Heikal (2020) [32]. Lesion formation was monitored daily, and the demineralization of the enamel, observed as homogeneous chalky white areas without cavitation on the sample surfaces, was recorded. The preparation of the toothpastes in solution form simulated the dilution of toothpaste with saliva during brushing [33]. In our study, the toothpastes were diluted with distilled water at a 1:3 ratio (toothpaste: distilled water); the same ratio was used in other studies [27,34]. The samples were immersed in the prepared toothpaste solutions for 1 min before the first demineralization cycle, before the second demineralization cycle, and after the second demineralization cycle during the 7-day pH cycle. This approach aimed to simulate brushing before and after dietary intake [35]. The abrasion on the tooth surface depends on factors such as the type, particle hardness, size, size distribution, and concentration of the abrasive in the toothpaste, as well as on the hardness, arrangement of the toothbrush bristles, the force applied during brushing, and the brushing technique [36,37]. The toothpastes were prepared in solution form to eliminate these variables and isolate the effects of the toothpastes’ abrasiveness. Applying the toothpastes in solution form enabled a standardized application across all samples.
This study revealed that the Group 4 (Naturalive) and Group 5 (Buccotherm) toothpastes resulted in significantly higher microhardness values measured after remineralization than those measured after demineralization. Naturalive contains calcium carbonate, which is added to toothpastes as an abrasive. It has been reported that calcium carbonate has low solubility at a neutral pH, and dissolves at a low pH; that calcium mineral provides remineralization; and that carbonate neutralizes plaque acids due to an increase in local pH [38]. One study evaluated the plaque pH effects of two toothpastes, one with carbonate and the other with alumina trihydrate, which did not contain fluoride and had the same abrasiveness. It was found that the buffering effect was better and longer, and that the plaque’s pH was significantly higher after using the toothpaste containing calcium carbonate [39]. Considering the SEM results of our study, it can be assumed that toothpastes with calcium carbonate may have remineralization efficiency. The Buccotherm toothpaste contains 500 ppm fluoride. One of the recommendations for reducing the potential risk of fluoridated toothpastes, as a risk factor for fluorosis in children under the age of six years, is using toothpastes with low fluoride concentrations. Studies on toothpastes containing fluoride at different concentrations have reported that 500 ppm fluoride in toothpaste may be sufficient to prevent dental caries [40,41]. Similar to the results obtained in other studies, our microhardness and SEM analysis results showed the remineralization effect of the fluoride contained in this toothpaste and supported the argument that it is a standard in terms of its caries-preventive effect [42,43]. The main action of mechanism of fluoride is its topical effect, which is caused by its continuous presence in oral fluids at low rates [44]. Fluoride, which exists in the plaque fluid through topical sources, is adsorbed onto the demineralized subsurface crystals, forms a fluorapatite-like structure with lower solubility than the original tooth mineral that is more resistant to acid attacks, inhibits demineralization, and provides remineralization [11].
Nano-hydroxyapatite is the most frequently studied biomaterial in the medical field due to its biocompatibility and role as the main mineral component of bones and teeth. Studies have reported that nano-hydroxyapatite forms a protective layer on the intact enamel surface, providing the demineralized enamel with the necessary minerals to recover [23]. One in vitro study evaluated the remineralization efficiency of toothpastes on primary teeth and reported that toothpaste containing only nano-hydroxyapatite provided more mineral gain than toothpaste groups containing only fluoride and those containing fluoride and nano-hydroxyapatite. However, the differences between these groups were not statistically significant [45]. Similar results were also reported in other in vitro studies on primary and permanent teeth [25,46]. In our study, according to the demineralization stage, a numerical increase was observed in the microhardness values of the Splat group. Splat was used as a hydroxyapatite-containing toothpaste, but this increase was not statistically significant. As reported in other studies, it may be stated that the property of hydroxyapatite to provide mineral deposition on the outer surface of lesions was in parallel with the findings of the SEM images of our study which presented the prisms becoming more prominent and the filling of the prism body compared to the demineralized enamel in the samples that were subjected to the Splat toothpaste [25,46].
There are limited studies on the remineralization efficiency of sea salt. Since trace elements can affect the physicochemical properties of enamel by incorporating it into its structure, the remineralization effect of sea salt has been associated with the elements it contains [47,48]. It has been shown to reduce dental caries due to its fluoride content [49]. In the group in which Logodent, containing sea salt, was applied, we observed a numerical increase in the microhardness value in the remineralization stage compared to that in the demineralization stage, but this increase was not statistically significant. Although we did not know the type of sea salt in Logodent and how much fluoride it contained, we observed in the SEM images that the prism bodies emptied in the demineralized enamel were filled after remineralization. Therefore, sea salt can be used as a remineralization agent thanks to the elements it contains.
Although Theodent toothpaste was used in studies on theobromine-containing toothpastes, the cost of this toothpaste was high, and its availability in Turkey was limited [50,51]. Therefore, the “Natural Chocolate and Mint Extract Toothpaste for Kids” introduced by the Eyup Sabri Tuncer Company, which was the only toothpaste for children containing theobromine in Turkey, was chosen for our study, even though it was for children aged 7–16 years. Due to the use of natural peppermint oil in its content for cosmetic purposes, care should be taken against the risk of swallowing by children. In our study, despite the numerical increase in the microhardness values in the remineralization stage compared to those in the demineralization stage, this increase was not statistically significant in the group in which this toothpaste was applied. Theobromine was reported to prevent the dissolution of apatites on the enamel surface by increasing the crystal size, and its effect increases in direct proportion to its concentration [22]. In their 28-day study, Amaechi et al. [52] observed a higher increase in surface microhardness in the 1.1 Mmol theobromine group than in the 1100 ppm fluoride-containing toothpaste group. On the other hand, Lippert [53] conducted a 5-day study and found that theobromine did not increase the surface hardness more than the groups containing 226 ppm and 452 ppm fluoride despite using the same amount of theobromine. These results may have been influenced by the duration of the application. Likewise, the fact that toothpastes were applied during a 7-day pH cycle in our study may have prevented us from sufficiently observing the remineralization effect of theobromine reported in previous studies. Additionally, we did not know the amount of theobromine contained in the toothpaste. However, the remineralizing effect of theobromine should be noted, considering the SEM images and the increase in microhardness values after the remineralization stage.
The toothpastes in the study groups contain agents associated with maintaining oral, dental, and gum health, providing freshness throughout the day, helping to prevent tooth decay, and exhibiting antibacterial, anti-inflammatory, antispasmodic, and analgesic effects, as promoted by the manufacturers of these toothpastes (Table 1). Upon reviewing the studies, it can be observed that plant extracts, such as aloe vera gel, chamomile, licorice root, grape seed, green tea, miswak, tea tree, propolis, peppermint oil, and eucalyptus oil are noted for their antimicrobial effects. However, research on the effectiveness of these agents for remineralization is limited [54,55,56,57,58,59]. In addition, some studies indicate that licorice root, grape seed extracts, and aloe vera may effectively promote teeth remineralization [54,55,58]. The cavity-preventive effect of xylitol, a natural sugar alcohol, is explained by its impact on saliva and plaque bacteria [60]. Arginine, a semi-essential amino acid, is metabolized by oral commensal Streptococcus species, resulting in ammonia that increases the pH of the environment and shifts the balance towards remineralization at the tooth–plaque interface [61]. Although there are studies evaluating the effects of Castéra-Verduzan thermal spring water in oral spray preparations for patients with xerostomia and its antibacterial activity, no research has been found regarding its remineralization efficacy [62,63] In our study, the effectiveness of the evaluated toothpastes may also be attributed to the effects of the other ingredients they contain or to their combined effects with remineralization agents.
SEM analysis revealed a homogeneous distribution of distinct enamel prisms on healthy surfaces. In artificially carious enamel, prisms appeared with intact peripheries and dissolved bodies and displayed a honeycomb appearance. These findings are parallel to the observations reported by Koçyiğit et al. [45]. Although pores and surface irregularities were observed in the prism body in the SEM images of enamel samples from Group 1 (Splat), they appeared to be filled compared to the demineralized state. In a study where the same toothpaste was evaluated using SEM-EDX, remineralization of the enamel surface was observed, and calcium fluoride deposits were noted [64]. On the surface of the enamel samples from Group 2, irregular deposits were noticeable. In the enamel samples from Group 3, although there were voids in the prism bodies, the interiors of the honeycomb-like prisms appeared to be filled. In a study evaluating the remineralization efficacy of fluoride-containing toothpaste, nano-hydroxyapatite-containing toothpaste, and toothpaste solutions containing theobromine at two different concentrations (100 and 200), SEM analyses showed surface coatings filling the pores. However, no significant differences between the groups were reported [65]. In the enamel samples from Group 4 (Naturalive), although small fillings were observed, the honeycomb appearance was noticeable. This result was confirmed in an in vitro study that examined the effect of toothpaste containing nano-calcium carbonate on the surface roughness of demineralized enamel. The study supported the finding of reduced surface roughness of enamel samples after application of this toothpaste, as well as the appearance of white spots on the enamel surface, and an increase in calcium and phosphate on the enamel surface, as observed in SEM-EDS analysis, indicating that it also enhanced remineralization [66]. In the enamel samples from Group 5 (Buccotherm), the enamel surface exhibited a diffuse, cloud-like structure. It has been reported that enamel samples exposed to a solution containing 500 ppm sodium fluoride showed a greater increase in microhardness than in the control group, which was confirmed by ESEM analysis [67].
The Splat, Logodent, Eyup Sabri Tuncer, and Buccotherm toothpastes contain hydrated silica as an abrasive agent, whereas Naturalive contains calcium carbonate. Carbonate group abrasives act as a natural buffer due to their alkaline pH. Silica, mainly composed of silicon bonded to two oxygen molecules, is added to the toothpaste structure both as an abrasive and thickener [68]. Although the upper limit of RDA and REA, commonly used measures to evaluate the toothpaste abrasiveness, has been reported for adult toothpastes, no limit has been set specifically for children [26]. There was no information on the abrasiveness of the toothpastes used in our study. The lower surface roughness values after remineralization compared to those after demineralization, almost reaching the initial values, and the SEM analysis results, may indicate that the remineralization agents in the toothpastes made the tooth surface smoother through precipitation on the tooth surface. The correlation analysis revealed that the toothpastes increased or decreased the surface roughness according to their remineralization potentials. Despite the significant increase in the microhardness values created by Naturalive after the remineralization stage, the lack of a significant difference between other toothpastes and Naturalive in terms of surface roughness may have been due to the lower abrasiveness of calcium carbonate in the Naturalive toothpaste than hydrated silica or its remineralization efficiency. One study evaluated the abrasiveness of silica and calcium carbonate with similar particle sizes using the radiometric method, and it was reported that silica had a higher RDA value than calcium carbonate; it was emphasized that the abrasiveness value could increase with the agent’s particle size [69]. It may be considered that the abrasive effect of the hydrated silica in Buccotherm is eliminated by the remineralization feature of its 500-ppm fluoride content.
This study was conducted under in vitro conditions. The obtained results should be evaluated further by in vivo studies. Evaluations of the toothpastes included in the study using different methodologies and for longer durations may lead to changes in remineralization effects. The increases in microhardness values may also have been due to the effects of other products in toothpastes or their effects together with remineralization agents. To evaluate toothpastes’ remineralization and abrasiveness activities, toothpaste manufacturers should provide more detailed information about toothpaste contents and concentrations, interactions, and the particle sizes of agents in toothpastes. Although providing quantitative information about the surface changes dependent on remineralization agents increases research costs, methods such as micro-CT and microradiography, which can measure lesion depth and mineral content, can be included in future studies when more detailed information is needed.
5. Conclusions
After applying only Group 4 (Naturalive) and Group 5 (Buccotherm), a significant increase in the microhardness values, which had decreased due to demineralization, was observed. All toothpastes in the study recovered the initial caries lesions on primary teeth in a 7-day pH cycle but did not provide complete remineralization. Therefore, further studies are needed to determine the long-term effectiveness of the toothpastes.
Author Contributions
All authors made a significant contribution to the reported work. G.O.A. and D.C. contributed to the conceptualization, methodology, validation, formal analysis, investigation, resources, writing—original draft preparation, writing—review and editing, visualization, and funding acquisition. G.O.A. performed the data curation. D.C. performed the project administration. All authors have read and agreed to the published version of the manuscript.
Funding
Our work was supported by the Academic Research Funding Programmes Directorate of Scientific and Technological Research Council of Turkey (TUBITAK), project number 121S027, within the scope of the 1002-Short Term Research and Development Funding Programme.
Institutional Review Board Statement
Approval to conduct the study was obtained from the Clinical Research Ethics Committee of the Faculty of Medicine at Suleyman Demirel University on 4 September 2020 (decision no. 17/242).
Informed Consent Statement
A total of 37 primary molar teeth that had been extracted for any reason and met with the inclusion criteria were collected from healthy patients after their written informed consent was obtained for the study from their parents.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to Suleyman Demirel University Innovative Technologies Application and Research Center (YETEM) for the laboratory and analysis stages of the study; to Isparta University of Applied Sciences Biomedical Engineering Plasma Medicine Laboratory for the solutions; and to the Academic Research Funding Programmes Directorate of Scientific and Technological Research Council of Turkey (TUBITAK) for the funding sources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kagihara, L.E.; Niederhauser, V.P.; Stark, M. Assessment, management, and prevention of early childhood caries. J. Am. Acad. Nurse Pract. 2009, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W. Predicting caries in permanent teeth from caries in primary teeth: An eight-year cohort study. J. Dent. Res. 2002, 81, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Cate, J.M.; Arends, J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977, 11, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef]
- Cury, J.A.; Tenuta, L.M. Enamel remineralization: Controlling the caries disease or treating early caries lesions? Braz. Oral Res. 2009, 23, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.M.; Featherstone, J.D. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 33–40. [Google Scholar] [CrossRef]
- Amaechi, B.T. Remineralization therapies for initial caries lesions. Curr. Oral Health Rep. 2015, 2, 95–101. [Google Scholar] [CrossRef]
- Torres, C.R.G. Modern Operative Dentistry, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- American Academy of Pediatric Dentistry. Fluoride therapy. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2021; pp. 302–305. [Google Scholar]
- Featherstone, J.D. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, 002278. [Google Scholar] [CrossRef]
- Santos, A.P.P.; Nadanovsky, P.; Oliveira, B.H. A systematic review and meta-analysis of the effects of fluoride toothpaste on the prevention of dental caries in the primary dentition of preschool children. Community Dent. Oral Epidemiol. 2013, 41, 112. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J.M.; Navada, R.; Walia, R. Low-levels of fluoride in plaque and saliva and their effects on the demineralisation and remineralisation of enamel; role of fluoride toothpastes. Int. Dent. J. 2004, 54, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E. The implications of the new paradigm of dental caries. J. Dent. 2011, 39, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.M.; Ellwood, R.P.; Davies, R.M. Fluoride ingestion from toothpaste by young children. Br. Dent. J. 1999, 186, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.A.; Ricomini-Filho, A.P.; Berti, F.L.P.; Tabchoury, C.P. Systemic effects (risks) of water fluoridation. Braz. Dent. J. 2019, 30, 421–428. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Chen, H.; Manthari, R.K.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Effects of fluoride on synapse morphology and myelin damage in mouse hippocampus. Chemosphere 2018, 194, 628–633. [Google Scholar] [CrossRef]
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of fluoride on endocrine tissues and their secretory functions—Review. Chemosphere 2020, 260, 127565. [Google Scholar] [CrossRef]
- Kargul, B.; Özcan, M.; Peker, S.; Nakamoto, T.; Simmons, W.B.; Falster, A.U. Evaluation of human enamel surfaces treated with theobromine: A pilot study. Oral Health Prev. Dent. 2012, 10, 275–282. [Google Scholar]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139. [Google Scholar]
- Lynch, R.J.; ten Cate, J.M. The anti-caries efficacy of calcium carbonate-based fluoride toothpastes. Int. Dent. J. 2005, 55, 175–178. [Google Scholar] [CrossRef]
- Haghgoo, R.; Ahmadvand, M.; Moshaverinia, S. Remineralizing effect of topical NovaMin and nano-hydroxyapatite on caries-like lesions in primary teeth. J. Contemp. Dent. Pract. 2016, 17, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Stovell, A.G.; Newton, B.M.; Lynch, R.J. Important considerations in the development of toothpaste formulations for children. Int. Dent. J. 2013, 63, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, M.; Itthagarun, A.; King, N.M. Comparison of the remineralizing potential of child formula dentifrices. Int. J. Paediatr. Dent. 2011, 21, 132–140. [Google Scholar] [CrossRef]
- Thaveesangpanich, P.; Itthagarun, A.; King, N.M.; Wefel, J.S. The effects of child formula toothpastes on enamel caries using two in vitro pH-cycling models. Int. Dent. J. 2005, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Shabanian, M.; Jabarifar, S.E.; Salavati, S.; Khosravi, K.; Tavakoli, N.; Akhavan, A. Effect of fluoride dentifrices on the microhardness of deciduous enamel surfaces. Oral Health Prev. Dent. 2012, 10, 59–64. [Google Scholar] [PubMed]
- Gümüs, H.; Aydınbelge, M.; Sönmez, H. Evaluation of the efficacy of different remineralizing agents on artificial early enamel lesions of primary teeth: An in vitro study. J. Adv. Oral Res. 2020, 11, 180–188. [Google Scholar] [CrossRef]
- Fanning, E.A. A longitudinal study of tooth formation and root resorption. N. Z. Dent. J. 1961, 57, 202–217. [Google Scholar]
- el Habashy, L.M.; Heikal, M. Effectiveness of casein phospopeptide amorphous calcium phosphate with or without fluoride on remineralization of enamel caries-like lesions in primary teeth. Egypt. Dent. J. 2020, 66, 799–808. [Google Scholar] [CrossRef]
- Kapoor, A.; Saraf, B.G.; Sheoran, N.; Sardana, D. Comparative evaluation of remineralizing potential of three pediatric dentifrices. Int. J. Clin. Pediatr. Dent. 2016, 9, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kiranmayi, M.; Nirmala, S.V.; Nuvvula, S. Appraisal of the remineralizing potential of child formula dentifrices on primary teeth: An in vitro pH cycling model. Contemp. Clin. Dent. 2015, 6, 81–85. [Google Scholar]
- Itthagarun, A.; Wei, S.H.Y.; Wefel, J.S. The effect of different commercial dentifrices on enamel lesion progression: An in vitro pH-cycling study. Int. Dent. J. 2000, 50, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Dwyer-Joyce, R.S.; Pickles, M.J. Interaction between toothbrushes and toothpaste abrasive particles in simulated tooth cleaning. Wear 2004, 257, 368–376. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, V.; Pratap, C.; Chaturvedi, T. Abrasivity of dentrifices: An update. S.R.M. J. Res. Dent. Sci. 2016, 7, 96–100. [Google Scholar]
- Cury, J.A.; Francisco, S.B.; Simões, G.S.; Del Bel Cury, A.A.; Tabchoury, C.P. Effect of a calcium carbonate-based dentifrice on enamel demineralization in situ. Caries Res. 2003, 37, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.A. Effect induced by a chalk-based toothpaste on the pH changes of plaque challenged by a high sugar diet over an 8-hour period. Caries Res. 1986, 20, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, E.; Altenburger, M.; Attin, T.; Lussi, A.; Buchalla, W. Remineralization of initial carious lesions in deciduous enamel after application of dentifrices of different fluoride concentrations. Clin. Oral Investig. 2010, 14, 265–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Damato, F.; Strang, R.; Stephen, K. Effect of fluoride concentration on remineralization of carious enamel: An in vitro ph-cycling study. Caries Res. 1990, 24, 174–180. [Google Scholar] [CrossRef]
- Rirattanapong, P.; Smutkeeree, A.; Surarit, R.; Saendsirinavin, C.; Kunanantsak, V. Effects of fluoride dentifrice on remineralization of demineralized primary enamel. Southeast Asıan J. Trop. Med. Public Health 2010, 41, 243–249. [Google Scholar]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, 007868. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 2011, 22, 97–114. [Google Scholar]
- Koçyigit, C.; Yüksel, B.N.; Özalp, N. Effects of nano-hydroxyapatite dentifrices with and without fluoride on primary teeth enamel: A micro-CT and a SEM study. Cumhur. Dent. J. 2020, 23, 191–199. [Google Scholar] [CrossRef]
- Vyavhare, S.; Sharma, D.; Kulkarni, V. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J. Clin. Pediatr. Dent. 2015, 39, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Qamar, Z.; Haji Abdul Rahim, Z.B.; Chew, H.P.; Fatima, T. Influence of trace elements on dental enamel properties: A review. J. Pak. Med. Assoc. 2017, 67, 116–120. [Google Scholar]
- Condò, S.G.; DeVizio, W.; Volpe, A.R. Gingiva, teeth and sea salt. Am. J. Dent. 1999, 12, 5–8. [Google Scholar]
- Hadjimarkos, D. Sea salt and dental caries. Nature 1962, 195, 392. [Google Scholar] [CrossRef] [PubMed]
- Premnath, P.; John, J.; Manchery, N.; Subbiah, G.K.; Nagappan, N.; Subramani, P. Effectiveness of theobromine on enamel remineralization: A comparative in-vitro study. Cureus 2019, 11, 5686. [Google Scholar] [CrossRef] [PubMed]
- Durhan, M.A.; Ozsalih, S.; Gokkaya, B.; Kulan, P.Y.; Kargul, B. Caries preventive effects of theobromine containing toothpaste on early childhood caries: Preliminary results. Acta Stomatol. Croat. 2021, 55, 18–27. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Porteous, N.; Ramalingam, K.; Mensinkai, P.K.; Ccahuana Vasquez, R.A.; Sadeghpour, A.; Nakamoto, T. Remineralization of artificial enamel lesions by theobromine. Caries Res. 2013, 47, 399–405. [Google Scholar] [CrossRef]
- Lippert, F. The effects of fluoride, strontium, theobromine and their combinations on caries lesion rehardening and fluoridation. Arch. Oral Biol. 2017, 80, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Oznurhan, F. Antibacterial efficacy and remineralization capacity of glycyrrhizic acid added casein phosphopeptide-amorphous calcium phosphate. Microsc. Res. Tech. 2020, 83, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Al Haddad, T.; Khoury, E.; Farhat Mchayleh, N. Comparison of the remineralizing effect of brushing with aloe vera versus fluoride toothpaste. Eur. J. Dent. 2021, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Szarka, S.; Ott, P.G.; Héthelyi, E.B.; Szoke, E.; Tyihák, E. Separation and identification of antibacterial chamomile components using OPLC, bioautography and GC-MS. Med. Chem. 2012, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rubel, M.; Prashhant, G.; Naveen, K. Effect of grape seed extract on remineralization of artificial caries: An in-vitro study. Asian J. Pharm. Clin. Res. 2016, 9, 174–176. [Google Scholar]
- Nordin, A.; Bin Saim, A.; Ramli, R.; Abdul Hamid, A.; Mohd Nasri, N.W.; Bt Hj Idrus, R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020, 27, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Policy on use of xylitol in pediatric dentistry. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2021; pp. 72–73. [Google Scholar]
- Chakraborty, B.; Burne, R.A. Effects of arginine on streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl. Environ. Microbiol. 2017, 83, e00496–17. [Google Scholar] [CrossRef] [PubMed]
- Alpöz, E.; Çankaya, H.; Güneri, P.; Epstein, J.B.; Boyacioglu, H.; Kabasakal, Y.; Ocakci, P.T. Impact of Buccotherm® on xerostomia: A single blind study. Spec. Care Dent. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Çakır, B.; Eden, E.; Turan, E. Evaluation of antibacterial effect of toothpastes with different contents: An in vitro study. Aydın Dent. J. 2017, 3, 13–22. [Google Scholar]
- Mutlu, E.; Ozdemir, M.; Gencay, K. Evaluation of remineralizing potential of hydroxyapatite, phosphopeptide-amorphous calcium phospahate and fluoride dentifrices using SEM/EDX analysis: A randomized controlled in-vitro study. Pediatr. Dent. J. 2022, 32, 176–185. [Google Scholar] [CrossRef]
- Taneja, V.; Nekkanti, S.; Gupta, K.; Hassija, J. Remineralization potential of theobromine on artificial carious lesions. J. Int. Soc. Prev. Community Dent. 2019, 9, 576–583. [Google Scholar] [PubMed]
- Detara, M.; Triaminingsih, S.; Irawan, B. Effects of nano calcium carbonate and siwak toothpaste on demineralized enamel surface roughness. J. Phys. Conf. Ser. 2018, 3, 1073. [Google Scholar] [CrossRef]
- Abdslam, A.; Farag, M.; Omer, S. Evaluation of the effect of two different concentration of arginine on fluoride uptake by demineralized enamel surfaces, in vitro study. Dent. Sci. Update 2022, 3, 199–208. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, I.; Shahid, F.; Hassan, U.; Zafar, M.S. Common toothpastes abrasives and methods of evaluating their abrasivity. J. Oral Res. 2020, 3, 9–15. [Google Scholar] [CrossRef]
- Camargo, I.M.; Saiki, M.; Vasconcellos, M.B.; Avila, D.M. Abrasiveness evaluation of silica and calcium carbonate used in the production of dentifrices. J. Cosmet. Sci. 2001, 52, 163–167. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).