Characterizations of Interfacial Solar Water Evaporation
Abstract
:1. Introduction
2. Intrinsic Properties
2.1. X-ray Diffraction
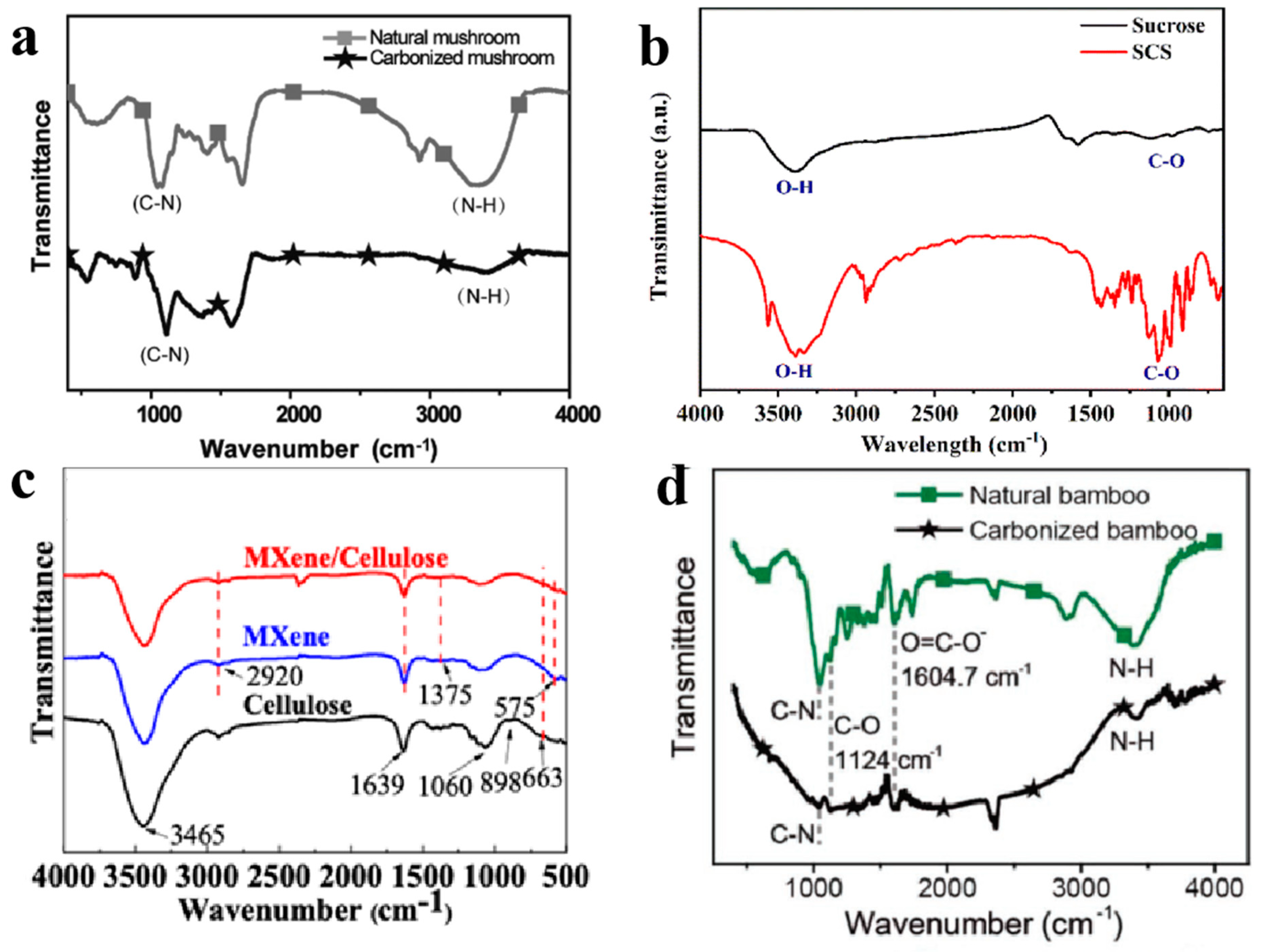
2.2. Fourier Transform Infrared Spectroscopy
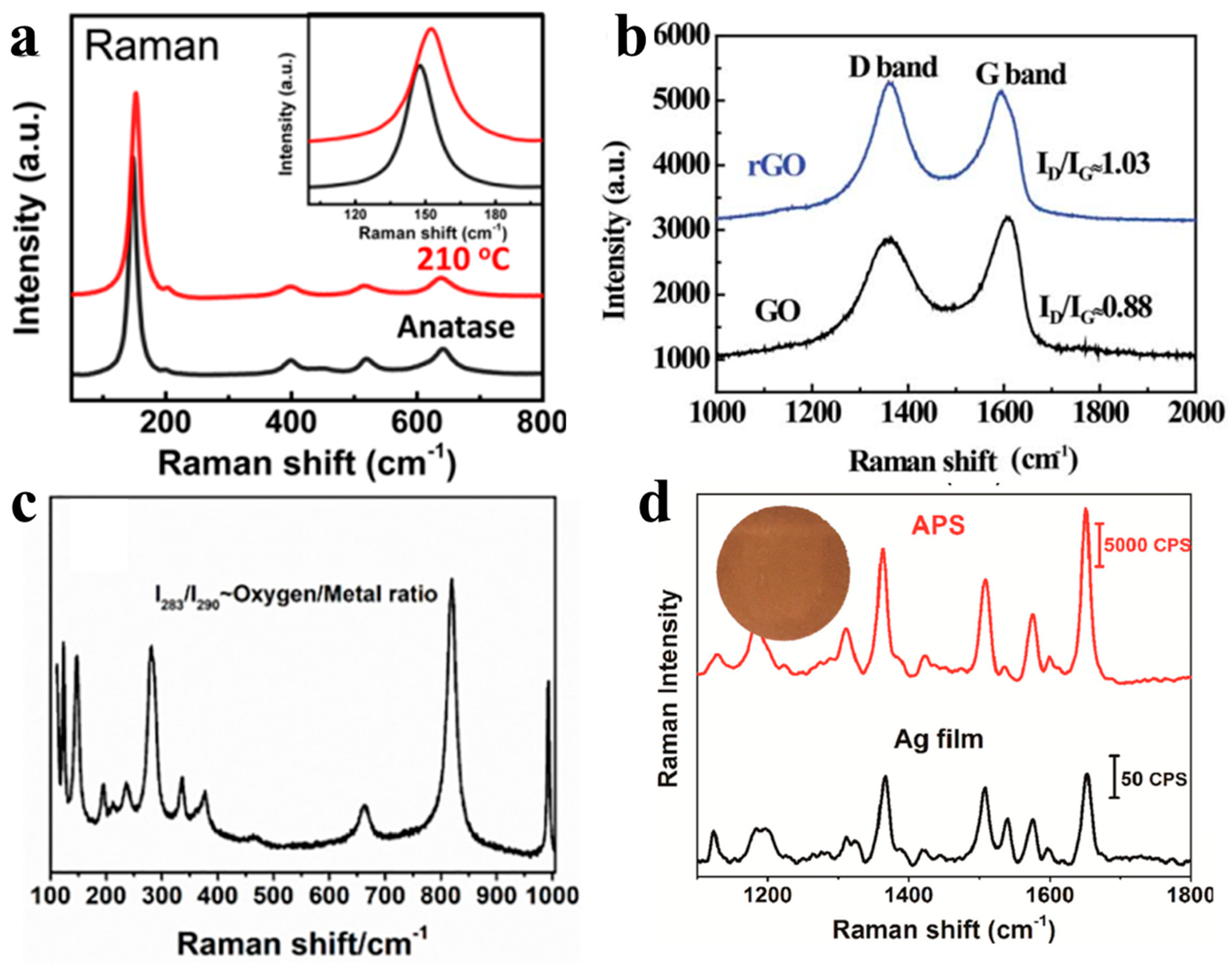
2.3. Raman Spectroscopy
2.4. X-ray Photoelectron Spectroscopy
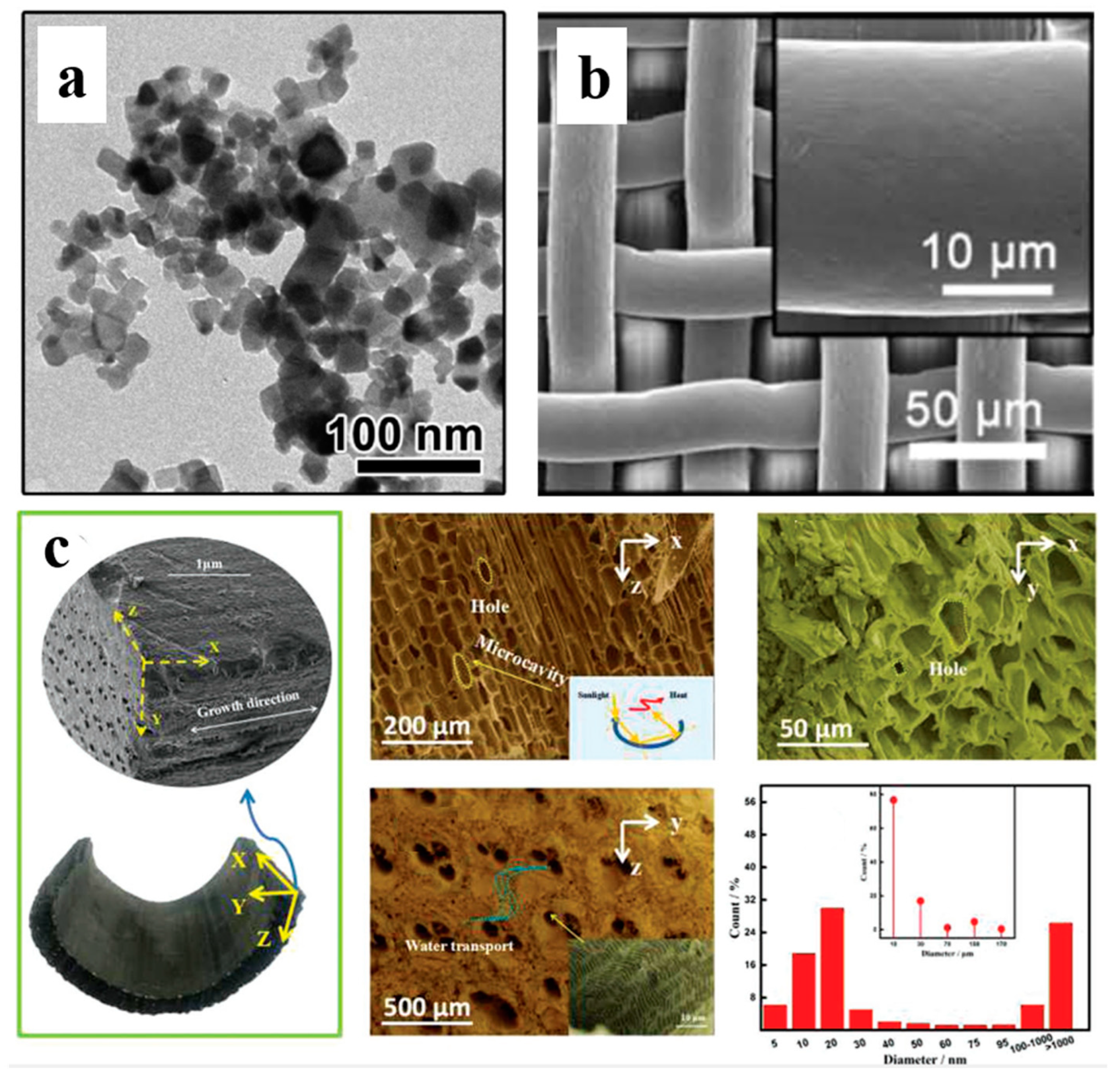
2.5. Scanning Electron Microscopy
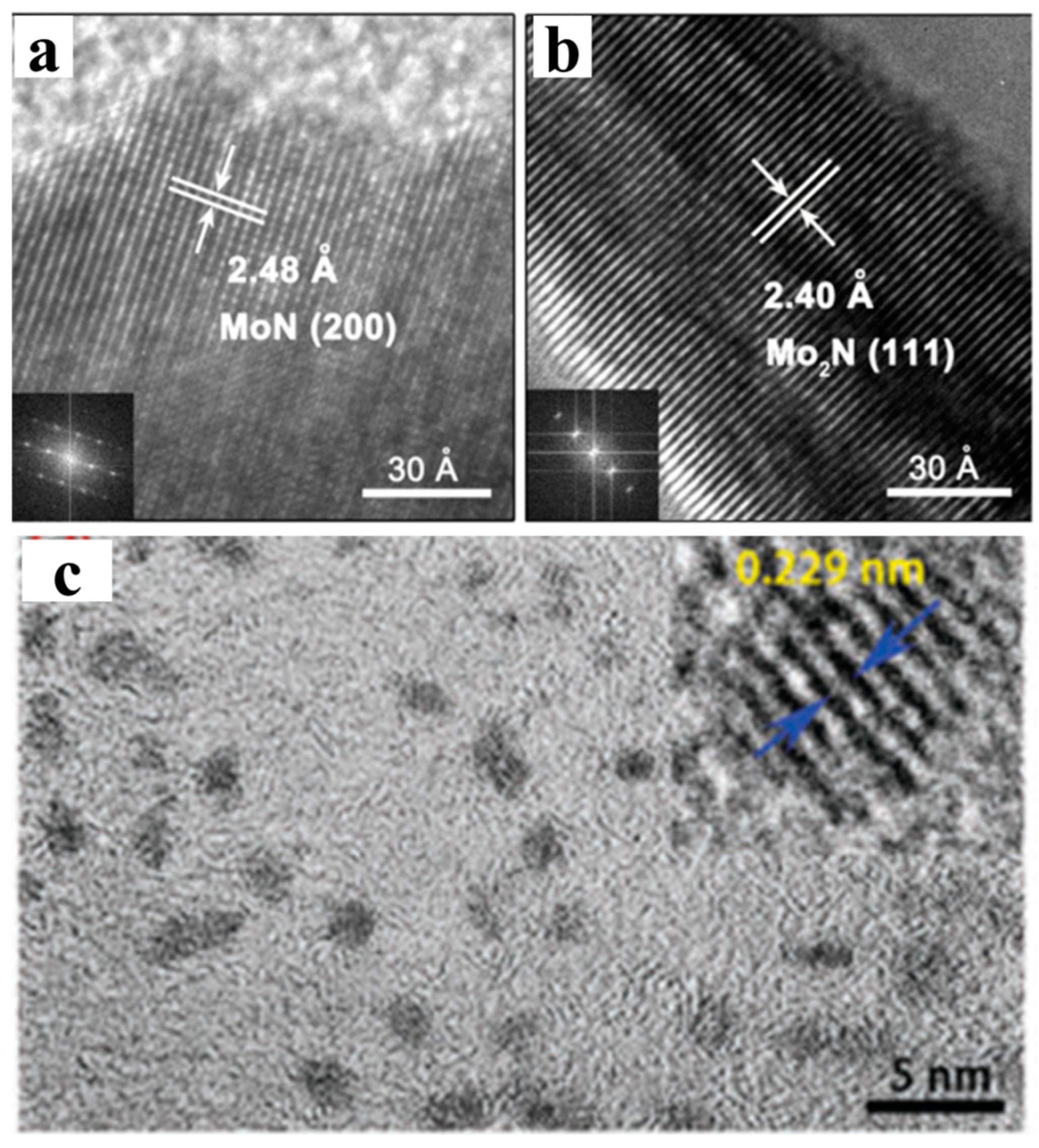
2.6. Transmission Electron Microscopy
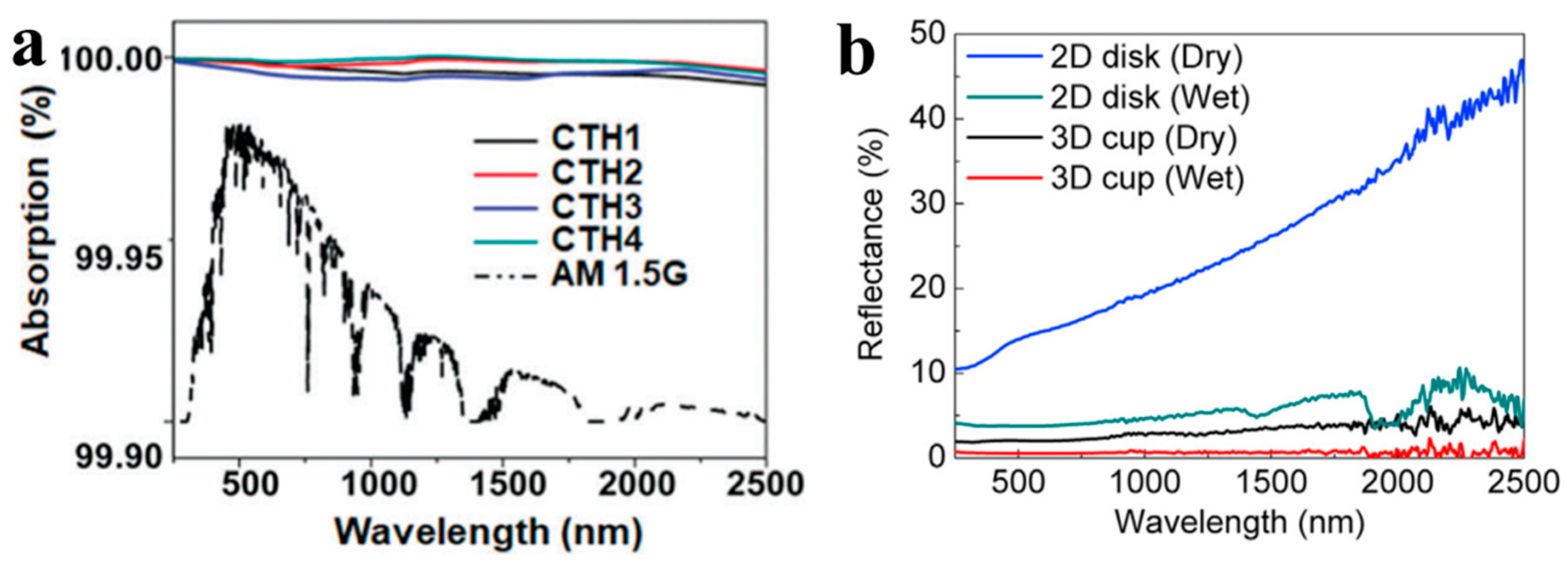
2.7. Diffuse Reflectance Spectroscopy
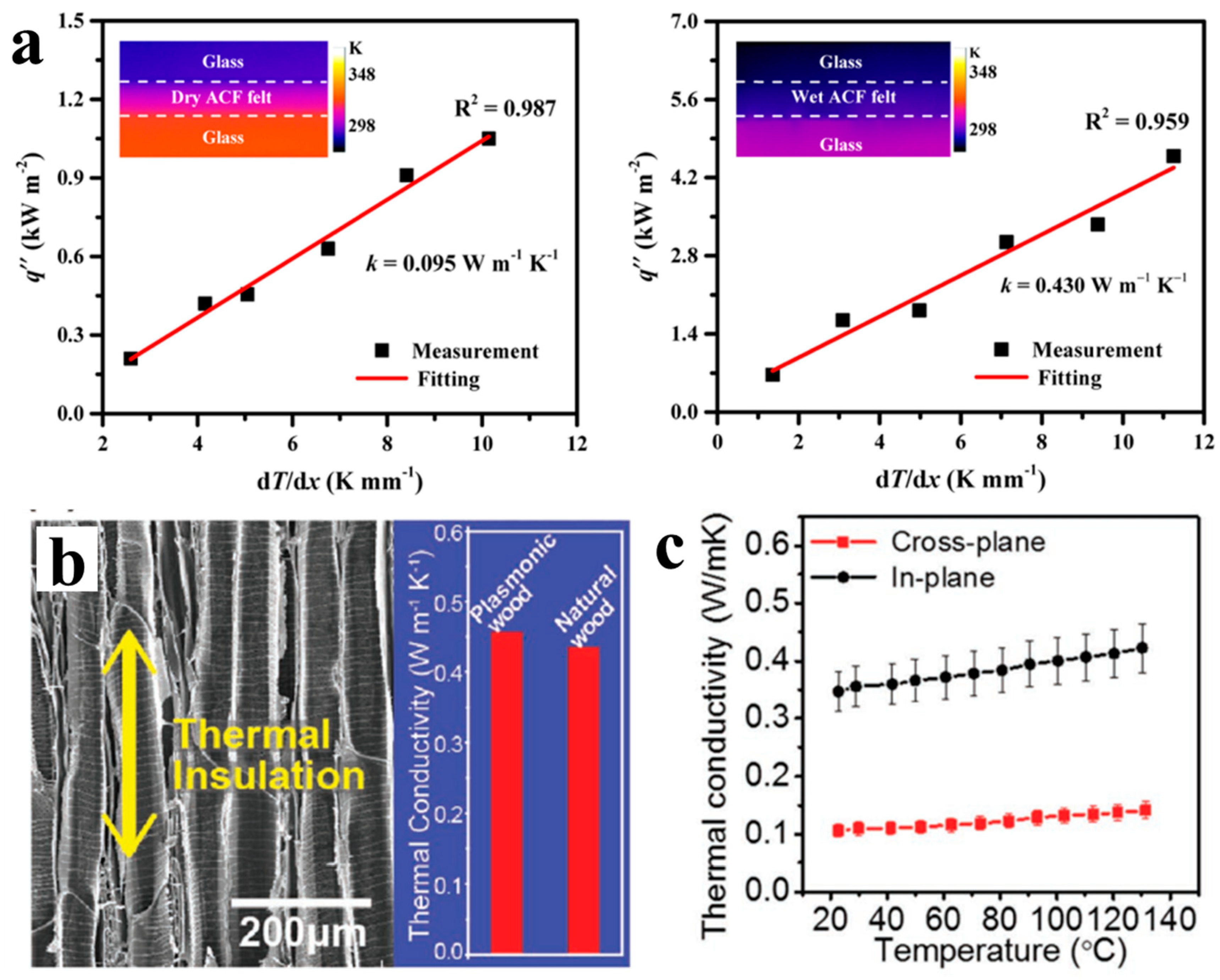
2.8. Thermal Conductivity
2.9. Contact Angle
3. Performance Evaluation
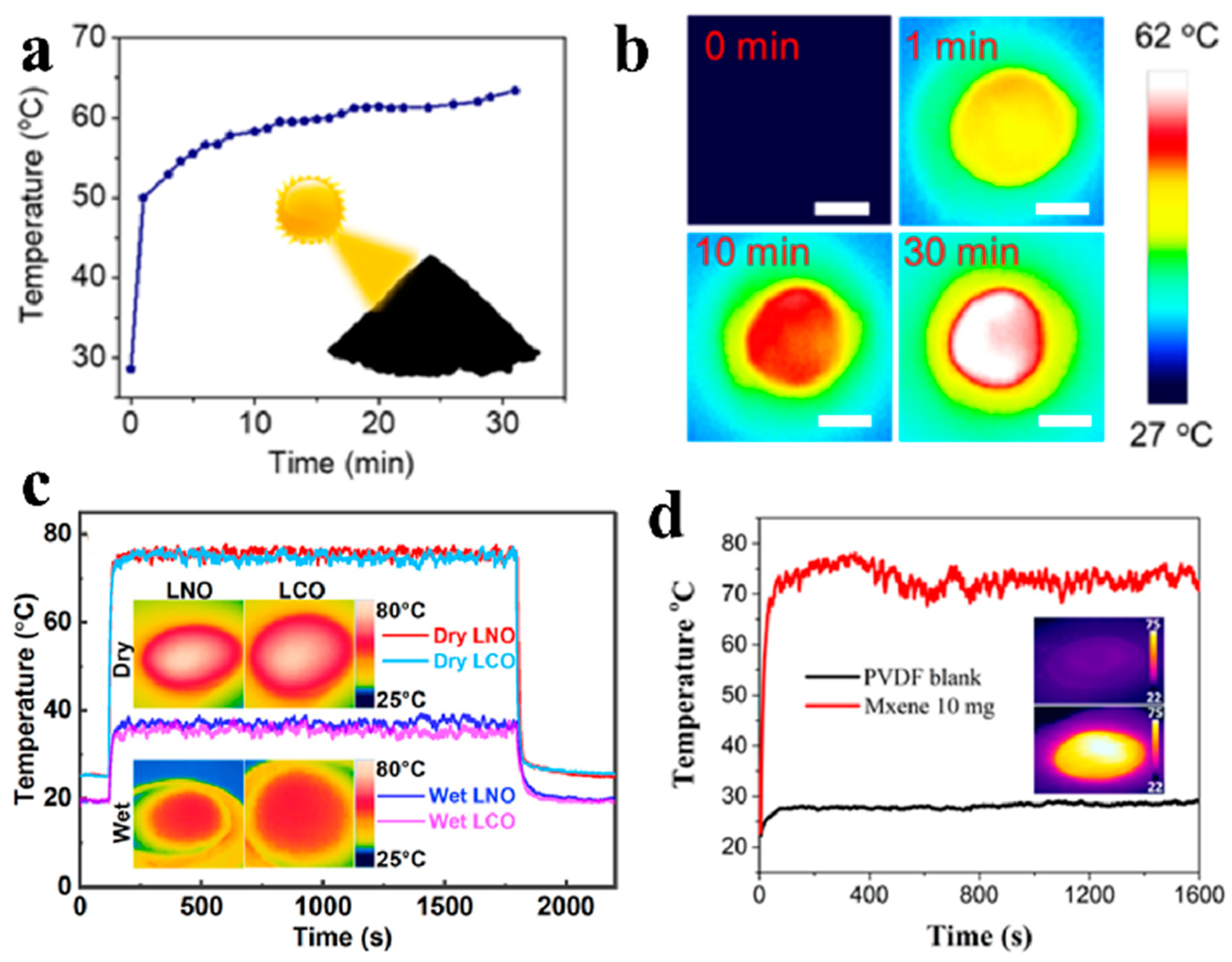
3.1. Surface Temperature
3.2. Evaporation Rate
3.3. Conversion Efficiency
3.4. Inductively Coupled Plasma Mass Spectrometry
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Shi, Y.; Wang, W.; Li, H.; Li, R.; Hong, S.; Wang, P. Distinct stage-wise environmental energy harvesting behaviors within solar-driven interfacial water evaporation coupled with convective airflow. Nano Energy 2023, 107, 108142. [Google Scholar] [CrossRef]
- Yu, H.; Wang, D.; Jin, H.; Wu, P.; Wu, X.; Chu, D.; Lu, Y.; Yang, X.; Xu, H. 2D MoN1.2-rGO Stacked Heterostructures Enabled Water State Modification for Highly Efficient Interfacial Solar Evaporation. Adv. Funct. Mater. 2023, 33, 2214828. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Su, R.; Wang, Y.; Li, Z.; Du, B.; Sun, Y.; Guan, P.; Besenbacher, F.; Yu, M. Phase-Transition Induced Conversion into a Photothermal Material: Quasi-Metallic WO(2.9) Nanorods for Solar Water Evaporation and Anticancer Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 10666–10671. [Google Scholar] [CrossRef]
- Ma, Q.; Yin, P.; Zhao, M.; Luo, Z.; Huang, Y.; He, Q.; Yu, Y.; Liu, Z.; Hu, Z.; Chen, B.; et al. MOF-Based Hierarchical Structures for Solar-Thermal Clean Water Production. Adv. Mater. 2019, 31, 1808249. [Google Scholar] [CrossRef]
- Li, Z.; Sun, L.; Liu, Y.; Zhu, L.; Yu, D.; Wang, Y.; Sun, Y.; Yu, M. SnSe@SnO2 core-shell nanocomposite for synchronous photothermal–photocatalytic production of clean water. Environ. Sci.-Nano 2019, 6, 1507–1515. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Nie, B.; Zhang, W.; Dou, X.; Meng, Y.; Zhao, X.; Wu, Y.; Li, H. A porous dome array evaporator for high-performance photothermal water evaporation and thermoelectric power generation. J. Mater. Chem. A 2024, 12, 293–302. [Google Scholar] [CrossRef]
- Zha, X.-J.; Zhao, X.; Pu, J.-H.; Tang, L.-S.; Ke, K.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Flexible Anti-Biofouling MXene/Cellulose Fibrous Membrane for Sustainable Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 36589–36597. [Google Scholar] [CrossRef]
- Bian, Y.; Du, Q.; Tang, K.; Shen, Y.; Hao, L.; Zhou, D.; Wang, X.; Xu, Z.; Zhang, H.; Zhao, L.; et al. Carbonized Bamboos as Excellent 3D Solar Vapor-Generation Devices. Adv. Mater. Technol. 2019, 4, 1800593. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, J.; Zhao, W.; Huang, F. Constructing Black Titania with Unique Nanocage Structure for Solar Desalination. ACS Appl. Mater. Interfaces 2016, 8, 31716–31721. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2017, 5, 16212–16219. [Google Scholar] [CrossRef]
- Huang, S.; Long, Y.; Yi, H.; Yang, Z.; Pang, L.; Jin, Z.; Liao, Q.; Zhang, L.; Zhang, Y.; Chen, Y.; et al. Multifunctional molybdenum oxide for solar-driven water evaporation and charged dyes adsorption. Appl. Surf. Sci. 2019, 491, 328–334. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.; Yu, J.; Wang, Y.; Nie, S.; Zhu, S.; Zhu, J. Dual functional asymmetric plasmonic structures for solar water purification and pollution detection. Nano Energy 2018, 51, 451–456. [Google Scholar] [CrossRef]
- Jiang, Q.; Tian, L.; Liu, K.-K.; Tadepalli, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Bilayered Biofoam for Highly Efficient Solar Steam Generation, Adv. Mater. 2016, 28, 9400–9407. [Google Scholar]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic Light-to-Heat Conversion Membranes with Self-Healing Ability for Interfacial Solar Heating, Adv. Mater. 2015, 27, 4889–4894. [Google Scholar]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of Black TiOx Nanoparticles by Mg Reduction of TiO2 Nanocrystals and their Application for Solar Water Evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Lei, T.; Ma, H.; Su, J.; Ling, S.; Wang, W. Arched Bamboo Charcoal as Interfacial Solar Steam Generation Integrative Device with Enhanced Water Purification Capacity. Adv. Sustain. Syst. 2019, 3, 1800144. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, L.; Zhang, H.; Yu, D.; Aslan, H.; Zhao, J.; Li, Z.; Yu, M.; Besenbacher, F.; Sun, Y. Dual-phase molybdenum nitride nanorambutans for solar steam generation under one sun illumination. Nano Energy 2019, 57, 842–850. [Google Scholar] [CrossRef]
- Ding, D.; Huang, W.; Song, C.; Yan, M.; Guo, C.; Liu, S. Non-stoichiometric MoO3−x quantum dots as a light-harvesting material for interfacial water evaporation. Chem. Commun. 2017, 53, 6744–6747. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, F.; Guo, Y.; Zhang, Y.; Yu, G. A hydrogel-based antifouling solar evaporator for highly efficient water desalination. Energy Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Jin, Y.; Zhuo, S.; Shi, L.; Chang, J.; Hong, S.; Ng, K.-C.; Wang, P. A 3D Photothermal Structure toward Improved Energy Efficiency in Solar Steam Generation. Joule 2018, 2, 1171–1186. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Hu, Y.; Wang, X. Commercially Available Activated Carbon Fiber Felt Enables Efficient Solar Steam Generation. ACS Appl. Mater. Interfaces 2018, 10, 9362–9368. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Chen, F.; Zhu, X.; Dai, J.; Li, Y.; Yang, Z.; Yan, X.; Song, J.; Wang, Y.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2018, 8, 1701028. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Zhao, X.; Chen, G.; Dai, J.; Pastel, G.; Jia, C.; Chen, C.; Hitz, E.; Siddhartha, D.; et al. Scalable and Highly Efficient Mesoporous Wood-Based Solar Steam Generation Device: Localized Heat, Rapid Water Transport. Adv. Funct. Mater. 2018, 28, 1707134. [Google Scholar] [CrossRef]
- Long, Y.; Huang, S.; Yi, H.; Chen, J.; Wu, J.; Liao, Q.; Liang, H.; Cui, H.; Ruan, S.; Zeng, Y.-J. Carrot-inspired solar thermal evaporator. J. Mater. Chem. A 2019, 7, 26911–26916. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Xiao, D.; Wang, P.; Luo, X.; Liu, W.; Liu, S.; Li, J.; Li, S.; Chen, Z. Melanin-Inspired Design: Preparing Sustainable Photothermal Materials from Lignin for Energy Generation. ACS Appl. Mater. Interfaces 2021, 13, 7600–7607. [Google Scholar] [CrossRef]
- Ahmad Wani, T.; Garg, P.; Bera, S.; Bhattacharya, S.; Dutta, S.; Kumar, H.; Bera, A. Narrow-Bandgap LaMO3 (M=Ni, Co) nanomaterials for efficient interfacial solar steam generation. J. Colloid Interface Sci. 2022, 612, 203–212. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, J.; Li, Z.; Xu, P.; Wang, X.; Yin, W.; Wang, M.; Ge, X.; Yin, Y. Space-Confined Seeded Growth of Black Silver Nanostructures for Solar Steam Generation. Nano Lett. 2019, 19, 400–407. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Wang, Y.; Duan, C. Highly efficient solar steam generation of supported metal–organic framework membranes by a photoinduced electron transfer process. Nanoscale 2019, 11, 11121–11127. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Ma, C.; Zhang, C.; Wu, D.; Zhu, H. Carbonized daikon for high efficient solar steam generation. Sol. Energy Mater. Sol. C 2019, 191, 83–90. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Wang, W.; Huang, J.; Xia, D.; Liao, J.; Wu, M.; Papavassiliou, D.V. Scalable, eco-friendly and ultrafast solar steam generators based on one-step melamine-derived carbon sponges toward water purification. Nano Energy 2019, 58, 322–330. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, R.; Zhang, T.; Zhao, K.; Xiao, P.; Ma, Y.; Ajayan, P.M.; Shi, G.; Chen, Y. Graphene-Based Standalone Solar Energy Converter for Water Desalination and Purification. ACS Nano 2018, 12, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zheng, T.; Song, Z.; Shao, Y.; Li, N.; Jia, K.; Tian, Y.; Song, Q.; Liu, H.; Xue, G. Realization of Low Latent Heat of a Solar Evaporator via Regulating the Water State in Wood Channels. ACS Appl. Mater. Interfaces 2020, 12, 18504–18511. [Google Scholar] [CrossRef]
- Wu, Y.; Kong, R.; Ma, C.; Li, L.; Zheng, Y.; Lu, Y.; Liang, L.; Pang, Y.; Wu, Q.; Shen, Z.; et al. Simulation-Guided Design of Bamboo Leaf-Derived Carbon-Based High-Efficiency Evaporator for Solar-Driven Interface Water Evaporation. Energy Environ. Mater. 2022, 5, 1323–1331. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Wang, X.; Huang, Y.; He, S.; Cui, J.; Wu, L.; Feng, R.; Huang, S. Characterizations of Interfacial Solar Water Evaporation. Appl. Sci. 2024, 14, 7523. https://doi.org/10.3390/app14177523
Yi H, Wang X, Huang Y, He S, Cui J, Wu L, Feng R, Huang S. Characterizations of Interfacial Solar Water Evaporation. Applied Sciences. 2024; 14(17):7523. https://doi.org/10.3390/app14177523
Chicago/Turabian StyleYi, Huan, Xiaoshuai Wang, Yijun Huang, Shucheng He, Jiao Cui, Liwei Wu, Ribao Feng, and Shaolong Huang. 2024. "Characterizations of Interfacial Solar Water Evaporation" Applied Sciences 14, no. 17: 7523. https://doi.org/10.3390/app14177523




