EEG-Based Methods for Diagnosing Color Vision Deficiency: A Comprehensive Review
Abstract
:1. Introduction
2. Neurophysiological Basis of Color Vision
| CVD Type | Cause | |
|---|---|---|
| Anomalous trichromacy | Protanomaly | Abnormal L-cone |
| Deuteranomaly | Abnormal M-cone | |
| Tritanomaly | Abnormal S-cone | |
| Dichromacy | Protanopia | Missing L-cone |
| Deuteranopia | Missing M-cone | |
| Tritanopia | Missing S-cone | |

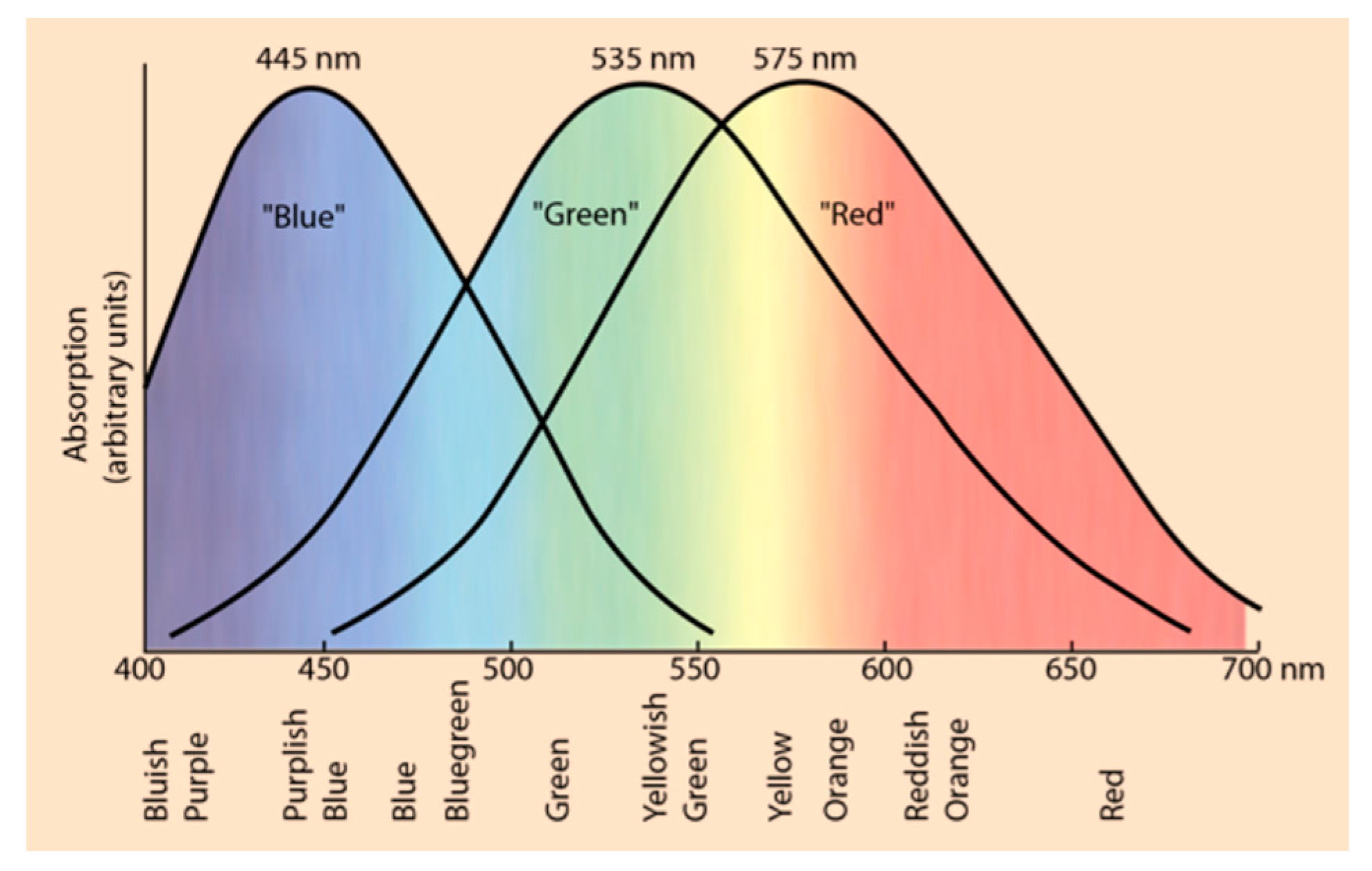
3. EEG as a Diagnostic Tool
4. Comparing Conventional and EEG-Based Methods for Diagnosing CVD
5. EEG-Based Approaches for Diagnosing CVD
5.1. ERP Approaches for Diagnosing CVD
5.2. SSVEP Approaches for Diagnosing CVD
| Reference | Year | Method/Analysis | Channels | Extracted Features | Findings |
|---|---|---|---|---|---|
| [71] | - | Silent substitution, vector voltmeter | Oz, O1, O2 | Amplitude | Differences in EEG were observed between color-deficient and healthy infants. |
| [77] | 2016 | Color stimulus, principal component analysis (PCA) | Cz, O1, Oz, O2 (10–20 international system) | Energy | Higher EEG energy for correct color choices compared with incorrect choices. |
| [78] | 2021 | Color stimulus, multifractal detrended fluctuation analysis (MFDFA) and multi-fractal detrended cross-correlation analysis (MFDXA) | F3, F4, F7, F8, Fz, P3, P4, O1, O2 | Spectral width | The complexity of the EEG varies across different perceived colors. |
| [79] | 2021 | Color stimulus, Fourier transform, Quadratic discriminant analysis, SVM, and KNN classifiers | 63 different channels | Conventional band power, inverse solution of EEG waves | Different electrodes showed a significant impact of different colors. |
| [80] | 2016 | Color stimulus, interval-Type II fuzzy classifier | F3, F4, Fz, P3, Pz, P4, O1, O2, T7, T8 | PSD | Different PSD for each color with the highest classification rate observed for red and the lowest for yellow. |
| [76] | 2017 | Color stimulus, Python, BeagleBoard | Cz, O1, Oz, O2 | Energy, entropy | There is more deviation in the energy of color-deficient people compared to healthy people. |
| [81] | 2020 | Ishihara plates stimulus, GPF, Brain Topography and ERP Analysis | Fp1, Fp2, F3, Fz, F4, C3, Cz, C4, P7, P3, Pz, P4, P8, O1, Oz, O2 (10–20 international system) | Amplitude, latency | Variations in latency and amplitudes between healthy and color-deficient people. |
| [82] | 2021 | Ishihara plates stimulus, FDR correction, KNN classifier | Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz (10–20 international system) | Frequency | People with CVD have different values for the frequency bands compared to individuals with normal color vision, and the best results showing differences were observed for the electrodes P4, T6, and O2. |
| [85] | 2021 | Sweep SSVEP, CCA, color vision severity index (ICVD) | PO3, PO4, POz, O1, O2, and Oz (10–20 international system) | Amplitude, frequency | The type and severity of CVD can be determined by ICVD. |
| [87] | 2021 | SSVEP, Metamer Identification Algorithm (metalD), CCA | F3, Fz, F4, T7, C3, CZ, C4, T8, CP3, CP4, P3, Pz, P4, PO7, PO8, Oz (10–10 international system) | Frequency | People with normal color vision have an SSVEP that is close to zero, while people with CVD do not. |
| [88] | 2022 | SSVEP, Gaussian process regression (metalD+) | O1, Oz, O2, PO7, PO3, POz, PO4, PO8, P5, P3, P1, Pz, P2, P4, P6, CPz | Frequency | This method can improve the measurement and reduce noise compared to the metalD method. |
| [89] | 2023 | SSVEP, CCA | O1, Oz, O2, PO7, PO3, POz, PO4, PO8, P5, P3, P1, Pz, P2, P4, P6, CPz | Amplitude | CVD assessment efficiency depends on the visual stimulation frequency. |
5.3. Pros and Cons of Both Techniques
6. Data Analysis and Interpretation
7. Clinical Applications and Challenges
8. Future Directions and Research Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Woldeamanuel, G.G.; Geta, T.G. Prevalence of Color Vision Deficiency Among School Children in Wolkite, Southern Ethiopia. BMC Res. Notes 2018, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, N.B.; AlShammari, R.Z.; AlOmar, R.S.; AlShamlan, N.A.; Alqahtani, A.A.; AlAmer, N.A. Prevalence of Color Vision Deficiency in Medical Students at a Saudi University. J. Fam. Community Med. 2021, 28, 196–201. [Google Scholar] [CrossRef]
- Manda, A. Color Blindness Prevalence. Available online: https://www.news-medical.net/health/Color-Blindness-Prevalence.aspx (accessed on 10 August 2024).
- National Eye Institute. Causes of Color Vision Deficiency. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/color-blindness/causes-color-vision-deficiency (accessed on 23 June 2024).
- Birch, J. Worldwide Prevalence of Red-Green Color Deficiency. J. Opt. Soc. Am. A 2012, 29, 313–320. [Google Scholar] [CrossRef]
- Male, S.R.; Shamanna, B.R.; Gandhi, R.; Theagarayan, B. Global Prevalence of Color Vision Deficiency: A Systematic Review and Meta-Analysis. J. Vis. Impair. Blind. 2023, 64, 1508. [Google Scholar]
- Osuobeni, E.P. Prevalence of Congenital Red-Green Color Vision Defects in Arab Boys from Riyadh, Saudi Arabia. Ophthalmic Epidemiol. 1996, 3, 167–170. [Google Scholar] [CrossRef]
- Alabdelmoneam, M. Prevalence of Congenital Color Vision Defects in Saudi Females of Arab Origin. Optom. Vis. Sci. 2011, 88, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Rajavi, Z.; Sabbaghi, H.; Baghini, A.S.; Yaseri, M.; Sheibani, K.; Norouzi, G. Prevalence of Color Vision Deficiency and Its Correlation with Amblyopia and Refractive Errors among Primary School Children. J. Ophthalmic Vis. Res. 2015, 10, 130–138. [Google Scholar] [CrossRef]
- Fareed, M.; Anwar, M.A.; Afzal, M. Prevalence and Gene Frequency of Color Vision Impairments among Children of Six Populations from North Indian Region. Genes Dis. 2015, 2, 211–218. [Google Scholar] [CrossRef]
- Fakorede, S.T.; Akpan, L.G.; Adekoya, K.O.; Oboh, B. Prevalence and Population Genetic Data of Colour Vision Deficiency among Students from Selected Tertiary Institutions in Lagos State, Nigeria. Egypt. J. Med. Hum. Genet. 2022, 23, 73. [Google Scholar] [CrossRef]
- Shah, A.; Hussain, R.; Fareed, M.; Afzal, M. Prevalence of Red-Green Color Vision Defects among Muslim Males and Females of Manipur, India. Iran. J. Public Health 2013, 42, 16–24. [Google Scholar]
- Chan, X.B.V.; Goh, S.M.S.; Tan, N.C. Subjects with Colour Vision Deficiency in the Community: What Do Primary Care Physicians Need to Know? Asia Pac. Fam. Med. 2014, 13, 10. [Google Scholar] [CrossRef]
- Osman, S.; Khalaf, S.; Mohammed, H.; El-Sebaity, D.; Osman, D. Prevalence and Predictors of Colour Vision Defects among Egyptian University Students. East. Mediterr. Health J. 2021, 27, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N. Colour Blindness. Public Health 1998, 112, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Fanlo Zarazaga, A.; Gutiérrez Vásquez, J.; Pueyo Royo, V. Review of the Main Colour Vision Clinical Assessment Tests. Arch. Soc. Esp. Oftalmol. 2019, 94, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Salvia, J.; Ysseldyke, J. An Analysis of the Reliability and Validity of the Ishihara Color Plates with Mentally Retarded Males. Percept. Mot. Ski. 1971, 33, 243–246. [Google Scholar] [CrossRef]
- Pandey, N.; Chandrakar, A.K.; Garg, M.L. Tests for Color Vision Deficiency: Is It Time to Revise the Standards? Indian J. Ophthalmol. 2015, 63, 752–753. [Google Scholar] [CrossRef]
- Das, J.M.; Anosike, K.; Asuncion, R.M.D. Locked-in Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559026/ (accessed on 10 August 2024).
- National Institute of Neurological Disorders and Stroke. Locked-in Syndrome. Available online: https://www.ninds.nih.gov/health-information/disorders/locked-syndrome (accessed on 11 August 2024).
- National Organization for Rare Disorders (NORD). Locked-in Syndrome—Symptoms, Causes, Treatment. Available online: https://rarediseases.org/rare-diseases/locked-in-syndrome/ (accessed on 9 August 2024).
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain Computer Interfaces, a Review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef]
- Newton, I.; Hemming, G.W. Opticks: Or, A Treatise of the Reflections, Refractions, Inflexions and Colours of Light; Also Two Treatises of the Species and Magnitude of Curvilinear Figures; Printed for Sam; Smith, S., Walford, B., Eds.; Burndy Library: London, UK, 1704; Available online: http://archive.org/details/optickstreatise00newta (accessed on 9 July 2024).
- Celesia, G.G. Chapter 13 Color Vision Deficiencies. In Handbook of Clinical Neurophysiology; Celesia, G.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5, pp. 251–269. [Google Scholar] [CrossRef]
- Thoreson, W.B.; Dacey, D.M. Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina. Physiol. Rev. 2019, 99, 1527–1573. [Google Scholar] [CrossRef]
- Conway, B.R. Color Vision, Cones, and Color-Coding in the Cortex. Neuroscientist 2009, 15, 274–290. [Google Scholar] [CrossRef]
- Werner, A. Spatial and Temporal Aspects of Chromatic Adaptation and Their Functional Significance for Colour Constancy. Vis. Res. 2014, 104, 80–89. [Google Scholar] [CrossRef]
- Balasundaram, R.; Reddy, S.C. Prevalence of Colour Vision Deficiency among Medical Students and Health Personnel. Malays. Fam. Physician 2006, 1, 52–53. [Google Scholar] [PubMed]
- Kagami, S.; Bradshaw, S.E.; Fukumoto, M.; Tsukui, I. Cataracts in Airline Pilots: Prevalence and Aeromedical Considerations in Japan. Aviat. Space Environ. Med. 2009, 80, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, D.; Georgalas, I.; Kalantzis, G.; Karmiris, E.; Koutsandrea, C.; Diagourtas, A.; Ladas, I.; Georgopoulos, G. Acquired Color Vision and Visual Field Defects in Patients with Ocular Hypertension and Early Glaucoma. Clin. Ophthalmol. 2009, 3, 251–257. [Google Scholar]
- Jafarzadehpur, E.; Hashemi, H.; Emamian, M.H.; Khabazkhoob, M.; Mehravaran, S.; Shariati, M.; Fotouhi, A. Color Vision Deficiency in a Middle-Aged Population: The Shahroud Eye Study. Int. Ophthalmol. 2014, 34, 1067–1074. [Google Scholar] [CrossRef]
- Weil, R.S.; Schrag, A.E.; Warren, J.D.; Crutch, S.J.; Lees, A.J.; Morris, H.R. Visual Dysfunction in Parkinson’s Disease. Brain 2016, 139, 2827–2843. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power Spectral Density and Coherence Analysis of Alzheimer’s EEG. Cogn. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tran, J.; Fabiano, N.; Dhir, V.; Larose, K.; Lasker, I.; Wong, S.; Mohammad, I.Y.Z.; Le, S.; Shorr, R.; et al. Colour Vision Defects in Schizophrenia Spectrum Disorders: A Systematic Review. Neurosci. Appl. 2024, 3, 104046. [Google Scholar] [CrossRef]
- Ascaso, F.J.; Cruz, N.; Del Buey, M.A.; Cristóbal, J.A. An Unusual Case of Cocaine-Induced Maculopathy. Eur. J. Ophthalmol. 2009, 19, 880–882. [Google Scholar] [CrossRef]
- Salomão, S.R.; Watanabe, S.E.S.; Berezovsky, A.; Motono, M. Multifocal Electroretinography, Color Discrimination and Ocular Toxicity in Tamoxifen Use. Curr. Eye Res. 2007, 32, 345–352. [Google Scholar] [CrossRef]
- Gobba, F.; Cavalleri, A. Color Vision Impairment in Workers Exposed to Neurotoxic Chemicals. Neurotoxicology 2003, 24, 693–702. [Google Scholar] [CrossRef]
- Simunovic, M.P. Colour Vision Deficiency. Eye 2010, 24, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Dohvoma, V.A.; Mvogo, S.R.E.; Kagmeni, G.; Emini, N.R.; Epee, E.; Mvogo, C.E. Color Vision Deficiency among Biomedical Students: A Cross-Sectional Study. Clin. Ophthalmol. 2018, 12, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- National Eye Institute. Types of Color Vision Deficiency. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/color-blindness/types-color-vision-deficiency (accessed on 17 July 2024).
- Klinke, T.; Hannak, W.; Böning, K.; Jakstat, H. A Comparative Study of the Sensitivity and Specificity of the Ishihara Test with Various Displays. Int. Dent. J. 2024, 74, 892–896. [Google Scholar] [CrossRef]
- Brain Stuff. How Is Color Vision Deficiency Different from Colorblindness? Available online: https://brainstuff.org/blog/how-is-color-vision-deficiency-different-from-colorblindness (accessed on 8 August 2024).
- American Academy of Ophthalmology. Rods. Available online: https://www.aao.org/eye-health/anatomy/rods (accessed on 12 August 2024).
- Neitz, M.; Neitz, J. Molecular Genetics of Color Vision and Color Vision Defects. Arch. Ophthalmol. 2000, 118, 691–700. [Google Scholar] [CrossRef]
- HyperPhysics. The Color-Sensitive Cones. Available online: http://hyperphysics.phy-astr.gsu.edu/hbase/vision/colcon.html (accessed on 3 July 2024).
- Tudor, M.; Tudor, L.; Tudor, K.I. Hans Berger (1873–1941)—The History of Electroencephalography. Acta Med. Croat. 2005, 59, 307–313. [Google Scholar]
- Kirschstein, T.; Köhling, R. What is the Source of the EEG? Clin. EEG Neurosci. 2009, 40, 146–149. [Google Scholar] [CrossRef]
- Britton, J.W.; Frey, L.C.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; St Louis, E.K. Introduction. In Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK390346/ (accessed on 8 August 2024).
- Jadhav, C.; Kamble, P.; Mundewadi, S.; Jaiswal, N.; Mali, S.; Ranga, S.; Suvvari, T.K.; Rukadikar, A. Clinical Applications of EEG as an Excellent Tool for Event-Related Potentials in Psychiatric and Neurotic Disorders. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 73–83. [Google Scholar]
- Gururangan, K.; Razavi, B.; Parvizi, J. Diagnostic Utility of Eight-Channel EEG for Detecting Generalized or Hemispheric Seizures and Rhythmic Periodic Patterns. Clin. Neurophysiol. Pract. 2018, 3, 65–73. [Google Scholar] [CrossRef]
- Britton, J.W.; Frey, L.C.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; St Louis, E.K. The Abnormal EEG. In Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK390357/ (accessed on 4 May 2024).
- Hsiao, C.-L.; Chen, P.-Y.; Chen, I.-A.; Lin, S.-K. The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units. Diagnostics 2024, 14, 1111. [Google Scholar] [CrossRef]
- Ryynänen, O.; Hyttinen, J.; Malmivuo, J. Effect of Measurement Noise and Electrode Density on the Spatial Resolution of Cortical Potential Distribution with Different Resistivity Values for the Skull. Trans. Biomed. Eng. 2006, 53, 1851–1858. [Google Scholar] [CrossRef]
- Liu, X.; Makeyev, O.; Besio, W. Improved Spatial Resolution of Electroencephalogram Using Tripolar Concentric Ring Electrode Sensors. Electr. Comput. Biomed. Eng. Fac. Publ. 2020, 2020, 6269394. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Q.-Q.; Chen, H.; Hu, X.-Q.; Li, W.-G.; Bai, Y.; Han, J.-X.; Wang, Y.; Liang, Z.-H.; Chen, D.; et al. The Applied Principles of EEG Analysis Methods in Neuroscience and Clinical Neurology. Mil. Med. Res. 2023, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Klein, K.M.; Hamer, H.M. Non-Invasive EEG Evaluation in Epilepsy Diagnosis. Expert Rev. Neurother. 2015, 15, 425–444. [Google Scholar] [CrossRef]
- Lenartowicz, A.; Loo, S.K. Use of EEG to Diagnose ADHD. Curr. Psychiatry Rep. 2014, 16, 498. [Google Scholar] [CrossRef]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression Biomarkers Using Non-Invasive EEG: A Review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Gomes, A. Analysis of Visual Patterns Through the EEG Signal: Color Study. In Proceedings of the Augmented Cognition: 17th International Conference, AC 2023, Held as Part of the 25th HCI International Conference, HCII 2023, Copenhagen, Denmark, 23–28 July 2023; pp. 46–53. [Google Scholar] [CrossRef]
- Khadir, A.; Maghareh, M.; Ghamsari, S.S.; Beigzadeh, B. Brain Activity Characteristics of RGB Stimulus: An EEG Study. Sci. Rep. 2023, 13, 18988. [Google Scholar] [CrossRef] [PubMed]
- Perkins School for the Blind. The Visual Pathway from the Eye to the Brain. Available online: https://www.perkins.org/the-visual-pathway-from-the-eye-to-the-brain/ (accessed on 10 May 2024).
- Plutino, A.; Armellin, L.; Mazzoni, A.; Marcucci, R.; Rizzi, A. Aging Variations in Ishihara Test Plates. Color Res. Appl. 2023, 48, 721–734. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kwon, O.-Y.; Kim, Y.-J.; Kim, H.-K.; Lee, Y.-E.; Williamson, J.; Fazli, S.; Lee, S.-W. EEG Dataset and OpenBMI Toolbox for Three BCI Paradigms: An Investigation into BCI Illiteracy. GigaScience 2019, 8, giz002. [Google Scholar] [CrossRef]
- Kotlewska, I.; Wójcik, M.J.; Nowicka, M.M.; Marczak, K.; Nowicka, A. Present and Past Selves: A Steady-State Visual Evoked Potentials Approach to Self-Face Processing. Sci. Rep. 2017, 7, 16438. [Google Scholar] [CrossRef]
- Mun, S.; Park, M.-C.; Park, S.; Whang, M. SSVEP and ERP Measurement of Cognitive Fatigue Caused by Stereoscopic 3D. Neurosci. Lett. 2012, 525, 89–94. [Google Scholar] [CrossRef]
- Boylan, M.R.; Figueria, J.S.; Elhamiasl, M.; da Silva Andrade, I.; Barry-Anwar, R.; Keil, A.; Scott, L.S. Assessing the Reliability of ERP, SSVEP, and Oscillatory Data Methodology for Visual Paradigms in Infant EEG. J. Vis. 2023, 23, 4914. [Google Scholar] [CrossRef]
- Tai, P.; Ding, P.; Wang, F.; Gong, A.; Li, T.; Zhao, L.; Su, L.; Fu, Y. Brain-Computer Interface Paradigms and Neural Coding. Front. Neurosci. 2024, 17, 1345961. [Google Scholar] [CrossRef]
- Cao, T.; Wan, F.; Mak, P.U.; Mak, P.-I.; Vai, M.I.; Hu, Y. Flashing Color on the Performance of SSVEP-Based Brain-Computer Interfaces. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August 2012; pp. 1819–1822. [Google Scholar] [CrossRef]
- Albahri, A.S.; Al-Qaysi, Z.T.; Alzubaidi, L.; Alnoor, A.; Albahri, O.S.; Alamoodi, A.H.; Bakar, A.A. A Systematic Review of Using Deep Learning Technology in the Steady-State Visually Evoked Potential-Based Brain-Computer Interface Applications: Current Trends and Future Trust Methodology. Int. J. Telemed. Appl. 2023, 2023, 7741735. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Fernandez-Vargas, J.; Kita, K.; Yu, W. Influence of Stimulus Color on Steady State Visual Evoked Potentials. Adv. Intell. Syst. Comput. 2017, 531, 499–509. [Google Scholar] [CrossRef]
- Bieber, M.L.; Knoblauch, K.; Werner, J.S. Detecting Colour Vision Deficiency in 4- and 8-Week-Old Human Infants. In The Colour Vision Deficiencies XIII, Proceedings of the Thirteenth Symposium of the International Research Group on Colour Vision Deficiencies, Held in Pau, France, 27–30 July 1995; Cavonius, C.R., Ed.; Springer: Dordrecht, The Netherlands, 1997; pp. 277–282. [Google Scholar] [CrossRef]
- Yan, W.; Xu, G.; Li, M.; Xie, J.; Han, C.; Zhang, S.; Luo, A.; Chen, C. Steady-State Motion Visual Evoked Potential (SSMVEP) Based on Equal Luminance Colored Enhancement. PLoS ONE 2017, 12, e0169642. [Google Scholar] [CrossRef]
- Spitschan, M.; Woelders, T. The Method of Silent Substitution for Examining Melanopsin Contributions to Pupil Control. Front. Neurol. 2018, 9, 941. [Google Scholar] [CrossRef]
- Hendrickson, A.; Bumsted-O’Brien, K.; Natoli, R.; Ramamurthy, V.; Possin, D.; Provis, J. Rod Photoreceptor Differentiation in Fetal and Infant Human Retina. Exp. Eye Res. 2008, 87, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Vinekar, A.; Mangalesh, S.; Jayadev, C.; Maldonado, R.S.; Bauer, N.; Toth, C.A. Retinal Imaging of Infants on Spectral Domain Optical Coherence Tomography. BioMed. Res. Int. 2015, 2015, 782420. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Rajendran, R.; Koganti, Y.; Maheswari, V.U. Portable Embedded Device to Analyse the Effect of Color Blindness on EEG. In Proceedings of the 2017 International Conference on Nextgen Electronic Technologies: Silicon to Software (ICNETS2), Chennai, India, 23–25 March 2017; pp. 270–274. [Google Scholar] [CrossRef]
- Thomas, B.; Umamaheswari, V. EEG Based Color Impairment Detection. Int. J. Recent Innov. Trends Comput. Commun. 2016, 4, 540–542. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, A.; Roy, C.; Nag, S.; Sanyal, S.; Sengupta, R.; Ghosh, D. Brain Response to Color Stimuli: An EEG Study with Nonlinear Approach. Cogn. Neurodyn. 2021, 15, 1023–1053. [Google Scholar] [CrossRef]
- Göksel Duru, D.; Alobaidi, M. Classification of Brain Electrophysiological Changes in Response to Colour Stimuli. Phys. Eng. Sci. Med. 2021, 44, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Lahiri, R. Discriminating Different Color from EEG Signals Using Interval-Type 2 Fuzzy Space Classifier (A Neuro-Marketing Study on the Effect of Color to Cognitive State). In Proceedings of the 2016 IEEE 1st International Conference on Power Electronics, Intelligent Control and Energy Systems (ICPEICES), Delhi, India, 4–6 July 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Wicaksono, A.; Mengko, T.; Iramina, K. Investigation of EEG Signal Response Using Event-Related Potential (ERP) Towards Ishihara Pseudo-Isochromatic Visual Stimulus. In Proceedings of the 2020 IEEE Signal, Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 5 December 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Ekhlasi, A.; Ahmadi, H.; Molavi, A.; Nia, M.; Nasrabadi, A.M. EEG Signal Analysis During Ishihara’s Test in Subjects with Normal Vision and Color Vision Deficiency. Biomed. Phys. Eng. Express 2021, 7, 025021. [Google Scholar] [CrossRef] [PubMed]
- Duart, X.; Quiles, E.; Suay, F.; Chio, N.; García, E.; Morant, F. Evaluating the Effect of Stimuli Color and Frequency on SSVEP. Sensors 2020, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Tello, R.J.M.G.; Müller, S.M.T.; Ferreira, A.; Bastos, T.F. Comparison of the Influence of Stimuli Color on Steady-State Visual Evoked Potentials. Res. Biomed. Eng. 2015, 31, 218–231. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, G.; Wang, Y.; Du, C.; Liang, R.; Zhang, K.; Jia, Y.; Du, Y. Quantitative and Objective Diagnosis of Color Vision Deficiencies Based on Steady-State Visual Evoked Potentials. Int. Ophthalmol. 2021, 41, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Ridder, W.; McCulloch, D.; Herbert, A. Stimulus Duration, Neural Adaptation, and Sweep Visual Evoked Potential Acuity Estimates. Invest. Ophthalmol. Vis. Sci. 1999, 39, 2759–2768. [Google Scholar]
- Norton, J.J.S.; DiRisio, G.F.; Carp, J.S.; Norton, A.E.; Kochan, N.S.; Wolpaw, J.R. Brain-Computer Interface-Based Assessment of Color Vision. J. Neural Eng. 2021, 18, 066015. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, H.; Long, K.J.; Atkins, A.E.; Zois, D.-S.; Norton, J.J.S. Improving BCI-Based Color Vision Assessment Using Gaussian Process Regression. In Proceedings of the ICASSP 2022 IEEE International Conference on Acoustics, Speech and Signal Process (ICASSP), Singapore, 23–27 May 2022; pp. 1306–1310. [Google Scholar] [CrossRef]
- Atkins, A.E.; Habibzadeh, H.; Vaughan, T.M.; Norton, J.J.S. Optimizing Stimulation Frequency for BCI-Based Color Vision Assessment: Preliminary Results. In Proceedings of the 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER), Baltimore, MD, USA, 24–27 April 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Shin, G.-H.; Lee, M.; Lee, S.-W. Mobile BCI Dataset of Scalp- and Ear-EEGs with ERP and SSVEP Paradigms While Standing, Walking, and Running. Sci. Data 2021, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.; Singh, J. EEG Signal Processing for Detection of Colour Vision Deficiencies. ECS Trans. 2022, 107, 10053–10060. [Google Scholar] [CrossRef]
- Fontanella, L.; Ippoliti, L. Karhunen–Loéve Expansion of Temporal and Spatio-Temporal Processes. In Handbook of Statistics; Elsevier: Amsterdam, The Netherlands, 2012; Volume 30, pp. 497–520. [Google Scholar]
- Jia, W.; Sun, M.; Lian, J.; Hou, S. Feature Dimensionality Reduction: A Review. Complex Intell. Syst. 2022, 8, 2663–2693. [Google Scholar] [CrossRef]
- Hosseini, M.-P.; Hosseini, A.; Ahi, K. A Review on Machine Learning for EEG Signal Processing in Bioengineering. Rev. Biomed. Eng. 2021, 14, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2017, 15, 41–51. [Google Scholar] [CrossRef]
- Uddin, S.; Haque, I.; Lu, H.; Moni, M.A.; Gide, E. Comparative Performance Analysis of K-Nearest Neighbour (KNN) Algorithm and Its Different Variants for Disease Prediction. Sci. Rep. 2022, 12, 6256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fort, J.M.; Giménez Mateu, L. Mini Review: Challenges in EEG Emotion Recognition. Front. Psychol. 2024, 14, 1289816. [Google Scholar] [CrossRef]
- Jiang, L.; Siriaraya, P.; Choi, D.; Zeng, F.; Kuwahara, N. Electroencephalogram Signals Emotion Recognition Based on Convolutional Neural Network-Recurrent Neural Network Framework with Channel-Temporal Attention Mechanism for Older Adults. Front. Aging Neurosci. 2022, 14, 945024. [Google Scholar] [CrossRef]
- Höller, Y.; Jónsdóttir, S.T.; Hannesdóttir, A.H.; Ólafsson, R.P. EEG-Responses to Mood Induction Interact with Seasonality and Age. Front. Psychiatry 2022, 13, 950328. [Google Scholar] [CrossRef]
- Eroğlu, K.; Kayıkçıoğlu, T.; Osman, O. Effect of Brightness of Visual Stimuli on EEG Signals. Behav. Brain Res. 2020, 382, 112486. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Brain Mechanisms of Vision. Sci. Am. 1979, 241, 150–163. [Google Scholar] [CrossRef]
- Rehman, A.; Al Khalili, Y. Neuroanatomy, Occipital Lobe. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://europepmc.org/article/nbk/nbk544320 (accessed on 11 August 2024).
- Warren, D.J.; Normann, R.A.; Finn, W.E.; LoPresti, P.G. Visual Neuroprostheses. In Neuroprosthetics: Theory and Practice; Finn, W.E., LoPresti, P.G., Normann, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 261–306. [Google Scholar]

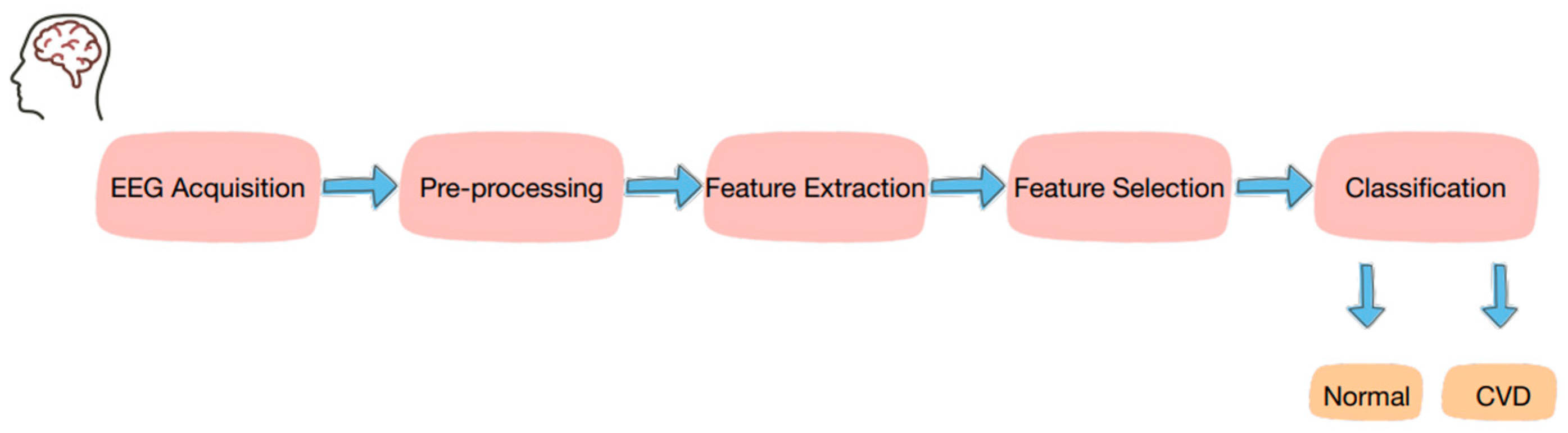
| Aspect | Conventional CVD Tests | EEG-Based Methods |
|---|---|---|
| Behavioral Requirement | Yes, requires active participation (e.g., verbal response) | No, relies on brain responses without behavioral input |
| Subjectivity | High, results can be influenced by individual perception and experience | Low, results are based on objective EEG data |
| Training Required | Yes, some tests require extensive training (e.g., anomaloscope) | Minimal, simple instructions and sensor placement |
| Susceptibility to Manipulation | Yes, possible through memorization (e.g., Ishihara plates) | No, EEG signals cannot be consciously controlled |
| Speed | Fast, typically completed within minutes | Slower, requires more time for setup and signal analysis |
| Suitability for Disabled Individuals | Limited, requires active participation | High, suitable for individuals with disabilities or communication challenges |
| Use Case | Quick screening, large population testing | Research, cases where traditional methods are not viable or require more detailed analysis |
| ERP Technique | SSVEP Technique | |
|---|---|---|
| Pros | Higher accuracy in measuring scalp EEG allows for more precise measurements of brain activity. | Greater SNR and less susceptibility to external environmental factors. |
| Comfortable stimulation process from the subjects’ point of view. | Rapid recording capabilities enable efficient data collection and analysis for tasks requiring quick responses. | |
| No limitations for individuals who can perform the recording. | Precise quantification of specific responses, as it enables accurate measurement of responses to visual stimuli at particular frequencies, aiding in identifying and analyzing brain activity patterns with high specificity. | |
| Numerous studies have utilized it, making its methods and analysis techniques clearer to other researchers. | Unbiased identification, as it relies on neural responses to visual stimuli without subjective interpretation. | |
| Cons | Easily affected by noise, eye movements, and body movements, which can impact the signal’s reliability. | Some subjects find it annoying due to the flickering stimulation. |
| May involve subjective judgment in identifying neural responses, potentially introducing biases or variations in the analysis process. | Limited usability for individuals with certain neurological disorders, such as epilepsy, who should avoid flickering stimuli. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlEssa, G.N.; Alzahrani, S.I. EEG-Based Methods for Diagnosing Color Vision Deficiency: A Comprehensive Review. Appl. Sci. 2024, 14, 7579. https://doi.org/10.3390/app14177579
AlEssa GN, Alzahrani SI. EEG-Based Methods for Diagnosing Color Vision Deficiency: A Comprehensive Review. Applied Sciences. 2024; 14(17):7579. https://doi.org/10.3390/app14177579
Chicago/Turabian StyleAlEssa, Ghada N., and Saleh I. Alzahrani. 2024. "EEG-Based Methods for Diagnosing Color Vision Deficiency: A Comprehensive Review" Applied Sciences 14, no. 17: 7579. https://doi.org/10.3390/app14177579





