Bench-Scale Gasification of Olive Cake in a Bubbling Fluidized Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Cake
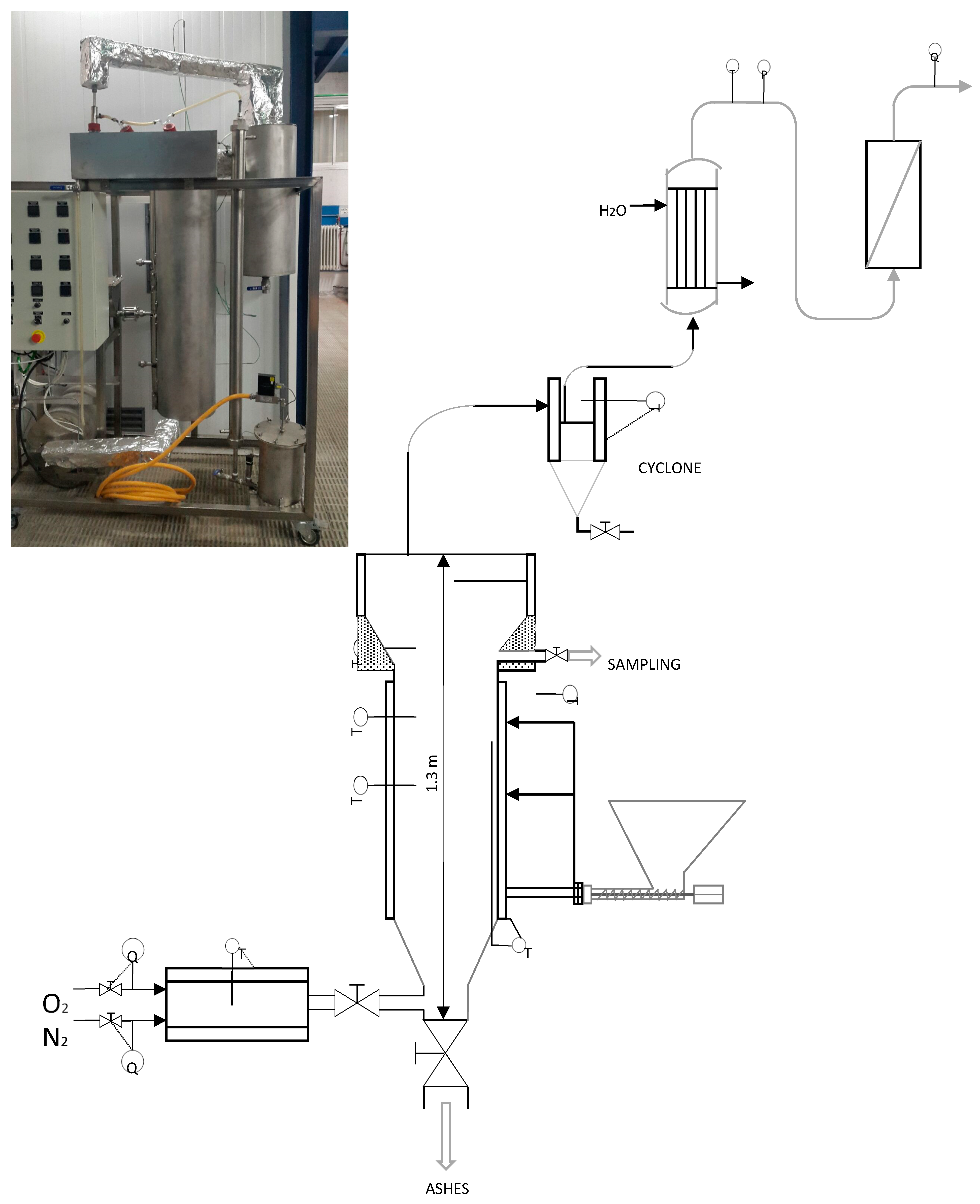
2.2. Brief Description of the Bench-Scale Plant
2.3. Description of the Operational Procedure
3. Results
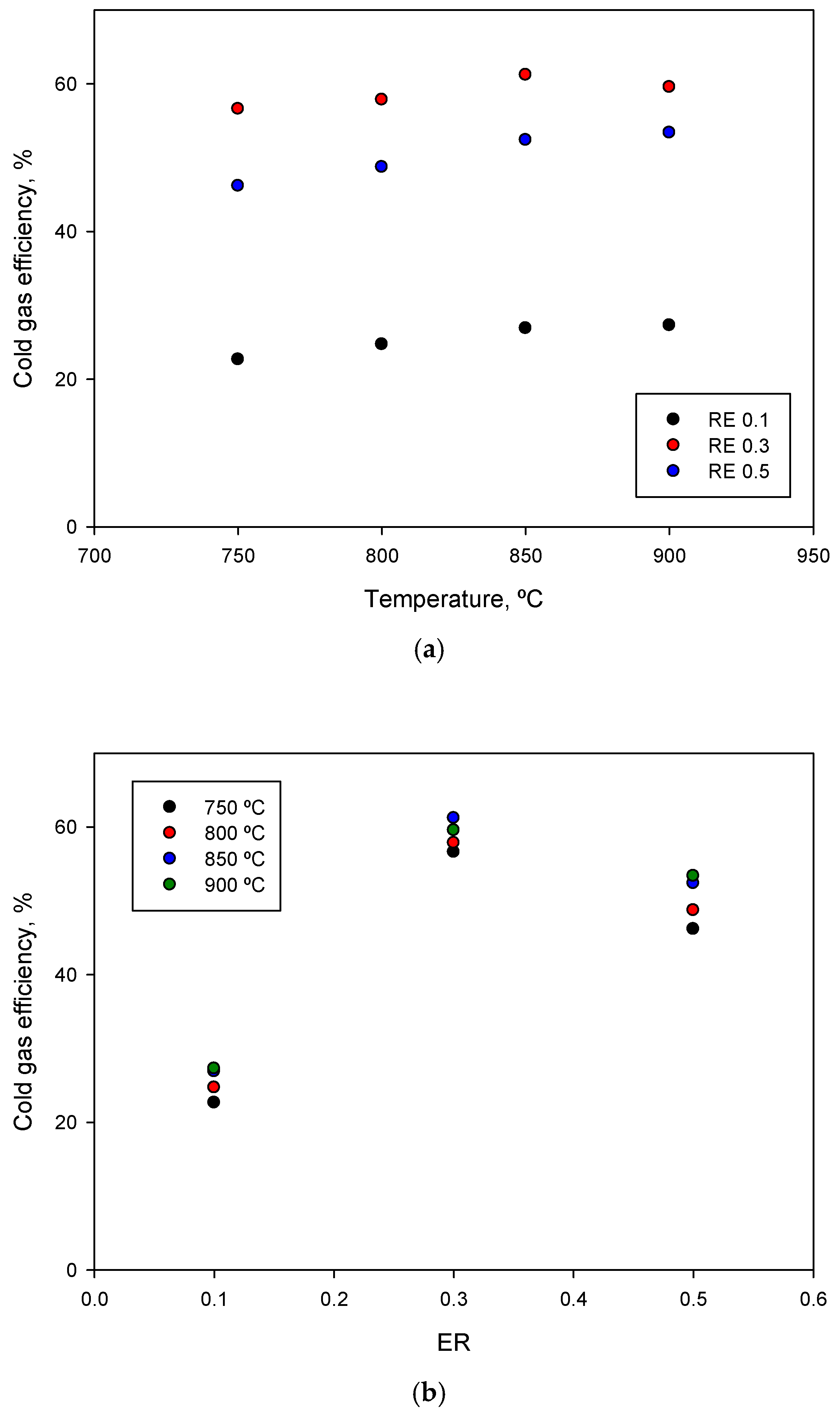
3.1. Influence of Temperature and ER on Cold Gas Efficiency
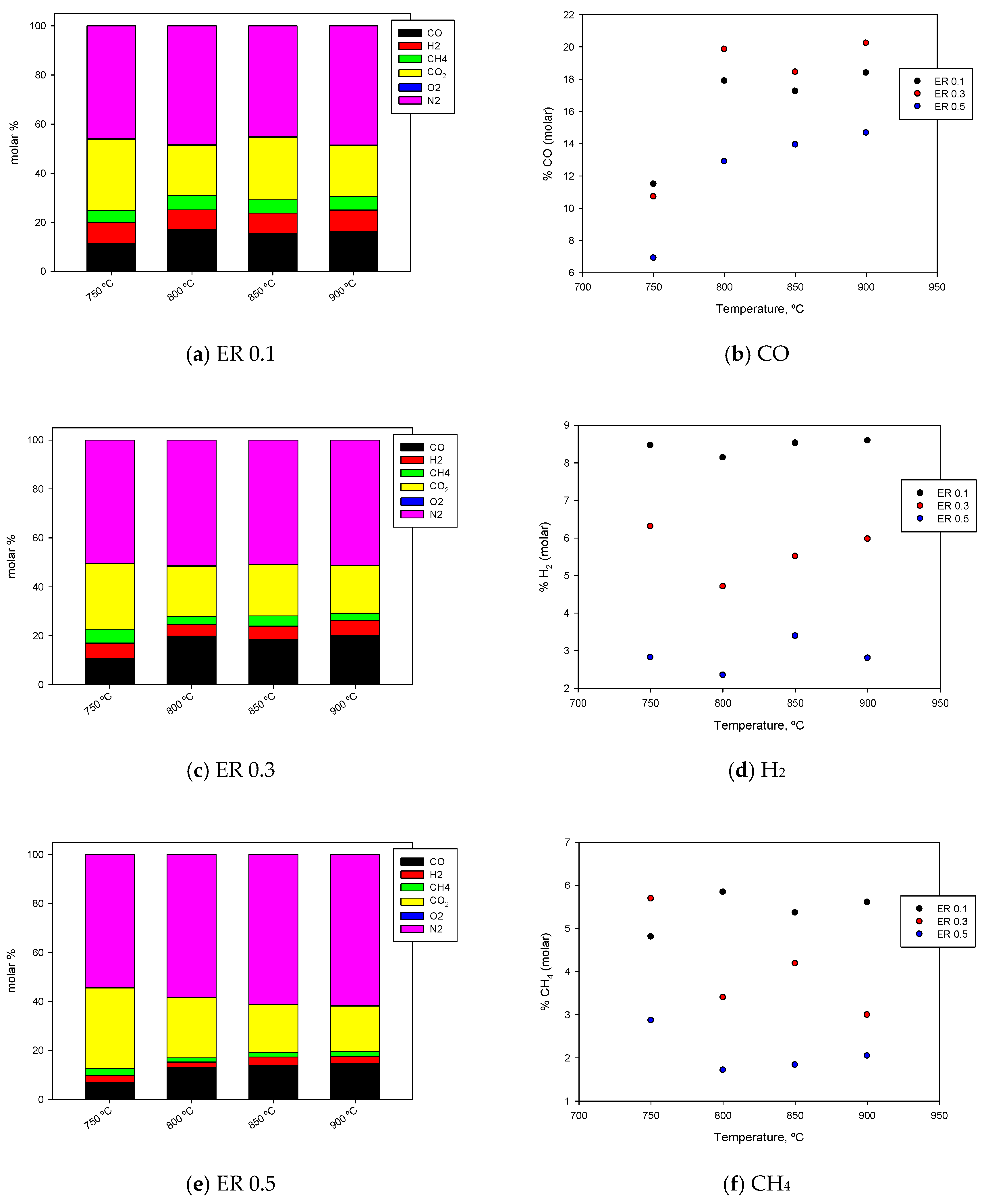
3.2. Influence of Temperature and ER on Gas Composition
3.3. Influence of Temperature and ER on the Lower Heating Value of the Gas and the Gas Flow
3.4. Influence of Temperature and ER on the Production of CO, H2, and CH4
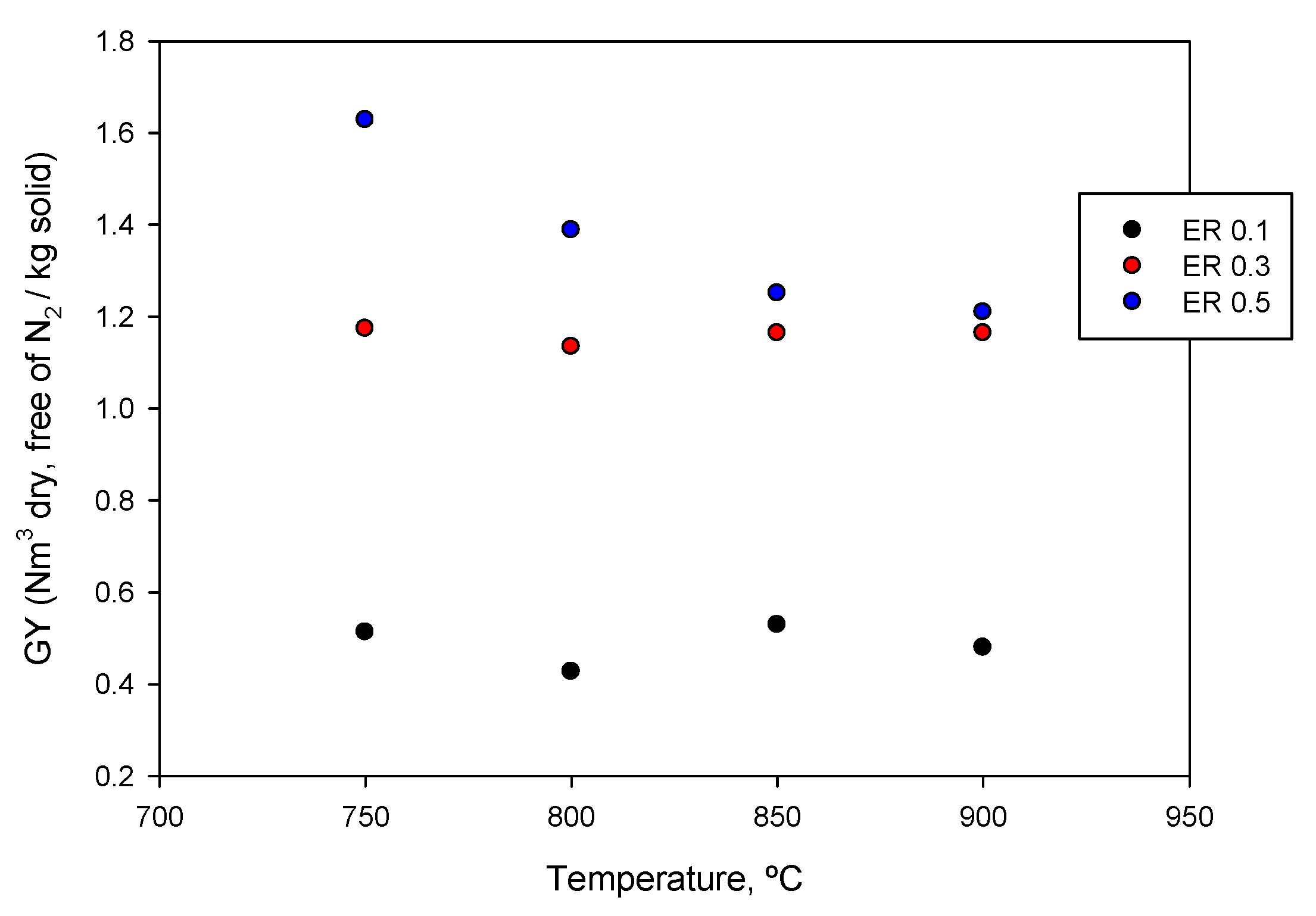
3.5. Influence of Temperature and ER on Gas Production
3.6. Solid and Liquid Residues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Olive Council, The world of Olive Oil. 2022. Available online: https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed on 2 February 2024).
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Gálvez-Pérez, A.; Martín-Lara, M.A.; Calero, M.; Pérez, A.; Canu, P.; Blázquez, G. Experimental investigation on the air gasification of olive cake at low temperatures. Fuel Process. Technol. 2021, 213, 106703. [Google Scholar] [CrossRef]
- Carmo-Calado, L.; Hermoso-Orzáez, M.J.; Diaz-Perete, D.; La Cal-Herrera, J.; Brito, P.; Terrados-Cepeda, J. Experimental Research on the Production of Hydrogen-Rich Synthesis Gas via the Air-Gasification of Olive Pomace: A Comparison between an Updraft Bubbling Bed and a Downdraft Fixed Bed. Hydrogen 2023, 4, 726–745. [Google Scholar] [CrossRef]
- IEA Bioenergy, Gasification for Multiple Purposes-Bioenergy Review 2023. 2023. Available online: https://www.ieabioenergyreview.org/gasification-for-multiple-purposes/ (accessed on 2 February 2024).
- Dogru, M.; Erdem, A. Autothermal Fixed Bed Updraft Gasification of Olive Pomace Biomass and Renewable Energy Generation via Organic Rankine Cycle Turbine: Green energy generation from waste biomass in the Mediterranean region. Johns. Matthey Technol. Rev. 2020, 64, 119–134. [Google Scholar] [CrossRef]
- Tezer, O.; Karabag, N.; Ozturk, M.U.; Ongen, A.; Ayol, A. Comparison of green waste gasification performance in updraft and downdraft fixed bed gasifiers. Int. J. Hydrogen Energy 2022, 47, 31864–31876. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Ayol, A. Gasification performance of olive pomace in updraft and downdraft fixed bed reactors. Int. J. Hydrogen Energy 2023, 48, 22909–22920. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef]
- Vera, D.; Jurado, F.; Margaritis, N.K.; Grammelis, P. Experimental and economic study of a gasification plant fuelled with olive industry wastes. Energy Sustain. Dev. 2014, 23, 247–257. [Google Scholar] [CrossRef]
- Borello, D.; De Caprariis, B.; De Filippis, P.; Di Carlo, A.; Marchegiani, A.; Pantaleo, A.M.; Shah, N.; Venturini, P. Thermo-Economic Assessment of a Olive Pomace Gasifier for Cogeneration Applications. Energy Procedia 2015, 75, 252–258. [Google Scholar] [CrossRef]
- Borello, D.; Pantaleo, A.; Caucci, M.; De Caprariis, B.; De Filippis, P.; Shah, N. Modeling and Experimental Study of a Small Scale Olive Pomace Gasifier for Cogeneration: Energy and Profitability Analysis. Energies 2017, 10, 1930. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Lara-Díaz, J.; Valverde, J.L.; Sanchez-Silva, L.; Sánchez, P. Dolomite effect on steam co-gasification of olive pomace, coal and petcoke: TGA-MS analysis, reactivity and synergistic effect. Fuel 2018, 234, 142–150. [Google Scholar] [CrossRef]
- Sert, M.; Gökkaya, D.S.; Cengiz, N.; Ballice, L.; Yüksel, M.; Sağlam, M. Hydrogen production from olive-pomace by catalytic hydrothermal gasification. J. Taiwan Inst. Chem. Eng. 2018, 83, 90–98. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D.; Carvalho, T.; Brito, P. Modelling and experimental analysis of a small-scale olive pomace gasifier for cogeneration applications: A techno-economic assessment. Chem. Ind. Chem. Eng. Q. 2019, 25, 329–339. [Google Scholar] [CrossRef]
- Quesada, L.; Pérez, A.; Calero, M.; Blázquez, G.; Martín-Lara, M.A. Kinetic study of thermal degradation of olive cake based on a scheme of fractionation and its behavior impregnated of metals. Bioresour. Technol. 2018, 261, 104–116. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Brachi, P.; Ruoppolo, G.; Di Fraia, S.; Vanoli, L. Experimental and Numerical Investigation of Biosolid Gasification: Equilibrium-Based Modeling with Emphasis on the Effects of Different Pretreatment Methods. Ind. Eng. Chem. Res. 2019, 59, 299–307. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Serrano, C. Co-Gasification of Biomass Wastes and Coal−Coke Blends in an Entrained Flow Gasifier: An Experimental Study. Energy Fuels 2010, 24, 2479–2488. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Oxygen Gasification of Municipal Solid Waste in a Fixed-bed Gasifier. Chin. J. Chem. Eng. 2014, 22, 1021–1026. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Xu, C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.; McKay, G. Techno-economic and sensitivity analysis of coconut coir pith-biomass gasification using ASPEN PLUS. Appl. Energy 2020, 261, 114350. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Osmieri, L.; Specchia, S.; Yusup, S.; Tavasoli, A.; Zamaniyan, A. H2-rich syngas production through mixed residual biomass and HDPE waste via integrated catalytic gasification and tar cracking plus bio-char upgrading. Chem. Eng. J. 2017, 308, 578–587. [Google Scholar] [CrossRef]
- Taba, L.E.; Irfan, M.F.; Daud, W.A.M.W.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Pohořelý, M.; Vosecký, M.; Hejdová, P.; Punčochář, M.; Skoblja, S.; Staf, M.; Vošta, J.; Koutský, B.; Svoboda, K. Gasification of coal and PET in fluidized bed reactor. Fuel 2006, 85, 2458–2468. [Google Scholar] [CrossRef]
- Sidek, F.; Samad, N.A.; Saleh, S. Review on effects of gasifying agents, temperature and equivalence ratio in biomass gasification process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 863, 012028. [Google Scholar] [CrossRef]
- Karatas, H.; Olgun, H.; Engin, B.; Akgun, F. Experimental results of gasification of waste tire with air in a bubbling fluidized bed gasifier. Fuel 2013, 105, 566–571. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yu, T.U.; Lee, J.W.; Moon, J.H.; Jeong, H.J.; Park, S.S.; Yang, W.; Lee, U.D. Gasification of Mixed Plastic Wastes in a Moving-Grate Gasifier and Application of the Producer Gas to a Power Generation Engine. Energy Fuels 2013, 27, 2092–2098. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Boerrigter, H.; Rauch, R. Review of Applications of Gases from Biomass Gasification; Report ECNRX-06-066; Energy and Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2006. [Google Scholar]
- Habibollahzade, A.; Ahmadi, P.; Rosen, M.A. Biomass gasification using various gasification agents: Optimum feedstock selection, detailed numerical analyses and tri-objective grey wolf optimization. J. Clean. Prod. 2021, 284, 124718. [Google Scholar] [CrossRef]
- Njuguna, F.I.; Ndiritu, H.M.; Gathitu, B.B.; Hawi, M.; Munyalo, J.M. Experimental investigation and optimization of the gasification parameters of macadamia nutshells in a batch-fed bubbling fluidized bed gasifier with air preheating. Energy Storage Sav. 2023, 2, 559–570. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Villanueva, A.L.; Ollero, P. Air−Steam Gasification of Biomass in a Fluidized Bed under Simulated Autothermal and Adiabatic Conditions. Ind. Eng. Chem. Res. 2008, 47, 5957–5965. [Google Scholar] [CrossRef]
- Jand, N.; Brandani, V.; Foscolo, P.U. Thermodynamic Limits and Actual Product Yields and Compositions in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2005, 45, 834–843. [Google Scholar] [CrossRef]
- Lv, P.; Chang, J.; Xiong, Z.; Huang, H.; Wu, C.; Chen, Y.; Zhu, J. Biomass Air−Steam Gasification in a Fluidized Bed to Produce Hydrogen-Rich Gas. Energy Fuels 2003, 17, 677–682. [Google Scholar] [CrossRef]
- Sadaka, S.S.; Ghaly, A.E.; Sabbah, M.A. Two-phase biomass air-steam gasification model for fluidized bed reactors: Part III—Model validation. Biomass Bioenergy 2002, 22, 479–487. [Google Scholar] [CrossRef]
| Elemental analysis (dry basis) | C (wt., %) | 44.0 |
| H (wt., %) | 7.0 | |
| N (wt., %) | 1.1 | |
| S (wt., %) | ND | |
| Proximate analysis | Moisture (wt., %) | 6.6 |
| Volatile (wt., %) | 60.8 | |
| Fixed carbon (wt., %) | 22.2 | |
| Ashes (wt., %) | 10.4 |
| Temperature, °C | ER | LHVgas, MJ/Nm3 | Gas Flow (0 °C, 1 atm), L/min | Gas Flow (0 °C, 1 atm), L/kg Feed | CGE, % |
|---|---|---|---|---|---|
| 750 | 0.1 | 4.09 | 3.95 | 948.0 | 22.67 |
| 0.3 | 4.08 | 9.88 | 2371.2 | 56.59 | |
| 0.5 | 2.21 | 14.89 | 3573.6 | 46.16 | |
| 800 | 0.1 | 5.11 | 3.44 | 825.6 | 24.70 |
| 0.3 | 4.24 | 9.72 | 2332.8 | 57.83 | |
| 0.5 | 2.50 | 13.89 | 3333.6 | 48.73 | |
| 850 | 0.1 | 4.77 | 4.01 | 962.4 | 26.90 |
| 0.3 | 4.43 | 9.84 | 2361.6 | 61.19 | |
| 0.5 | 2.79 | 13.39 | 3213.6 | 52.39 | |
| 900 | 0.1 | 5.01 | 3.88 | 931.2 | 27.29 |
| 0.3 | 4.28 | 9.92 | 2380.8 | 59.55 | |
| 0.5 | 2.89 | 13.15 | 3156.0 | 53.38 |
| T, °C | ER | Yield, g/kg | ||
|---|---|---|---|---|
| YH2 | YCO | YCH4 | ||
| 750 | 0.1 | 7.17 | 136.17 | 32.55 |
| 0.3 | 13.37 | 318.00 | 96.48 | |
| 0.5 | 9.02 | 309.08 | 73.25 | |
| 800 | 0.1 | 6.01 | 174.63 | 34.50 |
| 0.3 | 9.81 | 579.48 | 56.69 | |
| 0.5 | 6.99 | 537.63 | 40.89 | |
| 850 | 0.1 | 7.34 | 183.85 | 36.93 |
| 0.3 | 11.63 | 544.98 | 70.66 | |
| 0.5 | 9.74 | 560.32 | 42.18 | |
| 900 | 0.1 | 7.15 | 190.84 | 37.30 |
| 0.3 | 12.70 | 602.48 | 50.95 | |
| 0.5 | 7.90 | 579.23 | 46.18 | |
| T, °C | ER | Input | Output | |||||
|---|---|---|---|---|---|---|---|---|
| Gas, g/kg | Solid, g/kg | Total, g/kg | Gas, g/kg | Liquid, g/kg | Solid, g/kg | Total, g/kg | ||
| 750 | 0.1 | 712 | 1000 | 1712 | 1227 | 211 | 144 | 1582 |
| 750 | 0.3 | 1965 | 1000 | 2965 | 3023 | 163 | 133 | 3319 |
| 750 | 0.5 | 3190 | 1000 | 4190 | 4559 | 127 | 125 | 4811 |
| 800 | 0.1 | 655 | 1000 | 1655 | 1120 | 187 | 132 | 1439 |
| 800 | 0.3 | 1965 | 1000 | 2965 | 2857 | 149 | 114 | 3120 |
| 800 | 0.5 | 3190 | 1000 | 4190 | 4318 | 111 | 117 | 4546 |
| 850 | 0.1 | 712 | 1000 | 1712 | 1343 | 191 | 126 | 1671 |
| 850 | 0.3 | 1965 | 1000 | 2965 | 2880 | 135 | 121 | 3136 |
| 850 | 0.5 | 3219 | 1000 | 4219 | 4163 | 100 | 112 | 4375 |
| 900 | 0.1 | 741 | 1000 | 1741 | 1284 | 163 | 133 | 1580 |
| 900 | 0.3 | 1994 | 1000 | 2994 | 2867 | 128 | 109 | 3104 |
| 900 | 0.5 | 3190 | 1000 | 4190 | 4087 | 79 | 106 | 4272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blázquez, G.; Calero, M.; Gálvez-Pérez, Á.; Martín-Lara, M.Á.; Pérez, A. Bench-Scale Gasification of Olive Cake in a Bubbling Fluidized Bed Reactor. Appl. Sci. 2024, 14, 7282. https://doi.org/10.3390/app14167282
Blázquez G, Calero M, Gálvez-Pérez Á, Martín-Lara MÁ, Pérez A. Bench-Scale Gasification of Olive Cake in a Bubbling Fluidized Bed Reactor. Applied Sciences. 2024; 14(16):7282. https://doi.org/10.3390/app14167282
Chicago/Turabian StyleBlázquez, Gabriel, Mónica Calero, Ángela Gálvez-Pérez, María Ángeles Martín-Lara, and Antonio Pérez. 2024. "Bench-Scale Gasification of Olive Cake in a Bubbling Fluidized Bed Reactor" Applied Sciences 14, no. 16: 7282. https://doi.org/10.3390/app14167282
APA StyleBlázquez, G., Calero, M., Gálvez-Pérez, Á., Martín-Lara, M. Á., & Pérez, A. (2024). Bench-Scale Gasification of Olive Cake in a Bubbling Fluidized Bed Reactor. Applied Sciences, 14(16), 7282. https://doi.org/10.3390/app14167282










