In Vitro, Ex Vivo, and In Vivo Evaluation of Silver Nanoparticles Synthesized Using Green Tomato Extract: Perspectives on Topical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Tomato Extract Preparation and Characterization
2.3. Silver Nanoparticle Synthesis
2.4. Silver Nanoparticle Characterization
2.4.1. UV-Vis Spectrophotometry
2.4.2. Mean Size and Zeta Potential
2.4.3. Quantification of AgNP
2.4.4. Morphological Analysis
2.4.5. Chemical Characterization of AgNP
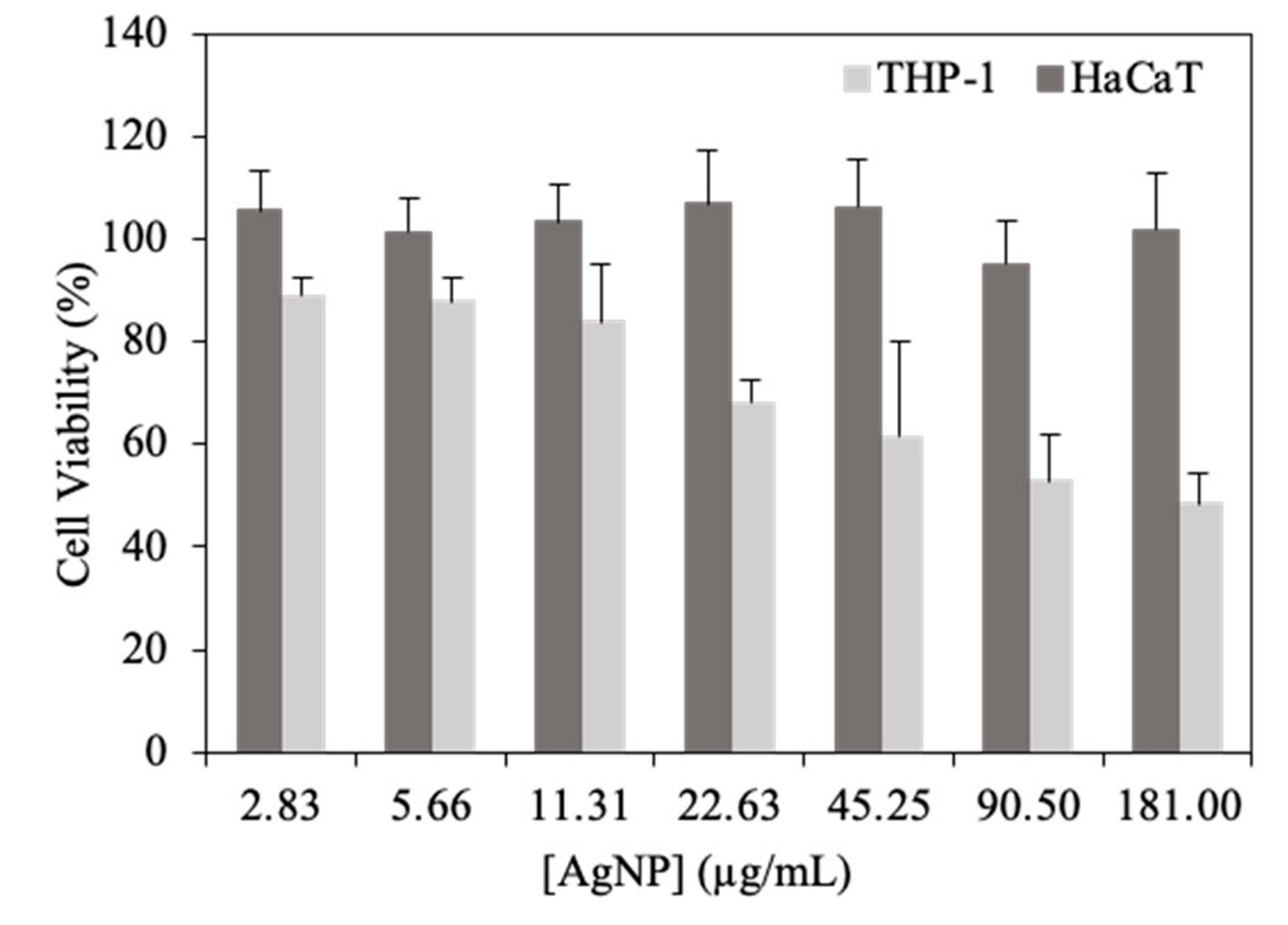
2.5. In Vitro Cell Viability in Human Cell Lines
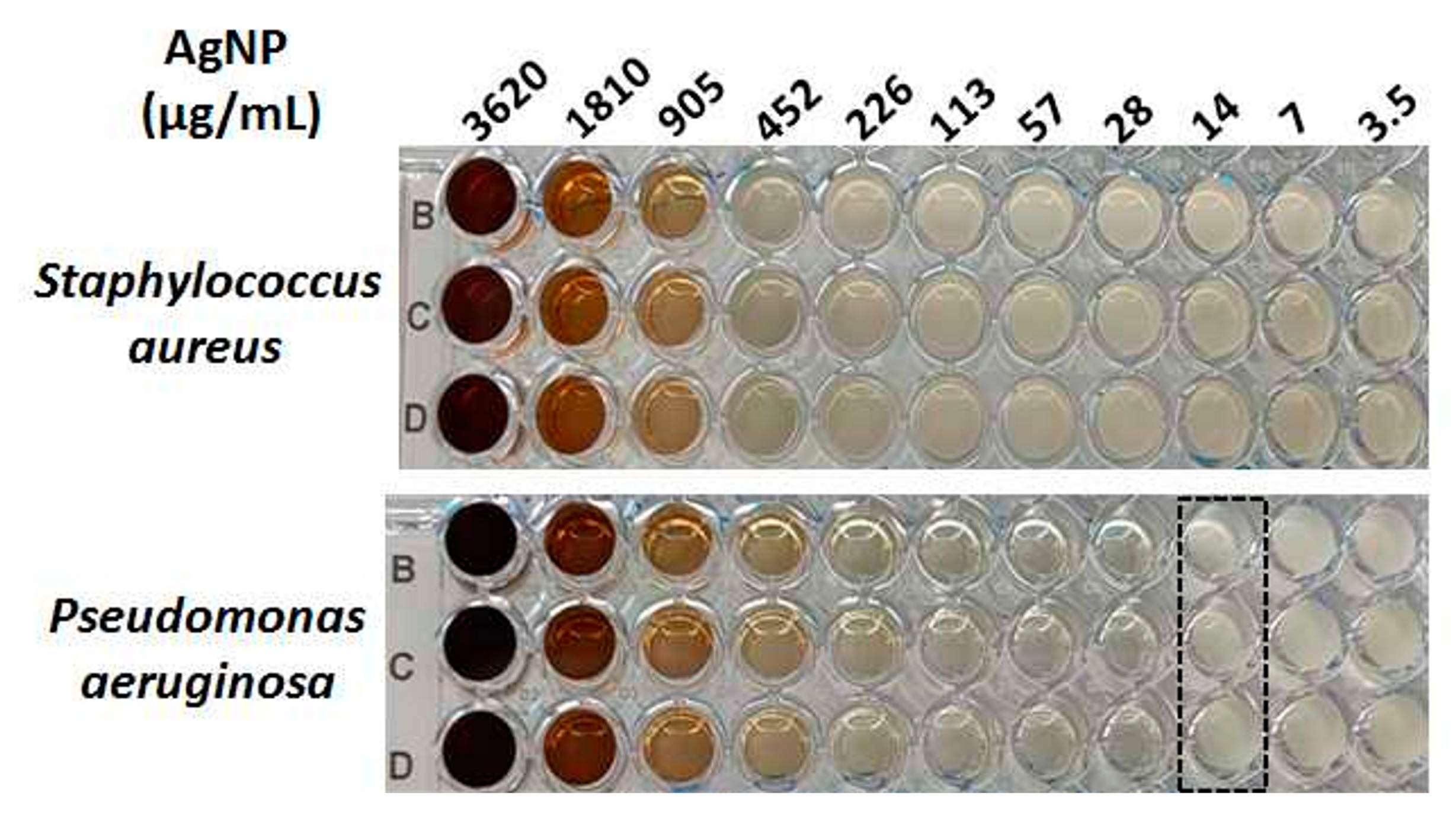
2.6. MIC Assessment by Broth Microdilution
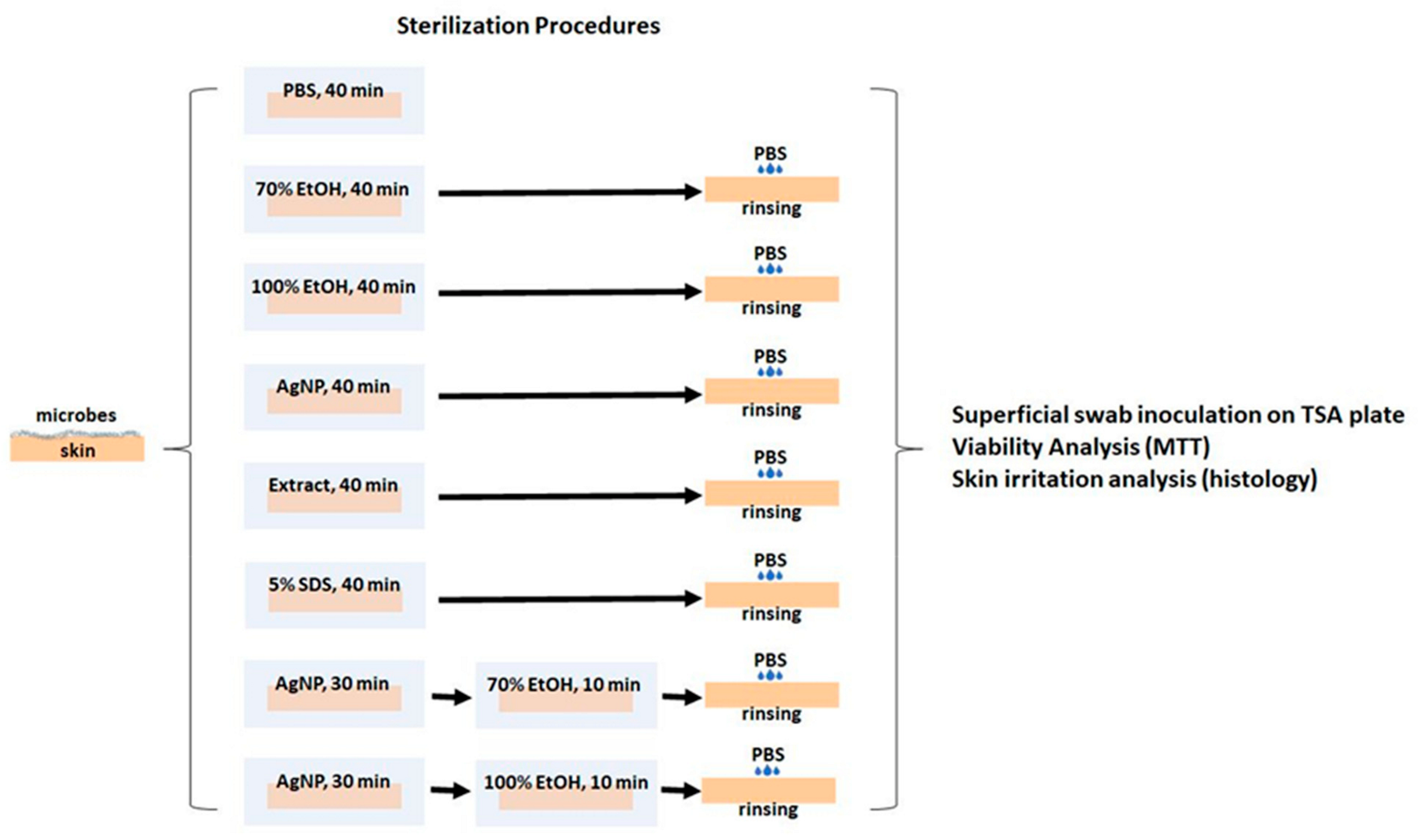
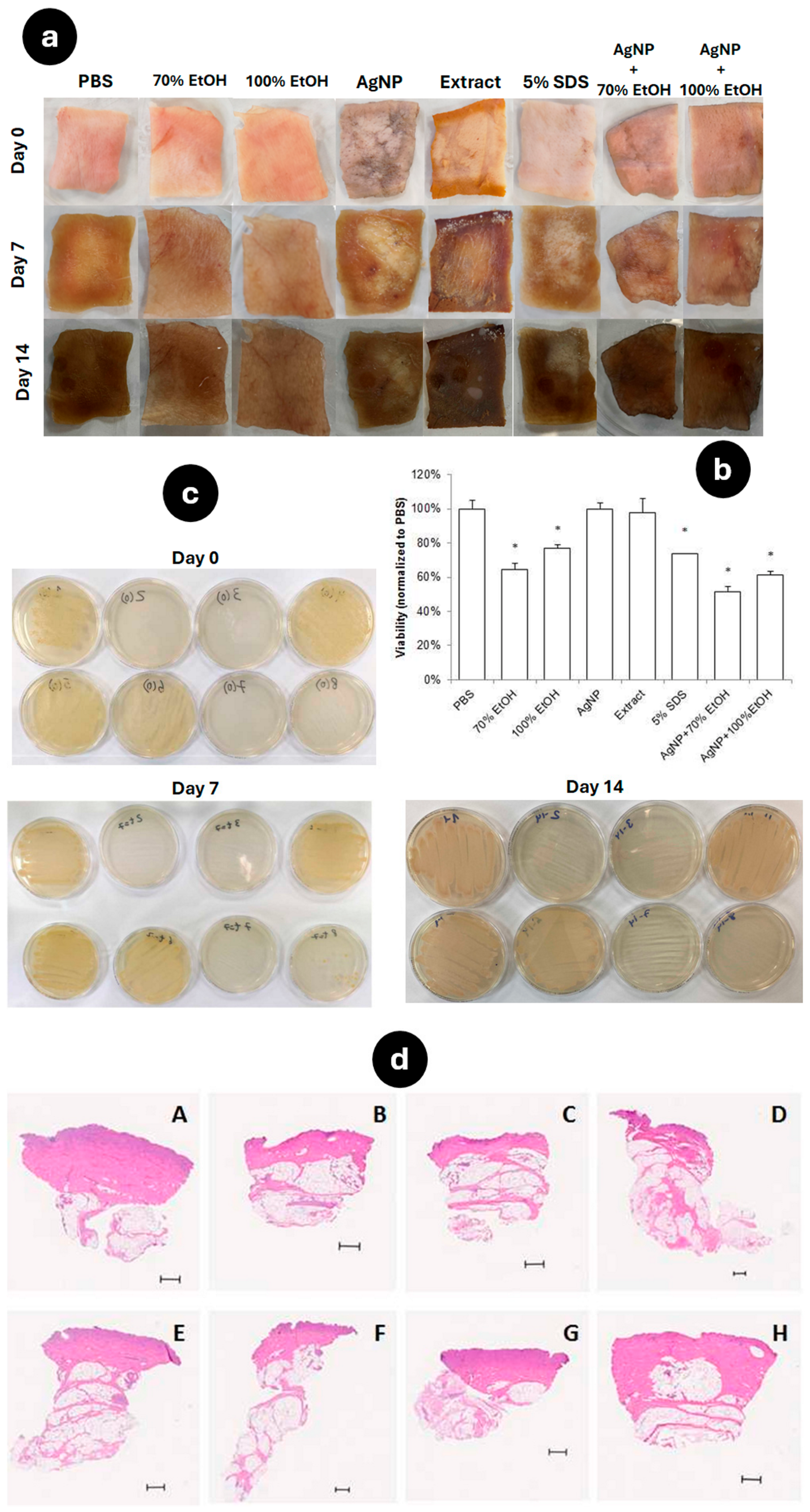
2.7. Antimicrobial Activity of AgNP Assessed in an Ex Vivo Model
2.8. Animal Model of Excisional Wound
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Tomato Extract
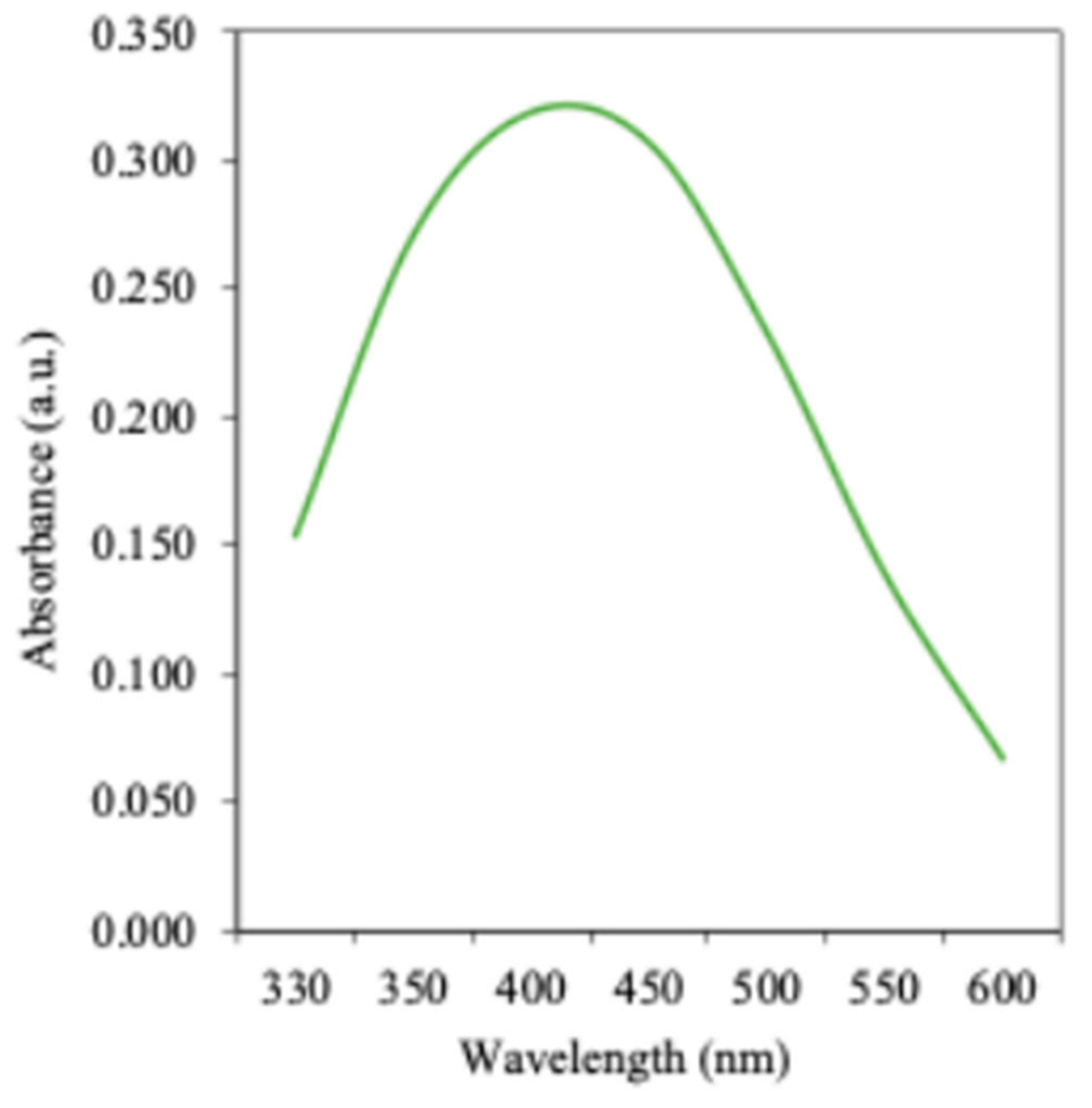
3.2. Biosynthesis of AgNP
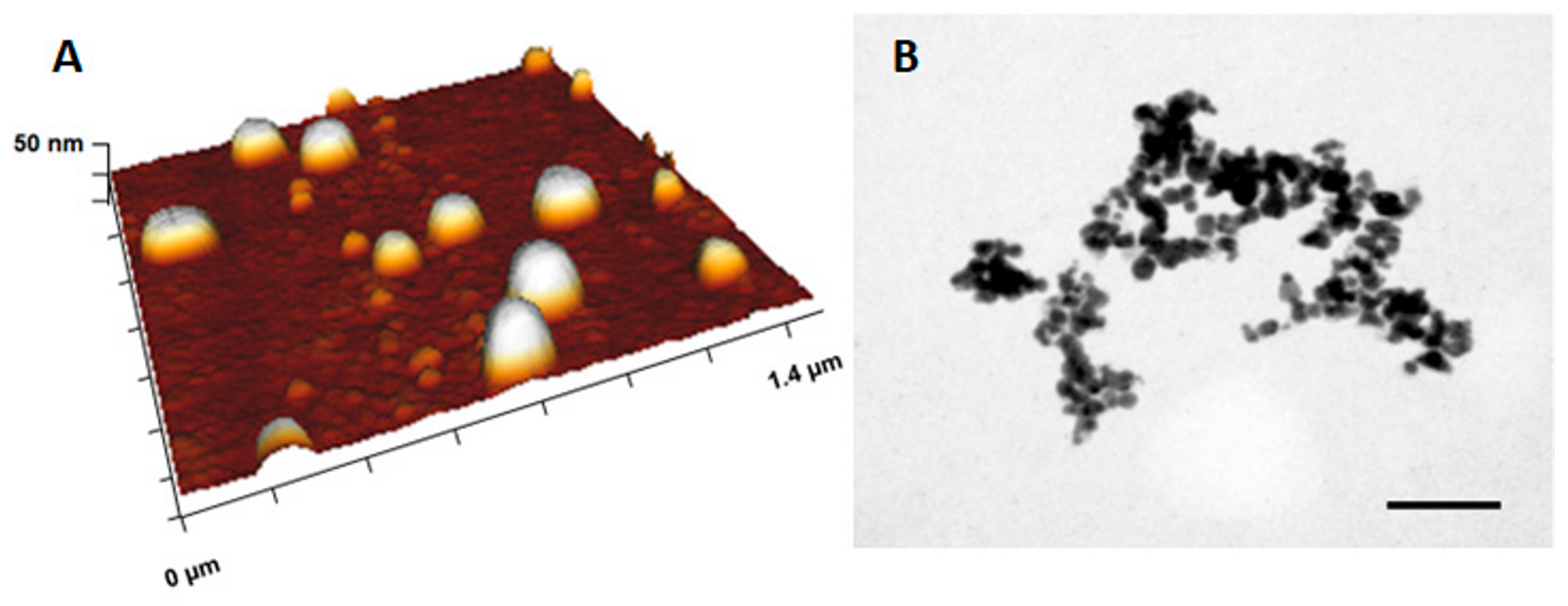
3.3. Physico-Chemical and Morphological Characterization of Optimized AgNP
3.4. In Vitro Biocompatibility Assays
3.5. In Vitro Antimicrobial Activity
3.6. Antimicrobial Activity of AgNP Evaluated in an Ex Vivo Skin Model
3.7. Wound-Healing Effect of AgNP in a Murine Incisional Acute Wound Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Catauro, M.; Raucci, M.G.; de Gaetano, F.; Marotta, A. Antibacterial and bioactive silver-containing Na2O·CaO·2SiO2 glass prepared by sol–gel method. J. Mater. Sci. Mater. Med. 2004, 15, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.H.; Burchette, R.J.; Siddiqi, R.A.; Huen, I.T.; Hadnott, L.L.; Fishman, A. The Efficacy of Silver-Ion Implanted Catheters in Reducing Peritoneal Dialysis-Related Infections. Perit. Dial. Int. 2003, 23, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green. Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications-A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Dhir, R.; Chauhan, S.; Subham, P.; Kumar, S.; Sharma, P.; Shidiki, A.; Kumar, G. Plant-mediated synthesis of silver nanoparticles: Unlocking their pharmacological potential-a comprehensive review. Front. Bioeng. Biotechnol. 2024, 11, 1324805. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Aramwit, P.; Bang, N.; Ratanavaraporn, J.; Ekgasit, S. Green synthesis of silk sericin-capped silver nanoparticles and their potent anti-bacterial activity. Nanoscale Res. Lett. 2014, 9, 79. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 90, 173–176. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Abdul Rahuman, A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.D.; Lekshmi, M.; Madhumitha, G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; de Sousa, M.; Carvalheiro, M.C.; Simões, P.; Simões, S. Alpha-tomatine and the two sides of the same coin: An anti-nutritional glycoalkaloid with potential in human health. Food Chem. 2022, 391, 133261. [Google Scholar] [CrossRef]
- Costa, A.; Marques, M.; Congiu, F.; Paiva, A.; Simões, P.; Ferreira, A.; Bronze, M.R.; Marto, J.; Ribeiro, H.M.; Simões, S. Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis. Appl. Sci. 2021, 11, 5120. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Eleutério, C.; Simões, P.; Carvalheiro, M.; Simões, S. A new method for quantification of tomatine-enriched extracts. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Galamba, N.; Paiva, A.; Barreiros, S.; Simões, P. Solubility of Polar and Nonpolar Aromatic Molecules in Subcritical Water: The Role of the Dielectric Constant. J. Chem. Theory Comput. 2019, 15, 6277–6293. [Google Scholar] [CrossRef] [PubMed]
- Balčiūnaitienė, A.; Viškelis, J.; Urbonavičienė, D.; Viškelis, P. Tomatoes By-Products Extracts Mediated Green Synthesis of Silver Nanoparticles and Their Application as Antimicrobial Agent. In Tomato—From Cultivation to Processing Technology; Viškelis, P., Urbonavičienė, D., Viškelis, J., Eds.; IntechOpen Limited: London, UK, 2022. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jayachandran, P.; Ilango, S.; Suseela, V.; Nirmaladevi, R.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Green Synthesized Silver Nanoparticle-Loaded Liposome-Based Nanoarchitectonics for Cancer Management: In Vitro Drug Release Analysis. Biomedicines 2023, 11, 217. [Google Scholar] [CrossRef]
- Wang, H.; Agrawal, A.; Wang, Y.; Crawford, D.W.; Siler, Z.D.; Peterson, M.L.; Woofter, R.T.; Labib, M.; Shin, H.Y.; Baumann, A.P.; et al. An ex vivo model of medical device-mediated bacterial skin translocation. Sci. Rep. 2021, 11, 5746. [Google Scholar] [CrossRef] [PubMed]
- Carbone, K.; de Angelis, A.; Mazzuca, C.; Santangelo, E.; Macchioni, V.; Cacciotti, I.; Petrella, G.; Cicero, D.; Micheli, L. Microwave-assisted synthesis of catalytic silver nanoparticles by hyperpigmented tomato skins: A green approach. LWT 2020, 133, 110088. [Google Scholar] [CrossRef]
- Mohamed, M.; Faraj, K.M.; Al-Jobori, K. Green Synthesis of Silver Nanoparticles Using Tomato (Lycopersicon esculentum) Extract and Evaluation of their Antifungal Activities. Plant Arch. 2020, 20, 5777–5786. [Google Scholar]
- Zia, M.; Gul, S.; Akhtar, J.; Haq, I.U.; Abbasi, B.H.; Hussain, A.; Naz, S.; Chaudhary, M.F. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol. 2017, 11, 193–199. [Google Scholar] [CrossRef]
- Santiago, T.R.; Bonatto, C.C.; Rossato, M.; Lopes, C.A.P.; Lopes, C.A.; G Mizubuti, E.S.; Silva, L.P. Green synthesis of silver nanoparticles using tomato leaf extract and their entrapment in chitosan nanoparticles to control bacterial wilt. J. Sci. Food Agric. 2019, 99, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, V.; Pastore, A.; Maisto, M.; Keivani, N.; Tenore, G.C.; Stornaiuolo, M.; Summa, V. Agri-Food Waste Recycling for Healthy Remedies: Biomedical Potential of Nutraceuticals from Unripe Tomatoes (Solanum lycopersicum L.). Foods 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Costa, A.; Faria-Silva, A.C.; Ascenso, A.; Marto, J.; Carvalheiro, M.; Gonçalves, L.M.; Marques, M.; Paiva, A.; Bento, M.; et al. Sustainable valorization of food-processing industry by-products: Challenges and opportunities to obtain bioactive compounds. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 619–644. [Google Scholar] [CrossRef]
- Ghosh, P.; Fawcett, D.; Sharma, S.; Poinern, G. Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste. Materials 2017, 10, 852. [Google Scholar] [CrossRef]
- Mehrdel, B.; Aziz, A.A. The Sensitivity of Surface Plasmon Resonance Damping for Colloidal Silver Nanoparticles. J. Phys. Conf. Ser. 2018, 1083, 012042. [Google Scholar] [CrossRef]
- Saha, P.; Mahiuddin, M.; Islam, A.B.M.N.; Ochiai, B. Biogenic Synthesis and Catalytic Efficacy of Silver Nanoparticles Based on Peel Extracts of Citrus macroptera Fruit. ACS Omega 2021, 6, 18260–18268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Handayani, W.; Ningrum, A.S.; Imawan, C. The Role of pH in Synthesis Silver Nanoparticles Using Pometia pinnata (Matoa) Leaves Extract as Bioreductor. J. Phys. Conf. Ser. 2020, 1428, 012021. [Google Scholar] [CrossRef]
- Ider, M.; Abderrafi, K.; Eddahbi, A.; Ouaskit, S.; Kassiba, A. Silver Metallic Nanoparticles with Surface Plasmon Resonance: Synthesis and Characterizations. J. Clust. Sci. 2017, 28, 1051–1069. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, S.; Jamzad, M.; Yari, M. Biologically synthesized silver nanoparticles by aqueous extract of Satureja intermedia C.A. Mey and the evaluation of total phenolic and flavonoid contents and antioxidant activity. J. Nanostruct. Chem. 2016, 6, 357–364. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid. Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Madiehe, A.M.; du Preez, M.G. The antimicrobial activity of biogenic silver nanoparticles synthesized from extracts of Red and Green European pear cultivars. Artif. Cells Nanomed. Biotechnol. 2021, 49, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Mak, T.N.; Shinohara, D.B.; Sfanos, K.S.; Meyer, T.F.; Brüggemann, H. Deciphering the Intracellular Fate of Propionibacterium acnes in Macrophages. BioMed Res. Int. 2013, 2013, 603046. [Google Scholar] [CrossRef] [PubMed]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.S.; Costa, S.F. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta 2015, 1850, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Ansell, D.M.; Campbell, L.; Thomason, H.A.; Brass, A.; Hardman, M.J. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair. Regen. 2014, 22, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.; Raposo, S.; Marto, J.; Silva, A.N.; Simões, S.; Ribeiro, H.M. Mometasone furoate hydrogel for scalp use: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2014, 19, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Neves, Â.; Gonçalves, L.; Pinto, P.; Almeida, C.; Simões, S. Rice Water: A Traditional Ingredient with Anti-Aging Efficacy. Cosmetics 2018, 5, 26. [Google Scholar] [CrossRef]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound healing applications of biogenic colloidal silver and gold nanoparticles: Recent trends and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 4305–4318. [Google Scholar] [CrossRef] [PubMed]

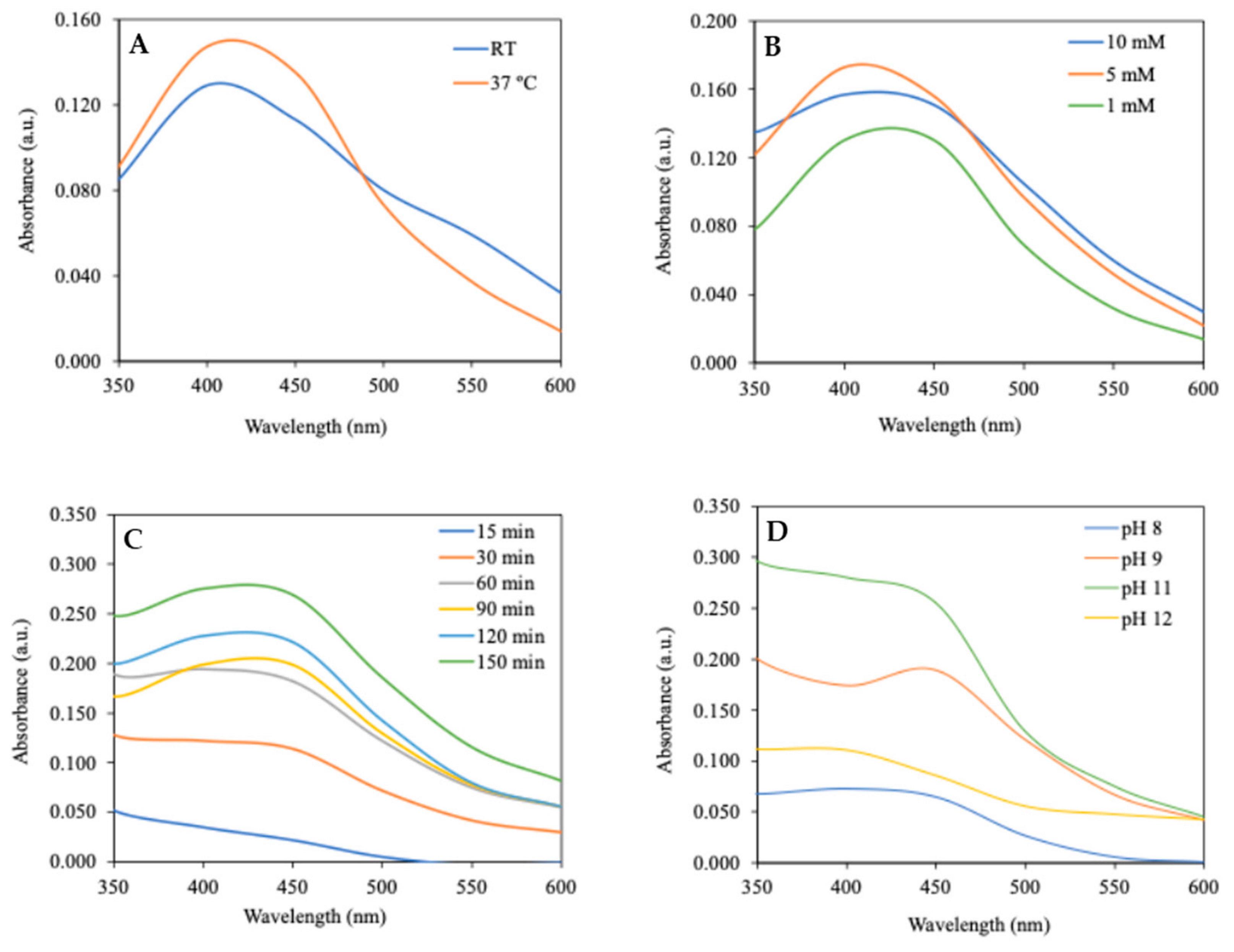


| Manipulated Parameter | Z-Average (nm) | PdI | Zeta Potential (mV) | |
|---|---|---|---|---|
| Time | 15 min | 163 ± 22 | 0.32 ± 0.08 | −22 ± 1 |
| 30 min | 235 ± 47 | 0.29 ± 0.04 | −28 ± 1 | |
| 60 min | 222 ± 25 | 0.30 ± 0.03 | −28 ± 1 | |
| 90 min | 191 ± 27 | 0.29 ± 0.02 | −29 ± 1 | |
| 120 min | 290 ± 35 | 0.36 ± 0.07 | −29 ± 3 | |
| 150 min | 246 ± 34 | 0.34 ± 0.04 | −32 ± 0 | |
| Temperature | Room | 51 ± 1 | 0.34 ± 0.05 | −29 ± 1 |
| 37 °C | 35 ± 0 | 0.31 ± 0.03 | −21 ± 3 | |
| AgNO3 | 1 mM | 23 ± 1 | 0.61 ± 0.01 | n.d. |
| 5 mM | 29 ± 6 | 0.50 ± 0.19 | n.d. | |
| 10 mM | >1000 | - | n.d. | |
| pH | pH 8 | 245 ± 5 | 0.43 ± 0.17 | −22 ± 2 |
| pH 9 | 226 ± 28 | 0.51 ± 0.02 | −22 ± 1 | |
| pH 11 | 160 ± 3 | 0.32 ± 0.03 | −31 ± 1 | |
| pH 12 | >1000 | - | n.d. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, D.; Faria-Silva, C.; Carvalho, F.A.; Ascensão, L.; Simões, P.; Carvalheiro, M.; Simões, S. In Vitro, Ex Vivo, and In Vivo Evaluation of Silver Nanoparticles Synthesized Using Green Tomato Extract: Perspectives on Topical Application. Appl. Sci. 2024, 14, 7309. https://doi.org/10.3390/app14167309
Cunha D, Faria-Silva C, Carvalho FA, Ascensão L, Simões P, Carvalheiro M, Simões S. In Vitro, Ex Vivo, and In Vivo Evaluation of Silver Nanoparticles Synthesized Using Green Tomato Extract: Perspectives on Topical Application. Applied Sciences. 2024; 14(16):7309. https://doi.org/10.3390/app14167309
Chicago/Turabian StyleCunha, Daniela, Catarina Faria-Silva, Filomena A. Carvalho, Lia Ascensão, Pedro Simões, Manuela Carvalheiro, and Sandra Simões. 2024. "In Vitro, Ex Vivo, and In Vivo Evaluation of Silver Nanoparticles Synthesized Using Green Tomato Extract: Perspectives on Topical Application" Applied Sciences 14, no. 16: 7309. https://doi.org/10.3390/app14167309






