Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Substrate Sampling
2.2.1. Surface Water
2.2.2. Wetland Bottom Sediment
- Elemental Composition: Analyzed using X-ray fluorescence (XRF), which identifies and quantifies the elemental composition by measuring the fluorescent X-rays emitted from the sample [46].
- Sediment Texture Analysis: Performed using the pipette method, which separates and classifies sediment particles by size to determine the texture [47].
- Microbiological Diversity: Assessed through 16s RNA sequencing, a technique used to identify and characterize bacterial communities by sequencing the ribosomal RNA genes [48].
2.2.3. Precipitated Mineral Salts
2.2.4. Macrophyte Roots and Periphyton Sampling
2.3. Analytical Methods
2.3.1. X-ray Fluorescence Spectrometer
2.3.2. Pipette Method for Soil Texture Analysis
2.3.3. 16S rRNA Sequencing
2.3.4. Inductively Coupled Plasma Optical Emission Spectroscopy
2.3.5. Scanning Electron Microscopy
2.4. Statistical Analysis
3. Results and Discussion
3.1. Surface Water Quality
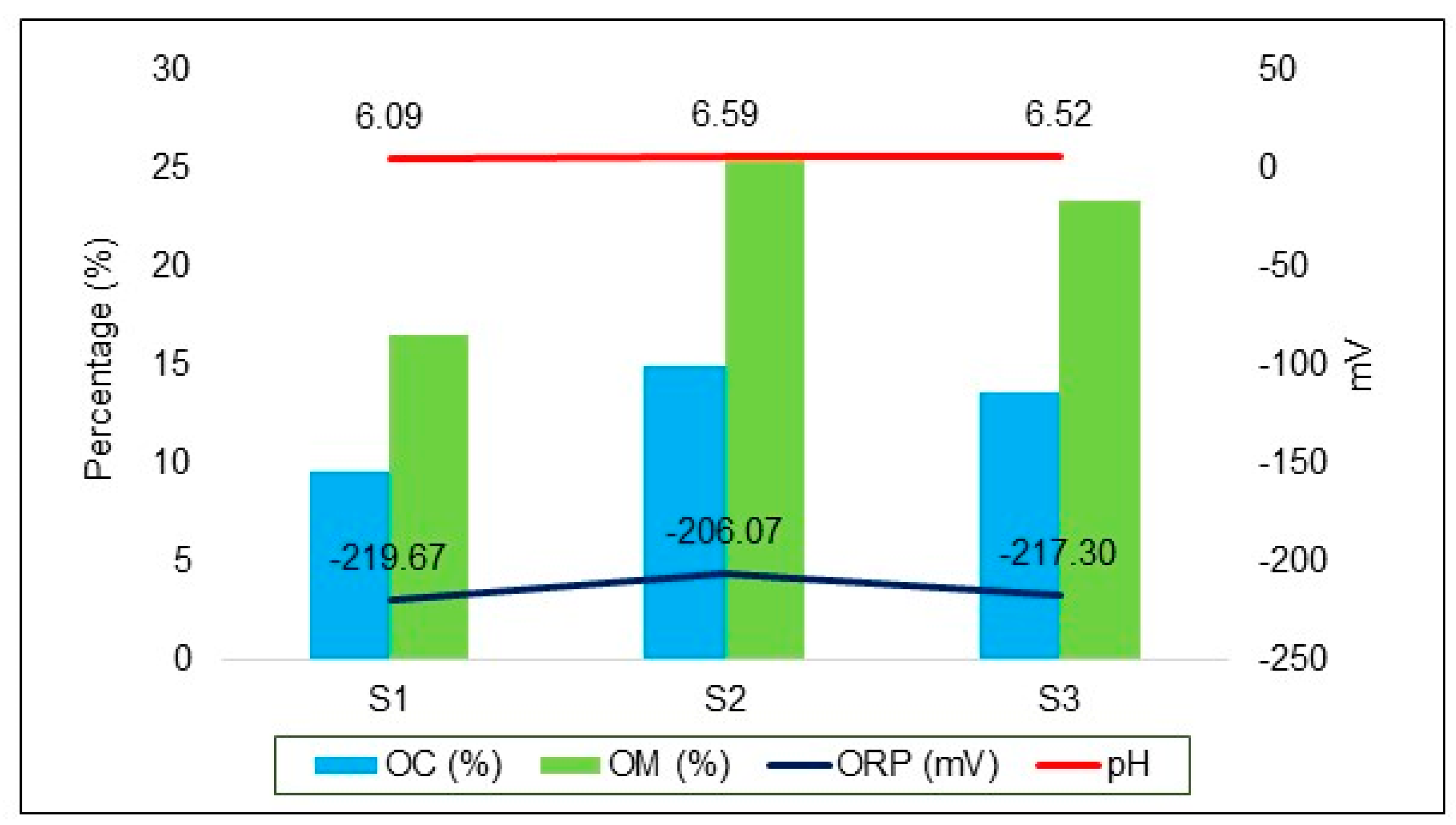
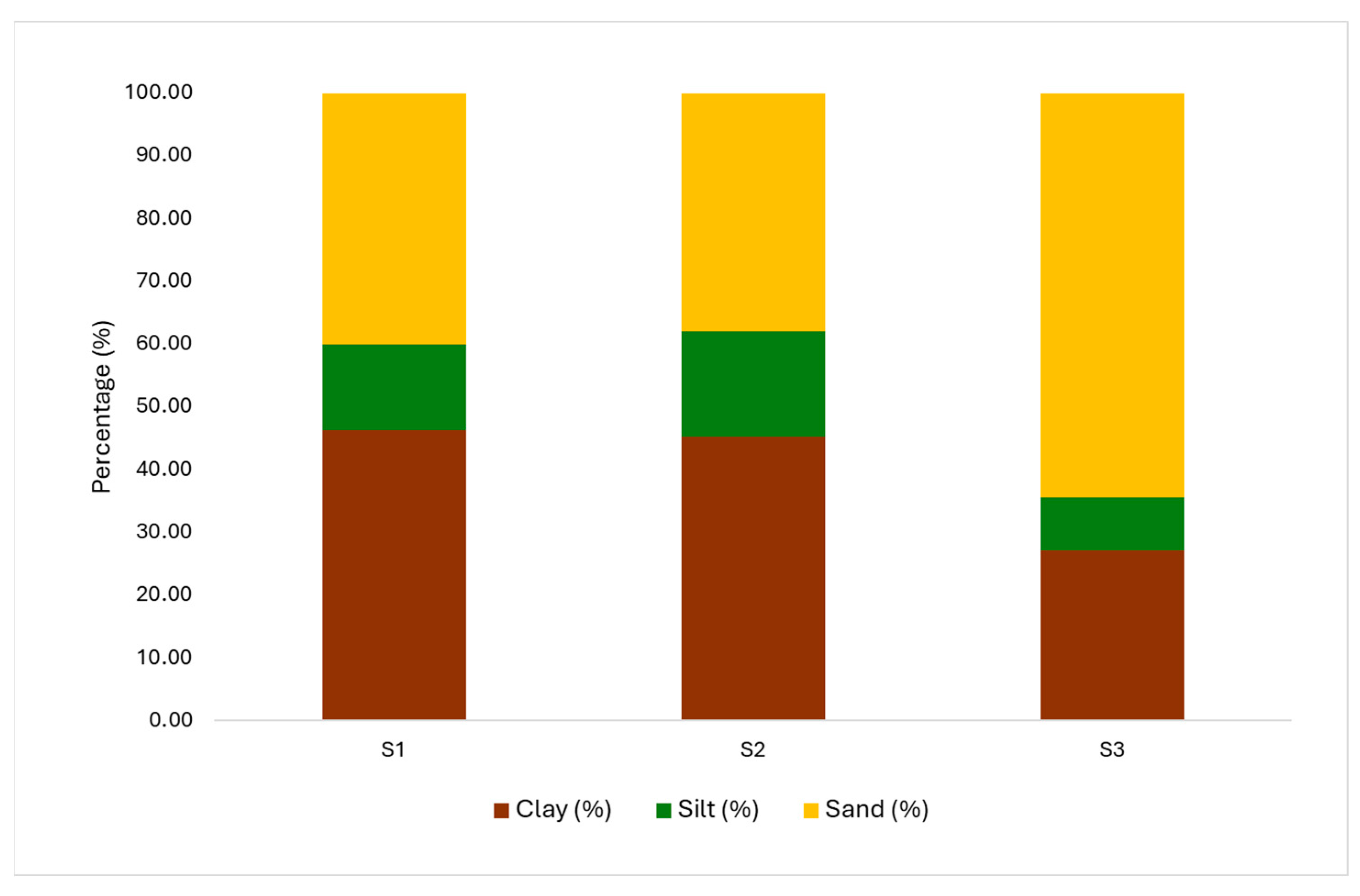
3.2. Bottom Sediment Characterization
3.3. Macrophyte Sorption Abilities
3.4. Periphyton Sorption Abilities
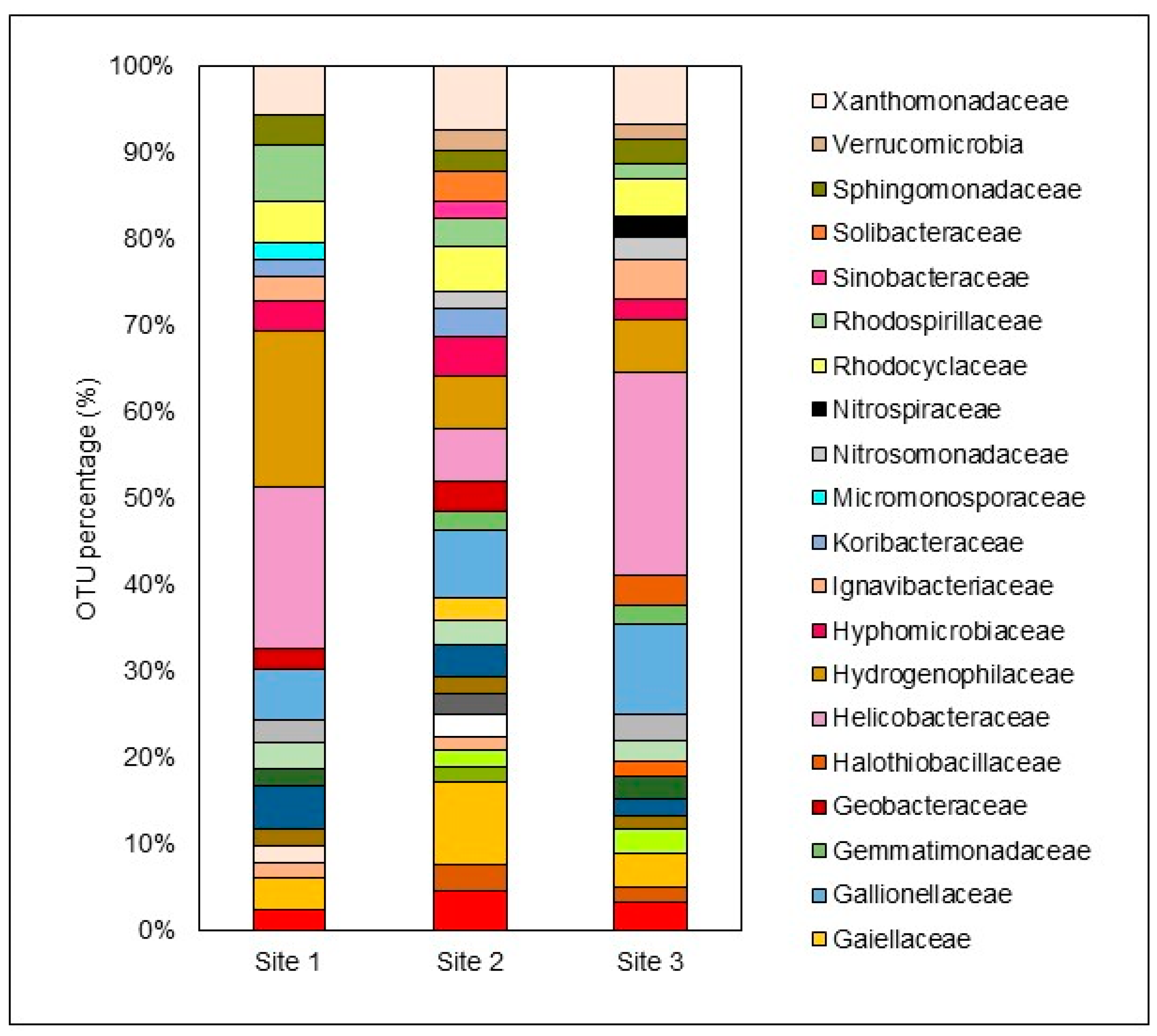
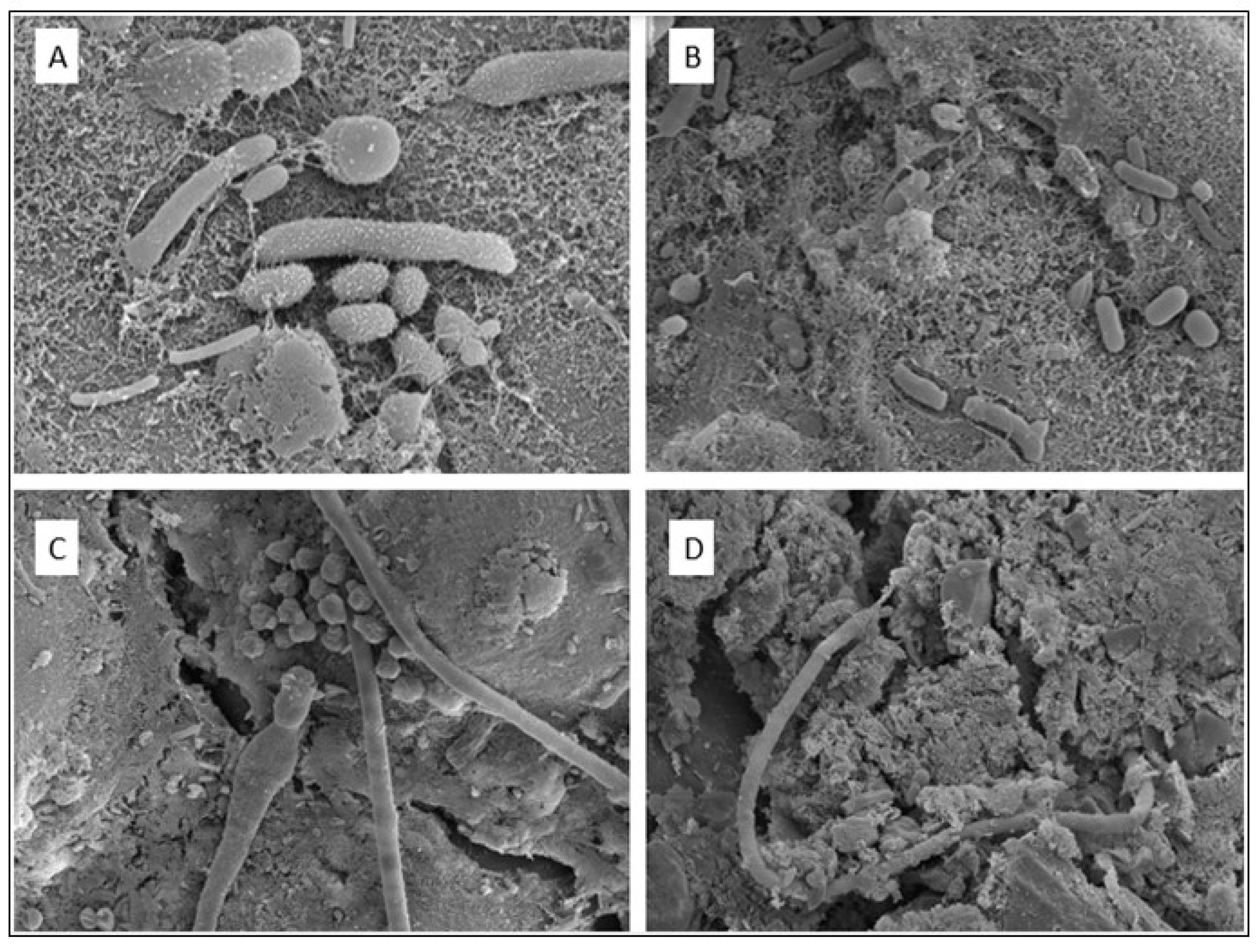
3.5. Microbiological Diversity
- S1:
- Helicobacteraceae > Hydrogenophilaceae > Rhodospirillaceae > Gallionellaceae > Xanthomonadaceae.
- S2:
- Acidobacteriaceae > Gallionellaceae > Xanthomonadaceae > Helicobacteraceae > Hydrogenophilaceae.
- S3:
- Helicobacteraceae > Gallionellaceae > Xanthomonadaceae > Hydrogenophilaceae > Rhodospirillaceae.
3.6. Precipitated Mineral Salts
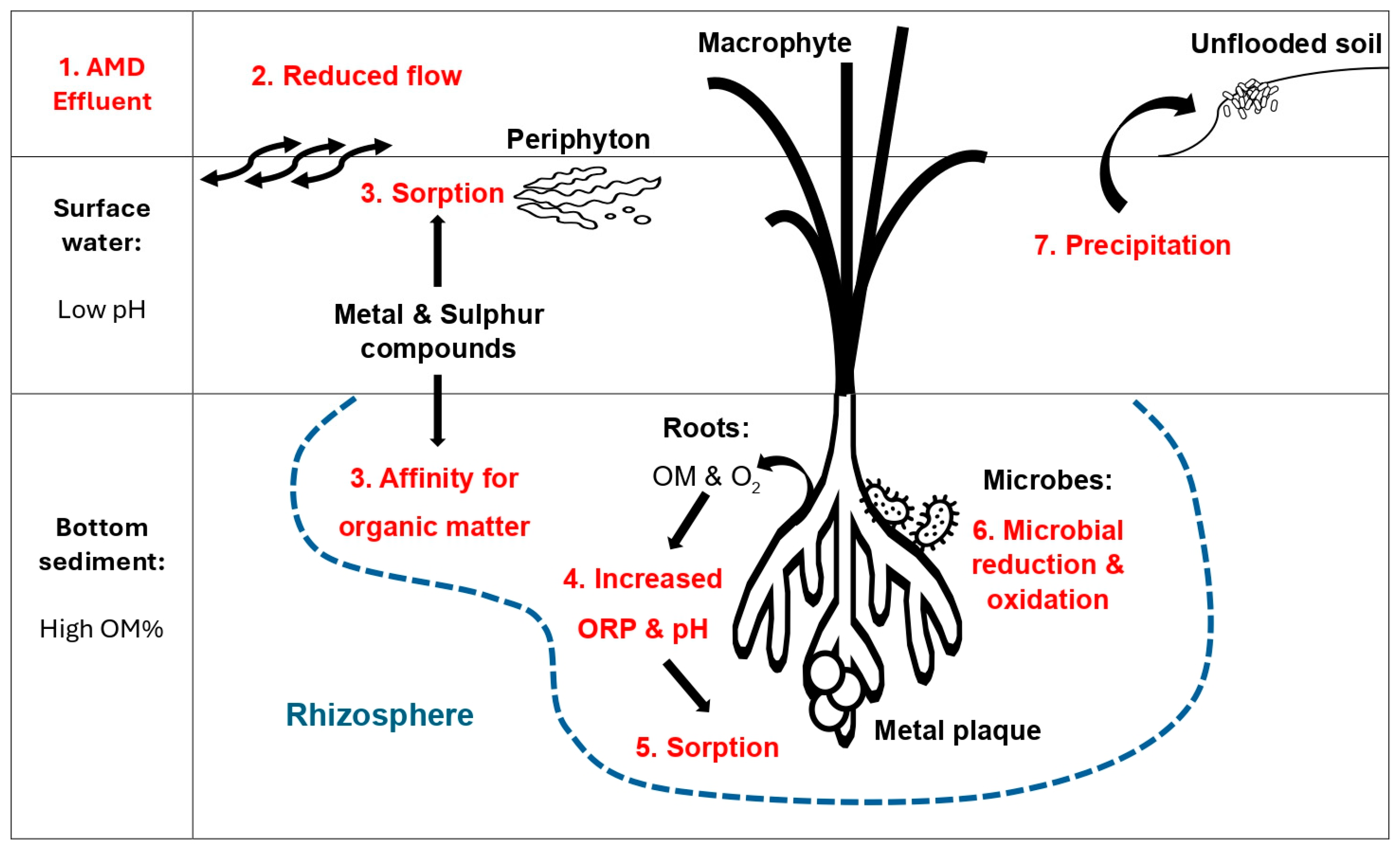
3.7. Ecological Engineering Interventions and Ecosystem Services
3.8. Integrated Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Acid mine drainage |
| EC | Electrical conductivity |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| OC | Organic carbon |
| OM | Organic matter |
| ORP | Oxidation−reduction potential |
| OTUs | Operational taxonomic units |
| SA | South Africa |
| SANAS | National Standard Accreditation System |
| SANBI | South African National Biodiversity Institute |
| SEM | Scanning electron microscopy |
| SRB | Sulfate-reducing bacteria |
| TDS | Total dissolved solids |
| US EPA | United States Environmental Protection Agency |
| XRF | X-ray fluorescence |
References
- Batty, L.C. Metal Removal Processes in Wetlands Receiving Acid Mine Drainage. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 1999. Available online: https://etheses.whiterose.ac.uk/15045/1/323042.pdf (accessed on 17 September 2023).
- MacFarlane, D.; Dlamini, B.; Marneweck, G.; Kassier, D.; Campbell, J.; Young, A.; Dini, J.A.; Holness, S.D.; De Klerk, A.R.; Oberholster, P.J.; et al. Wetland Rehabilitation in Mining Landscapes: An Introductory Guide. Wetlands in Working Landscapes; WRC Report No. TT 658/16; Water Research Commission: Pretoria, South Africa, 2016; Available online: https://www.wrc.org.za/wp-content/uploads/mdocs/TT%20658-16.pdf (accessed on 24 October 2023).
- Jackson, C.R.; Thompson, J.A.; Kolka, R.K. Wetland soils, hydrology and geomorphology. In Ecology of Freshwater and Estuarine Wetlands; Batzer, D., Sharitz, R., Eds.; University of California Press: Berkeley, CA, USA, 2014; pp. 23–60. Available online: https://www.fs.usda.gov/nrs/pubs/jrnl/2014/nrs_2014_jackson_001.pdf (accessed on 24 October 2023).
- Vymazal, J. Enhancing ecosystem services on the landscape with created, constructed and restored wetlands. Ecol. Eng. 2011, 37, 1–5. [Google Scholar] [CrossRef]
- Sobolewski, A. A review of processes responsible for metal removal in wetlands treating contaminated mine drainage. Int. J. Phytoremediat. 1999, 1, 9–51. [Google Scholar] [CrossRef]
- Matagi, S.; Swai, D.; Mugabe, R. A review of heavy metal removal mechanisms in wetlands. Afr. J. Trop. Hydrobiol. 1998, 8, 23–35. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- De Klerk, A.R.; Oberholster, P.J.; Van Wyk, J.H.; Truter, J.C.; Schaefer, L.M.; Botha, A.M. The effect of rehabilitation measures on ecological infrastructure in response to acid mine drainage from coal mining. Ecol. Eng. 2016, 95, 463–474. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes treatment case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Humphries, M.S.; McCarthy, T.S.; Pillay, L. Attenuation of pollution arising from acid mine drainage by a natural wetland on the Witwatersrand. S. Afr. J. Sci. 2017, 113, 9. [Google Scholar] [CrossRef]
- Petersen, J.F.; Sack, D.; Gabler, R.E. Physical Geography, 11th ed.; Cengage Learning: Boston, MA, USA, 2016; pp. 447–466. [Google Scholar]
- Hobbs, P.; Oelofse, S.H.; Rascher, J. Management of environmental impacts from coal mining in the upper Olifants River catchment as a function of age scale. Int. J. Water Resour. Dev. 2008, 24, 417–431. [Google Scholar] [CrossRef]
- Mdlalose, N.P.S. Evaluation of the Water Use Licensing Regime of the National Water Act in Advancing the Protection and Conservation of Water Resources. Master’s Dissertation, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2019. Available online: https://researchspace.ukzn.ac.za/handle/10413/18837 (accessed on 23 October 2023).
- Munnik, V.; Hochmann, G.; Hlabane, M.; Law, S. The Social and Environmental Consequences of Coal Mining in South Africa: A Case Study; Environmental Monitoring Group, Cape Town and Both ENDs: Amsterdam, The Netherlands, 2010; Available online: https://www.bothends.org/uploaded_files/uploadlibraryitem/1case_study_South_Africa_updated.pdf (accessed on 23 October 2023).
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Ford, K.L. Passive treatment systems for acid mine drainage Technical Note 409. U.S. Bur. Land Manag. 2003, 19. Available online: https://digitalcommons.unl.edu/usblmpub/19 (accessed on 23 March 2022).
- Gazea, B.; Adam, K.; Kontopoulos, A. A review of passive systems for the treatment of acid mine drainage. Miner. Eng. 1996, 9, 23–42. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Zipper, C.; Skousen, J. Passive treatment of acid mine drainage. In Acid Mine Drainage, Rock Drainage, and Acid Sulphate Soils: Causes, Assessment, Prediction, Prevention, and Remediation; Jacobs, J.A., Lehr, J.H., Testa, S.M., Eds.; Virginia State University: Petersburg, VA, USA, 2014; pp. 339–353. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation technologies for acid mine drainage: Recent trends and future perspectives. Chemosphere 2022, 311, 137089. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M. Passive mine water treatment: The correct approach? Ecol. Eng. 2004, 22, 299–304. [Google Scholar] [CrossRef]
- Grenfell, M.C.; Ellery, W.N.; Garden, S.E.; Dini, J.; Van der Valk, A.G. The language of intervention: A review of concepts and terminology in wetland ecosystem repair. Water SA 2007, 33, 43–50. [Google Scholar] [CrossRef][Green Version]
- Mander, Ü.; Mitsch, W.J. Biogeochemical aspects of ecosystem restoration and rehabilitation. Ecol. Eng. 2011, 37, 1003–1007. [Google Scholar] [CrossRef]
- Mitsch, W.J. What is ecological engineering? Ecol. Eng. 2012, 45, 5–12. [Google Scholar] [CrossRef]
- Odum, H.T.; Odum, B. Concepts and methods of ecological engineering. Ecol. Eng. 2003, 20, 339–361. [Google Scholar] [CrossRef]
- García, J. Advances in pollutant removal processes and fate in natural constructed wetlands. Ecol. Eng. 2011, 37, 663–805. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Lefeuvre, J.C.; Bouchard, V. Ecological engineering applied to river and wetland restoration. Ecol. Eng. 2002, 18, 529–541. [Google Scholar] [CrossRef]
- Madlala, T.; Kanyerere, T.; Oberholster, P.; Butler, M. Assessing the groundwater dependence of valley bottom wetlands in coal-mining environment using multiple environmental tracers, Mpumalanga, South Africa. Sustain. Water Resour. Manag. 2021, 7, 55. [Google Scholar] [CrossRef]
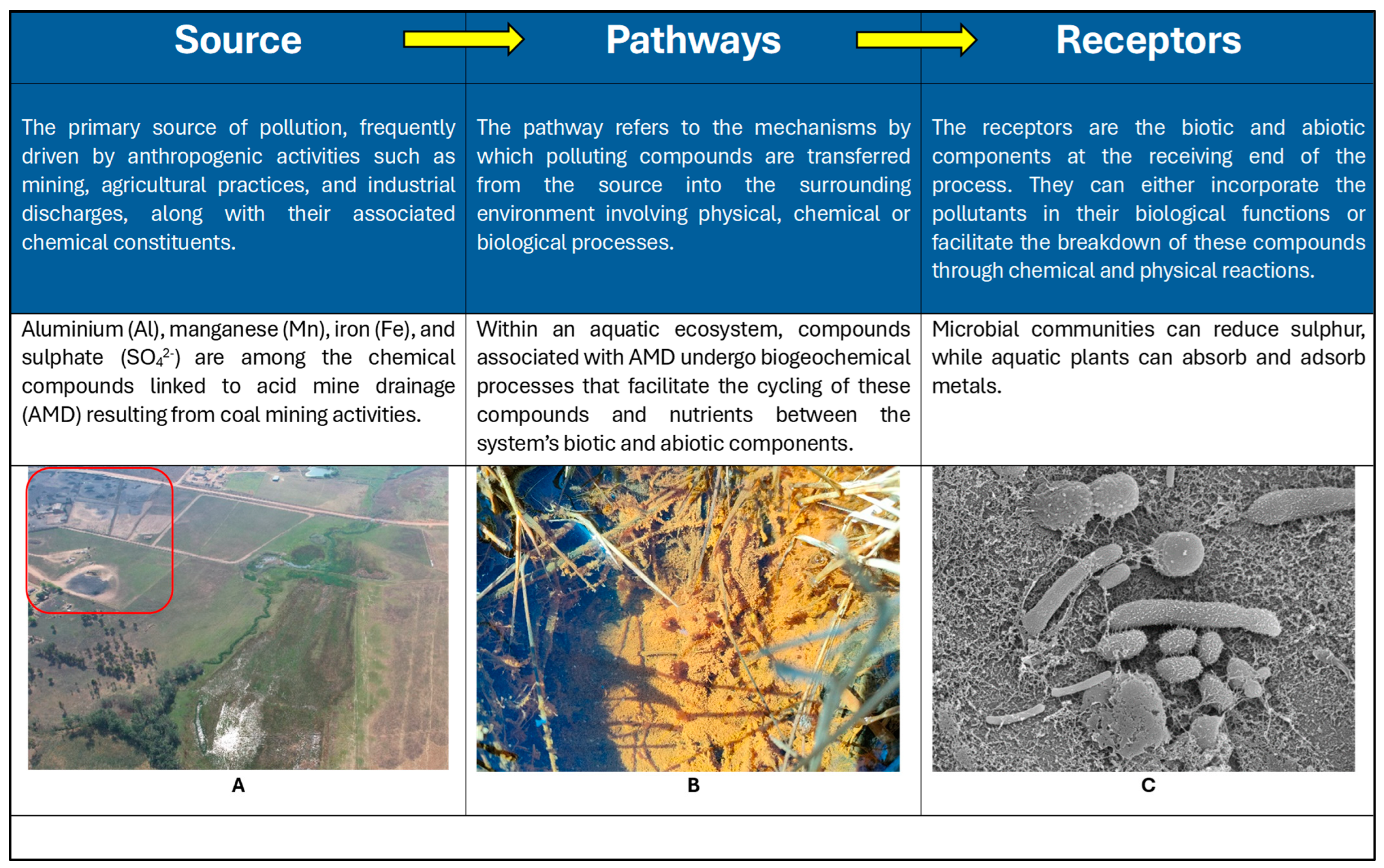
- Belle, G.; Schoeman, Y.; Oberholster, P.J. Potential toxic-element pollution in surface water and its implications for aquatic and human health: Source–pathway–receptor model. Water 2023, 15, 3100. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Botha, A.M.; Hill, L.; Strydom, W.F. River catchment responses to anthropogenic acidification in relationship with sewage effluent: An ecotoxicology screening application. Chemosphere 2017, 189, 407–417. [Google Scholar] [CrossRef]
- Van der Merwe, L.; Kempster, P.L.; Grobler, D.F. A note on the occurrence of metals in the Olifants River, Eastern Transvaal, South Africa. Water SA 1994, 20, 195–203. [Google Scholar] [CrossRef]
- Atangana, E.; Oberholster, P.J. Using heavy metal pollution indices to assess water quality of surface groundwater on catchment levels in South Africa. J. Afr. Earth Sci. 2021, 182, 104254. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; De Klerk, L.P. An assessment of the impact of different land use activities on water quality in the upper Olifants River catchment. Water SA 2013, 39, 231–244. [Google Scholar] [CrossRef]
- Nkhonjera, G.K. Understanding the impact of climate change on the dwindling water resources of South Africa, focusing mainly on Olifants River basin: A review. Environ. Sci. Policy 2017, 71, 19–29. [Google Scholar] [CrossRef]
- SANBI (South African National Biodiversity Institute). Biodiversity Geographic Information System. Map Data. 2018. Available online: http://bgisviewer.sanbi.org/Html5Viewer/Index.html?configBase=http://bgisviewer.sanbi.org/Geocortex/Essentials/REST/sites/Gauteng/viewers/Gauteng/virtualdirectory/Resources/Config/Default&user=&extent=&layerTheme=Gauteng%20C-Plan%203.3%20Terrestestrial%20CBAs (accessed on 24 August 2023).
- SANBI (South African National Biodiversity Institute). A Framework for Investing in Ecological Infrastructure in South Africa; SANBI: Pretoria, South Africa, 2014; Available online: https://www.sanbi.org/wp-content/uploads/2018/04/framework-ieimarch2014sanbi.pdf (accessed on 23 October 2023).
- SANBI (South African National Biodiversity Institute). The status of South Africa’s ecosystems and biodiversity. In National Biodiversity Assessments; SANBI: Pretoria, South Africa, 2018; Available online: https://biodiversityadvisor.sanbi.org/contentmanagement/index?guid=96f2b64e-da8f-48ec-8f1a-0d1ab770bf21 (accessed on 23 October 2023).
- Oberholster, P.J.; De Klerk, A.R.; Chamier, J.; Cho, M.; Crafford, J.; De Klerk, L.P.; Dini, J.A.; Harris, K.; Holness, S.D.; Le Roux, W.; et al. Assessment of the Ecological Integrity of the Zaalklapspruit Wetland in Mpumalanga (South Africa) before and after Rehabilitation: The Grootspruit Case Study; WRC Report 2230/2/16; Water Research Commission: Pretoria, South Africa, 2016; Available online: https://www.wrc.org.za/wp-content/uploads/mdocs/2230%20Volume%202%20-%20Jo.pdf (accessed on 23 October 2023).
- Department of Water Affairs and Forestry, South Africa. South African Water Quality Guidelines. In Volume 7: Aquatic Ecosystems, 2nd ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996. Available online: https://www.dws.gov.za/iwqs/wq_guide/edited/Pol_saWQguideFRESH_vol7_Aquaticecosystems.pdf (accessed on 24 October 2023).
- Defo, C.; Kaur, R.; Bharadwaj, A.; Lal, K.; Kumar, P. Modelling approaches for simulating wetland pollutant dynamics. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1371–1408. [Google Scholar] [CrossRef]
- Michelutti, B.; Wiseman, M. Engineered wetlands as a tailings rehabilitation strategy. In Restoration and Recovery of an Industrial Region; Gunn, J.M., Ed.; Springer: New York, NY, USA, 1995; pp. 135–141. [Google Scholar] [CrossRef]
- De Klerk, A.R.; De Klerk, L.P.; Chamier, J.; Wepener, V. Seasonal variations of water and sediment quality parameters in endorheic reed pans on the Mpumalanga Highveld. Water SA 2012, 38, 663–672. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual; Report no. EPA-823-F-01-023; US EPA: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/collectionmanual.pdf (accessed on 23 October 2023).
- APHA (American Public Health Association). Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 2006. [Google Scholar]
- Bucurică, I.; Dulama, I.; Radulescu, C.; Banica, A. Surface water quality assessment using electroanalytical methods and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Rom. J. Phys. 2022, 67, 1–14. [Google Scholar]
- Hennekam, R.; Sweere, T.; Tjallingii, R.; de Lange, G.J.; Reichart, G.-J. Trace metal analysis of sediment cores using a novel X-ray fluorescence core scanning method. Quat. Int. 2019, 514, 55–67. [Google Scholar] [CrossRef]
- Bansal, S.; Creed, I.F.; Tangen, B.A.; Bridgham, S.D.; Desai, A.R.; Krauss, K.W.; Neubauer, S.C.; Noe, G.B.; Rosenberry, D.O.; Trettin, C.; et al. Practical Guide to Measuring Wetland Carbon Pools and Fluxes. Wetlands 2023, 43, 105. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.; Marhova, M.; Kostadinova, S.; Gochev, V.; Tsankova, M.; Ivanova, A.; Yahubyan, G. Metagenomic analysis of the microbial community structure in protected wetlands in the Maritza River Basin. Biotechnol. Biotechnol. Equip. 2019, 33, 1721–1732. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T. Heavy metals in plants in constructed and natural wetlands: Concentration, accumulation and seasonality. Water Sci. Technol. 2015, 71, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.; Nascimento, D.; Curi, N.; Guilherme, L.; Carvalho, G.; Ribeiro, B. Comparison of portable X-ray fluorescence spectrometry and laboratory-based methods to assess the soil elemental composition: Applications for wetland soils. Environ. Technol. Innov. 2020, 19, 100826. [Google Scholar] [CrossRef]
- Shelton, L.R. Field Guide for Collecting and Processing Stream-Water Samples for the National Water-Quality Assessment Program; USGS Report no. 94-455; United States Geological Survey: Sacramento, CA, USA, 1994. [CrossRef]
- Hauer, F.R.; Lamberti, G.A. Methods in Stream Ecology, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2006; pp. 357–377. [Google Scholar]
- Oberholster, P.J.; Schoeman, Y.; Truter, J.C.; Botha, A.M. Using periphyton assemblage and water quality variables to assess the ecological recovery of an ecologically engineered wetland affected by acid mine drainage after a dry spell. Processes 2022, 10, 877. [Google Scholar] [CrossRef]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wedell, R.; Wolff, H. (Eds.) Handbook of Practical X-ray Fluorescence Analysis; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference Information Centre: Wageningen, The Netherlands, 2002; Available online: https://www.isric.org/sites/default/files/ISRIC_TechPap09.pdf (accessed on 23 October 2023).
- Azaroual, S.E.; Kasmi, Y.; Aasfar, A.; El Arroussi, H.; Zeroual, Y.; El Kadiri, Y.; Zrhridri, A.; Elfahime, E.; Sefiani, A.; Kadmiri, I.M. 16S Metagenomics investigation of bacterial diversity and prediction of its functionalities in Moroccan phosphate mine ecosystem. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.K.; Cheng, Y.; Zhang, S.; Zhao, H. A comparison of methods for clustering 16S rRNA sequences into OTUs. PLoS ONE 2013, 8, e70837. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Ion 16S Metagenomic Kit and Ion Reported Metagenomics Workflow Solution; Thermo Fisher Scientific: Waltham, MA, USA, 2016; Available online: https://www.thermofisher.com/za/en/home/life-science/sequencing/dna-sequencing/microbial-sequencing/microbial-identification-ion-torrent-next-generation-sequencing/ion-16s-metagenomics-solution.html (accessed on 23 October 2022).
- Williams, T.R. Analytical methods for atomic absorption spectrophotometry (Perkin-Elmer Corp). J. Chem. Educ. 1972, 49, A250. [Google Scholar] [CrossRef]
- Gómez-Arias, A.; Yesares, L.; Caraballo, M.A.; Maleke, M.; Vermeulen, D.; Nieto, J.M.; Van Heerden, E.; Castillo, J. Environmental and geochemical characterization of alkaline mine wastes from Phalaborwa (Palabora) Complex, South Africa. J. Geochem. Explor. 2021, 224, 106757. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for SEM of plant surfaces. Mater. Today 2010, 12, 32–43. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, J.; Zhang, P.; Wang, Q.; Wu, Y.; Ding, Y.; Wang, H.; Fu, C.; Sun, J. Review on strategies of close-to-natural wetland restoration and a brief case plan for a typical wetland in northern China. Chemosphere 2021, 285, 131534. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Sarma, H.P.; Devi, A. Sediment characterisation and spatial distribution of heavy metals in the sediment of a tropical freshwater wetland of Indo-Burmese province. Environ. Pollut. 2019, 250, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.S.; Paller, M.H.; Nelson, E.A.; Specht, W.L.; Halverson, N.V.; Gladden, J.B. Metal distribution stability in constructed wetland sediment. J. Environ. Qual. 2006, 35, 1948–1959. [Google Scholar] [CrossRef]
- O’Sullivan, A.D.; McCabe, O.M.; Murray, D.A.; Otte, M.L. Wetlands for rehabilitation of metal mine wastes. Biol. Environ. 1999, 99B, 11–17. [Google Scholar]
- Leenheer, J.A.; Croué, J.-P. Peer reviewed: Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Rao, Y.; Fu, T.; Wu, X. Influence of litter decomposition on iron and manganese in the sediments of wetlands for acid mine drainage treatments. Acta Geochim. 2019, 38, 68–77. [Google Scholar] [CrossRef]
- Palihakkara, P.D.B.J.; Vitharana, U.W.A. A time-efficient accurate texture analysing method for tropical soils. J. Agric. Value Addit. 2019, 2, 1–9. Available online: https://www.uwu.ac.lk/wp-content/uploads/1.palihakkara_and_vitharana_p_1-9.pdf (accessed on 23 October 2023).
- Sasaki, K.; Ogino, T.; Endo, Y.; Kurosawa, K. Field study on heavy metal accumulation in a natural wetland receiving acid mine drainage. Mater. Trans. 2001, 42, 1877–1884. [Google Scholar] [CrossRef]
- Lin, J.G.; Chen, S.Y. The relationship between adsorption of heavy metal and organic matter in river sediments. Environ. Int. 1998, 24, 345–352. [Google Scholar] [CrossRef]
- Engin, M.S.; Uyanik, A.; Kutbay, H.G. Accumulation of heavy metals in water, sediments and wetland plants of Kizilirmak Delta (Samsun, Turkey). Int. J. Phytoremediat. 2015, 17, 6–75. [Google Scholar] [CrossRef]
- Tarutis Jr, W.J.; Unz, R.F.; Brooks, R.P. Behavior of sedimentary Fe and Mn in a natural wetland receiving acidic mine drainage, Pennsylvania, USA. J. Appl. Geochem. 1992, 7, 77–85. [Google Scholar] [CrossRef]
- Mays, P.A.; Edwards, G.S. Comparison of heavy metal accumulation in a natural wetland and constructed wetlands receiving acid mine drainage. Ecol. Eng. 2001, 16, 487–500. [Google Scholar] [CrossRef]
- Scholz, M.; Lee, B.H. Constructed wetlands: A review. Int. J. Environ. Stud. 2005, 62, 421–447. [Google Scholar] [CrossRef]
- Fang, J.; Dong, J.; Li, C.; Chen, H.; Wang, L.; Lyu, T.; He, H.; Liu, J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern China. Appl. Soil Ecol. 2021, 168, 104141. [Google Scholar] [CrossRef]
- McNear, D.H., Jr. The rhizosphere—Roots, soil and everything in between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Crowder, A.A.; Coltman, D.W. Formation of manganese oxide plaque on rice roots in solution culture under varying pH and manganese (Mn2+) concentration conditions. J. Plant Nutr. 1993, 16, 589–599. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Cheng, P.H.; Botha, A.M.; Genthe, B. The potential of selected macroalgal species for treatment of AMD at different pH ranges in temperate regions. Water Res. 2014, 60, 82–92. [Google Scholar] [CrossRef][Green Version]
- Boekken, T.; Kroglund, F.; Lindstrom, E.-A.; Carvalho, L. Acidification of rivers and lakes. In Indicators and Methods for the Ecological Status Assessment under the Water Framework Directive: Linkage between Chemical and Biological Quality of Surface Waters; Solimini, A.G., Cardoso, A.C., Heiskanen, A.-S., Eds.; European Communities, Institute for Environment and Sustainability: Ispra, Italy, 2006. [Google Scholar]
- Novis, P.M.; Harding, J.S. Extreme Acidophiles. In Algae and Cyanobacteria in Extreme Environments. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 11, pp. 443–463. [Google Scholar] [CrossRef]
- Vymazal, J. Short-term uptake of heavy metals by periphyton algae. Hydrobiologia 1984, 119, 171–179. [Google Scholar] [CrossRef]
- Aguinaga, O.E.; Wakelin, J.F.; White, K.N.; Dean, A.P.; Pittman, J.K. The association of microbial activity with Fe, S and trace element distribution in sediment cores within a natural wetland polluted by acid mine drainage. Chemosphere 2019, 231, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.P.; Lynch, S.; Rowland, P.; Toft, B.D.; Pittman, J.K.; White, K.N. Natural wetlands are efficient at providing long-term metal remediation of freshwater systems polluted by acid mine drainage. Environ. Sci. Technol. 2013, 47, 12029–12036. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, L.; Xue, S.; Liu, B.; Jin, G. The recruitment of bacterial communities by the plant root system changed by acid mine drainage pollution in soils. FEMS Microbiol. Lett. 2020, 367, fnaa117. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A.; Brock, T. Brock Biology of Microorganisms, 14th ed.; Pearson: Upper Saddle River, NJ, USA, 2015. [Google Scholar]
- Drennan, D.; Lee, I.; Landkamer, L.; Figueroa, L.A.; Webb, S.; Sharp, J.O. Biogeochemistry of a Field-Scale Sulphate Reducing Bioreactor Treating Mining Influenced Water. In Proceedings of the American Geophysical Union, Fall Meeting, San Francisco, CA, USA, 3–7 December 2012; SAO/NASA Astrophysics Data System. Available online: https://ui.adsabs.harvard.edu/abs/2012AGUFM.B11A0394D/abstract (accessed on 18 February 2023).
- Drewniak, L.; Krawczyk, P.S.; Mielnicki, S.; Adamska, D.; Sobczak, A.; Lipinski, L.; Burec-Drewniak, W.; Sklodowska, A. Physiological and metagenomic analyses of microbial mats involved in self-purification of mine waters contaminated with heavy metals. Front. Microbiol. 2016, 7, 1252. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.C.; Adebusoye, S.A.; Ugoji, E.O.; Ilori, M.O.; Amund, O.O.; Hickey, W.J. Microbial communities in sediments of Lagos Lagoon, Nigeria: Elucidation of community structure and potential impacts of contamination by municipal and industrial wastes. Front. Microbiol. 2016, 7, 1213. [Google Scholar] [CrossRef]
- Zheng, T.; Lin, X.; Xu, J.; Ren, J.; Sun, D.; Gu, Y.; Huang, J. Enhanced nitrogen removal of steel rolling wastewater by constructed wetland combined with sulphur autotrophic denitrification. Sustainability 2021, 13, 1559. [Google Scholar] [CrossRef]
- Leitholf, I.M.; Fretz, C.E.; Mahanke, R.; Santangelo, Z.; Senko, J.M. An integrated microbiological and electrochemical approach to determine distributions of Fe metabolism in acid mine drainage-induced “iron mound” sediments. PLoS ONE 2019, 14, e0213807. [Google Scholar] [CrossRef]
- Biebl, H.; Pfennig, N. Isolation of members of the family Rhodospirillaceae. In The Prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria; Stolp, H., Trüper, H.G., Balows, A., Schlegel, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 267–273. [Google Scholar] [CrossRef]
- Oberholzer, M.M.; Oberholster, P.J.; Ndlela, L.L.; Botha, A.M.; Truter, J.C. Assessing alternative supporting organic materials for the enhancement of water reuse in subsurface constructed wetlands receiving acid mine drainage. Recycling 2022, 7, 41. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P.; Pédrot, M. Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma 2009, 154, 13–19. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Weber, K.P.; Gehder, M.; Legge, R.L. Assessment of changes in the microbial community of constructed wetland mesocosms in response to acid mine drainage exposure. Water Res. 2008, 42, 180–188. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Xu, C.; Huang, S.; Lin, Y.; Tolic, N.; Roscioli-Johnson, K.M.; Santschi, P.H.; Jaffe, P.R. Unique organic matter and microbial properties in the rhizosphere of a wetland soil. Environ. Sci. Technol. 2016, 50, 4169–4417. [Google Scholar] [CrossRef]




| Wetland Substrate | Assessment | Analytical Method |
|---|---|---|
| Surface water | Physical and chemical characteristics | Electrometric, conductimetric, inductively coupled plasma (ICP) spectrometry [44,45] |
| Bottom sediment | Elemental composition | X-ray fluorescence (XRF) [46] |
| Sediment texture analysis | Pipette method for sediment texture analysis [47] | |
| Microbiological diversity | 16s RNA sequencing [48] | |
| Macrophyte roots and periphyton | Metal adsorption and absorption | Inductively coupled plasma optical emission spectroscopy (ICP-OES) [49] |
| Unflooded soil precipitate | Mineralogy | X-ray fluorescence (XRF) [50] |
| Parameter | Unit | S1 | S2 | S3 | Change (S1:S3) | % Change (S1:S3) |
|---|---|---|---|---|---|---|
| pH | – | 3.90 | 3.84 | 7.18 | +3.28 | 84.10 |
| Alkalinity | mg/L CaCO3 | 22.00 | <5.00 | 143.00 | +121.00 | 550.00 |
| Electrical conductivity (EC) | mS/m | 178.6 | 180.50 | 152.10 | −26.5 | 14.84 |
| Total dissolved solids (TDSs) | mg/L | 1196.62 | 1209.35 | 1019.07 | −177.5 | 14.84 |
| Dissolved organic carbon (DOC) | mg/L | 5.18 | 5.17 | 16.00 | +10.82 | 208.88 |
| Aluminum (Al) | mg/L | 1.60 | 1.80 | <0.01 | −1.59 | 99.38 |
| Iron (Fe) | mg/L | 0.33 | 0.58 | 0.26 | −0.07 | 21.21 |
| Manganese (Mn) | mg/L | 10.80 | 10.90 | 37.10 | +26.30 | 243.52 |
| Sulfate (SO42−) | mg/L | 1310.00 | 579.00 | 774.00 | −536.00 | 40.07 |
| Sediment Characteristic | Unit | Average Concentration | Student’s t-Test (S1; S3) | ||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | Mean Difference | t | p Value | ||
| pH | – | 6.09 | 6.59 | 6.52 | −0.43 | −1.02 | 0.37 |
| Oxidation reduction potential (ORP) | mV | −219.67 | −206.07 | −217.30 | −2.37 | −0.02 | 0.99 |
| Organic carbon (OC) | % | 9.61 | 14.93 | 13.59 | −3.98 | −0.83 | 0.49 |
| Organic matter (OM) | % | 16.57 | 25.74 | 23.43 | −6.86 | −0.83 | 0.49 |
| Clay | % | 46.29 | 45.25 | 27.19 | 19.10 | 7.00 | 0.00 |
| Silt | % | 13.65 | 16.77 | 8.35 | 5.30 | 3.62 | 0.02 |
| Sand | % | 40.06 | 37.97 | 64.47 | −24.41 | −8.20 | 0.00 |
| Aluminum (Al2O3) | wt % | 5.19 | 5.87 | 4.55 | 0.64 | 1.29 | 0.29 |
| Iron (Fe2O3) | wt % | 4.36 | 4.79 | 4.41 | −0.05 | −0.08 | 0.94 |
| Manganese (MnO) | wt % | 0.06 | 0.03 | 0.07 | −0.02 | −2.24 | 0.11 |
| Sulfur (SO3) | wt % | 0.64 | 0.56 | 0.63 | 0.00 | 0.21 | 0.85 |
| Element | Unit | Average Concentration | Student’s t-Test (S1; S3) | ||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | Mean Difference | t | p Value | ||
| Adsorption | |||||||
| Aluminum (Al) | (mg/kg) | 1.77 | 0.56 | 0.22 | 1.55 | 14.98 | 0.00 |
| Iron (Fe) | (mg/kg) | 0.54 | 0.72 | 0.57 | −0.03 | −0.55 | 0.62 |
| Manganese (Mn) | (mg/kg) | 2.24 | 14.09 | 7.66 | −5.42 | −257.72 | 0.00 |
| Absorption | |||||||
| Aluminum (Al) | (mg/kg) | 192.87 | 154.82 | 178.74 | 14.13 | 8.92 | 0.00 |
| Iron (Fe) | (mg/kg) | 174.11 | 487.33 | 364.01 | −189.90 | −135.94 | 0.00 |
| Manganese (Mn) | (mg/kg) | 6.62 | 19.68 | 74.35 | −67.73 | −117.96 | 0.00 |
| Element | Unit | Average Concentration | Student’s t-Test (S1; S2) | |||
|---|---|---|---|---|---|---|
| S1 | S2 | Mean Difference | T | p Value | ||
| Adsorption | ||||||
| Aluminum (Al) | (mg/kg) | 0.48 | 0.44 | 0.04 | 0.38 | 0.73 |
| Iron (Fe) | (mg/kg) | 0.79 | 0.78 | 0.01 | 0.22 | 0.84 |
| Manganese (Mn) | (mg/kg) | 1.13 | 0.37 | 0.76 | 56.24 | 0.00 |
| Absorption | ||||||
| Aluminum (Al) | (mg/kg) | 170.3 | 38.61 | 131.68 | 572.71 | 0.00 |
| Iron (Fe) | (mg/kg) | 284.78 | 241.43 | 43.34 | 45.63 | 0.00 |
| Manganese (Mn) | (mg/kg) | 8.91 | 4.41 | 4.50 | 199.84 | 0.00 |
| Element | Unit | S1 | S2 | S3 |
|---|---|---|---|---|
| Aluminum (Al2O3) | (wt %) | 1.42 | 1.53 | 1.78 |
| Iron (Fe2O3) | (wt %) | 1.69 | 1.40 | 1.95 |
| Manganese (MnO) | (wt %) | 0.10 | 0.10 | 0.08 |
| Sulfur (SO3) | (wt %) | 18.50 | 18.56 | 13.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen van Vuuren, M.; Schoeman, Y.; Botha, A.-M.; Oberholster, P.J. Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa. Appl. Sci. 2024, 14, 7441. https://doi.org/10.3390/app14177441
Jansen van Vuuren M, Schoeman Y, Botha A-M, Oberholster PJ. Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa. Applied Sciences. 2024; 14(17):7441. https://doi.org/10.3390/app14177441
Chicago/Turabian StyleJansen van Vuuren, Mariette, Yolandi Schoeman, Anna-Maria Botha, and Paul J. Oberholster. 2024. "Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa" Applied Sciences 14, no. 17: 7441. https://doi.org/10.3390/app14177441
APA StyleJansen van Vuuren, M., Schoeman, Y., Botha, A.-M., & Oberholster, P. J. (2024). Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa. Applied Sciences, 14(17), 7441. https://doi.org/10.3390/app14177441









