Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis
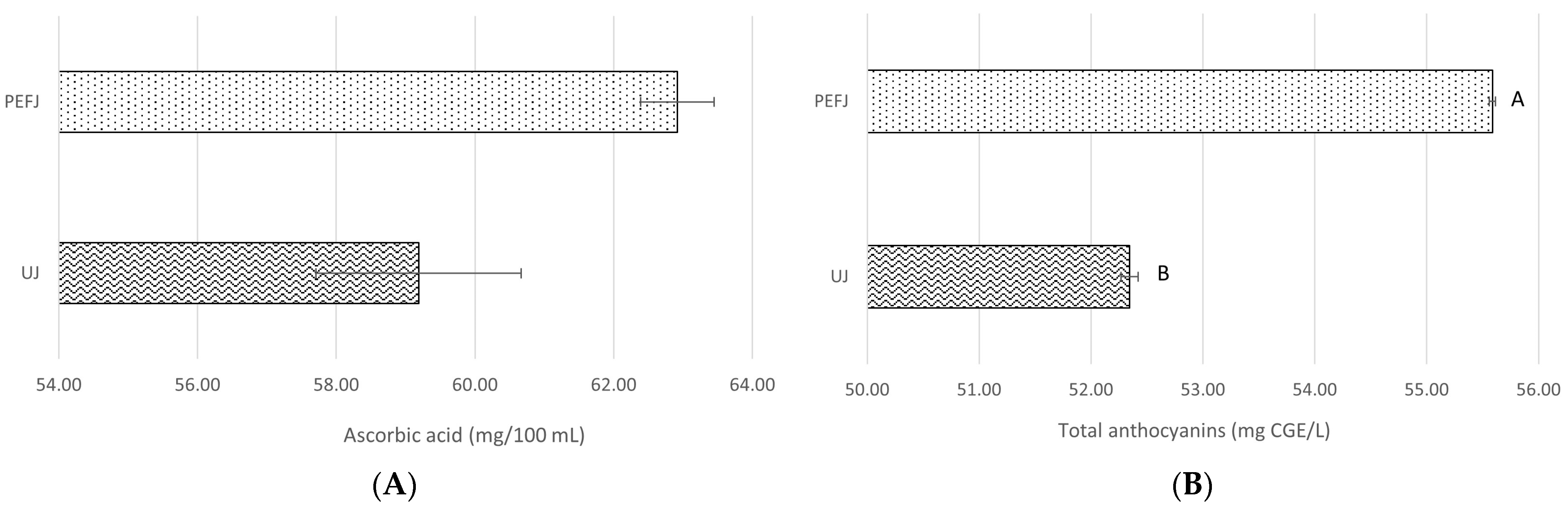
2.2. Antioxidant Components and Antioxidant Activity Assays
2.2.1. Antioxidant Components
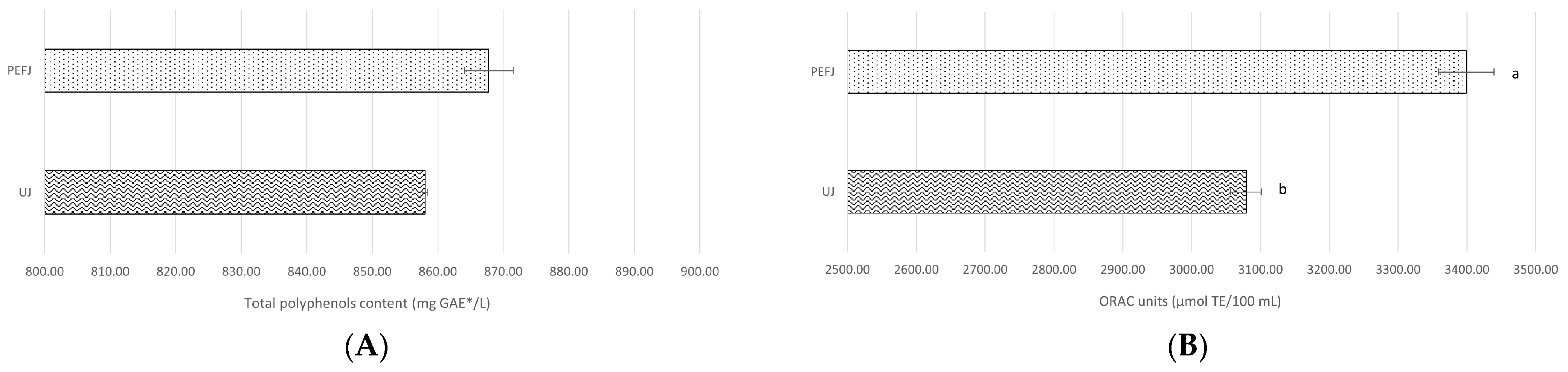
2.2.2. Antioxidant Activity Assays
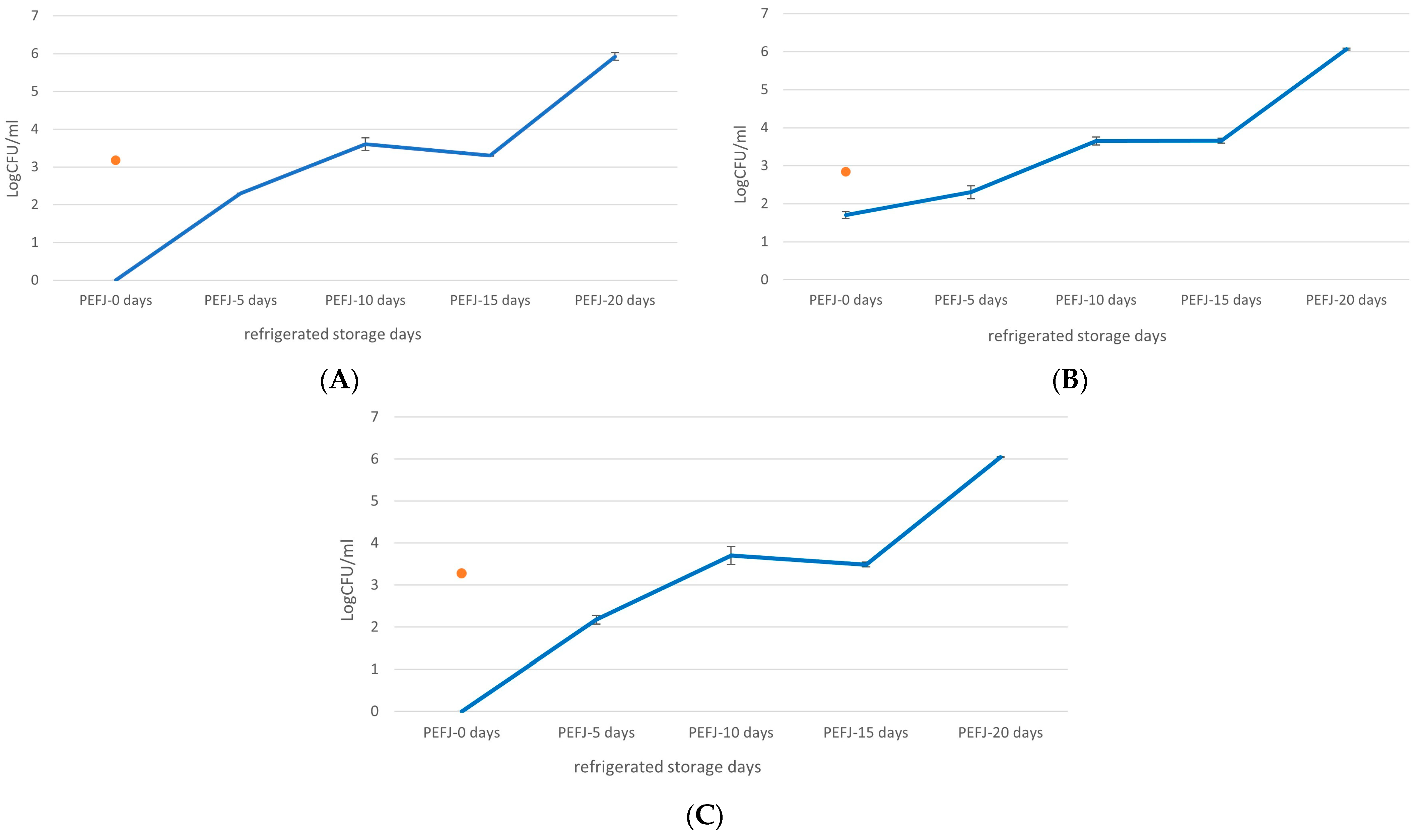
2.3. Microbial Analysis
2.4. Sensory Analysis
3. Materials and Methods
3.1. NFC Blood Orange Juice
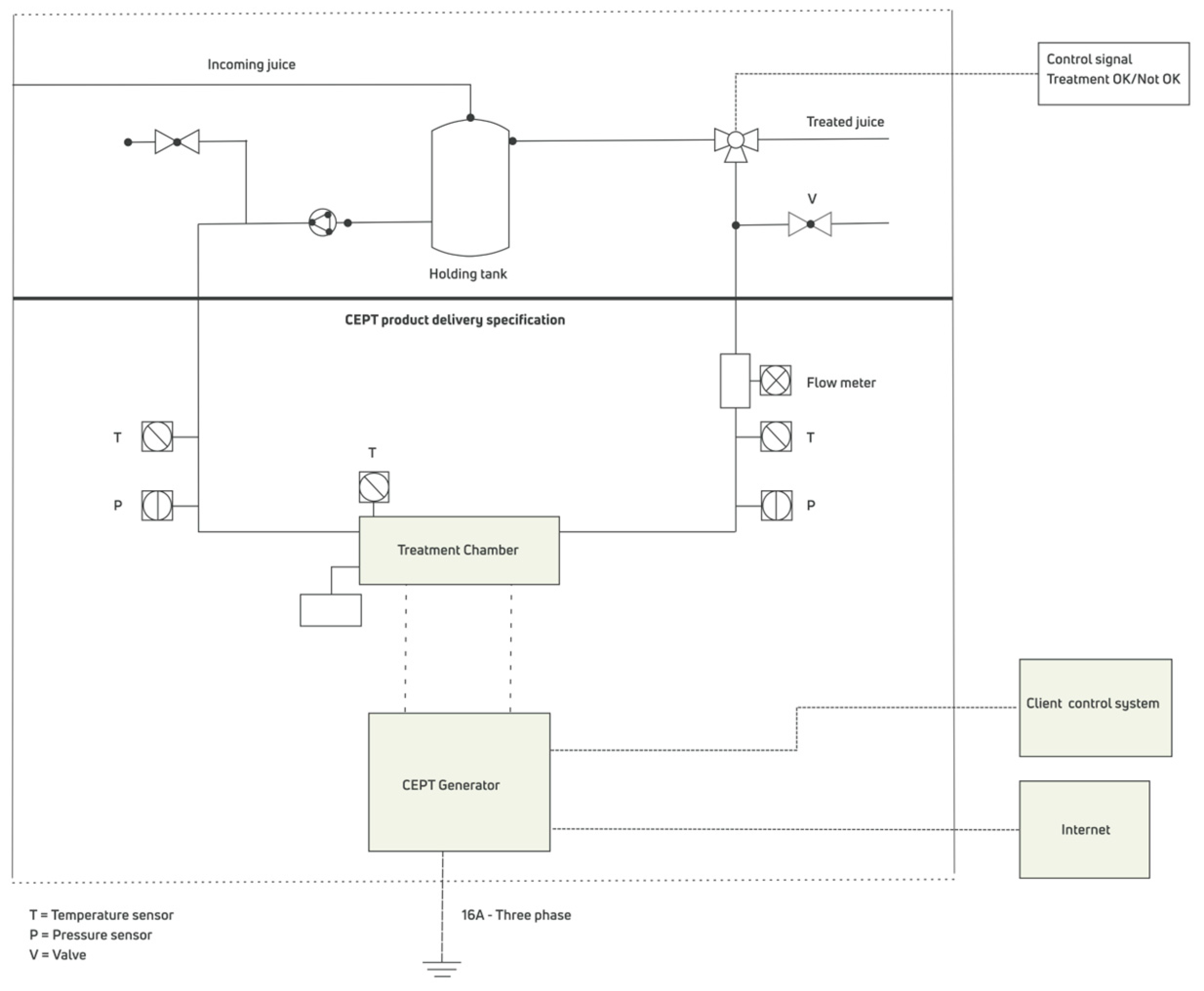
3.2. Pulsed Electric Field Equipment
3.3. Processing and Cleaning Procedures
3.4. Physicochemical Analysis
3.5. Antioxidant Components and Antioxidant Assays
3.6. Microbial Analysis
3.7. Sensory Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rapisarda, P.; Fabroni, S. Succhi. In Gli Agrumi Coltura e Cultura; ART Servizi Editoriali: Bologna, Italy, 2012; pp. 438–447. ISBN 978-88-6614-856-2. [Google Scholar]
- Rapisarda, P.; Lo Bianco, M.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Fallico, B.; Ballistreri, G.; Arena, E.; Brighina, S.; Rapisarda, P. Bioactive compounds in blood oranges (Citrus sinensis (L.) Osbeck): Level and intake. Food Chem. 2017, 215, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Timpanaro, N. Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules 2022, 27, 8675. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P. Qualità dei frutti e dei succhi. In Citrus. Trattato di Agrumicoltura; Vacante, V., Calabrese, F., Eds.; Edagricole-New Business Media: Milan, Italy, 2009; pp. 419–436. ISBN 9788850652723. [Google Scholar]
- Shomer, R.; Cogan, U.; Mannheim, C.H. Thermal death parameters of orange juice and effect of minimal heat treatment and carbon dioxide on shelf-life. J. Food Process. Preserv. 1994, 18, 305–315. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Non-enzymatic browning in citrus juice: Chemical markers, their detection and ways to improve product quality. J. Food Sci. Technol. 2014, 51, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
- Rodgers, S. Minimally Processed Functional Foods: Technological and Operational Pathways. J. Food Sci. 2016, 81, R2309–R2319. [Google Scholar] [CrossRef] [PubMed]
- Devlieghere, F.; Vermeiren, L.; Debevere, J. New preservation technologies: Possibilities and limitations. Int. Dairy J. 2004, 14, 273–285. [Google Scholar] [CrossRef]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 1999, 62, 1088–1096. [Google Scholar] [CrossRef]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M.P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016, 6, 19778. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J. Agric. Food Chem. 2000, 48, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O.; Cano, M.P. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in viscosity and pectolytic enzymes of tomato and strawberry juices processed by high-intensity pulsed electric fields. Int. J. Food Sci. Technol. 2009, 44, 2268–2277. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Akdemir Evrendilek, G. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Agcam, E.; Akyildiz, A.; Evrendilek, G.A. Effects of PEF and heat pasteurization on PME activity in orange juice with regard to a new inactivation kinetic model. Food Chem. 2014, 165, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Agcam, E.; Akyildiz, A.; Akdemir Evrendilek, G. A comparative assessment of long-term storage stability and quality attributes of orange juice in response to pulsed electric fields and heat treatments. Food Bioprod. Process. 2016, 99, 90–98. [Google Scholar] [CrossRef]
- Katiyo, W.; Yang, R.; Zhao, W. Effects of combined pulsed electric fields and mild temperature pasteurization on microbial inactivation and physicochemical properties of cloudy red apple juice (Malus pumila Niedzwetzkyana (Dieck)). J. Food Saf. 2017, 37, e12369. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Jäger, H.; Knorr, D.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Impact of pulsed electric fields, high hydrostatic pressure, and thermal pasteurization on selected characteristics of Opuntia dillenii cactus juice. LWT—Food Sci. Technol. 2017, 79, 534–542. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Berendsen, L.B.J.M.; Nederhoff, A.L.; Matser, A.M.; Van Boekel, M.A.J.S.; Nierop Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.-A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Elez-Martinez, P.; Soliva-Fortuny, R.C.; Martin-Belloso, O. Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment. Eur. Food Res. Technol. 2006, 222, 321–329. [Google Scholar] [CrossRef]
- Bates, R.P.; Morris, J.R.; Crandall, P.G. Principles and practices of small- and medium-scale fruit juice processing. FAO Agric. Serv. Bull. 2001, 146, 219–223. [Google Scholar]
- Fabroni, S.; Amenta, M.; Timpanaro, N.; Todaro, A.; Rapisarda, P. Change in taste-altering non-volatile components of blood and common orange fruit during cold storage. Food Res. Int. 2020, 131, 108916. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Bellomo, S.E.; Intelisano, S. Storage temperature effects on blood orange fruit quality. J. Agric. Food Chem. 2001, 49, 3230–3235. [Google Scholar] [CrossRef]
- Baker, R.A.; Cameron, R.G. Clouds of citrus juices and juice drinks. Food Technol. 1999, 53, 64–69. [Google Scholar]
- Fabroni, S.; Amenta, M.; Timpanaro, N.; Rapisarda, P. Supercritical carbon dioxide-treated blood orange juice as a new product in the fresh fruit juice market. Innov. Food Sci. Emerg. Technol. 2010, 11, 477–484. [Google Scholar] [CrossRef]
- Vervoort, L.; Van der Plancken, I.; Grauwet, T.; Timmermans, R.A.H.; Mastwijk, H.C.; Matser, A.M.; Hendrickx, M.E.; VanLoey, A. Comparing equivalent thermal, high pressureand pulsed electric field processes for mild pasteurization oforange juice. Part II. Impact on specific chemical andbiochemical quality parameters. Innov. Food Sci. Emerg. Technol. 2011, 12, 466–477. [Google Scholar] [CrossRef]
- Rivas, A.; Rodrigo, D.; Martinez, A.; Barbosa-Canovas, G.V.; Rodrigo, M. Effect of PEF and heat pasteurization on thephysical–chemical characteristics of blended orange andcarrot juice. Leb.-Wiss. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Rapisarda, P.; Fabroni, S.; Peterek, S.; Russo, G.; Mock, H.P. Juice of New Citrus Hybrids (Citrus clementina Hort ex Tan. × C. sinensis L. Osbeck) as a Source of Natural Antioxidants. Food Chem. 2009, 117, 212–218. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- FDA. FDA Nutritional Labeling Manual: A Guide for Developing and Using Databases; Center for Food Safety and Applied Nutrition: Washington, DC, USA, 1998. [Google Scholar]
- Hodgins, A.M.; Mittal, G.S.; Griffiths, M.W. Pasteurization of Fresh Orange Juice Using Low-Energy Pulsed Electrical Field. J. Food Sci. 2002, 67, 2294–2299. [Google Scholar] [CrossRef]
- Min, S.; Jin, Z.T.; Min, S.K.; Yeom, H.; Zhang, Q.H. Commercial-Scale Pulsed Electric Field Processing of Orange Juice. J. Food Sci. 2003, 68, 1265–1271. [Google Scholar] [CrossRef]
- Davey, M.W.; Van Montagu, M.; Inzè, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Torregrosa, F.; Cortes, C.; Esteve, M.J.; Frigola, A. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. J. Agric. Food Chem. 2005, 53, 9519–9525. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Influence of pulsed electric field processing on the quality of fruit juice beverages sweetened with stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Balaban, M.O.; Talcott, S.T. Microbial stability, phytochemical retention, and organoleptic attributes of dense phase CO2 processed muscadine grape juice. J. Agric. Food Chem. 2006, 54, 5468–5473. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.; Prior, R.L. The chemistry behind antioxidant capacity assay. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frigola, A. Ascorbic acid in orangejuice–milk beverage treated by high intensity pulsed electricfields and its stability during storage. Innov. Food Sci. Emerg. Technol. 2010, 11, 84–90. [Google Scholar] [CrossRef]
- Kincal, D.; Hill, W.S.; Balaban, M.O.; Marshall, M.R.; Wei, C.I. A continuous high pressure CO2 system for microbial reduction in orange juice. J. Food Sci. 2005, 70, M249–M254. [Google Scholar] [CrossRef]
- Kimbal, D. Citrus Processing. Quality Control and Technology; AVI Books: New York, NY, USA, 1999. [Google Scholar]
- Rouse, A.H.; Atkins, C.D. Pectinesterase and pectin in commercial citrus juices as determined by methods used at the citrus experimental station. In Agricultural Experiment Station Journal Series, No. 1141; University of Florida: Gainesville, FL, USA, 1955; pp. 271–275. [Google Scholar]
- Kincal, D.; Hill, W.S.; Balaban, M.O.; Portier, K.M.; Sims, C.A.; Wei, C.I. A continuous high-pressure carbon dioxide system for cloud and quality retention in orange juice. J. Food Sci. 2006, 71, C338–C344. [Google Scholar] [CrossRef]
- Rapisarda, P.; Intelisano, S. Sample preparation for vitamin C analysis of pigmented orange juice. Ital. J. Food Sci. 1996, 8, 251–256. [Google Scholar]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical method for determining anthocyanins in blood orange juice. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.L.; Martin, S.F.; Yotsey, C.O. Quantitative survey of narirutin, naringin, hesperidin and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin–Ciocalteu reagent. Methods Enzimol. 1999, 299, 152–178. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R. Developing and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- ISO 8586:2014; Sensory Analysis: General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2014.
- ISO 8589:2007/Amd 1:2014; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2014.
- ISO 4121:2003; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. ISO: Geneva, Switzerland, 2003.

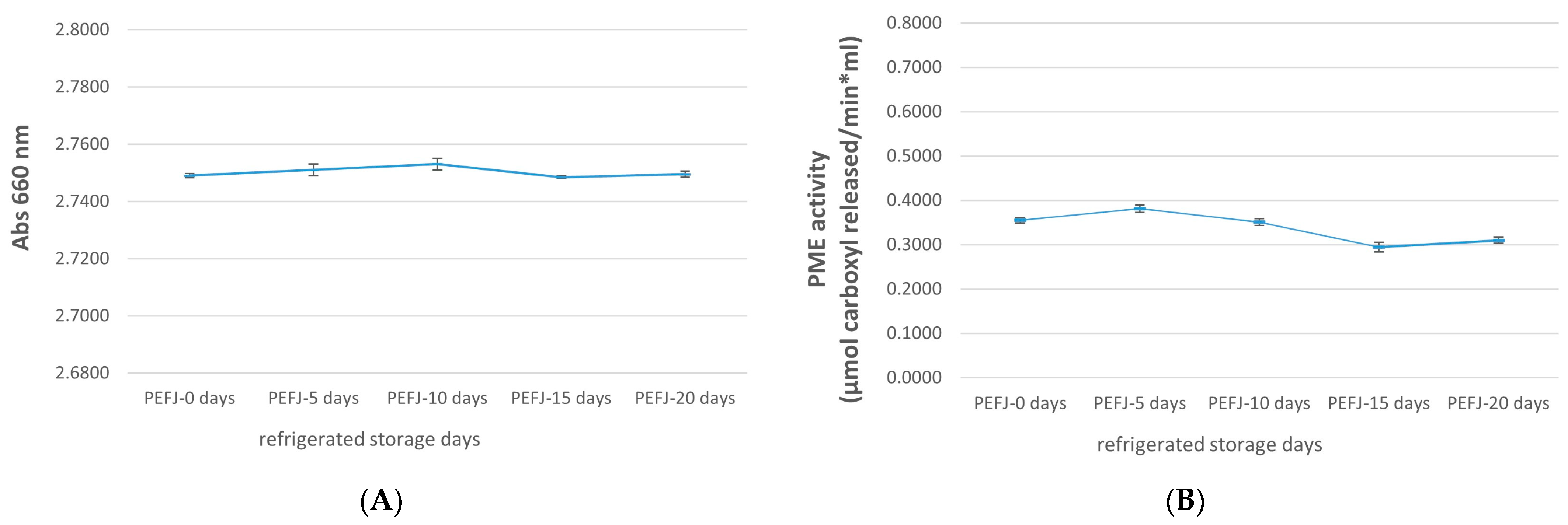


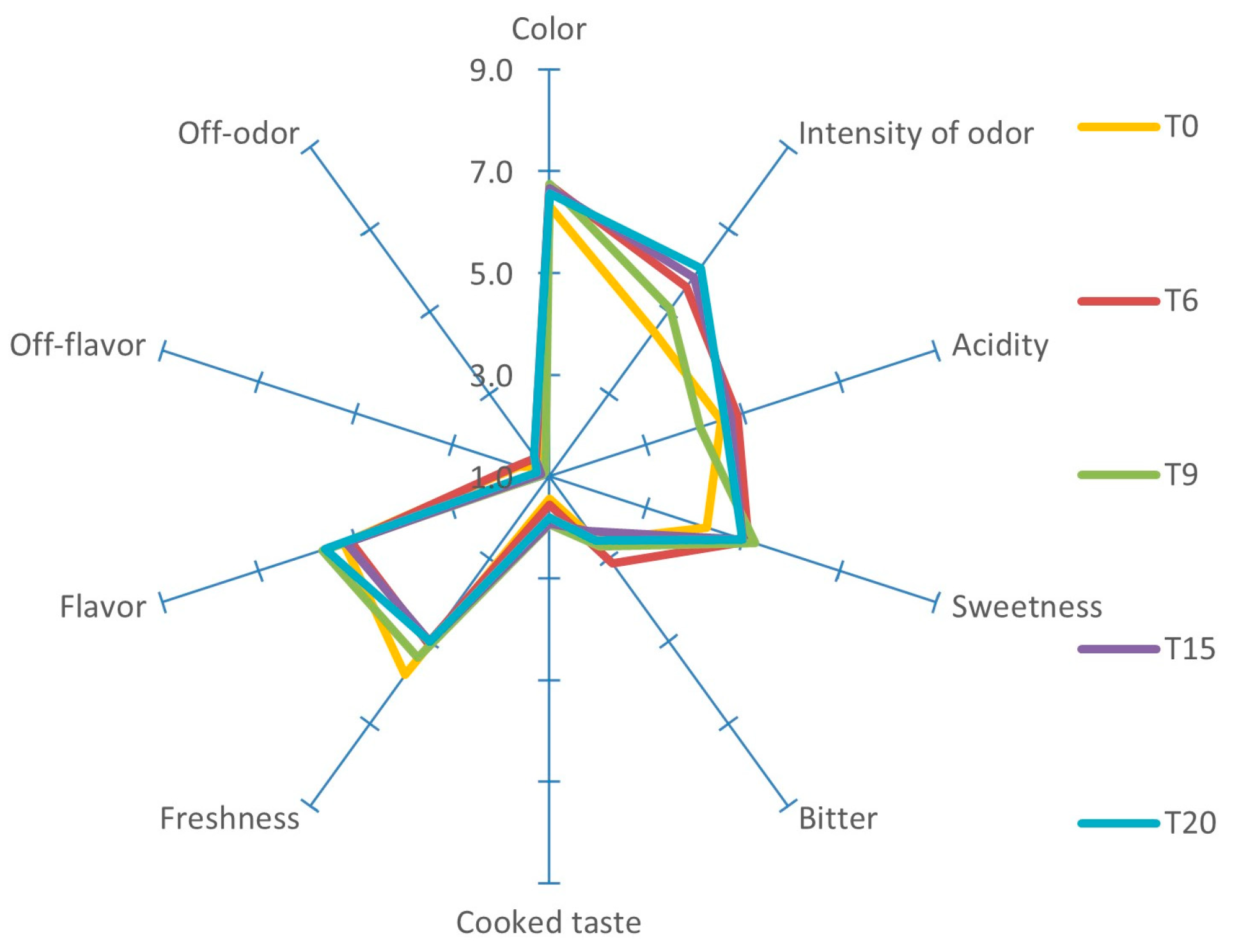
| TSS (°Brix) | TA (% Citric Acid) | pH | L* | a* | b* | C* | h | |
| UJ | 12.75 ± 0.00 B | 1.38 ± 0.02 | 3.32 ± 0.03 | 39.67 ± 0.02 b | 27.48 ± 1.71 | 17.37 ± 0.78 | 32.51 ± 1.87 | 32.33 ± 0.45 |
| PEFJ 0 days | 12.86 ± 0.01 A | 1.35 ± 0.11 | 3.30 ± 0.01 | 40.57 ± 0.13 a | 28.10 ± 1.31 | 17.79 ± 0.36 | 33.26 ± 1.30 | 32.35 ± 0.68 |
| REFRIGERATED STORAGE OF PEFJ | ||||||||
| TSS (°Brix) | TA (% citric acid) | pH | L* | a* | b* | C* | h | |
| PEFJ-0 days | 12.86 ± 0.01 C | 1.35 ± 0.11 b | 3.30 ± 0.01 | 40.57 ± 0.13 | 28.10 ± 1.31 | 17.79 ± 0.36 | 33.26 ± 1.30 | 32.35 ± 0.68 |
| PEFJ-5 days | 13.02 ± 0.01 B | 1.50 ± 0.08 ab | 3.29 ± 0.00 | 41.18 ± 0.12 | 26.47 ± 0.31 | 17.94 ± 0.09 | 31.98 ± 0.21 | 34.13 ± 0.44 |
| PEFJ-10 days | 13.02 ± 0.00 B | 1.63 ± 0.02 ab | 3.26 ± 0.01 | 41.18 ± 0.39 | 26.35 ± 0.58 | 17.82 ± 0.49 | 31.82 ± 0.21 | 34.08 ± 1.32 |
| PEFJ-15 days | 12.99 ± 0.00 B | 1.74 ± 0.00 a | 3.30 ± 0.00 | 40.58 ± 1.37 | 26.74 ± 1.55 | 17.89 ± 0.70 | 32.21 ± 0.90 | 33.85 ± 2.57 |
| PEFJ-20 days | 13.24 ± 0.02 A | 1.76 ± 0.02 a | 3.26 ± 0.01 | 41.73 ± 0.45 | 25.58 ± 0.93 | 18.31 ± 0.53 | 31.47 ± 0.44 | 35.63 ± 1.77 |
| Hesperidin (mg/L) | Narirutin (mg/L) | Total Flavanones (mg/L) | Ascorbic Acid (mg/100 mL) | Total Anthocyanins (mg CGE */L) | |
|---|---|---|---|---|---|
| PEFJ-0 days | 45.98 ± 0.81 ab | 27.15 ± 0.47 b | 73.12 ± 1.29 bc | 62.92 ± 4.82 | 55.59 ± 0.11 A |
| PEFJ-5 days | 44.18 ± 1.14 b | 27.17 ± 0.48 b | 71.36 ± 1.63 c | 58.65 ± 2.42 | 55.19 ± 0.07 AB |
| PEFJ-10 days | 44.63 ± 1.23 b | 27.52 ± 0.81 b | 72.15 ± 2.04 bc | 61.21 ± 1.32 | 53.92 ± 0.02 AB |
| PEFJ-15 days | 51.35 ± 1.74 a | 27.15 ± 0.58 b | 78.50 ± 2.32 ab | 53.22 ± 2.42 | 53.65 ± 0.54 AB |
| PEFJ-20 days | 50.60 ± 0.62 ab | 30.31 ± 0.48 a | 80.91 ± 1.09 a | 53.62 ±2.79 | 53.25 ± 0.04 B |
| Total Polyphenols Content (mg GAE */L) | ORAC Units (µmol TE/100 mL) | |
|---|---|---|
| PEFJ-0 days | 867.77 ± 0.63 AB | 3398.87 ± 31.71 |
| PEFJ-5 days | 862.28 ± 0.85 B | 3974.88 ± 140.97 |
| PEFJ-10 days | 872.99 ± 1.12 A | 3772.46 ± 54.57 |
| PEFJ-15 days | 828.30 ± 0.27 D | 3503.03 ± 309.02 |
| PEFJ-20 days | 836.16 ± 0.80 C | 3735.00 ± 23.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabroni, S.; Platania, G.M.; Amenta, M.; Ballistreri, G.; Galvano, F.; Nges, I.A.; Timpanaro, N. Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice. Appl. Sci. 2024, 14, 7275. https://doi.org/10.3390/app14167275
Fabroni S, Platania GM, Amenta M, Ballistreri G, Galvano F, Nges IA, Timpanaro N. Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice. Applied Sciences. 2024; 14(16):7275. https://doi.org/10.3390/app14167275
Chicago/Turabian StyleFabroni, Simona, Giusy Maria Platania, Margherita Amenta, Gabriele Ballistreri, Francesco Galvano, Ivo Achu Nges, and Nicolina Timpanaro. 2024. "Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice" Applied Sciences 14, no. 16: 7275. https://doi.org/10.3390/app14167275





