Combating Pathogens Using Carbon-Fiber Ionizers (CFIs) for Air Purification: A Narrative Review
Abstract
:1. Introduction
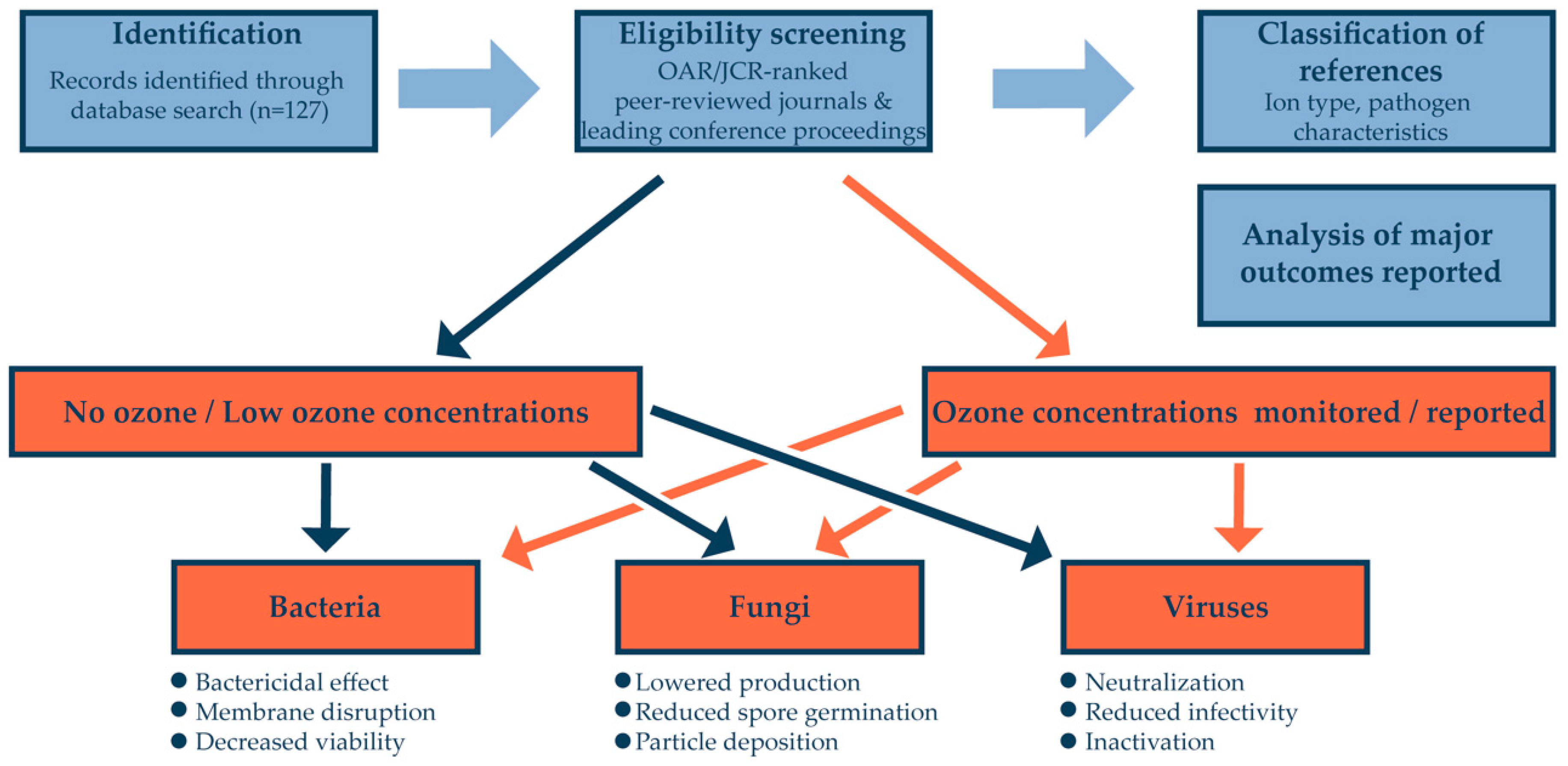
2. Material and Methods
3. Database Search Results
4. Discussion
4.1. Defining the Testing Protocols and Parameter Reporting for a Consistent Research Approach
4.2. Air Ionization and Microorganisms—A Familiar Interplay?
4.3. The Other Side of the Coin: How to Avoid Harmful Byproducts of Air Ionization?
4.4. Carbon-Fiber Ionizers: The Pros and Cons
4.5. Possible Challenges in the Development of Real-World CFI-Based Air Purification Systems
4.6. Effectiveness of Air Ionization in Comparison with Other Pathogen Mitigation Measures
4.7. Limitations
- Different particle sizes investigated;
- Varying degrees of ozone emissions (or simply a lack of O3 measurement in a particular study);
- Range of microorganisms studied (some studies report on microbes that are not infectious to humans; many others, although they analyze the effect of ions on human pathogens, do so on only bacteria or viruses; the spectrum of microorganisms analyzed per study is restricted to only several species; ultimately, some microorganisms may be inherently more resistant to ions than others);
- Vegetative bacterial forms (in this instance, we are still uncertain about how ions impact bacterial spores);
- Different methods to measure microorganism viability (which can negatively impact interstudy comparisons; viability was also not measured in every study);
- CFI-related parameters (voltage, material used, and flow velocity);
- Definition of ion source (an example is a study that does not define whether a CFI unit is utilized);
- Type of most efficient ions (the optimal type of ions is also disputed, as in several instances a frank contradiction between study results was observed (Cf. Table 1).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Yan, S.; Zhang, M.; Wang, C.; Xing, D. Air sampling and ATP bioluminescence for quantitative detection of airborne microbes. Talanta 2024, 274, 126025. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-P.; Lee, G.W.-M.; Lin, S.-Y.; Huang, C.P. Removal of bioaerosols by the combination of a photocatalytic filter and negative air ions. J. Aerosol Sci. 2008, 39, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Kolarž, P.; Ilić, A.Ž.; Janković, M.; Janićijević, A.; Trbovich, A.M. Estimating aerosol particle removal in indoor air by ion-enhanced deposition. J. Aerosol Sci. 2023, 173, 106199. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, S.G.; Hwang, J. Application of corona discharge-generated air ions for filtration of aerosolized virus and inactivation of filtered virus. J. Aerosol Sci. 2017, 107, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kim, H.J.; Kim, Y.J.; Sioutas, C. Unipolar Charging of Fine and Ultra-Fine Particles Using Carbon Fiber Ionizers. Aerosol Sci. Technol. 2008, 42, 793–800. [Google Scholar] [CrossRef]
- Han, B.; Hudda, N.; Ning, Z.; Kim, H.J.; Kim, Y.J.; Sioutas, C. A novel bipolar charger for submicron aerosol particles using carbon fiber ionizers. J. Aerosol Sci. 2009, 40, 285–294. [Google Scholar] [CrossRef]
- Ligotski, R.; Sager, U.; Schneiderwind, U.; Asbach, C.; Schmidt, F. Prediction of VOC adsorption performance for estimation of service life of activated carbon-based filter media for indoor air purification. Build. Environ. 2019, 149, 146–156. [Google Scholar] [CrossRef]
- Rashid, M.M.; Tushan, S.S.; Ahmed, S.; Tushar, S.I. Design and development of advanced Air Purifier Facial Mask. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Bangkok, Thailand, 5–7 March 2019; pp. 1–8. Available online: https://www.ieomsociety.org/ieom2019/papers/581.pdf (accessed on 22 June 2024).
- Rafique, M.S.; Tahir, M.B.; Rafique, M.S.; Shakil, M. Photocatalytic Nanomaterials for Air Purification and Self-Cleaning; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yoon, K.Y.; Park, J.H.; Hwang, J. Application of air ions for bacterial de-colonization in air filters contaminated by aerosolized bacteria. Sci. Total Environ. 2011, 409, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, K.Y.; Kim, Y.S.; Byeon, J.H.; Hwang, J. Removal of submicron aerosol particles and bioaerosols using carbon fiber ionizer assisted fibrous medium filter media. J. Mech. Sci. Technol. 2009, 23, 1846–1851. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, K.Y.; Noh, K.C.; Byeon, J.H.; Hwang, J. Removal of PM 2.5 entering through the ventilation duct in an automobile using a carbon fiber ionizer-assisted cabin air filter. J. Aerosol Sci. 2010, 41, 935–943. [Google Scholar] [CrossRef]
- Ratliff, K.M.; Oudejans, L.; Archer, J.; Calfee, W.; Gilberry, J.U.; Hook, D.A.; Schoppman, W.E.; Yaga, R.W.; Brooks, L.; Ryan, S. Large-scale evaluation of microorganism inactivation by bipolar ionization and photocatalytic devices. Build. Environ. 2023, 227, 109804. [Google Scholar] [CrossRef]
- Park, C.W.; Park, J.W.; Lee, S.H.; Hwang, J. Real-time monitoring of bioaerosols via cell-lysis by air ion and ATP bioluminescence detection. Biosens Bioelectron. 2014, 52, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wu, H.; Zhang, H.H.; Li, W.S.; Lai, A.C.K. Synergistic disinfection of aerosolized bacteria and bacteriophage by far-UVC (222-nm) and negative air ions. J. Hazard. Mater. 2023, 441, 129876. [Google Scholar] [CrossRef]
- Lee, S.G.; Hyun, J.Y.; Sung, H.L.; Jungho, H. One-pass antibacterial efficacy of bipolar air ions against aerosolized Staphylococcus epidermidis in a duct flow. J. Aerosol Sci. 2014, 69, 71–81. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Huang, G.; Lai, A.C.K. Numerical and experimental study on airborne disinfection by negative ions in air duct flow. Build. Environ. 2018, 127, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Sung, B.J.; Yoon, K.S.; Jeong, C.S. The bactericidal effect of an ionizer under low concentration of ozone. BMC Microbiol. 2016, 16, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woo, C.G.; Kim, H.J.; Kim, Y.J.; Han, B. Enhanced Antimicrobial Activity on Non-Conducting and Conducting Air Filters by Using Air Ions and Grapefruit Seed Extract. Aerosol Air Qual. Res. 2017, 17, 1917–1924. [Google Scholar] [CrossRef]
- Comini, S.; Mandras, N.; Iannantuoni, M.R.; Menotti, F.; Musumeci, A.G.; Piersigilli, G.; Allizond, V.; Banche, G.; Cuffini, A.M. Positive and Negative Ions Potently Inhibit the Viability of Airborne Gram-Positive and Gram-Negative Bacteria. Microbiol. Spectrum 2021, 9, e00651-21. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Iannantuoni, M.R.; Musumeci, A.; Allizond, V.; Cuffini, A.M. Use of Negative and Positive Ions for Reducing Bacterial Pathogens to Control Infections. ACTA Sci. Microbiol. 2019, 2, 35–38. [Google Scholar] [CrossRef]
- Marin, V.; Moretti, G.; Rassu, M. Effetti della ionizzazione dell’aria su alcuni ceppi batterici (Effects of ionization of the air on some bacterial strains). Ann Ig. 1989, 1, 1491–1500. [Google Scholar]
- Kanesaka, I.; Katsuse, A.; Takahashi, H.; Kobayashi, I. Evaluation of a bipolar ionization device in inactivation of antimicrobial-resistant bacteria, yeast, Aspergillus spp. and human coronavirus. J. Hosp. Infect. 2022, 126, 16–20. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Xu, Y.; Du, Y.; Wang, A.; Liu, Z.; Yan, K. Electrostatic precipitation (ESP) index driven bio-aerosol collection for a high biological viability sampling. J. Clean. Prod. 2023, 423, 138790. [Google Scholar] [CrossRef]
- Han, T.; Zhen, H.; Fennell, D.E.; Mainelis, G. Design and Evaluation of the Field-Deployable Electrostatic Precipitator with Superhydrophobic Surface (FDEPSS) with High Concentration Rate. Aerosol Air Qual. Res. 2015, 15, 2397–2408. [Google Scholar] [CrossRef]
- Mainelis, G.; Adhikari, A.; Willeke, K.; Lee, S.-A.; Reponen, T.; Grinshpun, S. Collection of airborne microorganisms by electrostatic precipitation. J. Aerosol Sci. 2002, 33, 1417–1432. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Song, J.; Hildebrand, P.D.; Forney, C.F. Interaction of ozone and negative air ions to control micro-organisms. J. Appl. Microbiol. 2002, 93, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Prombona, A.; Karanikolos, G.N. Ion-Based Metal/Graphene Antibacterial Agents Comprising Mono-Ionic and Bi-Ionic Silver and Copper Species. Langmuir 2018, 34, 11156–11166. [Google Scholar] [CrossRef] [PubMed]
- Zhuangbo, F.; Cao, S.J.; Wang, J.; Kumar, P.; Haghighat, F. Indoor airborne disinfection with electrostatic disinfector (ESD): Numerical simulations of ESD performance and reduction of computing time. Build. Environ. 2021, 200, 107956. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Lai, A.; Huang, G. Inactivation of airborne bacteria by cold plasma in air duct flow. Build. Environ. 2016, 106, 120–130. [Google Scholar] [CrossRef]
- Niveditha, A.S.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods—A review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Phillips, G.; Harris, G.J.; Jones, M.W. Effect of air ions on bacterial aerosols. Int. J. Biometeorol. 1964, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Hughes, J.F. Bactericidal Effects of Negative and Positive Ions Generated in Nitrogen on Starved Pseudomonas Veronii. J. Electrost. 2003, 57, 49–58. [Google Scholar] [CrossRef]
- Kampmann, Y.; Klingshirn, A.; Kloft, K.; Kreyenschmidt, J. The application of ionizers in domestic refrigerators for reduction in airborne and surface bacteria. J. Appl. Microbiol. 2009, 107, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Boothe, D.H.; Mitchell, B.W. Use of Negative Air Ionization for Reducing Bacterial Pathogens and Spores on Stainless Steel Surfaces. J. Appl. Poult. Res. 2004, 13, 200–206. [Google Scholar] [CrossRef]
- Fletcher, L.A.; Gaunt, L.F.; Beggs, C.B.; Shepherd, S.J.; Sleigh, P.A.; Noakes, C.J.; Kerr, K.G. Bactericidal action of positive and negative ions in air. BMC Microbiol. 2007, 7, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escombe, A.R.; Moore, D.A.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R.; et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009, 6, e43. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Al-Marzouqi, A.H.; Nazir, M.H.; Rizvi, T.A.; Zaneldin, E.; Khan, M. Comparative Experimental Investigation of Biodegradable Antimicrobial Polymer-Based Composite Produced by 3D Printing Technology Enriched with Metallic Particles. Int. J. Mol. Sci. 2022, 23, 11235. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Yu, V.L. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: Implications for the evaluation of other disinfection modalities. Infect. Control Hosp. Epidemiol. 2003, 24, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Lin, Y.S.; Goetz, A.M.; Muder, R.R. Controlling Legionella in hospital water systems: Experience with the superheat-and-flush method and copper-silver ionization. Infect Control Hosp. Epidemiol. 1998, 19, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Mitchell, B.W.; Holt, P.S. Application of negative air ionization for reducing experimental airborne transmission of Salmonella enteritidis to chicks. Poult. Sci. 1999, 78, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.S.; Mitchell, B.W.; Seo, K.-H.; Gast, R.K. Use of Negative Air Ionization for Reducing Airborne Levels of Salmonella enterica serovar enteritidis in a Room Containing Infected Caged Layers. J. Appl. Poult. Res. 1999, 8, 440–446. [Google Scholar] [CrossRef]
- Seo, K.H.; Mitchell, B.W.; Holt, P.S.; Gast, R.K. Bactericidal effects of negative air ions on airborne and surface Salmonella enteritidis from an artificially generated aerosol. J. Food Prot. 2001, 64, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.W., 3rd; Yost, M.G.; Barthakur, N.; Kreuger, A.P. Superoxide involvement in the bactericidal effects of negative air ions on Staphylococcus albus. Nature 1979, 281, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yermakov, M.; Grinshpun, S.A. Unipolar ion emission enhances respiratory protection against fine and ultrafine particles. J. Aerosol Sci. 2004, 35, 1359–1368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aplin, K.L.; Harrison, R.G. Electricity in the Atmosphere; Ions in the Atmosphere. In Encyclopedia of Atmospheric Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 9–13. [Google Scholar] [CrossRef]
- Pratt, R.; Barnard, R.W. Some effects of ionized air on Penicillium notatum. J. Pharm. Sci. 1960, 49, 643–646. [Google Scholar] [CrossRef]
- Shargawi, J.M.; Theaker, E.D.; Drucker, D.B.; MacFarlane, T.; Duxbury, A.J. Sensitivity of Candida albicans to negative air ion streams. J. Appl. Microbiol. 1999, 87, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Electronics, Sanyo Home Appliances Research Program. Sharp’s plasmacluster ions effectively deactivate H5N1 avian influenza virus. Asia Pac. Biotech. News. 2005, 6, 469. [Google Scholar]
- Bolashikov, Z.D.; Melikov, A.K. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009, 44, 1378–1385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soliman, M.Y.M.; Medema, G.; Bonilla, B.E.; Brouns, S.J.J.; van Halem, D. Inactivation of RNA and DNA viruses in water by copper and silver ions and their synergistic effect. Water Res. X 2020, 9, 100077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suwardi, A.; Ooi, C.C.; Daniel, D.; Tan, C.K.I.; Li, H.; Liang, O.Y.Z.; Tang, Y.K.; Chee, J.; Sadovoy, A.; Jiang, S.-Y.; et al. The Efficacy of Plant-Based Ionizers in Removing Aerosol for COVID-19 Mitigation. Research 2021, 2021, 2173642. [Google Scholar] [CrossRef] [PubMed]
- Van Egeren, D.; Stoddard, M.; Malakar, A.; Ghosh, D.; Acharya, A.; Mainuddin, S.; Majumdar, B.; Luo, D.; Nolan, R.P.; Joseph-McCarthy, D.; et al. No magic bullet: Limiting in-school transmission in the face of variable SARS-CoV-2 viral loads. Front. Public Health 2022, 10, 941773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Z.; Cao, S.J.; Haghighat, F. Removal of SARS-CoV-2 using UV+Filter in built environment. Sustain. Cities Soc. 2021, 74, 103226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Cui, H.; Zhang, C.; Chen, Z.; Jiang, X.; Liu, J.; Wan, Z.; Li, J.; Liu, J.; Gao, Y.; et al. Aerosol Transmission of the Pandemic SARS-CoV-2 and Influenza A Virus Was Blocked by Negative Ions. Front. Cell. Infect. Microbiol. 2022, 12, 897416. [Google Scholar] [CrossRef]
- Mitchell, B.W.; King, D.J. Effect of Negative Air Ionization on Airborne Transmission of Newcastle Disease Virus. Avian Diseases 1994, 38, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Estola, T.; Mäkelä, P.; Hovi, T. The effect of air ionization on the air-borne transmission of experimental Newcastle disease virus infections in chickens. J. Hygiene 1979, 83, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; An, S.H.; Lee, Y.; Kim, Y.J.; Han, B.; Kim, H.J. Development of On-Demand Antiviral Electrostatic Precipitators with Electrothermal-Based Antiviral Surfaces against Airborne Virus Particles. Toxics 2022, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Lee, J.; Park, D. Effects of Brush-Type Ionizer Materials on Virus Inactivation. Toxics 2022, 10, 611. [Google Scholar] [CrossRef]
- Jeong, S.B.; Shin, J.H.; Kim, S.W.; Seo, S.C.; Jung, J.H. Performance evaluation of an electrostatic precipitator with a copper plate using an aerosolized SARS-CoV-2 surrogate (bacteriophage phi 6). Environ. Technol. Innov. 2023, 30, 103124. [Google Scholar] [CrossRef]
- Hagbom, M.; Nordgren, J.; Nybom, R.; Hedlund, K.O.; Wigzell, H.; Svensson, L. Ionizing air affects influenza virus infectivity and prevents airborne-transmission. Sci. Rep. 2015, 5, 11431. [Google Scholar] [CrossRef]
- ISO 14698-1:2003; Cleanrooms and Associated Controlled Environments-Biocontamination Control: Part 1: General Principles and Methods. International Organization for Standardization: Geneva, Switzerland, 2003.
- Ouyang, H.; Wang, L.; Sapkota, D.; Yang, M.; Morán, J.; Li, L.; Olson, B.A.; Schwartz, M.; Hogan, C.J., Jr.; Torremorell, M. Control technologies to prevent aerosol-based disease transmission in animal agriculture production settings: A review of established and emerging approaches. Front. Vet. Sci. 2023, 10, 1291312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Streets, D.G.; Wang, C. How does decarbonization of the central heating industry affect employment? A spatiotemporal analysis from the perspective of urbanization. Energy Build. 2024, 306, 113912. [Google Scholar] [CrossRef]
- Engel-Cox, J.A.; Van Houten, B.; Phelps, J.; Rose, S.W. Conceptual model of comprehensive research metrics for improved human health and environment. Environ. Health Perspect. 2008, 116, 583–592. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Ist. Super. Sanita 2013, 49, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, Y.; Chen, C. Air purifiers: A supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020, 177, 106918. [Google Scholar] [CrossRef]
- Berry, G.; Parsons, A.; Morgan, M.; Rickert, J.; Cho, H. A review of methods to reduce the probability of the airborne spread of COVID-19 in ventilation systems and enclosed spaces. Environ. Res. 2022, 203, 111765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Block, S.S. Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Zhao, H.; Kong, X.; Yao, W.; Fei, X.; Zhao, J.; Zhao, S.; Feng, T. Control technology of pathogenic biological aerosol: Review and prospect. Build. Environ. 2023, 243, 110679. [Google Scholar] [CrossRef]
- Park, D.H.; Hwang, J.; Shin, D.; Kim, Y.; Lee, G.; Park, I.; Kim, S.B.; Hong, K.; Han, B. Developing an Optimal Antiviral Method for the Air-filtration System of Subway Stations. Aerosol. Air Qual. Res. 2023, 23, 230088. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Adhikari, A.; Honda, T.; Kim, K.Y.; Toivola, M.; Rao, K.S.R.; Reponen, T. Control of aerosol contaminants in indoor air: Combining the particle concentration reduction with microbial inactivation. Environ. Sci. Technol. 2007, 41, 606–612. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Ma, A.; Ramachandran, S. Negative Air Ions and Their Effects on Human Health and Air Quality Improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef]
- Cappa, C. Air Pollutant Emissions and Possible Health Effects Associated with Electronic Air Cleaners. 2023. Available online: https://ww2.arb.ca.gov/sites/default/files/2023-09/CARB%2022RD003%20White%20Paper%20Sept%2020%202023.pdf (accessed on 9 July 2024).
- Asadgol, Z.; Nadali, A.; Arfaeinia, H.; Gholi, M.K.; Fateh, R.; Fahiminia, M. Evaluation of Negative Air Ions in Bioaerosol Removal: Indoor Concentration of Airborne Bacterial and Fungal in Residential Building in Qom City, Iran. Int. J. Earth Energy Environ. Sci. 2018, 12, 300–311. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Mainelis, G.; Trunov, M.; Adhikari, A.; Reponen, T.; Willeke, K. Evaluation of ionic air purifiers for reducing aerosol exposure in confined indoor spaces. Indoor Air 2005, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Han, B.; Kim, Y.-J.; Oda, T.; Won, H. Submicrometer particle removal indoors by a novel electrostatic precipitator with high clean air delivery rate, low ozone emissions, and carbon fiber ionizer. Indoor Air 2013, 23, 369–378. [Google Scholar] [CrossRef]
- Wang, C.; Lu, S.; Zhang, Z. Inactivation of airborne bacteria using different UV sources: Performance modeling, energy utilization, and endotoxin degradation. Sci. Total Environ. 2019, 655, 787–795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyagi, A.K.; Nirala, B.K.; Malik, A.; Singh, K. The effect of negative air ion exposure on Escherichia coli and Pseudomonas fluorescens. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2008, 43, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Guo, C.; Gao, Z.; Shen, J. Emission rates of indoor ozone emission devices: A literature review. Build. Environ. 2019, 158, 302–318. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, S.; Lee, E.S.; Zhao, B.; Zhu, Y. Performance of wearable ionization air cleaners: Ozone emission and particle removal. Aerosol Sci. Technol. 2016, 50, 211–221. [Google Scholar] [CrossRef]
- WHO Global Air Quality Guidelines. In Particulate Matter (PM2.5 And Pmio), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021.
- WHO Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project. Available online: https://www.ncbi.nlm.nih.gov/books/NBK361805/ (accessed on 19 June 2024).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/ozone-pollution-and-your-patients-health/course-outline-and-key-points-ozone (accessed on 3 July 2024).
- Yehia, A.; Abdel-Salam, M.; Mizuno, A. On assessment of ozone generation in dc coronas. J. Phys. D Appl. Phys. 2000, 33, 831. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, M.; Kim, Y.J.; Han, B.; Hong, K.J. Ultrafine particle cleaning performance of an ion spray electrostatic air cleaner emitting zero ozone with diffusion charging by carbon fiber. Build. Environ. 2019, 165, 106422. [Google Scholar] [CrossRef]
- Selvaprakash, K.; Chen, Y.C. Using an insulating fiber as the sampling probe and ionization substrate for ambient ionization–mass spectrometric analysis of volatile, semi-volatile, and polar analytes. Anal. Bioanal. Chem. 2022, 414, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Yu, K.; Lu, G.; Cui, S.; Mao, S.; Chen, J. Vertically oriented graphene sheets grown on metallic wires for greener corona discharges: Lower power consumption and minimized ozone emission. Energy Environ. Sci. 2011, 4, 2525–2528. [Google Scholar] [CrossRef]
- Hobbs, P.C.D.; Gross, V.P.; Murray, K.D. Suppression of particle generation in a modified clean room corona air ionizer. J. Aerosol Sci. 1990, 21, 463–465. [Google Scholar] [CrossRef]
- Saxena, A.; Khare, D.; Agrawal, S.; Singh, A.; Dubey, A.K. Recent advances in materials science: A reinforced approach toward challenges against COVID-19. Emerg. Mater. 2021, 4, 57–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.; Lim, G.T.; Kim, Y.-J.; Han, B.; Woo, C.; Kim, H. A novel electrostatic precipitator-type small air purifier with a carbon fiber ionizer and an activated carbon fiber filter. J. Aerosol Sci. 2017, 117, 63–73. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update; WHO Regional Office for Europe: Copenhagen, Denmark, 2006. [Google Scholar]
- National Ambient Air Quality Standards for Ozone, EPA-HQ-OAR. United States Environmental Protection Agency; p. 2015, [Online], 2016-0202. Available online: https://www.govinfo.gov/content/pkg/FR-2018-12-06/pdf/2018-25424.pdf (accessed on 22 June 2024).
- Young, T.M.; Sobek, E.; Farahi, F. Quantifying the Natural Variation of ‘Data Signatures’ from Aerosols Using Statistical Control Bands. Mathematics 2022, 10, 2103. [Google Scholar] [CrossRef]
- First, M.W. HEPA Filters. J. Am. Biol. Saf. Assoc. 1998, 3, 33–42. [Google Scholar] [CrossRef]
- Ehsan, S.M.; Krystal, J.G.P.; Jodi, S.; Richard, A.M. Performance analysis of portable HEPA filters and temporary plastic anterooms on the spread of surrogate coronavirus. Build. Environ. 2020, 183, 107186. [Google Scholar] [CrossRef]
- Pawar, S.D.; Khare, A.B.; Keng, S.S.; Kode, S.S.; Tare, D.S.; Singh, D.K.; More, R.L.; Mullick, J. Selection and application of biological safety cabinets in diagnostic and research laboratories with special emphasis on COVID-19. Rev. Sci. Instrum. 2021, 92, 081401. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Contal, P.; Renaudin, V.; Penicot, P.; Leclerc, D.; Vendel, J. Modelling pressure drop in HEPA filters during dynamic filtration. J. Aerosol Sci. 1999, 30, 235–246. [Google Scholar] [CrossRef]
- Himanshu, M.; Simon, R.P.; Thomas, P.; James, T.W.; Allan, M.B. Survival of Microorganisms on HEPA Filters. Appl. Biosafety 2011, 16, 163–166. [Google Scholar] [CrossRef]
- Christiane, S.H.; Ulrich, W.A.; Leonie, S.; Ulf, D.; Oliver, W.; Dongliang, Y.; Xin, Z.; Kathrin, S.; Mirko, T.; Mira, A.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Cont. 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Fabio, P.S.; Caetano, P.S.; Fernanda, V.C.; Martha, S.R. A Systematic scoping review of ultraviolet C (UVC) light systems for SARS-CoV-2 inactivation. J. Photochem. Photobiol. 2021, 8, 100068. [Google Scholar] [CrossRef]
- Matsie, M.; Ashwin, S.D.; Paul, A.J.; Stephen, N.R.; Tobias, H.R.; Marcello, A.P.; Wilhelm, L.; Tim, A.S.; Sonya, P.M.; Martie, W.; et al. Institutional Tuberculosis Transmission, Controlled Trial of Upper Room Ultraviolet Air Disinfection: A Basis for New Dosing Guidelines. Am. J. Respir. Crit. Care Med. 2015, 192, 477–484. [Google Scholar] [CrossRef]
- Sung, M.; Kato, S. Estimating the germicidal effect of upper-room UVGI system on exhaled air of patients based on ventilation efficiency. Build. Environ. 2011, 46, 2326–2332. [Google Scholar] [CrossRef]
- Edward, N.; Richard, V.; David, H.S. Upper-Room Ultraviolet Germicidal Irradiation (UVGI) for Air Disinfection: A Symposium in Print. Photochem. Photobiol. 2013, 89, 764–769. [Google Scholar] [CrossRef]
- Jangra, R.; Ahlawat, K.; Dixit, A.; Prakash, R. Efficient deactivation of aerosolized pathogens using a dielectric barrier discharge based cold-plasma detergent in environment device for good indoor air quality. Sci. Rep. 2023, 13, 10295. [Google Scholar] [CrossRef]
- Chu, C.H.; Chen, S.R.; Wu, C.H.; Cheng, Y.C.; Cho, Y.M.; Chang, Y.K. The effects of negative air ions on cognitive function: An event-related potential (ERP) study. Int. J. Biometeorol. 2019, 63, 1309–1317. [Google Scholar] [CrossRef]
| Microorganism | Outcome of Ionization * | Ion Type and Performance | Ozone Levels | References |
|---|---|---|---|---|
| Surrogates (particle sizes equivalent to pathogen dimensions) | Particle deposition | − ions better than bipolar ions | <5 ppb | [3] |
| Bacteria | ||||
| Staphylococcus epidermidis, Escherichia coli | Microbial destruction b (membrane disruption) | + ions better than − ions | <10 ppb | [10] |
| Staphylococcus epidermidis, Escherichia coli | Microbial destruction (membrane disruption) | + ions | 21.8–26.0 ppb | [14] |
| Staphylococcus epidermidis, Escherichia coli (see also viruses) | Microorganism inactivation | − ions | 3.0–3.5 ppb (emission rate 0.026 mg/h) | [15] |
| Staphylococcus epidermidis | Antibacterial effect Cell contraction | Bipolar ions eliminate bacteria Unipolar (+) activity not antibacterial | <25 ppb | [16] |
| Staphylococcus epidermidis, Serratia marcescens | Microbial inactivation | − ions | 68 ppb c | [17] |
| Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Enterococcus faecalis | Potent bactericidal effect | − and + air ions | ~35 ppb | [18] |
| Staphylococcus aureus | Microbial deposition Microbial destruction | − ions better than + ions | Not measured | [19] |
| Staphylococcus aureus, Escherichia coli | Decreased viability -On Petri dishes (104 CFU/mL): S. aureus up to 86% after 3 h exposure and 95% after 8–12 h; E. coli up to 51% after 3 h exposure and 70% after 8–12 h; -On filters soaked with 104 CFU/mL: S. aureus up to 78% on PP filters and 82% on PET filters after 3 h; E. coli up to 52% on PP filters and PET filters after 3 h. | − and + air ions | N/A | [20] |
| Staphylococcus aureus, Escherichia coli | Agglutination of microbial products, removed from air, microbicidal effects. More noticeable effect on gram + bacteria Exceptional antibacterial activity via oxidative damage | − and + air ions | Only passively mentioned | [21] |
| Staphylococcus aureus, Escherichia coli | Bactericidal, more noticeable effect on gram-bacteria | − air ionization with oxidation effect | N/A | [22] |
| Escherichia coli | Enhanced pathogenic removal efficiency a | + ions | N/A | [11] |
| Escherichia coli (see also viruses) | Complete inactivation with more than a 5-log reduction (99.999%) in 90 min | bipolar | <24 ppb | [23] |
| Escherichia coli (see also fungi) | Pre-charging enhances collection efficiency | − ions | N/A | [24] |
| Pseudomonas fluorescens, Bacillus atropheus (see also fungi) | Particle deposition (microorganism viability not measured) | − ions | 39 ppb | [25] |
| Pseudomonas fluorescens, Bacillus anthracis (see also fungi) | Easier and more efficient collection (from 70% without charge to 80–90%) | + ions | N/A | [26] |
| Pseudomonas fluorescens | Synergistic bactericidal action (decreased microbial load), morphologically deformed | Combined − air ions and C. citratus essential oil vapor | N/A | [27] |
| Pseudomonas fluorescens, Erwinia carotovora, Escherichia coli | Bactericidal effect, P. fluorescens most vulnerable | − air ions | Yes (noted synergy between ozone and NAIs) | [28] |
| Escherichia coli | Antibacterial | Released + ions from copper/silver (metals proved to be synergistic) | N/A | [29] |
| Escherichia coli | Disinfection | − ions generated by a cold plasma tube | No ozone, but oxygen species and oxygen-containing radicals, UV-C, and short-term heating of microorganisms | [30,31] |
| Escherichia coli | Inactivate and decontaminate E. coli via oxidation | − and + ions, free radicals, all fall into the category of the fourth state of matter (cold plasma) | Harnessed ozone as part of the study to maximize disinfection | [32] |
| Serratia marcescens | Significant bactericidal effects | − and + air ions (NAIs showing slightly stronger repercussions) | N/A | [33] |
| Pseudomonas veronii | Destroyed cells in a starved, and thus highly impenetrable, state via the predicted ionic porous formation of the cell wall (due to ion accumulation on surface) | Both − and + ions of electric corona | N/A | [34] |
| Bacillus subtilis | Antimicrobial effects, reduced number of bioaerosols | − air ions created by ionizer, tested with concurrent ozone | Yes | [35] |
| Campylobacter jejuni, E. coli, Salmonella enteritidis, Listeria monocytogenes, Staphylococcus aureus, Bacillus stearothermophilu | Significantly decreased microbial load (levels in biofilm) | Supercharged − air ions | N/A | [36] |
| Mycobacterium parafortuitum | Cell inactivation and biocidal qualities via electroporous mechanisms | − air ions | Yes, but not the principal cause of destruction | [37] |
| Mycobacterium tuberculosis | Prevented most airborne TB | − air ions | N/A | [38] |
| Legionella | Ions find the − charged cell walls of pathogens and destroy them | + ions in water systems | N/A | [39,40,41,42] |
| Salmonella enteritidis | Statistically significant decrease in infection via airborne transmission, attracted to ground surfaces, direct organism killing | − air ions | N/A | [43,44,45] |
| Staphylococcus albus | Bactericidal effects | − air ions in synergy with superoxide radical anion | Inadvertently, as superoxide radicals may be chain carriers for ozonation (O3 decomposition) and since it has been remarked that ozone and superoxide combine in corona discharge | [46] |
| Clostridioides difficile, drug-resistant strains of Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae (see also fungi and viruses) | Reduction Bacteria 94.4–99.9%; Virus 94%; | bipolar | 22–66 ppb | [23] |
| Bacterial/viral agents | Same polarity of ions on respiratory protective masks (N95 and surgical) leads to electrostatic protection | − ions | N/A | [47] |
| Bacteria (review) | Bactericidal effects | − ions | N/A | [48] |
| Fungi | ||||
| Penicillium notatum | Lowered penicillin production (mostly by − ions), reduced germination of spores (mostly by + ions), lowered CO2 production | − and + air ions | N/A | [49] |
| Penicillium chrysogenum (see also bacteria) | Particle deposition (microorganism viability not measured) | − ions | 39 ppb | [25] |
| Penicillium brevicompactum (see also bacteria) | Easier and more efficient collection (from 70% without charge to 80–90%) | + ions | N/A | [26] |
| Aspergillus fumigatus, Candida albicans (see also bacteria and viruses) | Reduction Fungi 32.4–87.3% | bipolar | 22–66 ppb | [23] |
| Candida albicans (see also bacteria) | Pre-charging enhances collection efficiency | − ions | N/A | [24] |
| Candida albicans (10 strains) | Inhibited growth | − air ions | Yes, additionally hypothesizes potential microbicidal role of ozone | [50] |
| Viruses | ||||
| H5N1 avian influenza virus | Neutralizes up to 26% of airborne pathogens | − ions | <50 ppb | [51,52] |
| RNA/DNA Viruses | Free ionic Ag+ inactivated ssRNA MS2 and ssDNA PhiX 174 (specifically in neutral and alkaline environments) | + charged copper/silver ions in water, synergistic effect | N/A | [53] |
| SARS-CoV-2 | Reduced aerosolized pathogens | − ions created by plant-based ionizer | No ozone detected | [54] |
| SARS-CoV-2 | Pathogens agglutinate and ‘fall’ down | − ions | Varies between generations/low concentration | [55,56] |
| SARS-CoV-2 and Influenza A virus | Inactivation—fixed on surfaces: >99.98% after 1 h of exposure; Disinfection—aerosolized: after 10 min of exposure at a 30 cm height—89.96% for SARS-CoV-2 and 91.27% for influenza A virus. At a 50 cm height, 87.77% for SARS-CoV-2 and 89.50% for the influenza A virus. | − ions | <50 ppb | [57] |
| Human coronavirus 229E | Reduction Virus 94%; | bipolar | 22–66 ppb | [23] |
| Newcastle disease virus | Facilitate pathogenic aerosol decay, wire-gauze completely prevented transmission | − ions | N/A | [58,59] |
| MS2 phage (see also bacteria and fungi) | Complete inactivation with more than a 5-log reduction (99.999%) in 30 min | bipolar | <24 ppb | [23] |
| MS2 bacteriophage, H1N1 influenza virus | Particle deposition | − ions | ~10 ppb (varying) | [60] |
| MS2 bacteriophage | Microbial inactivation | − ions | 1.6 ppb | [61] |
| MS2 bacteriophage | Reduction unipolar ions: 46.1%, 78.8%, and 83.7% after 15, 30, and 45 min of exposure, respectively, and up to 97.4% for bipolar ions | bipolar better than unipolar | unipolar ions: 2–10 ppb; bipolar ions: ~30 ppb | [4] |
| virus (P22 and Φ6 bacteriophages) (see also bacteria) | Microorganism inactivation | − ions | 3.0–3.5 ppb (emission rate 0.026 mg/h) | [15] |
| Φ6 bacteriophage (SARS-CoV-2 surrogate) | Particle removal Antiviral performance | − ions | Did not measure d | [62] |
| Canine calicivirus (CaCV), rhesus rotavirus (RRV), influenza A virus (H3N2) | Reduced infectivity of aerosolized CaCV and RRV (>97%); Active ionizer prevented 100% of guinea pigs from infection by H3N2. | − ions | <2 ppb | [63] |
| Air contaminants Removal Technology | Benefits | Limitations | Representative Examples |
|---|---|---|---|
| HEPA (High Efficiency Particulate Air) filters |
|
| [100,101,102,103,104] |
| UVGI (UltraViolet Germicidal Irradiation) |
|
| [105,106,107,108,109] |
| Ionizers also producing ozone |
|
| [18,28,37,61,99] |
| Ionizers with negligible ozone production |
|
| [10,62,63,110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radalj, A.; Nikšić, A.; Trajković, J.; Knezević, T.; Janković, M.; De Luka, S.; Djoković, S.; Mijatović, S.; Ilić, A.; Arandjelović, I.; et al. Combating Pathogens Using Carbon-Fiber Ionizers (CFIs) for Air Purification: A Narrative Review. Appl. Sci. 2024, 14, 7311. https://doi.org/10.3390/app14167311
Radalj A, Nikšić A, Trajković J, Knezević T, Janković M, De Luka S, Djoković S, Mijatović S, Ilić A, Arandjelović I, et al. Combating Pathogens Using Carbon-Fiber Ionizers (CFIs) for Air Purification: A Narrative Review. Applied Sciences. 2024; 14(16):7311. https://doi.org/10.3390/app14167311
Chicago/Turabian StyleRadalj, Andrea, Aleksandar Nikšić, Jelena Trajković, Tara Knezević, Marko Janković, Silvio De Luka, Stefan Djoković, Stefan Mijatović, Andjelija Ilić, Irena Arandjelović, and et al. 2024. "Combating Pathogens Using Carbon-Fiber Ionizers (CFIs) for Air Purification: A Narrative Review" Applied Sciences 14, no. 16: 7311. https://doi.org/10.3390/app14167311








