Easy Fabrication of Ultrafiltration Membrane via Polyethersulfone-Fumed Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Pore Characterization
3. Results and Discussion
3.1. Membrane Morphology
3.2. Pure Water Flux Test Experiments
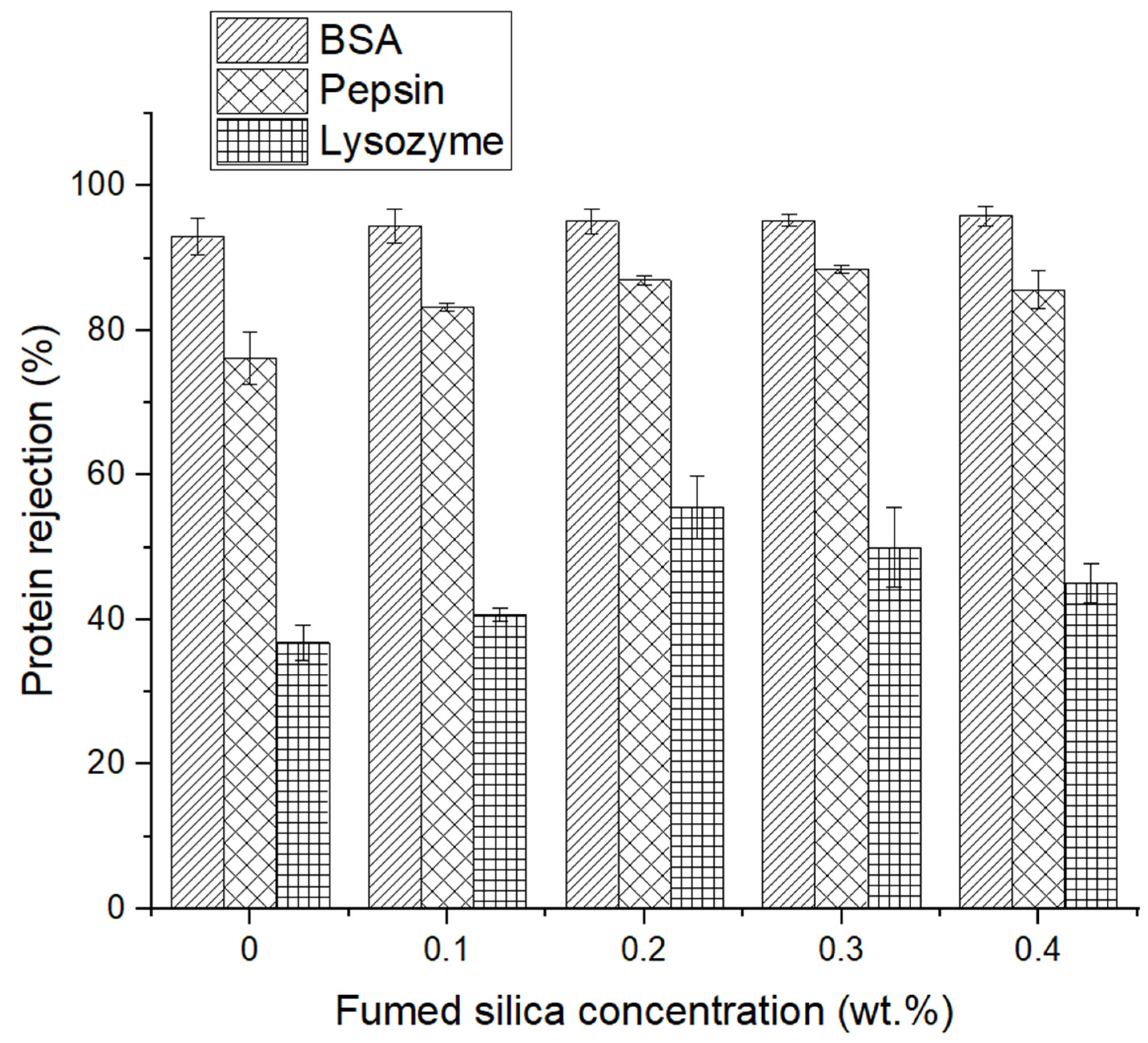
3.3. Protein Separation Performance Analysis
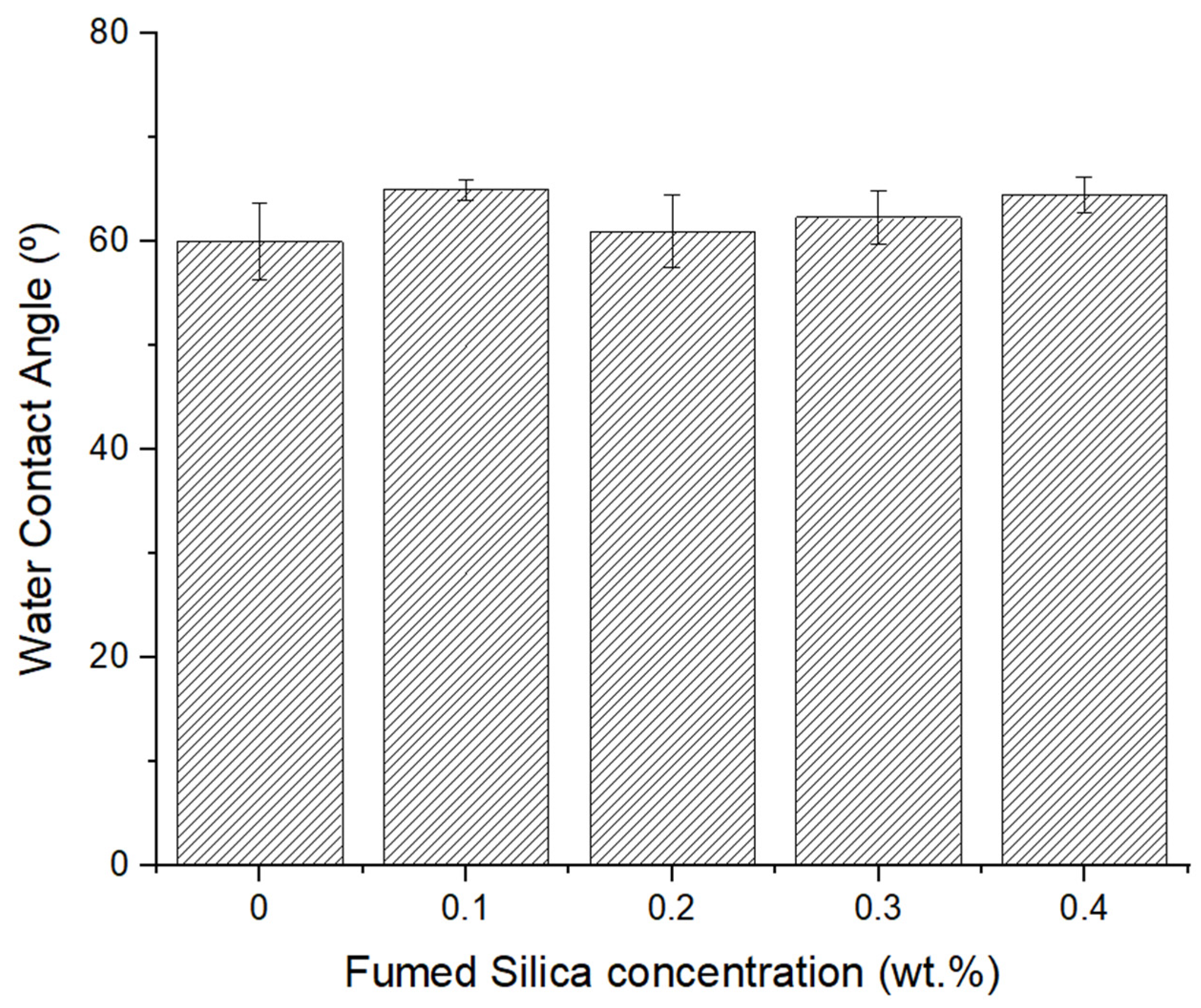
3.4. Water Contact Angle Analysis
3.5. Pore Statistic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Educational, Scientific, and Cultural Organization. United Nations World Water Development Report 2020: Water and Climate Change; United Nations Educational, Scientific, and Cultural Organization: Paris, France, 2020. [Google Scholar]
- Chen, Z.; Ngo, H.H.; Guo, W. A critical review on the end-uses of recycled water. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1446–1516. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Fang, G.; Li, Z.; Liu, Y. The growing water crisis in Central Asia and the driving force behind it. J. Clean. Prod. 2022, 378, 134574. [Google Scholar] [CrossRef]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; Boogaert, I.V.D.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simos, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Mou, Y.; Chen, L.; Li, X.; Wang, J.; Shu, Y.; Zhao, Y.; Huang, N. Improving hemocompatibility and antifouling performance of polyethersulfone membrane by in situ incorporation of phosphorylcholine polymers. Appl. Surf. Sci. 2024, 656, 159646. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Pang, W.Y.; Shafie, Z.M.H.M.; Zaulkiflee, N.D. PES/PVP/TiO2 matrix hollow fiber membrane with antifouling properties for humic acid removal. J. Water Proc. Eng. 2019, 31, 100827. [Google Scholar] [CrossRef]
- Nabeeh, A.; Abdalla, O.; Rehman, A.; Ghouri, Z.K.; Wahab, A.A.; Mahmoud, K.; Abdalla, A. Ultrafiltration polyethersulfone-MXene mixed matrix membranes with enhanced air dehumidification and oil-water separation performance. Sep. Purif. Technol. 2024, 346, 127285. [Google Scholar] [CrossRef]
- Kallem, P.; Bharath, G.; Rambabu, K.; Srinivasakannan, C.; Banat, F. Improved permeability and antifouling performance of polyethersulfone ultrafiltration membranes tailored by hydroxyapatite/boron nitride nanocomposites. Chemosphere 2021, 268, 129306. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, C.K. Fabrication and properties of ultrafiltration membranes composed of polysulfone and poly(1-vinylpyrrolidone) grafted silica nanoparticles. J. Memb. Sci. 2013, 444, 318–326. [Google Scholar] [CrossRef]
- Li, X.; Nayak, K.; Stamm, M.; Tripathi, B.P. Zwitterionic silica nanogel-modified polysulfone nanoporous membrane by in-situ method for water treatment. Chemosphere 2021, 280, 130615. [Google Scholar] [CrossRef] [PubMed]
- Eden, C.L.; Daramola, M.O. Evaluation of silica sodalite infused polysulfone mixed matrix membranes during H2/CO2 separation. Mater. Today Proc. 2021, 38522–38527. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathy, B.P. High permeation and antifouling polysulfone ultrafiltration membranes with in situ synthesized silica nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Suhail, F.; Batool, M.; Anjum, T.; Shah, A.T.; Tabassum, S.; Khan, A.L.; AlMohamadi, H.; Najam, M.; Gilani, M.A. Enhanced CO2 separation performance of polysulfone membranes via incorporation of pyrazole modified MCM-41 mesoporous silica as a nano-filler. Fuel 2023, 350, 128840. [Google Scholar] [CrossRef]
- Barthel, H.; Rosch, L.; Weis, J. Fumed Silica—Production, Properties, and Applications. Organosilicon Chem. 1996, 11, 761–778. [Google Scholar]
- Mavukkandy, M.O.; Bilad, M.R.; Kujawa, J.; Al-Gharabli, S.; Arafat, H.A. On the effect of fumed silica particles on the structure, properties and application of PVDF membranes. Sep. Purif. Technol. 2017, 187, 365–373. [Google Scholar] [CrossRef]
- Rutkevičius, M.; Pirzada, T.; Geiger, M.; Khan, S.A. Creating superhydrophobic, abrasion-resistant and breathable coatings from water-borne polydimethylsiloxane-polyurethane Co-polymer and fumed silica. J. Colloid Interface Sci. 2021, 596, 479–492. [Google Scholar] [CrossRef]
- Samei, M.; Iravaninia, M.; Mohammadi, T.; Asadi, A.A. Solution diffusion modeling of a composite PVA/fumed silica ceramic supported membrane. Chem. Eng. Process. 2016, 109, 11–19. [Google Scholar] [CrossRef]
- Isanejad, M.; Mohammadi, T. Effect of amine modification on morphology and performance of poly(ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 2018, 205, 303–314. [Google Scholar] [CrossRef]
- Deng, X.; Yang, F.; Ma, J.; Li, Y.; Dang, J.; Ouyang, M. Ternary phase field model and characterization of water/NMP/polysulfone membrane prepared by non-solvent induced phase separation. Sep. Purif. Technol. 2024, 330, 125307. [Google Scholar] [CrossRef]
- Saraswathi, M.S.S.A.; Rana, D.; Alwarappan, S.; Gowrishankar, S.; Kanimozhi, P.; Nagendran, A. Cellulose acetate ultrafiltration membranes customized with bio-inspired polydopamine coating and in situ immobilization of silver nanoparticles. New J. Chem. 2019, 43, 4216–4225. [Google Scholar] [CrossRef]
- Kumar, M.; Lawler, J. Preparation and characterization of negatively charged organic-inorganic hybrid ultrafiltration membranes for protein separation. Sep. Purif. Technol. 2014, 130, 112–123. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T.; Neelakandan, S.; Malarvizhi, K. Effects of Polyvinylpyrrolidone on the permeation and fouling-resistance properties of Polyetherimide ultrafiltration membranes. Ind. Eng. Chem. Res. 2015, 54, 4832–4838. [Google Scholar] [CrossRef]
- Manorma Ferreira, I.; Alves, P.; Gil, M.H.; Gando-Ferreira, L.M. Lignin separation from black liquor by mixed matrix polysulfone nanofiltration membrane filled with multiwalled carbon nanotubes. Sep. Purif. Technol. 2021, 260, 118231. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.R.; Hosseini, S.M.; Ray, M.B.; Parvizian, F.; Van der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Ramamoorthy, M.; Arthanareeswaran, G.; Raju, D.M. Cellulose acetate-poly(ether sulfone) blend ultrafiltration membranes. II. Application studies. J. Appl. Polym. Sci. 2004, 92, 3659–3665. [Google Scholar]
- Sarbolouki, M.N. A general diagram for estimating pore size of ultrafiltration and reverse osmosis membranes. Sep. Sci. Technol. 1982, 17, 381–386. [Google Scholar] [CrossRef]
- Hackett, C.; Hale, D.; Bair, B.; Manson-Endeboh, G.D.; Hao, X.; Qian, X.; Wickramasinghe, S.R.; Thompson, A. Polysulfone ultrafiltration membranes fabricated from green solvents: Significance of coagulation bath composition. Sep. Purif. Technol 2024, 332, 125752. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposute membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Memb. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Prihandana, G.S.; Maulana, S.S.; Soedirjo, R.S.; Tanujaya, V.; Pramesti, D.M.A.; Sriani, T.; Jamaludin, M.F.; Yusof, F.; Mahardika, M. Preparation and characterization of Polyethersulfone/activated carbon composite membranes for water filtration. Membranes 2023, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, L.; Mao, S.; Rohani, S.; Ching, C.; Lu, J. Zwitterionic chitosan-silica-PVA hybrid ultrafiltration membranes for protein separation. Sep. Purif. Technol. 2015, 152, 55–63. [Google Scholar] [CrossRef]
- Sen, D.; Ghosh, A.K.; Mazumder, S.; Bindal, R.C.; Tewari, P.K. Novel polysulfone-spray-dried silica composite membrane for water purification: Preparation, characterization and performance evaluation. Sep. Purif. Technol. 2014, 123, 79–86. [Google Scholar] [CrossRef]
- Sabir, A.; Islam, A.; Shafiq, M.; Shafeeq, A.; Butt, M.T.Z.; Ahmad, N.M.; Sanaullah, K.; Jamil, T. Novel polymer matrix composite membrane doped with fumed silica particles for reverse osmosis desalination. Desalination 2015, 368, 159–170. [Google Scholar] [CrossRef]
- Bowen, W.R.; Doneva, T.A. Atomic force microscopy studies of membranes: Effect of surface roughness on double-layer interactions and particle adhesion. J. Colloid Interface Sci. 2000, 229, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.H.D.; Hubadillah, S.K.; Adam, M.R.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Chapter 7—Silica-Based Hollow Fiber Membrane for Water Treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 157–180. ISBN 9780444638663. [Google Scholar]
- Liu, X.; Ng, H.Y. Fabrication of layered silica-polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Memb. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Lee, J.Y.; Qi, S.; Liu, X.; Li, Y.; Huo, F.; Tang, C.Y. Synthesis and characterization of silica gel–polyacrylonitrile mixed matrix forward osmosis membranes based on layer-by-layer assembly. Sep. Purif. Technol. 2014, 124, 207–216. [Google Scholar] [CrossRef]



| Membrane Code | Porosity, ε (%) | Mean Pore Size, rm (Å) | MWCO, α (kDa) |
|---|---|---|---|
| FS-0.0 | 6.59 | 40.87 | 38 |
| FS-0.1 | 6.95 | 30.02 | 25 |
| FS-0.2 | 7.20 | 28.74 | 25 |
| FS-0.3 | 7.24 | 28.25 | 25 |
| FS-0.4 | 7.18 | 29.18 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriani, T.; Arifvianto, B.; Baskoro, A.S.; Whulanza, Y.; Yusof, F.; Prihandana, G.S.; Mahardika, M. Easy Fabrication of Ultrafiltration Membrane via Polyethersulfone-Fumed Silica. Appl. Sci. 2024, 14, 7290. https://doi.org/10.3390/app14167290
Sriani T, Arifvianto B, Baskoro AS, Whulanza Y, Yusof F, Prihandana GS, Mahardika M. Easy Fabrication of Ultrafiltration Membrane via Polyethersulfone-Fumed Silica. Applied Sciences. 2024; 14(16):7290. https://doi.org/10.3390/app14167290
Chicago/Turabian StyleSriani, Tutik, Budi Arifvianto, Ario Sunar Baskoro, Yudan Whulanza, Farazila Yusof, Gunawan Setia Prihandana, and Muslim Mahardika. 2024. "Easy Fabrication of Ultrafiltration Membrane via Polyethersulfone-Fumed Silica" Applied Sciences 14, no. 16: 7290. https://doi.org/10.3390/app14167290
APA StyleSriani, T., Arifvianto, B., Baskoro, A. S., Whulanza, Y., Yusof, F., Prihandana, G. S., & Mahardika, M. (2024). Easy Fabrication of Ultrafiltration Membrane via Polyethersulfone-Fumed Silica. Applied Sciences, 14(16), 7290. https://doi.org/10.3390/app14167290






