From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes
Abstract
:1. Introduction and Focus of the Review: From the Environment to Molecular Interactions of Plastic Particles
2. Definition and Characterization of Plastics
3. Presence of Micro- and Nanoplastics in Environmental Matrices and Food
3.1. Water
3.2. Soil
3.3. Air
3.4. Food
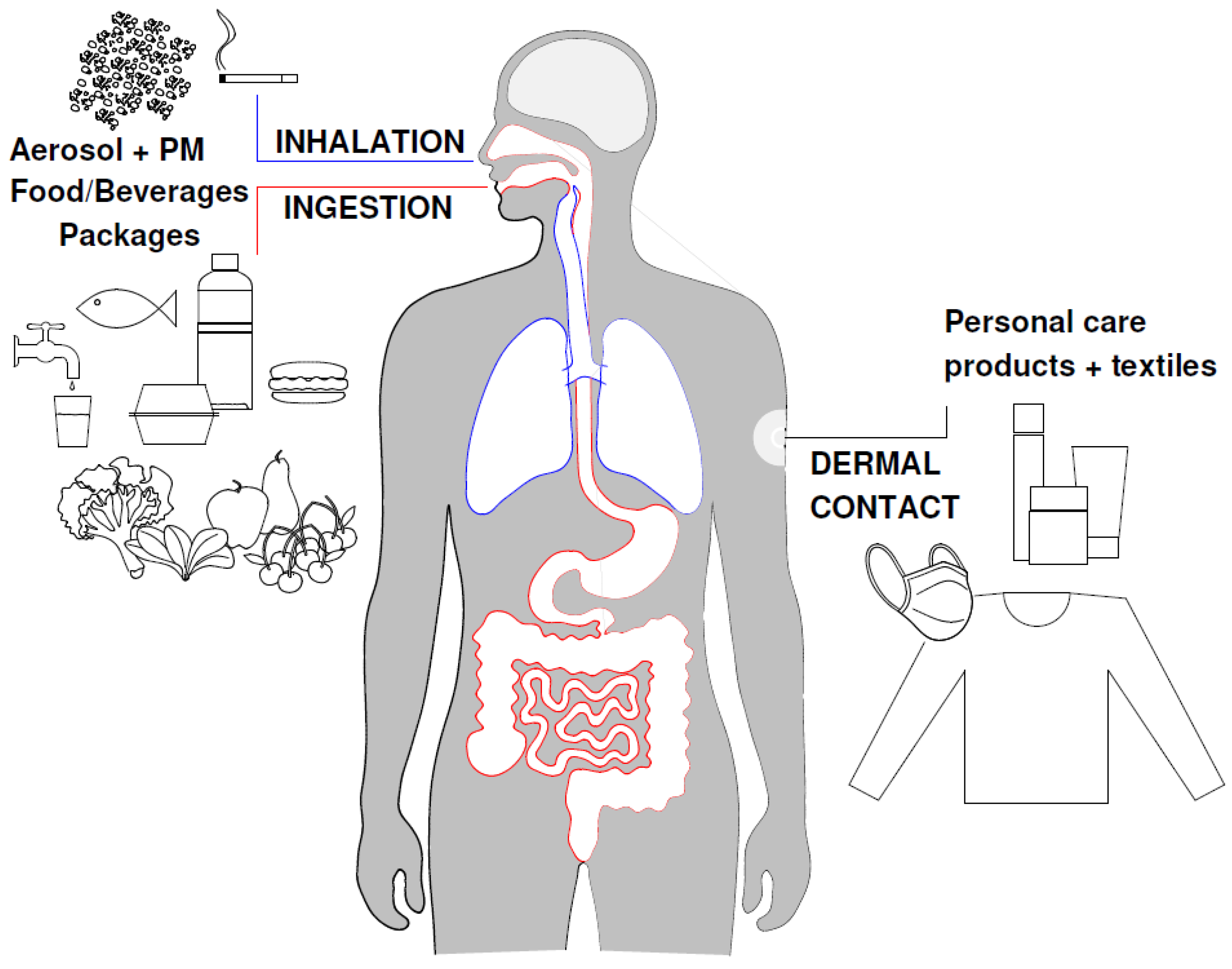
4. Human Exposure through Different Routes
5. Neurodegenerative Diseases and Environmental Factors
6. Mechanisms of Plastic Particles’ Transfer to the Central Nervous System (CNS) and Effects on the Blood–Brain Barrier
7. Molecular Effects of Nanoplastics in Processes of Neurotoxicity and Neurodegeneration
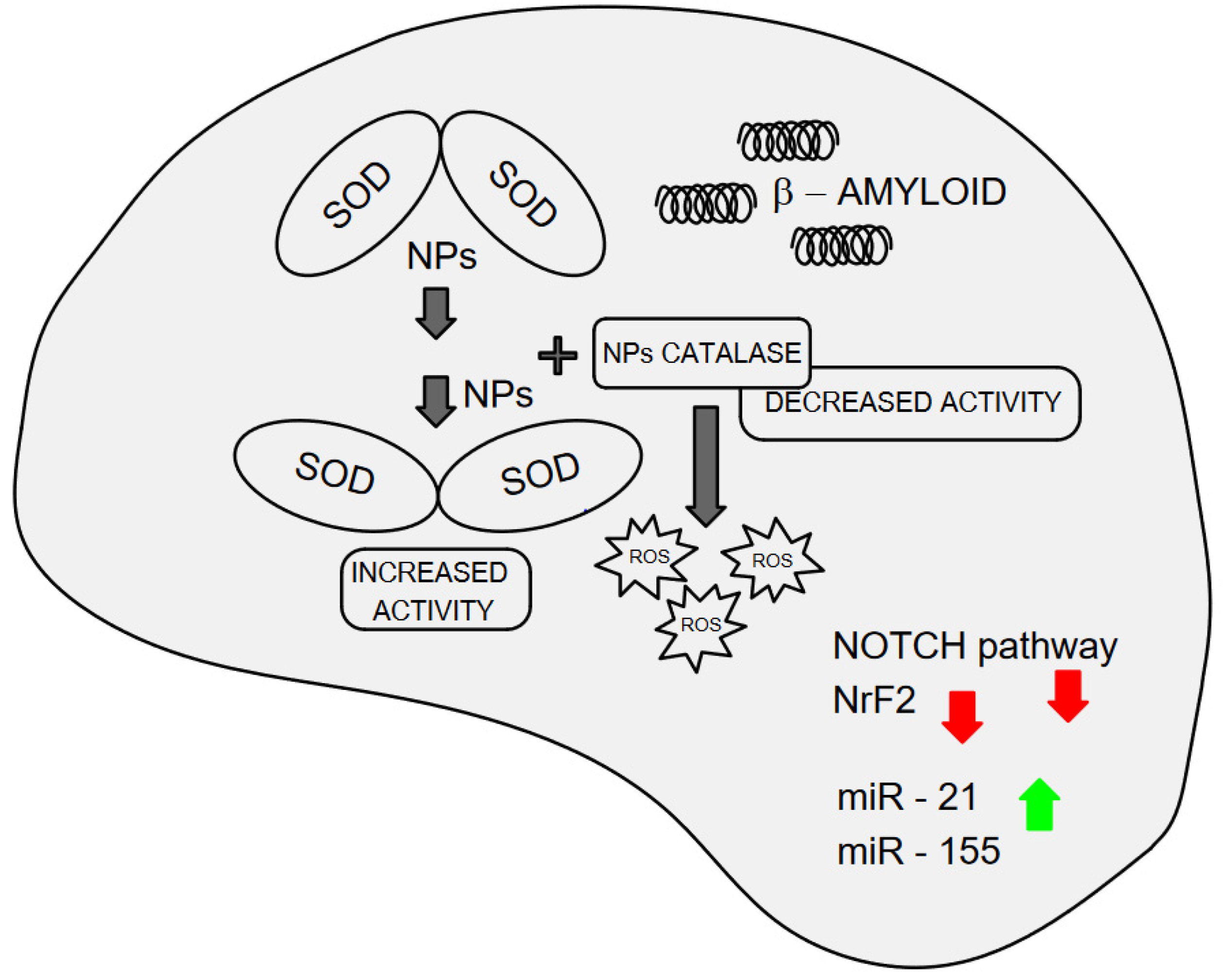
7.1. In Vitro and In Silico Interaction of Nanoplastics with Superoxide Dismutase and Amyloid Protein Formation: Implications for Oxidative Stress and Neurodegeneration
7.2. Nanoplastics Affect the Transcriptomics and Epigenomic in Neuronal Models
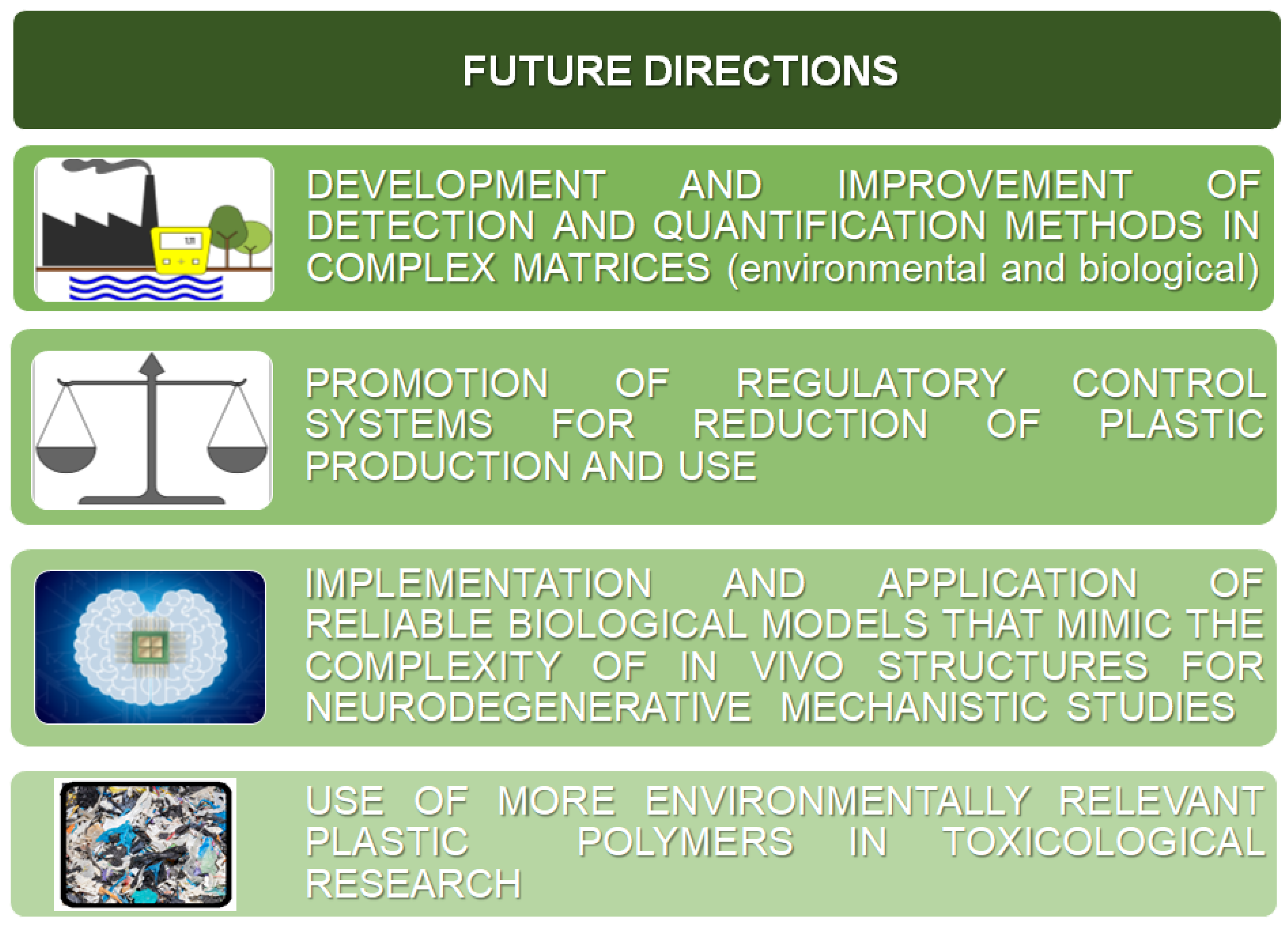
8. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Liu, C. Exploring Plastic-Management Policy in China: Status, Challenges and Policy Insights. Sustainability 2023, 15, 9087. [Google Scholar] [CrossRef]
- European Chemical Agency. Available online: https://echa.europa.eu/hot-topics/microplastics (accessed on 18 July 2024).
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An Overview of Microplastic and Nanoplastic Pollution in Agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Stapleton, P.A. Microplastic and Nanoplastic Transfer, Accumulation, and Toxicity in Humans. Curr. Opin. Toxicol. 2021, 28, 62–69. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). Official Journal of the European Union L 275/38- L 275/40. 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:275:0038:0040:EN:PDF (accessed on 18 July 2024).
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). 2021. Available online: https://www.efsa.europa.eu/en/events/event/update-scientific-colloquium-25-coordinated-approach-assess-human-health (accessed on 18 July 2024).
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood–Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.A.; Gan, N.; Wang, E.; Merrill, M.; Xu, W. Materials, Surfaces, and Interfacial Phenomena in Nanoplastics Toxicology Research. Environ. Pollut. 2022, 292, 118442. [Google Scholar] [CrossRef] [PubMed]
- Petters, S.S.; Kjærgaard, E.R.; Hasager, F.; Massling, A.; Glasius, M.; Bilde, M. Morphology and Hygroscopicity of Nanoplastics in Sea Spray. Phys. Chem. Chem. Phys. 2023, 25, 32430–32442. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Mohan, S.; Tatarchuk, T. Microplastics in Water: Types, Detection, and Removal Strategies. Environ. Sci Pollut Res 2023, 30, 84933–84948. [Google Scholar] [CrossRef]
- Plastic—The Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 19 July 2024).
- European Parliament. Environmental Determinants of Health, Including Those Caused by Climate Change. 2024. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2024/754209/IPOL_STU(2024)754209_EN.pdf (accessed on 6 August 2024).
- Mortensen, N.P.; Fennell, T.R.; Johnson, L.M. Unintended Human Ingestion of Nanoplastics and Small Microplastics through Drinking Water, Beverages, and Food Sources. NanoImpact 2021, 21, 100302. [Google Scholar] [CrossRef]
- Sewwandi, M.; Wijesekara, H.; Rajapaksha, A.U.; Soysa, S.; Vithanage, M. Microplastics and Plastics-Associated Contaminants in Food and Beverages; Global Trends, Concentrations, and Human Exposure. Environ. Pollut. 2023, 317, 120747. [Google Scholar] [CrossRef]
- Yang, L.; Kang, S.; Luo, X.; Wang, Z. Microplastics in Drinking Water: A Review on Methods, Occurrence, Sources, and Potential Risks Assessment. Environ. Pollut. 2024, 348, 123857. [Google Scholar] [CrossRef] [PubMed]
- Negrete Velasco, A.; Ramseier Gentile, S.; Zimmermann, S.; Le Coustumer, P.; Stoll, S. Contamination and Removal Efficiency of Microplastics and Synthetic Fibres in a Conventional Drinking Water Treatment Plant in Geneva, Switzerland. Sci. Total Environ. 2023, 880, 163270. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Praharaj, J.K.; Mahajan, D.K.; Purokait, B.; Mohanty, T.R.; Mohanty, D.; Gogoi, P.; Kumar, V.S.; et al. Microplastics Removal Efficiency of Drinking Water Treatment Plant with Pulse Clarifier. J. Hazard. Mater. 2021, 413, 125347. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wong, K.K.; Li, W.; Zhao, H.; Wang, T.; Stanescu, S.; Boult, S.; Van Dongen, B.; Mativenga, P.; Li, L. Characteristics of Nano-Plastics in Bottled Drinking Water. J. Hazard. Mater. 2022, 424, 127404. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of Nanoplastics in the Aquatic Environment and Possible Impacts on Aquatic Organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Ding, R.; Tong, L.; Zhang, W. Microplastics in Freshwater Environments: Sources, Fates and Toxicity. Water Air Soil Pollut. 2021, 232, 181. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic Pollution in the Surface Waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of Microplastic Mass and Removal Rates at Wastewater Treatment Plants Applying Focal Plane Array (FPA)-Based Fourier Transform Infrared (FT-IR) Imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Telles Silveira, I.; Chua, A.; Leusch, F.D.L. An Audit of Microplastic Abundance throughout Three Australian Wastewater Treatment Plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Karbalaei, S.; Maselli, V.; Walker, T.R. Micro(Nano)Plastics Sources, Fate, and Effects: What We Know after Ten Years of Research. J. Hazard. Mater. Adv. 2022, 6, 100057. [Google Scholar] [CrossRef]
- Materić, D.; Kjær, H.A.; Vallelonga, P.; Tison, J.-L.; Röckmann, T.; Holzinger, R. Nanoplastics Measurements in Northern and Southern Polar Ice. Environ. Res. 2022, 208, 112741. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Monira, S.; Roychand, R.; Hai, F.I.; Bhuiyan, M.; Dhar, B.R.; Pramanik, B.K. Nano and Microplastics Occurrence in Wastewater Treatment Plants: A Comprehensive Understanding of Microplastics Fragmentation and Their Removal. Chemosphere 2023, 334, 139011. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, Classification and Removal Efficiency of Microplastics in Wastewater Treatment Plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the Fragmentation of Microplastics into Nano-Plastics and Removal of Nano/Microplastics from Wastewater Using Membrane, Air Flotation and Nano-Ferrofluid Processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do Wastewater Treatment Plants Act as a Potential Point Source of Microplastics? Preliminary Study in the Coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled Wastewater as a Potential Source of Microplastics in Irrigated Soils from an Arid-Insular Territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]
- Rolf, M.; Laermanns, H.; Kienzler, L.; Pohl, C.; Möller, J.N.; Laforsch, C.; Löder, M.G.J.; Bogner, C. Flooding Frequency and Floodplain Topography Determine Abundance of Microplastics in an Alluvial Rhine Soil. Sci. Total Environ. 2022, 836, 155141. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Hashemi, S.H.; Flury, M. Micro- and Mesoplastics in Farmlands with Different Irrigation Water Sources. Water Air Soil Pollut. 2023, 234, 267. [Google Scholar] [CrossRef]
- Schell, T.; Hurley, R.; Buenaventura, N.T.; Mauri, P.V.; Nizzetto, L.; Rico, A.; Vighi, M. Fate of Microplastics in Agricultural Soils Amended with Sewage Sludge: Is Surface Water Runoff a Relevant Environmental Pathway? Environ. Pollut. 2022, 293, 118520. [Google Scholar] [CrossRef] [PubMed]
- Astner, A.F.; Gillmore, A.B.; Yu, Y.; Flury, M.; DeBruyn, J.M.; Schaeffer, S.M.; Hayes, D.G. Formation, Behavior, Properties and Impact of Micro- and Nanoplastics on Agricultural Soil Ecosystems (A Review). NanoImpact 2023, 31, 100474. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Bläsing, M.; Amelung, W. Plastics in Soil: Analytical Methods and Possible Sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Maddela, N.R.; Reddy, K.V.; Ranjit, P. (Eds.) Micro and Nanoplastics in Soil: Threats to Plant-Based Food; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-21194-2. [Google Scholar]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- European Commission. Caring for Soil Is Caring for Life—Ensure 75% of Soils Are Healthy by 2030 for Food, People, Nature and Climate. Caring for Soil is Caring for Life—Publications Office of the EU (europa.eu). 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/4ebd2586-fc85-11ea-b44f-01aa75ed71a1/ (accessed on 8 August 2024).
- Mathissen, M.; Scheer, V.; Vogt, R.; Benter, T. Investigation on the Potential Generation of Ultrafine Particles from the Tire–Road Interface. Atmos. Environ. 2011, 45, 6172–6179. [Google Scholar] [CrossRef]
- Luo, Y.; Awoyemi, O.S.; Naidu, R.; Fang, C. Detection of Microplastics and Nanoplastics Released from a Kitchen Blender Using Raman Imaging. J. Hazard. Mater. 2023, 453, 131403. [Google Scholar] [CrossRef]
- Byrley, P.; Boyes, W.K.; Rogers, K.; Jarabek, A.M. 3D Printer Particle Emissions: Translation to Internal Dose in Adults and Children. J. Aerosol Sci. 2021, 154, 105765. [Google Scholar] [CrossRef]
- Kim, J.; Pham, D.T.; Park, H.-J.; Chae, M.; Lee, S.-H.; Hong, S.; Kim, J.-Y.; Jung, J.; Lee, B.-T.; Kwon, J.-H. Development and Validation of Analytical Methods for Detecting and Identifying Microplastics in Salts, Soy Sauce, and Salted Pollock Roe. J. Food Compos. Anal. 2022, 114, 104856. [Google Scholar] [CrossRef]
- Lazăr, N.-N.; Călmuc, M.; Milea, Ș.-A.; Georgescu, P.-L.; Iticescu, C. Micro and Nano Plastics in Fruits and Vegetables: A Review. Heliyon 2024, 10, e28291. [Google Scholar] [CrossRef]
- Sohail, M.; Urooj, Z.; Noreen, S.; Baig, M.M.F.A.; Zhang, X.; Li, B. Micro- and Nanoplastics: Contamination Routes of Food Products and Critical Interpretation of Detection Strategies. Sci. Total Environ. 2023, 891, 164596. [Google Scholar] [CrossRef] [PubMed]
- Tympa, L.-E.; Katsara, K.; Moschou, P.N.; Kenanakis, G.; Papadakis, V.M. Do Microplastics Enter Our Food Chain Via Root Vegetables? A Raman Based Spectroscopic Study on Raphanus Sativus. Materials 2021, 14, 2329. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Wan, B.; Guo, L.-H.; Zhao, L. Microplastics from Consumer Plastic Food Containers: Are We Consuming It? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Wang, M.; Ying, R.; Li, X.; Hu, Z.; Zhang, Y. Disposable Plastic Materials Release Microplastics and Harmful Substances in Hot Water. Sci. Total Environ. 2022, 818, 151685. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and Other Harmful Substances Released from Disposable Paper Cups into Hot Water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, L.; Kavanagh, R.; Xiao, L.; Shi, Y.; Kehoe, D.K.; Sheerin, E.D.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Sampling, Identification and Characterization of Microplastics Release from Polypropylene Baby Feeding Bottle during Daily Use. JoVE 2021, 173, e62545. [Google Scholar] [CrossRef]
- De Boever, S.; Devisscher, L.; Vinken, M. Unraveling the Micro- and Nanoplastic Predicament: A Human-Centric Insight. Sci. Total Environ. 2024, 916, 170262. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET Microplastics Affect Human Gut Microbiota Communities during Simulated Gastrointestinal Digestion, First Evidence of Plausible Polymer Biodegradation during Human Digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of Human Exposure to Microplastics, and Estimation of the Total Burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to Microplastics (<10 Μm) Associated to Plastic Bottles Mineral Water Consumption: The First Quantitative Study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Panel on Dietetic Products, Nutrition, and Allergies: Scientific Opinion on Dietary Reference Values for Water. EFSA Journal 8:1459. Scientific Opinion on Dietary Reference Values for Water—2010–EFSA Journal—Wiley Online Library. 2010. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/1459 (accessed on 8 August 2024).
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, H.-J.; Kim, S.-K.; Kim, H.-J. Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kunz, A.; Shim, W.J.; Walther, B.A. Microplastic Contamination of Table Salts from Taiwan, Including a Global Review. Sci. Rep. 2019, 9, 10145. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- World Health Organization (WHO) 2015 Guideline: Sugars Intake for Adults and Children. Geneva. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 19 July 2024).
- Zarus, G.M.; Muianga, C.; Hunter, C.M.; Pappas, R.S. A Review of Data for Quantifying Human Exposures to Micro and Nanoplastics and Potential Health Risks. Sci. Total Environ. 2021, 756, 144010. [Google Scholar] [CrossRef]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the Release of Micro/Nanoplastics from Disposable Face Masks at Times of COVID-19. J. Hazard. Mater. 2021, 419, 126507. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and Microplastics: A Comprehensive Review on Their Exposure Routes, Translocation, and Fate in Humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, S.-Y.; Ni, H.-G. Incidence of Microplastics in Personal Care Products: An Appreciable Part of Plastic Pollution. Sci. Total Environ. 2020, 742, 140218. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Filippini, T.; Hatch, E.E.; Vinceti, M. Residential Exposure to Electromagnetic Fields and Risk of Amyotrophic Lateral Sclerosis: A Dose–Response Meta-Analysis. Sci. Rep. 2021, 11, 11939. [Google Scholar] [CrossRef]
- Tesauro, M.; Consonni, M.; Filippini, T.; Mazzini, L.; Pisano, F.; Chiò, A.; Esposito, A.; Vinceti, M. Incidence of Amyotrophic Lateral Sclerosis in the Province of Novara, Italy, and Possible Role of Environmental Pollution. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 284–290. [Google Scholar] [CrossRef]
- Vasta, R.; Callegaro, S.; Sgambetterra, S.; Cabras, S.; Di Pede, F.; De Mattei, F.; Matteoni, E.; Grassano, M.; Bombaci, A.; De Marco, G.; et al. Presymptomatic Geographical Distribution of ALS Patients Suggests the Involvement of Environmental Factors in the Disease Pathogenesis. J. Neurol. 2023, 270, 5475–5482. [Google Scholar] [CrossRef]
- Filippini, T.; Mandrioli, J.; Malagoli, C.; Costanzini, S.; Cherubini, A.; Maffeis, G.; Vinceti, M. Risk of Amyotrophic Lateral Sclerosis and Exposure to Particulate Matter from Vehicular Traffic: A Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 973. [Google Scholar] [CrossRef]
- Van Wijngaarden, E.; Rich, D.Q.; Zhang, W.; Thurston, S.W.; Lin, S.; Croft, D.P.; Squizzato, S.; Masiol, M.; Hopke, P.K. Neurodegenerative Hospital Admissions and Long-Term Exposure to Ambient Fine Particle Air Pollution. Ann. Epidemiol. 2021, 54, 79–86.e4. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.-C.; Dao, K.; Roque, P. Neurotoxicants Are in the Air: Convergence of Human, Animal, and In Vitro Studies on the Effects of Air Pollution on the Brain. BioMed. Res. Int. 2014, 2014, 736385. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.M.; Lampinen, R.; Rantanen, L.M.; Odendaal, L.; Jalava, P.; Chew, S.; White, A.R. Olfactory Cell Cultures to Investigate Health Effects of Air Pollution Exposure: Implications for Neurodegeneration. Neurochem. Int. 2020, 136, 104729. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Bruschi, M.; Filippini, T.; D’Alfonso, S.; Mazzini, L.; Corrado, L.; Consonni, M.; Vinceti, M.; Fusi, P.; Urani, C. Metal(Loid)s Role in the Pathogenesis of Amyotrophic Lateral Sclerosis: Environmental, Epidemiological, and Genetic Data. Environ. Res. 2021, 192, 110292. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Bishop, D.P.; Kum Jew, S.; Doble, P.A. Age-Related Accumulation of Toxic Metals in the Human Locus Ceruleus. PLoS ONE 2018, 13, e0203627. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Heavy Metals in Locus Ceruleus and Motor Neurons in Motor Neuron Disease. Acta Neuropathol. Commun. 2013, 1, 81. [Google Scholar] [CrossRef] [PubMed]
- Pan, I.; Umapathy, S.; Issac, P.K.; Rahman, M.M.; Guru, A.; Arockiaraj, J. The Bioaccessibility of Adsorped Heavy Metals on Biofilm-Coated Microplastics and Their Implication for the Progression of Neurodegenerative Diseases. Environ. Monit. Assess. 2023, 195, 1264. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lee, Y.-H.; Chiu, I.-J.; Lin, Y.-F.; Chiu, H.-W. Potent Impact of Plastic Nanomaterials and Micromaterials on the Food Chain and Human Health. Int. J. Mol. Sci. 2020, 21, 1727. [Google Scholar] [CrossRef] [PubMed]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation Strategies for Improving Intestinal Permeability of Drugs: A More Precise Look at Permeability Assessment Methods and Pharmacokinetic Properties Changes. J. Control Release 2020, 321, 669–709. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and Human Health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular Internalization and Release of Polystyrene Microplastics and Nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A Comparative Review of Microplastics and Nanoplastics: Toxicity Hazards on Digestive, Reproductive and Nervous System. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, W.; Jing, J.; Huang, D.; Zhang, L.; Wang, J.; Han, L.; Liu, Z.; Wang, Z.; Gao, A. The Gut-Brain Axis Involved in Polystyrene Nanoplastics-Induced Neurotoxicity via Reprogramming the Circadian Rhythm-Related Pathways. J. Hazard. Mater. 2023, 458, 131949. [Google Scholar] [CrossRef] [PubMed]
- Barrenschee, M.; Zorenkov, D.; Böttner, M.; Lange, C.; Cossais, F.; Scharf, A.B.; Deuschl, G.; Schneider, S.A.; Ellrichmann, M.; Fritscher-Ravens, A.; et al. Distinct Pattern of Enteric Phospho-Alpha-Synuclein Aggregates and Gene Expression Profiles in Patients with Parkinson’s Disease. Acta Neuropathol. Commun. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Baumuratov, A.S.; Antony, P.M.A.; Ostaszewski, M.; He, F.; Salamanca, L.; Antunes, L.; Weber, J.; Longhino, L.; Derkinderen, P.; Koopman, W.J.H.; et al. Enteric Neurons from Parkinson’s Disease Patients Display Ex Vivo Aberrations in Mitochondrial Structure. Sci. Rep. 2016, 6, 33117. [Google Scholar] [CrossRef]
- Pellegrini, C.; Daniele, S.; Antonioli, L.; Benvenuti, L.; D’Antongiovanni, V.; Piccarducci, R.; Pietrobono, D.; Citi, V.; Piragine, E.; Flori, L.; et al. Prodromal Intestinal Events in Alzheimer’s Disease (AD): Colonic Dysmotility and Inflammation Are Associated with Enteric AD-Related Protein Deposition. Int. J. Mol. Sci. 2020, 21, 3523. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The Bowel and beyond: The Enteric Nervous System in Neurological Disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Tang, B.L. Commentary: Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Front. Environ. Sci. 2017, 5, 63. [Google Scholar] [CrossRef]
- Windheim, J.; Colombo, L.; Battajni, N.C.; Russo, L.; Cagnotto, A.; Diomede, L.; Bigini, P.; Vismara, E.; Fiumara, F.; Gabbrielli, S.; et al. Micro- and Nanoplastics’ Effects on Protein Folding and Amyloidosis. Int. J. Mol. Sci. 2022, 23, 10329. [Google Scholar] [CrossRef] [PubMed]
- Gałęcka, I.; Szyryńska, N.; Całka, J. Influence of Polyethylene Terephthalate (PET) Microplastic on Selected Active Substances in the Intramural Neurons of the Porcine Duodenum. Part. Fibre Toxicol. 2024, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Paing, Y.M.M.; Eom, Y.; Song, G.B.; Kim, B.; Choi, M.G.; Hong, S.; Lee, S.H. Neurotoxic Effects of Polystyrene Nanoplastics on Memory and Microglial Activation: Insights from in Vivo and in Vitro Studies. Sci. Total Environ. 2024, 924, 171681. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Du, Z.; Geng, X.; Li, M.; Yang, X.; Bo, C.; Jia, Q.; Yu, G.; Shi, L. Inhibiting Ferroptosis in Brain Microvascular Endothelial Cells: A Potential Strategy to Mitigate Polystyrene Nanoplastics–induced Blood–brain Barrier Dysfunction. Environ. Res. 2024, 250, 118506. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A Delicate Balance: Iron Metabolism and Diseases of the Brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Shang, L.; Yin, J.; Qian, Z.; Chen, C.; Yang, Y. Size-Dependent Neurotoxicity of Micro- and Nanoplastics in Flowing Condition Based on an in Vitro Microfluidic Study. Chemosphere 2022, 303, 135280. [Google Scholar] [CrossRef]
- González-Acedo, A.; García-Recio, E.; Illescas-Montes, R.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J. Evidence from in Vitro and in Vivo Studies on the Potential Health Repercussions of Micro- and Nanoplastics. Chemosphere 2021, 280, 130826. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of Acute Effects of Polystyrene and Polyvinyl Chloride Micro- and Nanoplastics in an Advanced in Vitro Triple Culture Model of the Healthy and Inflamed Intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Rubio, L.; Marcos, R.; Cortés, C. Interactions of Polystyrene Nanoplastics with in Vitro Models of the Human Intestinal Barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef] [PubMed]
- Grodzicki, W.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Kruszewski, M. Nanoplastic Impact on the Gut-Brain Axis: Current Knowledge and Future Directions. Int. J. Mol. Sci. 2021, 22, 12795. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Siwicka-Gieroba, D.; Kotfis, K.; Zaid, S.; Terpilowska, S.; Robba, C.; Siwicki, A.K. The Brain-Gut Axis-Where Are We Now and How Can We Modulate These Connections? Curr. Neuropharmacol. 2021, 19, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Cory-Slechta, D.A.; Sobolewski, M.; Marvin, E.; Conrad, K.; Merrill, A.; Anderson, T.; Jackson, B.P.; Oberdorster, G. The Impact of Inhaled Ambient Ultrafine Particulate Matter on Developing Brain: Potential Importance of Elemental Contaminants. Toxicol. Pathol. 2019, 47, 976–992. [Google Scholar] [CrossRef] [PubMed]
- Sunderman, F.W. Nasal Toxicity, Carcinogenicity, and Olfactory Uptake of Metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar]
- Zhang, Y.; Tian, L.; Chen, J.; Liu, X.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Lin, B.; Xi, Z. Selective Bioaccumulation of Polystyrene Nanoplastics in Fetal Rat Brain and Damage to Myelin Development. Ecotoxicol. Environ. Saf. 2024, 278, 116393. [Google Scholar] [CrossRef]
- Buizza, L.; Cenini, G.; Lanni, C.; Ferrari-Toninelli, G.; Prandelli, C.; Govoni, S.; Buoso, E.; Racchi, M.; Barcikowska, M.; Styczynska, M.; et al. Conformational Altered P53 as an Early Marker of Oxidative Stress in Alzheimer’s Disease. PLoS ONE 2012, 7, e29789. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Yamada, S. A Novel Hypothesis on Metal Dyshomeostasis and Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis: Potential Pathogenetic Mechanism and Therapeutic Implications. Eur. J. Pharmacol. 2021, 892, 173737. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial Defects and Oxidative Stress in Alzheimer Disease and Parkinson Disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef]
- Hu, S.; Xu, M.; Cui, Z.; Xiao, Y.; Liu, C.; Liu, R.; Li, X. Study on the Binding of Polystyrene Microplastics with Superoxide Dismutase at the Molecular Level by Multi-Spectroscopy Methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122511. [Google Scholar] [CrossRef] [PubMed]
- Peter, Y.; Rotman, G.; Lotem, J.; Elson, A.; Shiloh, Y.; Groner, Y. Elevated Cu/Zn-SOD Exacerbates Radiation Sensitivity and Hematopoietic Abnormalities of Atm-Deficient Mice. EMBO J. 2001, 20, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, M.; Cui, Z.; Xiao, Y.; Liu, C.; Liu, R.; Zhang, G. Probing the Molecular Mechanism of Interaction between Polystyrene Nanoplastics and Catalase by Multispectroscopic Techniques. Chem.-Biol. Interact. 2023, 382, 110648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, H.; Li, T.; Yu, L.; Qi, Y.; Tian, G.; He, F.; Li, X.; Sun, N.; Liu, R. Size-Dependent Effects of Nanoplastics on Structure and Function of Superoxide Dismutase. Chemosphere 2022, 309, 136768. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.P.; Hearn, A.S.; Cabelli, D.E.; Nick, H.S.; Tainer, J.A.; Silverman, D.N. Contribution of Human Manganese Superoxide Dismutase Tyrosine 34 to Structure and Catalysis. Biochemistry 2009, 48, 3417–3424. [Google Scholar] [CrossRef]
- Bovio, F.; Melchioretto, P.; Forcella, M.; Fusi, P.; Urani, C. Cadmium Promotes Glycolysis Upregulation and Glutamine Dependency in Human Neuronal Cells. Neurochem. Int. 2021, 149, 105144. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein Aggregation and Neurodegenerative Disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Gabbrielli, S.; Colnaghi, L.; Mazzuoli-Weber, G.; Redaelli, A.C.L.; Gautieri, A. In Silico Analysis of Nanoplastics’ and β-Amyloid Fibrils’ Interactions. Molecules 2023, 28, 388. [Google Scholar] [CrossRef] [PubMed]
- Hollóczki, O.; Gehrke, S. Nanoplastics Can Change the Secondary Structure of Proteins. Sci. Rep. 2019, 9, 16013. [Google Scholar] [CrossRef]
- Gou, X.; Fu, Y.; Li, J.; Xiang, J.; Yang, M.; Zhang, Y. Impact of Nanoplastics on Alzheimer ’s Disease: Enhanced Amyloid-β Peptide Aggregation and Augmented Neurotoxicity. J. Hazard. Mater. 2024, 465, 133518. [Google Scholar] [CrossRef]
- Hoelting, L.; Scheinhardt, B.; Bondarenko, O.; Schildknecht, S.; Kapitza, M.; Tanavde, V.; Tan, B.; Lee, Q.Y.; Mecking, S.; Leist, M.; et al. A 3-Dimensional Human Embryonic Stem Cell (hESC)-Derived Model to Detect Developmental Neurotoxicity of Nanoparticles. Arch. Toxicol. 2013, 87, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Yun, S.-M.; Jo, C.; Jeong, J.; Park, M.H.; Han, C.; Koh, Y.H. Altered Expression of Notch1 in Alzheimer’s Disease. PLoS ONE 2019, 14, e0224941. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Huang, Y.; Zhong, Y.; Li, Z.; Ye, R.; Wang, B.; Zhang, B.; Meng, H.; Lin, X.; Du, J.; et al. Brain Single-Nucleus Transcriptomics Highlights That Polystyrene Nanoplastics Potentially Induce Parkinson’s Disease-like Neurodegeneration by Causing Energy Metabolism Disorders in Mice. J. Hazard. Mater. 2022, 430, 128459. [Google Scholar] [CrossRef] [PubMed]
- Martin-Folgar, R.; González-Caballero, M.C.; Torres-Ruiz, M.; Cañas-Portilla, A.I.; De Alba González, M.; Liste, I.; Morales, M. Molecular Effects of Polystyrene Nanoplastics on Human Neural Stem Cells. PLoS ONE 2024, 19, e0295816. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.; Park, S.J.; Lee, E.J.; Kim, K.W. Nanoplastics Exacerbate Parkinson’s Disease Symptoms in C. Elegans and Human Cells. J. Hazard. Mater. 2024, 465, 133289. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.; Morales Pantoja, I.E.; Smirnova, L. Brain Organoids and Organoid Intelligence from Ethical, Legal, and Social Points of View. Front. Artif. Intell. 2024, 6, 1307613. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Gao, Y.; Han, B.; Wang, T.; Dong, H.; Chen, L. Toxic Effects and Mechanisms of Nanoplastics on Embryonic Brain Development Using Brain Organoids Model. Sci. Total Environ. 2023, 904, 166913. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Montecinos-Oliva, C.; Fuenzalida, M. Wnt Signaling: Role in Alzheimer Disease and Schizophrenia. J. Neuroimmune Pharmacol. 2012, 7, 788–807. [Google Scholar] [CrossRef] [PubMed]
- Martín, D.; Salinas, M.; López-Valdaliso, R.; Serrano, E.; Recuero, M.; Cuadrado, A. Effect of the Alzheimer Amyloid Fragment Aβ(25–35) on Akt/PKB Kinase and Survival of PC12 Cells. J. Neurochem. 2001, 78, 1000–1008. [Google Scholar] [CrossRef]
- Peters, S.; Zitzelsperger, E.; Kuespert, S.; Iberl, S.; Heydn, R.; Johannesen, S.; Petri, S.; Aigner, L.; Thal, D.R.; Hermann, A.; et al. The TGF-β System As a Potential Pathogenic Player in Disease Modulation of Amyotrophic Lateral Sclerosis. Front. Neurol. 2017, 8, 669. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.-M.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.-L.; Zhang, Y.; Baker, T.R. Nanoplastics Impact the Zebrafish (Danio rerio) Transcriptome: Associated Developmental and Neurobehavioral Consequences. Environ. Pollut. 2020, 266, 115090. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, M.; De Alba González, M.; Morales, M.; Martin-Folgar, R.; González, M.C.; Cañas-Portilla, A.I.; De La Vieja, A. Neurotoxicity and Endocrine Disruption Caused by Polystyrene Nanoparticles in Zebrafish Embryo. Sci. Total Environ. 2023, 874, 162406. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Aromolaran, K.A.; Zukin, R.S. The Emerging Field of Epigenetics in Neurodegeneration and Neuroprotection. Nat. Rev. Neurosci. 2017, 18, 347–361. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, S.; Han, M.H.; Lee, S.B. Epigenetic Changes in Neurodegenerative Diseases. Mol. Cells 2016, 39, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, M.; Ortuño Guzmán, F.M.; Han, D.; Stojkovic, P.; Dopazo, J.; Stankovic, K.M. Polystyrene Nanoplastics Affect Transcriptomic and Epigenomic Signatures of Human Fibroblasts and Derived Induced Pluripotent Stem Cells: Implications for Human Health. Environ. Pollut. 2023, 320, 120849. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.J.; Suomi, F.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Emerging Roles of ATG7 in Human Health and Disease. EMBO Mol. Med. 2021, 13, e14824. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Ballesteros, S.; Rubio, L.; Marcos, R.; Hernández, A. Long-Term Exposure to Nanoplastics Alters Molecular and Functional Traits Related to the Carcinogenic Process. J. Hazard. Mater. 2022, 438, 129470. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA Dysregulation in Neurodegenerative Diseases: A Systematic Review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Wang, J.; Xu, G.; Yu, Y. Quantification of Nanoplastics Uptake and Transport in Lettuce by Pyrolysis Gas Chromatography-Mass Spectrometry. Talanta 2023, 265, 124837. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, Y.; Yin, J.; Zhu, Q.; Liao, C.; Jiang, G. Neurotoxicities Induced by Micro/Nanoplastics: A Review Focusing on the Risks of Neurological Diseases. J. Hazard. Mater. 2024, 469, 134054. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P. Animal Models of CNS Disorders. Biochem. Pharmacol. 2014, 87, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A. Concise Review: Patient-Derived Olfactory Stem Cells: New Models for Brain Diseases. Stem Cells 2012, 30, 2361–2365. [Google Scholar] [CrossRef]
- Paparella, M.; Bennekou, S.H.; Bal-Price, A. An Analysis of the Limitations and Uncertainties of in Vivo Developmental Neurotoxicity Testing and Assessment to Identify the Potential for Alternative Approaches. Reprod. Toxicol. 2020, 96, 327–336. [Google Scholar] [CrossRef]
| Plastic Particle Size | Plastic Type | Model Used | Target and Effect | Reference |
|---|---|---|---|---|
| 5 nm | PS | Computational model | Gastrointestinal barrier and BBB crossing | [8] |
| 30–50 nm | PS | Mouse | Brain accumulation (hippocampus) | [113] |
| 30–50 nm | PS | Rat primary culture of microglia | Internalization, microglia activation, and neuroinflammation | [113] |
| 25 and 50 nm | PS | Pregnant rat | Transgenerational accumulation in the brains of fetuses | [125] |
| 50 nm | PS | RBL-2H3 cells (rat basophilic leukemia cells) | Cell membrane crossing and delivery to lysosomes | [103] |
| 50 nm | PS | Mouse | Brain accumulation | [115] |
| 50 nm | PS | hCMEC/D3 human cerebral microvascular endothelial cells (BBB model) | Internalization, tight junction disturbance, decreased occludin expression, necroptosis | [115] |
| 50 nm | PS | Mouse | Increased permeability and BBB disruption | [114] |
| 50 nm | PS | Mouse b.End.3 endothelial cells (BBB model) | BBB disruption by ferroptosis contribution | [114] |
| 100 nm | PS | Mouse hippocampal neuronal HT22 cells | Internalization and decreased viability | [117] |
| 293 nm * | PS | Mouse | Presence of particles in brain tissues | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urani, C.; Barbieri, R.; Alloisio, S.; Tesauro, M. From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes. Appl. Sci. 2024, 14, 7280. https://doi.org/10.3390/app14167280
Urani C, Barbieri R, Alloisio S, Tesauro M. From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes. Applied Sciences. 2024; 14(16):7280. https://doi.org/10.3390/app14167280
Chicago/Turabian StyleUrani, Chiara, Raffaella Barbieri, Susanna Alloisio, and Marina Tesauro. 2024. "From the Environment to Molecular Interactions of Nanoplastics: Unraveling the Neurotoxic Impacts and the Implications in Neurodegenerative Processes" Applied Sciences 14, no. 16: 7280. https://doi.org/10.3390/app14167280






