Spray Dried Cashew (Anacardium occidentale L.) Juice Ingredients as an Upcycling Strategy for Abundant Cashew Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Spray-Dried Cashew Juice
2.3. Physicochemical Characterization of Spray-Dried Cashew Particles
2.3.1. Particle Size, True Density, Water Activity (aw), and pH
2.3.2. Solubility and Hygroscopicity
2.3.3. Instrumental Color
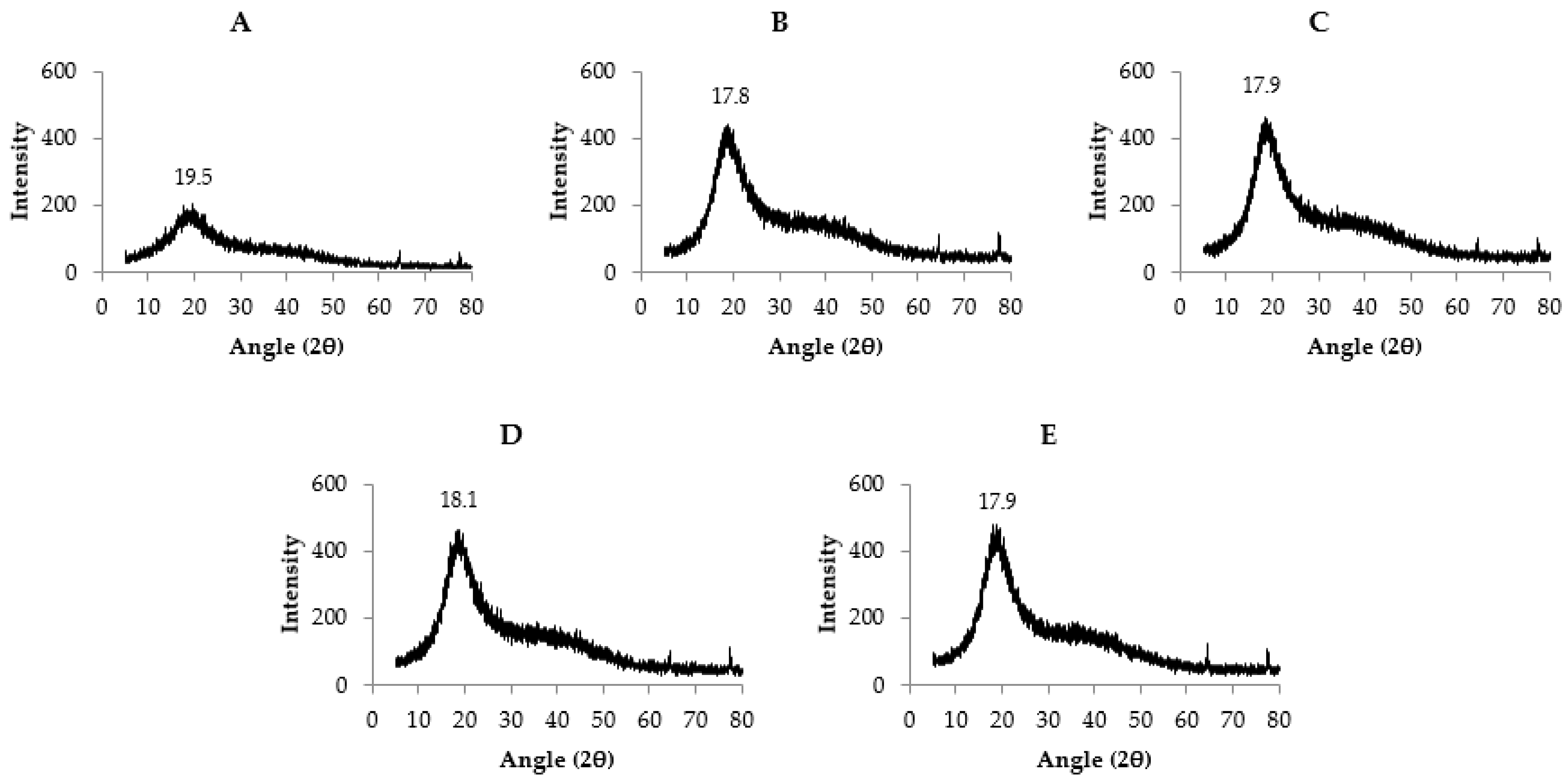
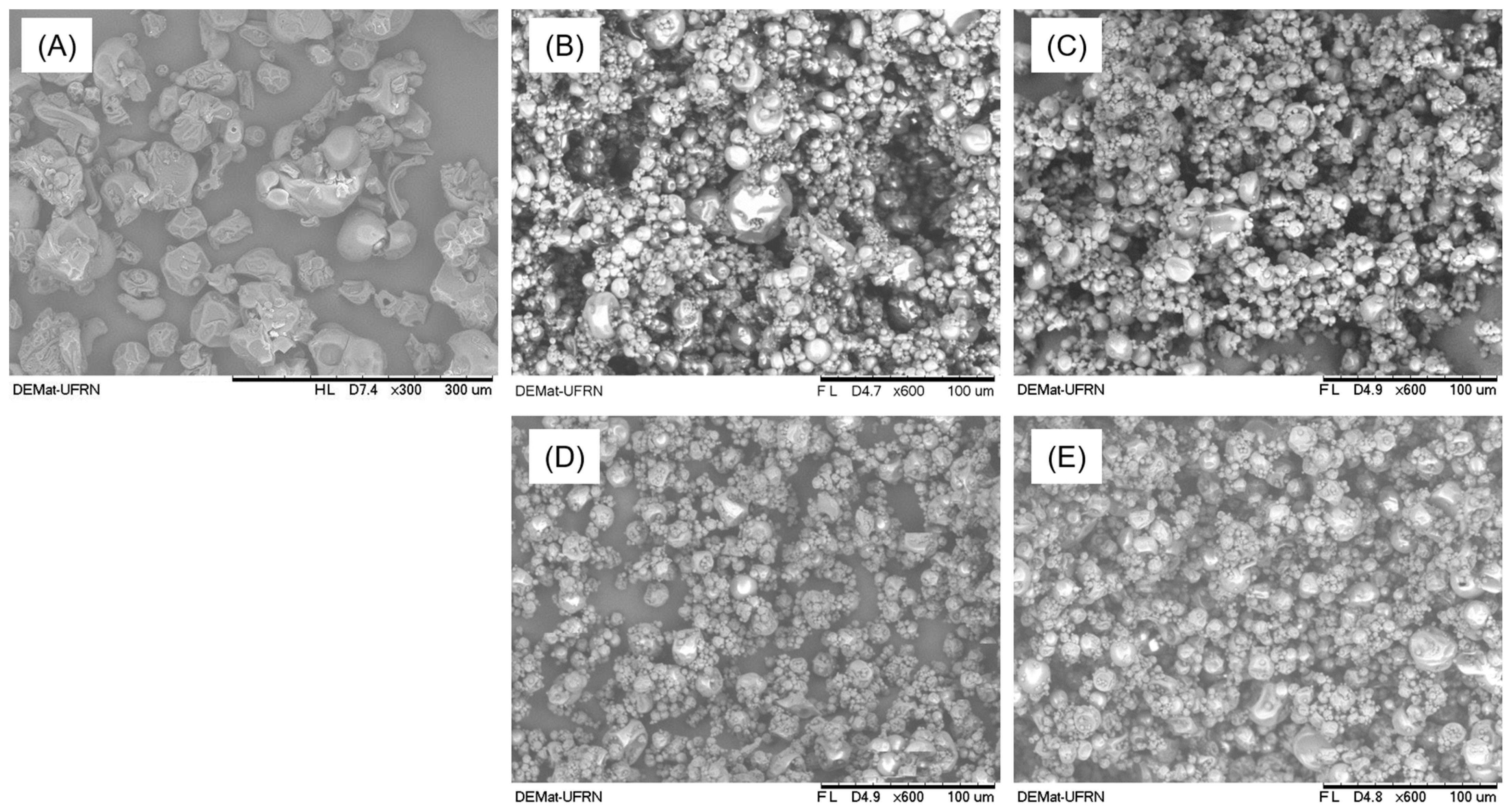
2.3.4. X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM)
2.4. Phytochemical Characterization
2.4.1. Total Phenolic Content (TPC)
2.4.2. Ascorbic Acid Determination
2.4.3. Carotenoid Quantification
2.4.4. Antioxidant Activity
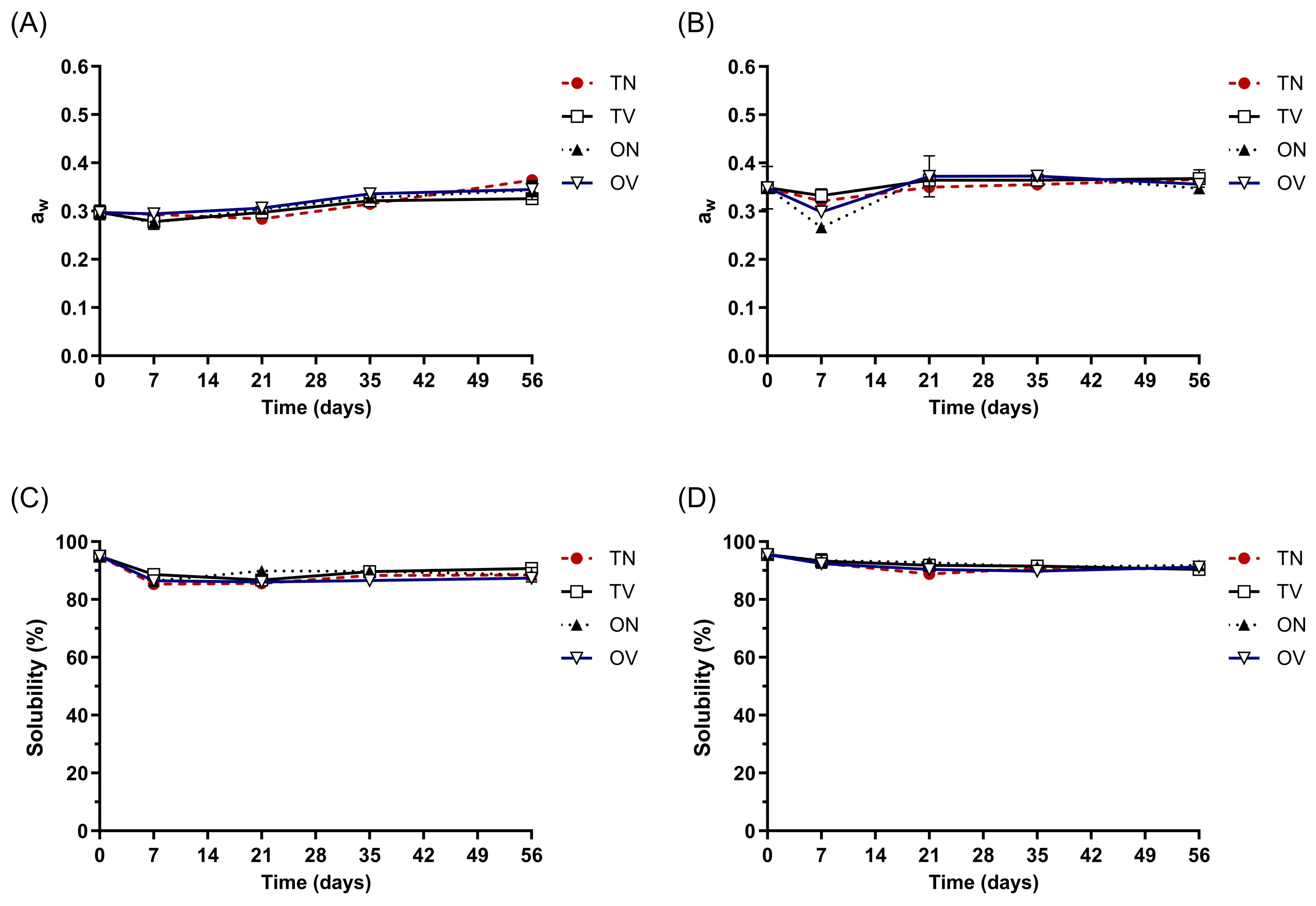
2.5. Evaluation of Storage Stability
2.6. Statistical Analyses
3. Results and Discussion
3.1. Spray Drying Performance: Solids Recovery
3.2. Physicochemical Attributes of Spray-Dried Cashew Particles
3.3. Microstructure and Morphology
3.4. Phytochemical Attributes
3.5. Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loureiro, K.C.; Jäger, A.; Pavlova, E.; Lima-Verde, I.B.; Štěpánek, P.; Sangenito, L.S.; Santos, A.L.S.; Chaud, M.V.; Barud, H.S.; Soares, M.F.L.R.; et al. Cashew gum (Anacardium occidentale) as a potential source for the production of tocopherol-loaded nanoparticles: Formulation, release profile and cytotoxicity. Appl. Sci. 2021, 11, 8467. [Google Scholar] [CrossRef]
- Dakuyo, R.; Konaté, K.; Kaboré, K.; Sanou, A.; Konkobo, F.A.; Bazié, D.; Sama, H.; Dicko, M.H. Ascorbic acid, pigments, anti-nutritional factors, and nutraceutical potential of Anacardium occidentale fruits as affected by temperature. Int. J. Food Prop. 2023, 26, 471–488. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, L.; Zhang, B.; Huang, W.; Zhang, Z.; An, B. Integrated analysis of the metabolome and transcriptome provides insights into anthocyanin biosynthesis of cashew apple. Food Res. Int. 2024, 175, 113711. [Google Scholar] [CrossRef]
- Oliveira, N.N.; Mothé, C.G.; Mothé, M.G.; de Oliveira, L.G. Cashew nut and cashew apple: A scientific and technological monitoring worldwide review. J. Food Sci. Technol. 2020, 57, 12–21. [Google Scholar] [CrossRef]
- Kham, N.N.N.; Phovisay, S.; Unban, K.; Kanpiengjai, A.; Saenjum, C.; Lumyong, S.; Shetty, K.; Khanongnuch, C. Valorization of Cashew Apple Waste into a Low-Alcohol, Healthy Drink Using a Co-Culture of Cyberlindnera rhodanensis DK and Lactobacillus pentosus A14-6. Foods 2024, 13, 1469. [Google Scholar] [CrossRef]
- Sobhana, A.; Mini, C. Entrepreneurship Ventures in Cashew Processing. In Entrepreneurship and Skill Development in Horticultural Processing; New India Publishing Agency: New Delhi, India, 2021; pp. 139–159. ISBN 9781000507775. [Google Scholar]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Kannan, V.; Rangarajan, V.; Manjare, S.D.; Pathak, P.V. Microbial production of value-added products from cashew apples—An economical boost to cashew farmers. J. Pure Appl. Microbiol. 2021, 15, 1816–1832. [Google Scholar] [CrossRef]
- Sahie, L.B.C.; Soro, D.; Kone, K.Y.; Assidjo, N.E.; Yao, K.B. Some Processing Steps and Uses of Cashew Apples: A Review. Food Nutr. Sci. 2023, 14, 38–57. [Google Scholar] [CrossRef]
- Jeyavishnu, K.; Thulasidharan, D.; Shereen, M.F.; Arumugam, A. Increased Revenue with High Value-Added Products from Cashew Apple (Anacardium occidentale L.)—Addressing Global Challenges. Food Bioprocess Technol. 2021, 14, 985–1012. [Google Scholar] [CrossRef]
- de Sena Andrade, R.A.M.; da Silva, D.C.; de Souza, M.M.B.; de Oliveira, R.L.; Maciel, M.I.S.; Porto, A.L.F.; de Almeida Melo, E.; de Andrade Lima Arruda, L.L.; Porto, T.S. Microencapsulation of phenolic compounds from cashew apple (Anacardium occidentale L.) agro-food waste: Physicochemical characterization, antioxidant activity, biodisponibility and stability. Food Chem. Adv. 2023, 3, 100364. [Google Scholar] [CrossRef]
- Santos, N.H.; Zapata, J.; Dereix, J.D.; Escobar, J.; de Almeida, A.B.; Silva, F.G.; Egea, M.B. The Active Aroma of “Cerrado” Cashew and Cagaita Fruits: Comparison between Two Extraction Methods. Appl. Sci. 2022, 12, 3330. [Google Scholar] [CrossRef]
- Akyereko, Y.G.; Yeboah, G.B.; Wireko-Manu, F.D.; Alemawor, F.; Mills-Robertson, F.C.; Odoom, W. Nutritional value and health benefits of cashew apple. JSFA Rep. 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Adegunwa, M.O.; Kayode, B.I.; Kayode, R.M.O.; Akeem, S.A.; Adebowale, A.A.; Bakare, H.A. Characterization of wheat flour enriched with cashew apple (Anacardium occidentale L.) fiber for cake production. J. Food Meas. Charact. 2020, 14, 1998–2009. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.T.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Use of Cashew Apple Pomace Powder in Pasta Making: Effects of Powder Ratio on the Product Quality. Polish J. Food Nutr. Sci. 2023, 73, 50–58. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Silva, E.S.; Moncada, M.; Perkins-Veazie, P.; Lila, M.A.; Greenlief, C.M.; Thomas, A.L.; Hoskin, R.T.; Krishnaswamy, K. Spray drying to produce novel phytochemical-rich ingredients from juice and pomace of American elderberry. Food Biosci. 2023, 55, 102981. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Grace, M.H.; Guiotto, A.; Pecorelli, A.; Valacchi, G.; Lila, M.A. Development of Spray Dried Spirulina Protein-Berry Pomace Polyphenol Particles to Attenuate Pollution-Induced Skin Damage: A Convergent Food-Beauty Approach. Antioxidants 2023, 12, 1431. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Grace, M.H.; Xiong, J.; Lila, M.A. Spray-drying microencapsulation of blackcurrant and cocoa polyphenols using underexplored plant-based protein sources. J. Food Sci. 2023, 88, 2665–2678. [Google Scholar] [CrossRef]
- Arumugham, T.; Krishnamoorthy, R.; AlYammahi, J.; Hasan, S.W.; Banat, F. Spray dried date fruit extract with a maltodextrin/gum arabic binary blend carrier agent system: Process optimization and product quality. Int. J. Biol. Macromol. 2023, 238, 124340. [Google Scholar] [CrossRef]
- de Jesus Silva, J.; Costa, T.V.; Santos, M.L.; Silva e Silva, L.T.; Santos, P.H.; de Carvalho Tavares, I.M.; Chaves, M.A. Encapsulation of açaí (Euterpe oleracea) pulp with whey protein isolate by spray-drying: An optimization study using response surface methodology (RSM). Food Humanit. 2023, 1, 1539–1546. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum Arabic, maltodextrin and inulin as coating materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Maia, G.A.; de Figueiredo, R.W.; de Souza, A.C.R.; de Brito, E.S.; de Azeredo, H.M.C. Addition of cashew tree gum to maltodextrin-based carriers for spray drying of cashew apple juice. Int. J. Food Sci. Technol. 2009, 44, 641–645. [Google Scholar] [CrossRef]
- Maia, P.D.D.S.; dos Santos Baião, D.; da Silva, V.P.F.; de Araújo Calado, V.M.; Queiroz, C.; Pedrosa, C.; Valente-Mesquita, V.L.; Pierucci, A.P.T.R. Highly Stable Microparticles of Cashew Apple (Anacardium occidentale L.) Juice with Maltodextrin and Chemically Modified Starch. Food Bioprocess Technol. 2019, 12, 2107–2119. [Google Scholar] [CrossRef]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild blueberry polyphenol-protein food ingredients produced by three drying methods: Comparative physico-chemical properties, phytochemical content, and stability during storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Araújo, A.D.A.; Coelho, R.M.D.; Fontes, C.P.M.L.; Silva, A.R.A.; Da Costa, J.M.C.; Rodrigues, S. Production and spouted bed drying of acerola juice containing oligosaccharides. Food Bioprod. Process. 2015, 94, 565–571. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The impact of hot air drying on the physical-chemical characteristics, bioactive compounds and antioxidant activity of acerola (Malphigia emarginata) residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Cheplick, S.; Kwon, Y.I.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour. Technol. 2010, 101, 404–413. [Google Scholar] [CrossRef]
- Moraes, F.P.d.; Gonçalves, A.C.; Miguel, T.B.V.; Borges, K.C.; Correia, R.T.P. Freeze Dried Acerola (Malpighia emarginata) Pulp and Pomace: Physicochemical Attributes, Phytochemical Content and Stability during Storage. J. Food Ind. 2017, 1, 17. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschman, C. Chorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, Supplement 1 Unit F4.3, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Baltacıoğlu, C.; Keskin, O.; Baltacıoğlu, H.; Ağçam, E. Encapsulation and drying methods in the production of powdered red cabbage (Brassica oleracea L.): Chemometrics and Fourier transform infrared spectroscopy. Food Sci. Technol. Int. 2024, 1–12. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res. Int. 2012, 48, 478–483. [Google Scholar] [CrossRef]
- Heidari, M.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Ahmadzadeh Nobari Azar, F.; Mohammadi, M.; Ghorbani, M. Microencapsulation of Vitis vinifera grape pulp phenolic extract using maltodextrin and its application in gummy candy enrichment. Food Sci. Nutr. 2024, 12, 3405–3416. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Rohini, C.; Kanchana, S.; Geetha, P.S.; Mini, M.L.; Arul, A.P. Optimize the process for encapsulated spray dried Lemon juice powder to enhance the Vitamin C. World J. Adv. Res. Rev. 2024, 21, 2064–2072. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried beetroot juice concentrate. Food Biosci. 2016, 14, 21–27. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Almeida, F.D.L.; Lima, M.A.; da Costa, J.M.C.; Rodrigues, S. Spray-Drying of Probiotic Cashew Apple Juice. Food Bioprocess Technol. 2014, 7, 2492–2499. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of fingered citron extract with gum arabic, modified starch, whey protein, and maltodextrin using spray drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Martin, L.G.P.; Fakhouri, F.M.; Oliveira, R.A. de Microencapsulation of blackberry pulp with arrowroot starch and gum arabic mixture by spray drying. J. Microencapsul. 2018, 35, 482–493. [Google Scholar] [CrossRef]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Mas, M.A.; Dewi, D.P.A.P.; Surjawan, I.; Arista, D.; Amelia, V.; Surjadi, A.; Kho, K. Production of red fruit (Pandanus conoideus) oil powder using spray drying and freeze drying. Int. J. Food Eng. 2023, 19, 211–224. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Rajkumar, H.; Ganesan, N.D. Effects of freeze-drying process on the production of cashew apple powder: Determination of bioactive compounds and fruit powder properties. J. Food Process. Preserv. 2021, 45, e15466. [Google Scholar] [CrossRef]
- Saikia, S.; Kumar Mahnot, N.; Lata Mahanta, C.; Chattopadhyay, P.; Agnihotri, A. Optimisation of a carambola pomace fibre fortified mix fruit beverage powder, its characterization and in vivo study. J. Saudi Soc. Agric. Sci. 2020, 19, 14–21. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray drying orange juice concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Saadat, S.; Movahhed, S.; Ahmadi Chenarbon, H. Effect of guar and arabic gums on qualitative properties of extruded rice. J. Food Process Eng. 2019, 42, e12959. [Google Scholar] [CrossRef]
- Bashir, O.; Hussain, S.Z.; Ameer, K.; Amin, T.; Beenish; Ahmed, I.A.M.; Aljobair, M.O.; Gani, G.; Mir, S.A.; Ayaz, Q.; et al. Influence of Anticaking Agents and Storage Conditions on Quality Characteristics of Spray Dried Apricot Powder: Shelf Life Prediction Studies Using Guggenheim-Anderson-de Boer (GAB) Model. Foods 2023, 12, 171. [Google Scholar] [CrossRef]
- Šavikin, K.; Nastić, N.; Janković, T.; Bigović, D.; Miličević, B.; Vidović, S.; Menković, N.; Vladić, J. Effect of Type and Concentration of Carrier Material on the Encapsulation of Pomegranate Peel Using Spray Drying Method. Foods 2021, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Aragüez-Fortes, Y.; Robaina-Morales, L.M.; Pino, J.A. Optimization of the spray-drying parameters for developing guava powder. J. Food Process Eng. 2019, 42, e13230. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Matos Jorge, L.M.; Jorge, R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef]
- Henao-Ardila, A.; Quintanilla-Carvajal, M.X.; Moreno, F.L. Combination of freeze concentration and spray drying for the production of feijoa (Acca sellowiana b.) pulp powder. Powder Technol. 2019, 344, 190–198. [Google Scholar] [CrossRef]
- Darniadi, S.; Ho, P.; Murray, B.S. Comparison of blueberry powder produced via foam-mat freeze-drying versus spray-drying: Evaluation of foam and powder properties. J. Sci. Food Agric. 2018, 98, 2002–2010. [Google Scholar] [CrossRef]
- Vargas-Muñoz, D.P.; Kurozawa, L.E. Influence of combined hydrolyzed collagen and maltodextrin as carrier agents in spray drying of cocona pulp. Brazilian J. Food Technol. 2020, 23, e2019254. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Rodrigues, S.; Narain, N. Effect of freeze- and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Rigon, R.T.; Noreña, C.P.Z. Microencapsulation by spray-drying of bioactive compounds extracted from blackberry (rubus fruticosus). J. Food Sci. Technol. 2016, 53, 1515–1524. [Google Scholar] [CrossRef]
- Bastos, D.D.S.; Gonçalves, M.D.P.; de Andrade, C.T.; Araújo, K.G.D.L.; da Rocha Leão, M.H.M. Microencapsulation of cashew apple (Anacardium occidentale, L.) juice using a new chitosan-commercial bovine whey protein isolate system in spray drying. Food Bioprod. Process. 2012, 90, 683–692. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, S.Y. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020, 269, 109744. [Google Scholar] [CrossRef]
- Zhang, C.; Quek, S.Y.; Fu, N.; Liu, B.; Kilmartin, P.A.; Chen, X.D. A study on the structure formation and properties of noni juice microencapsulated with maltodextrin and gum acacia using single droplet drying. Food Hydrocoll. 2019, 88, 199–209. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Pino, J.A.; Aragüez-Fortes, Y.; Bringas-Lantigua, M. Optimization of spray-drying process for concentrated orange juice. Acta Aliment. 2018, 47, 417–424. [Google Scholar] [CrossRef]
- Singh, C.S.; Paswan, V.K.; Rai, D.C. Process optimization of spray dried Jamun (Syzygium cumini L.) pulp powder. Lwt 2019, 109, 1–6. [Google Scholar] [CrossRef]
- Jafari, S.; Karami, Z.; Shiekh, K.A.; Kijpatanasilp, I.; Worobo, R.W.; Assatarakul, K. Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods 2023, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Labuza, T.P.; Altunakar, B. Water Activity Prediction and Moisture Sorption Isotherms. In Water Activity in Foods; Wiley: Hoboken, NJ, USA, 2020; Volume 100, pp. 161–205. ISBN 9781118765982. [Google Scholar]
- Kardile, N.B.; Nema, P.K.; Kaur, B.P.; Thakre, S.M. A Comparative Study of Suitability of Low-Density Polyethylene and Coextruded Laminate Pouches for Storage Stability and Shelf Life Prediction of Instant Puran Powder. J. Packag. Technol. Res. 2019, 3, 223–233. [Google Scholar] [CrossRef]
- Upadhyay, R.; Febin Prabhu Dass, J. Physicochemical analysis, microbial survivability, and shelf life study of spray-dried synbiotic guava juice powder. J. Food Process. Preserv. 2021, 45, e15103. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2023, 63, 6983–7015. [Google Scholar] [CrossRef]
- Toledo, M.E.A.; Ueda, Y.; Imahori, Y.; Ayaki, M. L-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biol. Technol. 2003, 28, 47–57. [Google Scholar] [CrossRef]
- Kapoor, S.; Aggarwal, P. Drying method affects bioactive compounds and antioxidant activity of carrot. Int. J. Veg. Sci. 2015, 21, 467–481. [Google Scholar] [CrossRef]
- Permatasari, N.A.; Sari, F. Quality changes of natural dye powder from red leaf amaranth (Alternanthera amoena Voss) during storage. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012005. [Google Scholar] [CrossRef]
- Bashir, O.; Hussain, S.Z.; Amin, T.; Jan, N.; Gani, G.; Bhat, S.A.; Jabeen, A. The optimization of spray-drying process for the development of apricot powder using response surface methodology. Br. Food J. 2022, 124, 3724–3747. [Google Scholar] [CrossRef]
- Daisy, L.L.; Nduko, J.M.; Joseph, W.M.; Richard, S.M. Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 2020, 57, 79–85. [Google Scholar] [CrossRef]
- Tan, S.L.; Sulaiman, R.; Rukayadi, Y.; Ramli, N.S. Physical, chemical, microbiological properties and shelf life kinetic of spray-dried cantaloupe juice powder during storage. LWT 2021, 140, 110597. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of horseradish (Armoracia rusticana L.) juice using spray-drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]

| Samples | C1 | C2 | C3 | C4 |
|---|---|---|---|---|
| Solids recovery, % | 88.8 ± 4.5 a | 88.8 ± 4.4 a | 70.1 ± 1.5 b | 71.8 ± 5.8 b |
| Average diameter, µm | 17.9 ± 0.4 bc | 15.1 ± 0.1 c | 20.4 ± 1.1 b | 29.5 ± 1.5 a |
| Density, g cm3 | 1.434 ± 0.001 b | 1.458 ± 0.000 a | 1.370 ± 0.001 c | 1.374 ± 0.001 d |
| aw | 0.262 ± 0.014 a | 0.296 ± 0.009 a | 0.266 ± 0.008 a | 0.245 ± 0.018 a |
| pH | 4.2 ± 0.0 a | 4.3 ± 0.0 a | 4.2 ± 0.0 a | 4.2 ± 0.0 a |
| Solubility, % | 97.1 ± 0.5 a | 95.5 ± 1.0 a | 94.9 ± 0.5 a | 97.5 ± 0.9 a |
| Hygroscopicity, % | 7.4 ± 0.4 ab | 7.5 ± 0.2 a | 6.0 ± 0.4 c | 6.5 ± 0.1 bc |
| ΔE | 22.3 ± 0.2 b | 22.2 ± 0.1 b | 23.9 ± 0.1 a | 23.7 ± 0.2 a |
| BI, % | 7.3 ± 0.1 c | 7.7 ± 0.0 b | 7.7 ± 0.0 b | 8.1 ± 0.2 a |
| Ascorbic Acid (mg 100 g−1) | Carotenoids (mg 100 g−1) | TPC (mg GAE 100 g−1) | DPPH Antioxidant Activity (µmol TE g−1) | |
|---|---|---|---|---|
| C1 | 673.2 ± 37.3 a | 0.4 ± 0.1 a | 393.4 ± 23.8 a | 16.4 ± 0.2 a |
| C2 | 656.9 ± 43.0 a | 0.2 ± 0.0 b | 330.9 ± 7.4 b | 14.8 ± 0.3 ab |
| C3 | 496.0 ± 65.0 b | 0.5 ± 0.0 a | 233.6 ± 22.2 c | 13.0 ± 0.7 b |
| C4 | 468.0 ± 42.6 b | 0.4 ± 0.0 a | 229.3 ± 11.9 c | 16.0 ± 1.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, F.P.d.; Costa, J.d.P.d.; da Silva, E.S.; Rocha, P.M.; Medeiros, F.G.M.d.; Costa, J.M.C.d.; Hoskin, R.T. Spray Dried Cashew (Anacardium occidentale L.) Juice Ingredients as an Upcycling Strategy for Abundant Cashew Apple. Appl. Sci. 2024, 14, 7485. https://doi.org/10.3390/app14177485
Moraes FPd, Costa JdPd, da Silva ES, Rocha PM, Medeiros FGMd, Costa JMCd, Hoskin RT. Spray Dried Cashew (Anacardium occidentale L.) Juice Ingredients as an Upcycling Strategy for Abundant Cashew Apple. Applied Sciences. 2024; 14(17):7485. https://doi.org/10.3390/app14177485
Chicago/Turabian StyleMoraes, Francisca Pereira de, Janaína de Paula da Costa, Edilene Souza da Silva, Patrícia Maria Rocha, Fábio Gonçalves Macêdo de Medeiros, José Maria Correia da Costa, and Roberta Targino Hoskin. 2024. "Spray Dried Cashew (Anacardium occidentale L.) Juice Ingredients as an Upcycling Strategy for Abundant Cashew Apple" Applied Sciences 14, no. 17: 7485. https://doi.org/10.3390/app14177485






