Degradation and Migration in Olive Oil Packaged in Polyethylene Terephthalate under Thermal Treatment and Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Oil Samples
2.2. Olive Oil Treatments
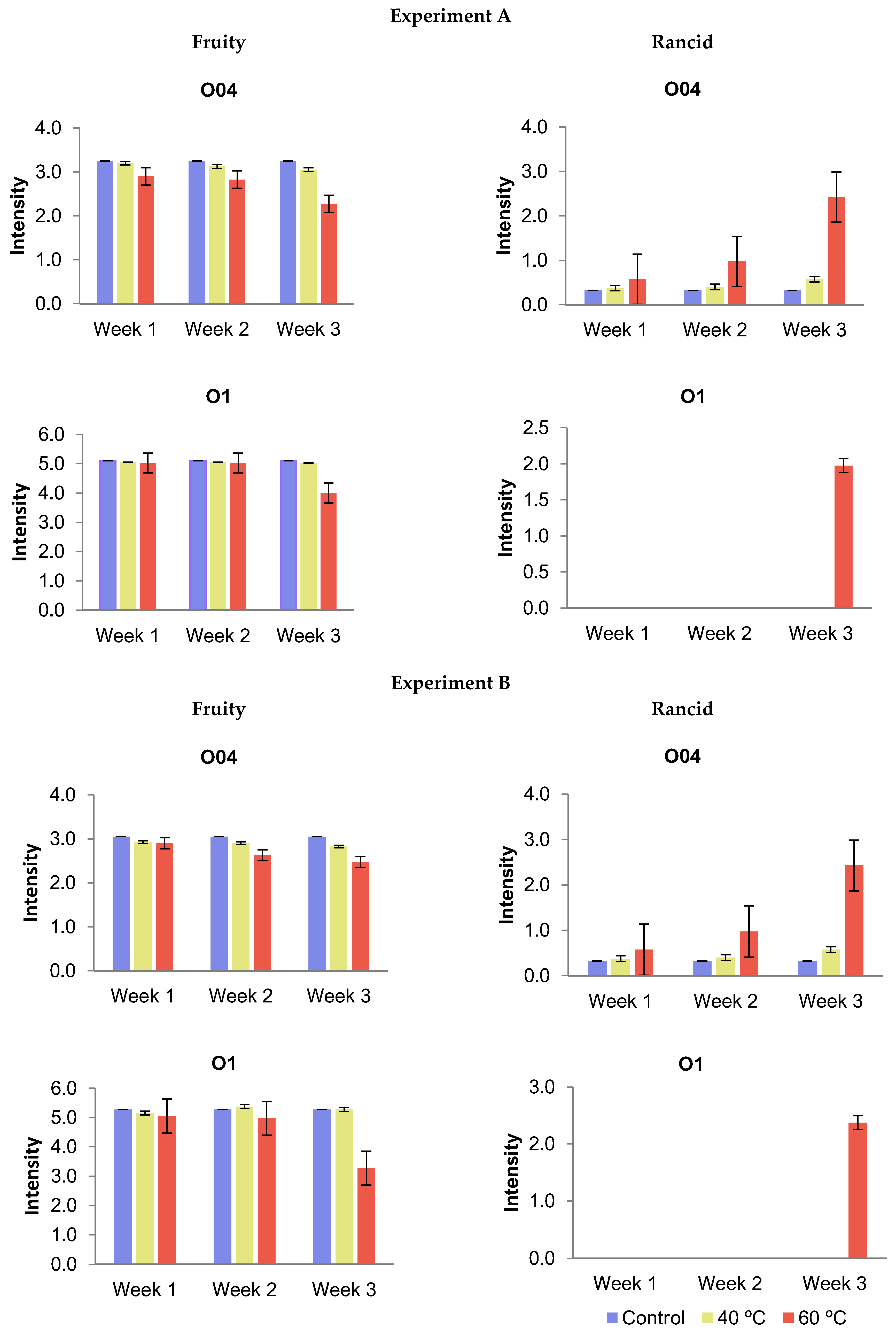
- Experiment A: This experiment focused on the physicochemical and organoleptic analysis of the oils and PET degradation (groups T0a to T6 in Table 1). The thermal study was carried out at temperatures of 40 °C and 60 °C for periods of 1, 2, and 3 weeks, respectively. After the thermal treatments, the samples were transferred to opaque glass jars and analyzed immediately;
- Experiment B: This experiment also involved the physicochemical and organoleptic analysis of the oils and PET degradation (groups T0b to T12 in Table 1). The methodology was similar to that of Experiment A; however, the oil samples were stored in darkness at room temperature (25 °C) for a period of 12 months following the thermal treatments. After this time, the samples were analyzed to assess their quality.
2.3. Olive Oil Physicochemical Parameters
- Cp: pigment concentration (mg pigment/kg oil);
- Aγ: absorbance at 470 nm (carotenoids) or 670 nm (chlorophylls);
- ε1%: specific absorbance measurement of a 1% (w/v) solution. εi (phaeophytin α): 613 or εi (luteolin): 2000;
- ma: mass of oil sample (g);
- Vf: final volume of solution (mL).
2.4. PET Degradation
2.5. Determination of Heavy Metals
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.1.1. Acidity Index (AG)
3.1.2. Peroxide Value (PV)
3.1.3. Extinction Coefficients K232 y K268
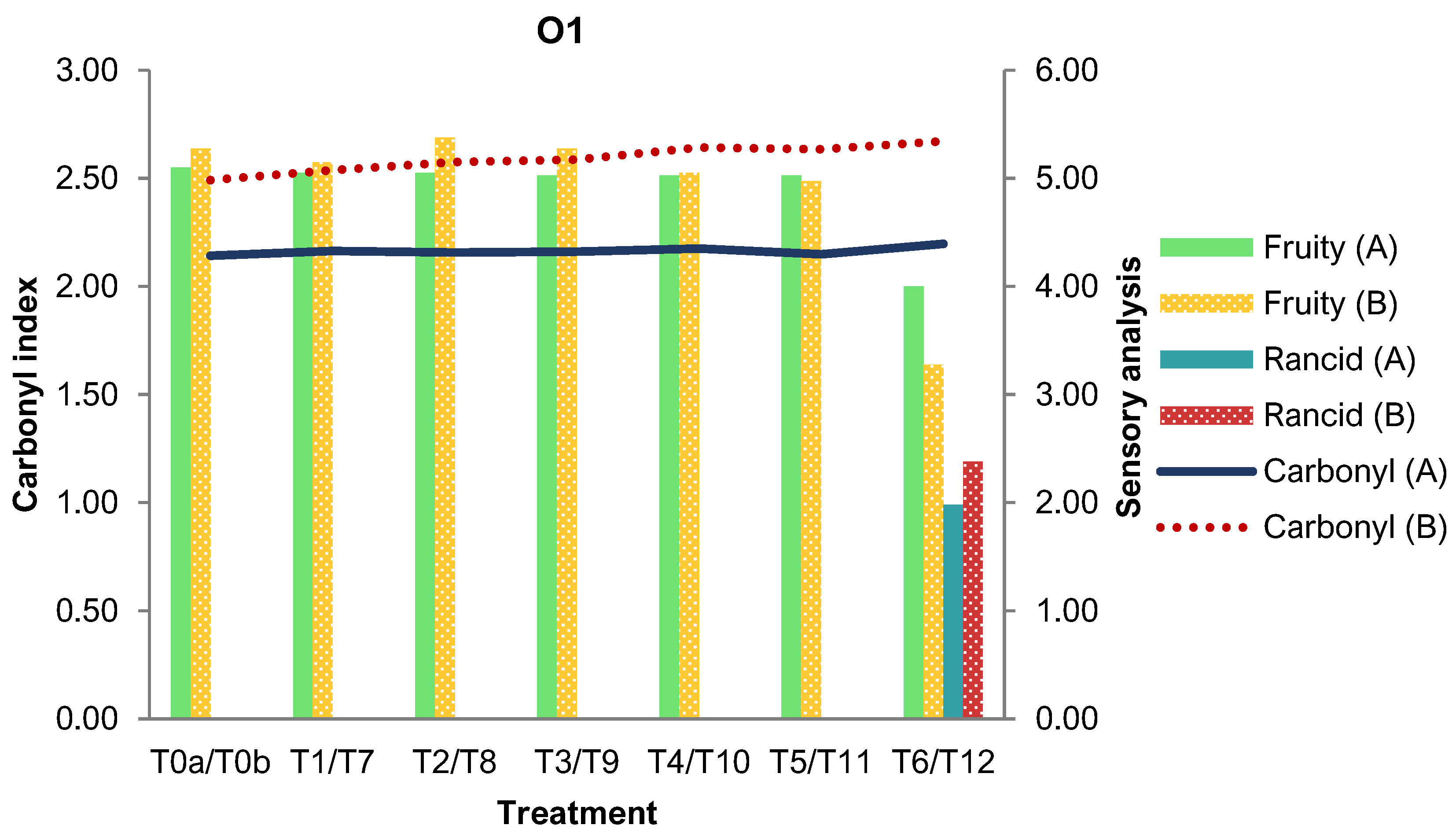
3.1.4. Sensory Analysis
3.1.5. Photosynthetic Pigments
3.1.6. Influence of Type of Oil, Temperature, Time Exposure, and Storage
3.2. Analysis of PET Degradation
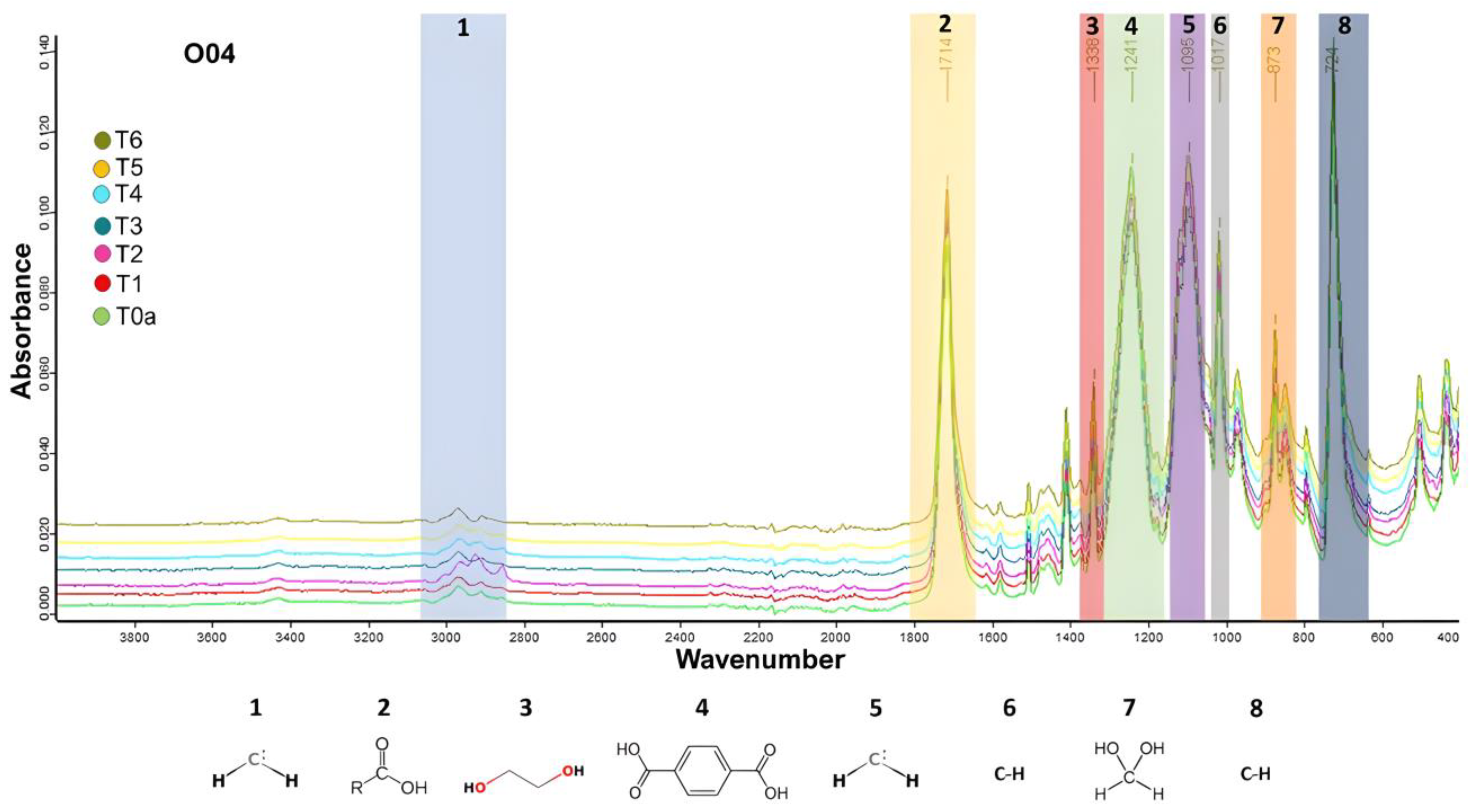
3.2.1. Fourier Transform Infrared Spectroscopy Results
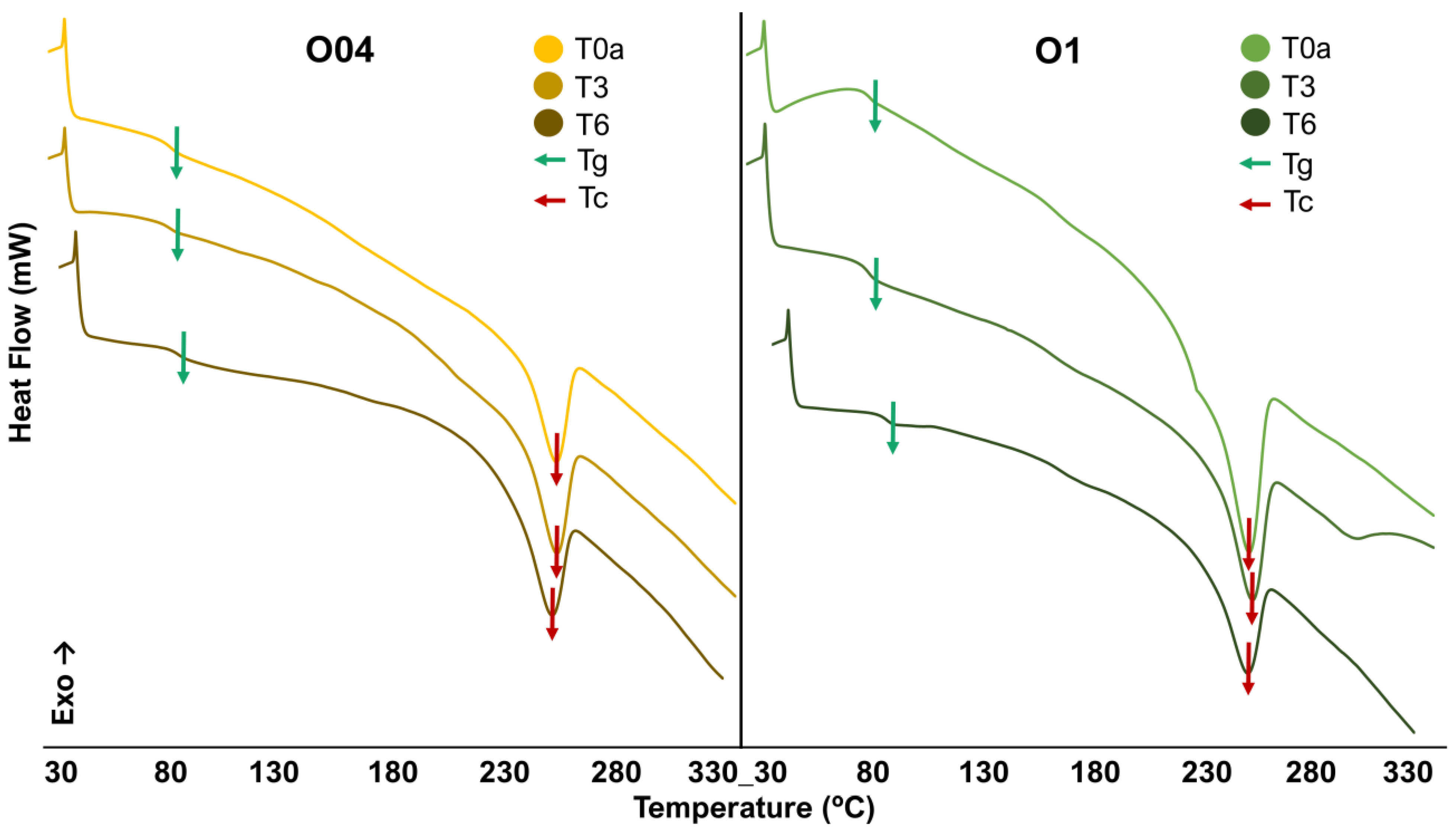
3.2.2. Differential Scanning Calorimetry Analysis
3.3. Heavy Metal Analysis
3.4. Influence of Commercial PET Packaging on Olive Oil Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. An Introduction to the Basic Concepts of Food Security. Available online: https://www.fao.org/3/al936s/al936s00.pdf (accessed on 5 July 2024).
- Food Safety in Compulsory Secondary Education. Spanish Agency for Food Safety. Ministry of Health and Consumption (AECOSAN). 2003. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/publicaciones/seguridad_alimentaria/seguridad_edu_secundaria.pdf (accessed on 5 July 2024).
- Food and Agriculture Organization of the United Nations (FAO). In a New United Nations Report Focused on Food Security and Nutrition in Europe and Central Asia, the Path Towards More Affordable and Sustainable Diets is Highlighted. Available online: https://www.fao.org/newsroom/detail/new-un-report-focuses-on-food-security-and-nutrition-in-europe-and-central-asia-points-way-towards-more-affordable-and-sustainable-diets/es (accessed on 5 July 2024).
- Food Poisoning. Available online: https://www.medlineplus.gov/spanish/ency/article/001652.htm (accessed on 5 July 2024).
- Vásquez de Plata, G. La Contaminación de Los Alimentos, Un Problema Por Resolver. Salud UIS 2003, 35, 48–57. [Google Scholar]
- EFSA Cadmium in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.980 (accessed on 5 July 2024).
- Food and Agriculture Organization of the United Nations (FAO) Sustainable Development Goals Helpdesk. Available online: https://www.fao.org/sustainable-development-goals-helpdesk/en (accessed on 5 July 2024).
- González, M. Determinación de La Migración de Monómeros y Aditivos Plásticos de Envases Alimentarios; Universidad de Granada: Granada, España, 2006. [Google Scholar]
- Torre, A. Migración de Diferentes Simulantes Alimentarios de Productos de Degradación de Poli(Ácido Láctico) Reciclado; Universidad Politécnica de Madrid: Madrid, España, 2017. [Google Scholar]
- Cobos, R. El Polietilén Tereftalato (PET) Como Envase de Aguas Minerales. Boletín Soc. Española Hidrol. Medica 2016, 31, 179–190. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023, on Maximum Limits for Certain Contaminants in Foodstuffs, and Repealing Regulation (EC) No 1881/2006. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2023-80614 (accessed on 5 July 2024).
- Commission Regulation (EU) 2020/1245 of 2 September 2020, Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2020-81322 (accessed on 5 July 2024).
- FAO/WHO Codex Alimentarius Commission Joint Food Standards Programme, Forty-Fifth Session, FAO Headquarters, Roma (Italia). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/zh/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-701-45%252FFinal%252520Report%252520CAC45%252FREP22_CACs.pdf (accessed on 5 July 2024).
- European Commission (EC) Statistics on Oilseeds and Protein Crops. Available online: https://agriculture.ec.europa.eu/data-and-analysis/markets/overviews/market-observatories/crops/oilseeds-and-protein-crops_es (accessed on 5 July 2024).
- European Commission (EC) Olive Oil. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/olive-oil_es (accessed on 5 July 2024).
- Cinquanta, L.; Esti, M.; Di Matteo, M. Oxidative Stability of Virgin Olive Oils. J. Am. Oil Chem. Soc. 2001, 78, 1197–1202. [Google Scholar] [CrossRef]
- Okogeri, O.; Tasioula-Margari, M. Changes occurring in phenolic compounds and alpha-tocopherol of virgin olive oil during storage. J. Agric. Food Chem. 2002, 50, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Di Giovacchino, L.; Sestili, S.; Di Vincenzo, D. Influence of Olive Processing on Virgin Olive Oil Quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Evolution of Major and Minor Components and Oxidation Indices of Virgin Olive Oil during 21 Months Storage at Room Temperature. Food Chem. 2007, 100, 36–42. [Google Scholar] [CrossRef]
- Morelló, J.-R.; Motilva, M.-J.; Tovar, M.-J.; Romero, M.-P. Changes in Commercial Virgin Olive Oil (Cv Arbequina) during Storage, with Special Emphasis on the Phenolic Fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- Fadda, C.; Del Caro, A.; Sanguinetti, A.M.; Urgeghe, P.P.; Vacca, V.; Arca, P.P.; Piga, A. Changes during Storage of Quality Parameters and in Vitro Antioxidant Activity of Extra Virgin Monovarietal Oils Obtained with Two Extraction Technologies. Food Chem. 2012, 134, 1542–1548. [Google Scholar] [CrossRef]
- Krichene, D.; Haddada, F.M.; Fernandez, X.; Cuvelier, L.L.; Zarrouk, M. Volatile Compounds Characterising Tunisian Virgin Olive Oils: The Influence of Cultivar. J. Food Sci. Technol. 2010, 45, 944–950. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R2104 (accessed on 5 July 2024).
- Boukandoul, S.; Zaidi, F.; Santos, C.S.P.; Casal, S. Moringa Oleifera Oil Nutritional and Safety Impact on Deep-Fried Potatoes. Foods 2023, 12, 4416. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.S.; Cho, H.; Hwang, K.T. Physicochemical Properties and Oxidative Stability of Frying Oils during Repeated Frying of Potato Chips. Food Sci. Biotechnol. 2018, 27, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Royal Decree 760/2021 of 31 August 2021, Approving the Quality Standard for Olive Oils and Olive Pomace Oils. Available online: https://www.boe.es/boe/dias/2021/09/01/pdfs/BOE-A-2021-14318.pdf (accessed on 5 July 2024).
- González-Torres, P.; Puentes, J.G.; Moya, A.J.; La Rubia, M.D. Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils. Appl. Sci. 2023, 13, 3020. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils. Determination of Acid Value and Acidity. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31991R2568 (accessed on 5 July 2024).
- ISO 3960:2017; Animal and Vegetable Fats and Oils. Determination of Peroxide Value. Iodometric (Visual) Endpoint Determination. International Organization for Standardization (ISO): Geneva, Switzerland, 2017. Available online: https://cdn.standards.iteh.ai/samples/71268/578d213fc79042e0badd1e9f699bf1d6/ISO-3960-2017.pdf (accessed on 5 July 2024).
- COI/T.20/Doc. No 5/Rev. 1. Sensory Analysis Of Olive Oil Standard. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-5-Rev1-2007-Eng.pdf (accessed on 5 July 2024).
- Puentes, J.G. Estudio Del Deterioro de Envases de PET y Su Relación Con La Calidad de Aceites de Oliva; Universidad de Jaén: Jaén, España, 2021. [Google Scholar]
- Abid, U.; Sun, G.; Soong, Y.-H.V.; Williams, A.; Chang, A.C.; Ayafor, C.; Patel, A.; Wong, H.-W.; Sobkowicz, M.J.; Xie, D. Evaluation of Enzymatic Depolymerization of PET, PTT, and PBT Polyesters. Biochem. Eng. J. 2023, 199, 109074. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Rharrabti, Y. Effect of Storage Time and Conditions on the Quality Characteristics of ‘Moroccan Picholine’ Olive Oil. Biocatal. Agric. Biotechnol. 2022, 39, 102244. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Lukić, I.; Brkić Bubola, K. Extra Virgin Olive Oil under Simulated Consumption Conditions: Evaluation of Quality, Health, and Flavour Properties. J. Food Compos. Anal. 2022, 110, 104570. [Google Scholar] [CrossRef]
- Escudero, A. Estudios de Migración En Aceites de Oliva Vírgenes Extra, Envasados En PET; Universidad de Jaén: Jaén, España, 2019. [Google Scholar]
- Zaroual, H.; Chèné, C.; Mestafa El Hadrami, E.; Karoui, R. Comparison of Four Classification Statistical Methods for Characterising Virgin Olive Oil Quality during Storage up to 18 Months. Food Chem. 2022, 370, 131009. [Google Scholar] [CrossRef]
- Albi, T.; Lanzón, A.; Guinda, A.; Pérez-Camino, M.C.; León, M. Microwave and Conventional Heating Effects on Some Chemical Parameters of Edible Fats. J. Agric. Food Chem. 1997, 45, 3000–3003. [Google Scholar] [CrossRef]
- Cossignani, L.; Simonetti, M.S.; Neri, A.; Damiani, P. Changes in Olive Oil Composition Due to Microwave Heating. J. Am. Oil Chem. 1998, 75, 931–937. [Google Scholar] [CrossRef]
- Ayton, J.; Mailer, R.; Grahamn, K. The Effect of Storage Conditions on Extra Virgin Olive Oil Quality. Available online: https://1.oliveoiltimes.com/library/Olive-Oil-Storage-Conditions.pdf (accessed on 5 July 2024).
- Khaneghah, A.M.; Shoeibi, S.; Ameri, M. Effects of Storage Conditions and PET Packaging on Quality of Edible Oils in Iran. Adv. Environ. Biol. 2012, 6, 694–701. [Google Scholar]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive Oil Stability under Deep-Frying Conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Rodrigues, N.; Renard, C.M.G.C.; Pereira, J.A. Volatile Changes in Cv. Verdeal Transmontana Olive Oil: From the Drupe to the Table, Including Storage. Food. Res. Int. 2018, 106, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Marx, Í.M.G.; Rodrigues, N.; Veloso, A.C.A.; Casal, S.; Pereira, J.A.; Peres, A.M. Effect of Malaxation Temperature on the Physicochemical and Sensory Quality of Cv. Cobrançosa Olive Oil and Its Evaluation Using an Electronic Tongue. LWT 2021, 137, 110426. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(Ethylene Terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef]
- Feng, X.; Hu, X.; Yu, J.; Zhao, M.; Yang, F.; Wang, X.; Zhang, C.; Weng, Y.; Han, J. A Hydrotalcite-Based PET Composites with Enhanced Properties for Liquid Milk Packaging Applications. Materials 2023, 16, 1857. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.F.; Papai, R.; Luz, M.S.; Gaubeur, I. Analytical Extraction Procedure Combined with Atomic and Mass Spectrometry for the Determination of Tin in Edible Oil Samples, and the Potential Application to Other Chemical Elements. J. Food Compos. Anal. 2021, 96, 103759. [Google Scholar] [CrossRef]
- Saxena, D.; Rana, D.; Bhoje Gowd, E.; Maiti, P. Improvement in Mechanical and Structural Properties of Poly(Ethylene Terephthalate) Nanohybrid. SN Appl. Sci. 2019, 1, 1363. [Google Scholar] [CrossRef]
- Roungpaisan, N.; Srisawat, N.; Rungruangkitkrai, N.; Chartvivatpornchai, N.; Boonyarit, J.; Kittikorn, T.; Chollakup, R. Melt Spinning Process Optimization of Polyethylene Terephthalate Fiber Structure and Properties from Tetron Cotton Knitted Fabric. Polymers 2023, 15, 4364. [Google Scholar] [CrossRef]
- Ibor, O.R.; Mpama, N.-O.L.; Okoli, C.P.; Ogarekpe, D.M.; Edet, U.O.; Ajang, R.O.; Onyezobi, C.E.; Anyanti, J.; Idogho, O.; Aizobu, D.; et al. Occurrence, Identification and Characterization of Plastic Pollution from an Open Solid Waste Dumpsite in Calabar, Southern Nigeria. Environ. Adv. 2023, 11, 100338. [Google Scholar] [CrossRef]
- Pal, S.K.; Prabhudesai, V.S.; Vinu, R. Catalytic Upcycling of Post-Consumer Multilayered Plastic Packaging Wastes for the Selective Production of Monoaromatic Hydrocarbons. J. Environ. Manag. 2024, 351, 119630. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, C.; Zhong, J.; Gao, W.; Li, J.; Liu, J. Co-Pyrolytic Interactions and Products of Brominated Epoxy Resin and Polyethylene Terephthalate: TG-FTIR Analysis and Machine Learning Prediction. J. Anal. Appl. Pyrolysis 2023, 175, 106223. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Z.; Chen, Q.; Lin, Y.; Zhou, Y.; Xu, Q. Determination of Key Factors Affecting Biofilm Formation on the Aged Poly(Ethylene Terephthalate) during Anaerobic Digestion. Chemosphere 2023, 344, 140435. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The Degradation Potential of PET Bottles in the Marine Environment: An ATR-FTIR Based Approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Chen, G.; Wang, Q.; Sun, T.; Zhang, M.; Du, Z.; Wu, M.; Guo, S.; Lei, T.; et al. Synergistic Effects and Products Yield Analyses Based on Co-Pyrolysis of Poplar Tree and Rape Stalks with Polyethylene Terephthalate and Polypropylene. J. Energy Inst. 2024, 112, 101461. [Google Scholar] [CrossRef]
- Eti, S.A.; Islam, M.S.; Shourove, J.H.; Saha, B.; Ray, S.K.; Sultana, S.; Ali Shaikh, M.A.; Rahman, M.M. Assessment of Heavy Metals Migrated from Food Contact Plastic Packaging: Bangladesh Perspective. Heliyon 2023, 9, e19667. [Google Scholar] [CrossRef]
- Chércoles Asensio, R.; San Andrés Moya, M.; De La Roja, J.M.; Gómez, M. Analytical Characterization of Polymers Used in Conservation and Restoration by ATR-FTIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR Investigation on the Co-Pyrolysis of the Waste HDPE, PP, PS and PET under High Heating Conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Zhang, B.; Wang, Z. Preparation and characterization of CO2-selective Pebax/NaY mixed matrix membranes. J. Appl. Polym. Sci. 2020, 137, 48398. [Google Scholar] [CrossRef]
- Romão, W.; Spinacé, M.A.S.; Paoli, M.-A. De Poli(Tereftalato de Etileno), PET: Uma Revisão Sobre Os Processos de Síntese, Mecanismos de Degradação e Sua Reciclagem. Polímeros 2009, 19, 121–132. [Google Scholar] [CrossRef]
- Liu, J.; Bian, S.G.; Xiao, M.; Wang, S.J.; Meng, Y.Z. Thermal Degradation and Isothermal Crystalline Behavior of Poly(Trimethylene Terephthalate). Chin. Chem. Lett. 2009, 20, 487–491. [Google Scholar] [CrossRef]
- McNeill, I.C.; Bounekhel, M. Thermal Degradation Studies of Terephthalate Polyesters: 1. Poly(Alkylene Terephthalates). Polym. Degrad. Stab. 1991, 34, 187–204. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Clark, F.R.S.; Wiles, D.M. Photo-Oxidation of Polypropylene Monofilaments. I. Chemical Changes and Mechanical Deterioration. Text. Res. J. 1976, 46, 590–599. [Google Scholar] [CrossRef]
- Ashwinder, C.; Malini, S.; Gupta, R.; Gupta, A. Evaluation on the Thermo-Oxidative Degradation of PET Using Prodegradant Additives. Indian J. Sci. Technol. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Bayo, J.; Rojo, D.; Olmos, S. Weathering Indices of Microplastics along Marine and Coastal Sediments from the Harbor of Cartagena (Spain) and Its Adjoining Urban Beach. Mar. Pollut. Bull. 2022, 178, 113647. [Google Scholar] [CrossRef]
- Xiaoyan, Q.; Li, X.; Yue, W.; Yin, C.; Xu, Y.; Hui, N.; Zhou, N.-Y. Composting-Based Degradation of Poly (Ethylene Terephthalate) Microplastics and Its Enhancement with Exogenous PET Hydrolase Supplementation. Environ. Technol. Innov. 2023, 32, 103347. [Google Scholar] [CrossRef]
- Azeem, M.; Fournet, M.B.; Attallah, O.A. Ultrafast 99% Polyethylene Terephthalate Depolymerization into Value Added Monomers Using Sequential Glycolysis-Hydrolysis under Microwave Irradiation. Arab. J. Chem. 2022, 15, 103903. [Google Scholar] [CrossRef]
- Viora, L.; Combeau, M.; Pucci, M.F.; Perrin, D.; Liotier, P.-J.; Bouvard, J.-L.; Combeaud, C. A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers 2023, 15, 4613. [Google Scholar] [CrossRef]
- Saabome, S.M.; Lee, J.E.; Hong, J.-S.; Kim, D.H.; Ahn, K.H. Mechanical Degradation of Poly(Ethylene Terephthalate) and Its Structural Modification by Chain Extender. Korea-Austral. Rheol. J. 2023, 35, 203–212. [Google Scholar] [CrossRef]
- Kanavouras, A. Alterations of PET Material Physical Properties during Storage of Olive Oil. Food Packag. Shelf Life 2019, 21, 100336. [Google Scholar] [CrossRef]
- Sánchez, M.; Rosales, A.; Maldonado, H. Estudio de Catalizadores En La Degradación de PET Reciclado. Ciencia UANL 2011, XIV, 39–45. [Google Scholar]
- Taghizadeh, S.F.; Rezaee, R.; Boskabady, M.; Mashayekhi Sardoo, H.; Karimi, G. Exploring the Carcinogenic and Non-Carcinogenic Risk of Chemicals Present in Vegetable Oils. Int. J. Environ. Anal. Chem. 2022, 102, 5756–5784. [Google Scholar] [CrossRef]
- Luka, M.F.; Akun, E. Investigation of Trace Metals in Different Varieties of Olive Oils from Northern Cyprus and Their Variation in Accumulation Using ICP-MS and Multivariate Techniques. Environ. Earth Sci. 2019, 78. [Google Scholar] [CrossRef]
- Zhuravlev, A.; Zacharia, A.; Gucer, S.; Chebotarev, A.; Arabadji, M.; Dobrynin, A. Direct Atomic Absorption Spectrometry Determination of Arsenic, Cadmium, Copper, Manganese, Lead and Zinc in Vegetable Oil and Fat Samples with Graphite Filter Furnace Atomizer. J. Food Compos. Anal. 2015, 38, 62–68. [Google Scholar] [CrossRef]
- Mohammed Alrajhi, I.; Idriss, H. Concentration of Trace Metals in Some Major Edible Oils of Riyadh. Rev. Intern. Contam. Ambient. 2020, 36, 977–984. [Google Scholar] [CrossRef]
- Haq, H.; Bibi, R.; Arain, M.; Safi, F.; Ullah, S.; Castro-Muñoz, R.; Boczkaj, G. Deep Eutectic Solvent (DES) with Silver Nanoparticles (Ag-NPs) Based Assay for Analysis of Lead (II) in Edible Oils. Food Chem. 2022, 379, 132085. [Google Scholar] [CrossRef]
- Khemani, K.C. Novel Approach for Studying the Thermal Degradation, and for Estimating the Rate of Acetaldehyde Generation by the Chain Scission Mechanism in Ethylene Glycol Based Polyesters and Copolyesters. Polym. Degrad. Stab. 2000, 67, 91–99. [Google Scholar] [CrossRef]

| Group | Temperature (°C) | Exposure Time (Weeks) | Storage (Months) |
|---|---|---|---|
| T0a * | 0 | 0 | 0 |
| T1 | 40 | 1 | 0 |
| T2 | 40 | 2 | 0 |
| T3 | 40 | 3 | 0 |
| T4 | 60 | 1 | 0 |
| T5 | 60 | 2 | 0 |
| T6 | 60 | 3 | 0 |
| T0b * | 0 | 0 | 12 |
| T7 | 40 | 1 | 12 |
| T8 | 40 | 2 | 12 |
| T9 | 40 | 3 | 12 |
| T10 | 60 | 1 | 12 |
| T11 | 60 | 2 | 12 |
| T12 | 60 | 3 | 12 |
| Stage | Pwr Level a | Pw (%) b | Ramp Time | Temperature | Hold Time |
|---|---|---|---|---|---|
| 1 | 800 | 100 | 5:00 | 50 | 3:00 |
| 2 | 800 | 100 | 5:00 | 120 | 3:00 |
| 3 | 800 | 100 | 5:00 | 150 | 3:00 |
| 4 | 1600 | 100 | 5:00 | 180 | 3:00 |
| Instrumental and Validation Parameters | ICP-MS | HR-ICP-MS |
|---|---|---|
| LOD (µg/kg) | 0.1 | 0.01 |
| LOQ (µg/kg) | 0.3 | 0.03 |
| Precision (RSD, %) | 3.5 | 2 |
| Accuracy (% recovery) | 95–105 | 98–102 |
| Full procedure precision (RSD, %) | 4 | 2.5 |
| Metal | CRM (µg/kg) | Results (µg/kg) | RSD (%) | Precision (µg/kg) | Repeatability (%) |
|---|---|---|---|---|---|
| Cd | 1 | 0.001 | 4 | 0.04 | 3.8 |
| Pb | 20 | 0.021 | 3.5 | 0.07 | 3.6 |
| Cu | 100 | 0.127 | 3 | 0.15 | 2.9 |
| Fe | 150 | 0.148 | 2.8 | 0.2 | 2.7 |
| Sb | 10 | 1.35 | 2 | 0.05 | 2.1 |
| O04 | AG | PV | K232 | K268 | Chlorophylls | Carotenoids |
|---|---|---|---|---|---|---|
| T0a | 0.28 ± 0.00 | 5.12 ± 0.22 | 2.73 ± 0.05 | 0.59 ± 0.00 | 0.33 ± 0.46 | 1.62 ± 0.21 |
| T1 | 0.28 ± 0.01 | 4.91 ± 0.08 | 2.69 ± 0.06 | 0.58 ± 0.00 | 0.05 ± 0.08 | 1.25 ± 0.17 |
| T2 | 0.27 ± 0.00 | 5.50 ± 0.08 | 2.92 ± 0.12 | 0.60 ± 0.02 | 1.14 ± 0.22 | 1.16 ± 0.11 |
| T3 | 0.27 ± 0.01 | 5.44 ± 0.04 | 3.10 ± 0.72 | 0.59 ± 0.02 | 1.08 ± 0.31 | 0.68 ± 0.59 |
| T4 | 0.28 ± 0.00 | 5.43 ± 0.01 | 2.95 ± 0.13 | 0.60 ± 0.02 | 0.98 ± 0.15 | 1.11 ± 0.02 |
| T5 | 0.28 ± 0.01 | 5.65 ± 0.05 | 2.82 ± 0.09 | 0.60 ± 0.01 | 0.49 ± 0.69 | 1.00 ± 0.05 |
| T6 | 0.28 ± 0.01 | 5.15 ± 0.27 | 2.67 ± 0.13 | 0.61 ± 0.01 | 0.93 ± 0.08 | 1.09 ± 0.03 |
| T0b | 0.14 ± 0.01 | 6.36 ± 0.06 | 2.39 ± 0.01 | 0.65 ± 0.01 | 2.20 ± 0.01 | 1.82 ± 0.03 |
| T7 | 0.12 ± 0.02 | 5.19 ± 0.00 | 3.03 ± 0.04 | 0.66 ± 0.01 | 1.62 ± 0.15 | 1.63 ± 0.04 |
| T8 | 0.13 ± 0.00 | 5.90 ± 0.72 | 2.52 ± 0.02 | 0.70 ± 0.03 | 2.12 ± 0.40 | 1.85 ± 0.25 |
| T9 | 0.14 ± 0.00 | 7.12 ± 0.35 | 2.80 ± 0.45 | 0.62 ± 0.01 | 2.12 ± 0.38 | 1.81 ± 0.16 |
| T10 | 0.13 ± 0.00 | 7.77 ± 0.27 | 2.91 ± 0.41 | 0.70 ± 0.05 | 1.96 ± 0.30 | 1.75 ± 0.21 |
| T11 | 0.14 ± 0.00 | 7.05 ± 0.18 | 2.90 ± 0.39 | 0.73 ± 0.08 | 2.30 ± 0.79 | 1.85 ± 0.26 |
| T12 | 0.14 ± 0.00 | 7.28 ± 0.92 | 2.82 ± 0.50 | 0.75 ± 0.00 | 2.67 ± 0.22 | 1.94 ± 0.14 |
| O1 | AG | PV | K232 | K268 | Chlorophylls | Carotenoids |
| T0a | 0.23 ± 0.04 | 5.91 ± 0.03 | 2.39 ± 0.36 | 0.58 ± 0.00 | 7.51 ± 0.23 | 3.51 ± 0.37 |
| T1 | 0.23 ± 0.01 | 6.79 ± 0.19 | 2.84 ± 0.15 | 0.57 ± 0.00 | 7.97 ± 0.98 | 3.77 ± 0.30 |
| T2 | 0.23 ± 0.01 | 6.82 ± 0.29 | 2.76 ± 0.02 | 0.60 ± 0.01 | 6.45 ± 1.17 | 3.16 ± 0.48 |
| T3 | 0.23 ± 0.01 | 6.58 ± 0.75 | 2.70 ± 0.29 | 0.63 ± 0.06 | 6.53 ± 0.54 | 3.39 ± 0.45 |
| T4 | 0.23 ± 0.00 | 6.27 ± 0.49 | 2.79 ± 0.26 | 0.60 ± 0.02 | 6.41 ± 0.32 | 3.00 ± 0.13 |
| T5 | 0.23 ± 0.01 | 6.40 ± 0.23 | 2.95 ± 0.27 | 0.61 ± 0.02 | 5.13 ± 0.36 | 3.34 ± 0.43 |
| T6 | 0.24 ± 0.01 | 6.32 ± 0.33 | 2.75 ± 0.02 | 0.58 ± 0.00 | 6.11 ± 0.76 | 3.14 ± 0.33 |
| T0b | 0.30 ± 0.01 | 5.93 ± 0.04 | 2.68 ± 0.06 | 0.41 ± 0.01 | 3.01 ± 0.04 | 3.89 ± 0.05 |
| T7 | 0.30 ± 0.00 | 7.21 ± 0.35 | 2.58 ± 0.33 | 0.42 ± 0.00 | 4.38 ± 0.00 | 3.79 ± 0.00 |
| T8 | 0.29 ± 0.01 | 7.32 ± 0.89 | 2.54 ± 0.43 | 0.40 ± 0.01 | 3.59 ± 1.37 | 3.50 ± 0.06 |
| T9 | 0.31 ± 0.01 | 7.42 ± 0.10 | 2.70 ± 0.37 | 0.43 ± 0.04 | 3.38 ± 1.54 | 3.72 ± 0.02 |
| T10 | 0.30 ± 0.01 | 7.65 ± 0.84 | 2.69 ± 0.11 | 0.45 ± 0.02 | 5.10 ± 0.61 | 3.81 ± 0.07 |
| T11 | 0.31 ± 0.01 | 7.36 ± 0.98 | 2.70 ± 0.45 | 0.43 ± 0.05 | 3.42 ± 1.92 | 3.75 ± 0.07 |
| T12 | 0.31 ± 0.01 | 5.97 ± 0.91 | 2.47 ± 0.03 | 0.62 ± 0.03 | 3.39 ± 1.56 | 3.25 ± 0.01 |
| Parameters | Acidity P | PV P | K232 P | K268 P | Chlorophylls P | Carotenoids P |
|---|---|---|---|---|---|---|
| Main effects | ||||||
| Type of oil | 0.000 | 0.000 | 0.055 | 0.000 | 0.000 | 0.000 |
| Temperature | 0.104 | 0.208 | 0.572 | 0.000 | 0.562 | 0.450 |
| Time exposure | 0.068 | 0.807 | 0.899 | 0.008 | 0.807 | 0.297 |
| Storage | 0.000 | 0.000 | 0.344 | 0.008 | 0.000 | 0.000 |
| Interactions | ||||||
| Type of oil—Temperature | 0.698 | 0.000 | 0.886 | 0.881 | 0.147 | 0.093 |
| Type of oil—Time exposure | 0.779 | 0.049 | 0.554 | 0.002 | 0.005 | 0.536 |
| Type of oil—Storage | 0.000 | 0.011 | 0.210 | 0.000 | 0.000 | 0.006 |
| Temperature—Time exposure | 0.610 | 0.004 | 0.588 | 0.012 | 0.508 | 0.299 |
| Temperature—Storage | 0.389 | 0.030 | 0.445 | 0.000 | 0.078 | 0.331 |
| Time exposure—Storage | 0.258 | 0.645 | 0.589 | 0.088 | 0.827 | 0.691 |
| Post Hoc Tukey HSD | ||||||
| Type of oil | ||||||
| O04 vs. 01 | −** | −** | + | +** | −** | −** |
| Temperature (°C) | ||||||
| 0 vs. 40/60 | −/− | −*/−** | −/− | −/−** | −/+ | +*/+* |
| 40 vs. 0/60 | +/− | +*/− | +/− | +/−** | +/+ | −*/+ |
| 60 vs. 0/40 | +/+ | +**/+ | +/+ | +**/+** | −/− | −*/− |
| Time exposure (weeks) | ||||||
| 0 vs. 1/2/3 | +/−/− | −*/−*/−* | −/−/− | −/−/−** | −/+/− | +/+/+* |
| 1 vs. 0/2/3 | −/−/− | +*/−/− | +/+/+ | +/−/−* | +/+/+ | −/+/+ |
| 2 vs. 0/1/3 | +/+/− | +*/+/+ | +/−/− | +/+/− | −/−/− | −/−/+ |
| 3 vs. 0/1/2 | +/+/+ | +*/+/− | +/−/+ | +**/+*/+ | +/−/+ | −*/−/− |
| Storage (months) | ||||||
| 0 vs. 12 | +** | −** | + | +* | + | −** |
| Wavenumber (cm−1) | Functional Group Description | Reference |
|---|---|---|
| 1714 | Stretching of the C=O bond of the carbonyl group | [46,47,48,49] |
| 1338 | Stretching of the C–O bond and bonds of the ethylene glycol compound | [37,50] |
| 1242 | Associated with terephthalate (OOCC6H4–COO) | [37] |
| 1096 | Symmetric vibration of the ester bond and methylene group (CH2) | [51,52] |
| 1017 | Stretching of the C–H bond and aromatic rings | [53,54,55] |
| 873 | Associated with aromatic rings | [52,53,54,55,56,57,58] |
| 724 | C–H bond rocking movements | [58] |
| Spectral Band (cm−1) | Functional Group Description | Reference |
|---|---|---|
| 3200–2850 | Symmetric and asymmetric stretching of the methylene group (CH₂) | [58] |
| 1850–1600 | Stretching of the C=O bond and appearance of the carbonyl group | [59] |
| 1450–1350 | Presence of alkenes | [59] |
| 1300–1200 | Stretching of the C(=O)–O bond and the presence of terephthalate | [50] |
| 1095–1040 | Symmetric and asymmetric stretching of the C–O–C bond and the presence of ether and ester groups | [52,60] |
| 900–800 | Stretching of the C–H bond and the presence of aromatic rings | [53,55] |
| 780–650 | Out-of-plane bending and the presence of aromatic rings | [58] |
| Group | O04 | O1 |
|---|---|---|
| T0a | 2.14 ± 0.08 | 2.14 ± 0.07 |
| T1 | 2.22 ± 0.05 | 2.16 ± 0.05 |
| T2 | 2.23 ± 0.04 | 2.16 ± 0.07 |
| T3 | 2.17 ± 0.08 | 2.16 ± 0.03 |
| T4 | 2.16 ± 0.06 | 2.17 ± 0.36 |
| T5 | 2.15 ± 0.05 | 2.15 ± 0.04 |
| T6 | 2.27 ± 0.15 | 2.20 ± 0.09 |
| t-Student (α = 0.05) | 0.797 | 0.520 |
| T0b | 2.48 ± 0.21 | 2.49 ± 0.15 |
| T7 | 2.52 ± 0.26 | 2.54 ± 0.23 |
| T8 | 2.56 ± 0.26 | 2.57 ± 0.19 |
| T9 | 2.63 ± 0.16 | 2.59 ± 0.18 |
| T10 | 2.65 ± 0.17 | 2.64 ± 0.26 |
| T11 | 2.67 ± 0.25 | 2.63 ± 0.27 |
| T12 | 2.69 ± 0.27 | 2.67 ± 0.22 |
| t-Student (α = 0.05) | 0.249 | 0.250 |
| O04 | Tg 1 | Tm 2 | Tc 3 | ΔHm 4 | ΔHc 5 | Wc 6 |
|---|---|---|---|---|---|---|
| T0a | 79.44 ± 0.01 | 264.06 ± 0.85 | 252.22 ± 0.81 | 24.84 ± 0.08 | 19.92 ± 0.06 | 6.85 |
| T3 | 83.44 ± 0.01 | 263.72 ± 0.85 | 252.52 ± 0.80 | 21.71 ± 0.07 | 20.05 ± 0.06 | 6.89 |
| T6 | 94.44 ± 0.01 | 257.19 ± 0.83 | 252.29 ± 0.76 | 26.28 ± 0.08 | 22.59 ± 0.07 | 7.76 |
| t-Student (α = 0.05) T0a vs. T3 | 0.00 | 0.65 | 0.67 | 0.00 | 0.07 | |
| t-Student (α = 0.05) T0a vs. T6 | 0.00 | 0.00 | 0.92 | 0.00 | 0.00 | |
| t-Student (α = 0.05) T3 vs. T6 | 0.00 | 0.00 | 0.75 | 0.00 | 0.00 | |
| O1 | Tg | Tm | Tc | ΔHm | ΔHc | Wc |
| T0a | 79.61 ± 0.10 | 255.93 ± 0.82 | 249.79 ± 0.80 | 27.83 ± 0.09 | 26.42 ± 0.08 | 9.08 |
| T3 | 82.43 ± 0.58 | 254.89 ± 0.82 | 251.35 ± 0.81 | 26.19 ± 0.08 | 20.78 ± 0.07 | 7.14 |
| T6 | 91.44 ± 4.62 | 252.27 ± 0.81 | 252.71 ± 0.81 | 19.17 ± 0.06 | 17.53 ± 0.06 | 6.02 |
| t-Student (α = 0.05) T0a vs. T3 | 0.01 | 0.20 | 0.08 | 0.00 | 0.00 | |
| t-Student (α = 0.05) T0a vs. T6 | 0.13 | 0.01 | 0.01 | 0.00 | 0.00 | |
| t-Student (α = 0.05) T3 vs. T6 | 0.38 | 0.02 | 0.11 | 0.00 | 0.00 |
| O04 | Cd | Pb | Cu | Fe | Sb |
|---|---|---|---|---|---|
| T0a | 0.001 ± 0.000 | 0.024 ± 0.002 | 0.107 ± 0.008 | 0.156 ± 0.021 | 1.422 ± 0.012 |
| T6 | 0.001 ± 0.000 | 0.022 ± 0.002 | 0.134 ± 0.010 | 0.152 ± 0.027 | 0.885 ± 0.017 |
| t-Student (α = 0.05) | 0.005 | 0.236 | 0.007 | 0.676 | 0.000 |
| T0b | 0.001 ± 0.000 | 0.139 ± 0.011 | 0.126 ± 0.010 | 1.538 ± 0.041 | 1.075 ± 0.032 |
| T12 | 0.003 ± 0.000 | 0.132 ± 0.010 | 0.118 ± 0.009 | 1.410 ± 0.096 | 1.317 ± 0.018 |
| t-Student (α = 0.05) | 0.000 | 0.358 | 0.291 | 0.167 | 0.011 |
| O1 | Cd | Pb | Cu | Fe | Sb |
| T0a | 0.001 ± 0.000 | 0.020 ± 0.002 | 0.182 ± 0.014 | 0.197 ± 0.015 | 0.769 ± 0.060 |
| T6 | 0.001 ± 0.000 | 0.017 ± 0.001 | 0.156 ± 0.012 | 0.392 ± 0.030 | 1.117 ± 0.087 |
| t-Student (α = 0.05) | 0.000 | 0.060 | 0.035 | 0.000 | 0.001 |
| T0b | 0.001 ± 0.000 | 0.202 ± 0.016 | 0.157 ± 0.012 | 1.800 ± 0.140 | 1.201 ± 0.094 |
| T12 | 0.004 ± 0.000 | 0.145 ± 0.011 | 0.130 ± 0.010 | 1.551 ± 0.121 | 1.643 ± 0.128 |
| t-Student (α = 0.05) | 0.000 | 0.002 | 0.014 | 0.004 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Torres, P.; García-Ruiz, Á.; La Rubia, M.D. Degradation and Migration in Olive Oil Packaged in Polyethylene Terephthalate under Thermal Treatment and Storage Conditions. Appl. Sci. 2024, 14, 7507. https://doi.org/10.3390/app14177507
González-Torres P, García-Ruiz Á, La Rubia MD. Degradation and Migration in Olive Oil Packaged in Polyethylene Terephthalate under Thermal Treatment and Storage Conditions. Applied Sciences. 2024; 14(17):7507. https://doi.org/10.3390/app14177507
Chicago/Turabian StyleGonzález-Torres, Pablo, Ángeles García-Ruiz, and M. Dolores La Rubia. 2024. "Degradation and Migration in Olive Oil Packaged in Polyethylene Terephthalate under Thermal Treatment and Storage Conditions" Applied Sciences 14, no. 17: 7507. https://doi.org/10.3390/app14177507
APA StyleGonzález-Torres, P., García-Ruiz, Á., & La Rubia, M. D. (2024). Degradation and Migration in Olive Oil Packaged in Polyethylene Terephthalate under Thermal Treatment and Storage Conditions. Applied Sciences, 14(17), 7507. https://doi.org/10.3390/app14177507








