Selected Soluble Dietary Fibres as Replacers of Octenyl Succinic Anhydride (OSA) Starch in Spray-Drying Production of Linseed Oil Powders Applied to Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Emulsions Preparation and Spray-Drying
2.3. Food Model Preparation
2.4. Characterization of Emulsions
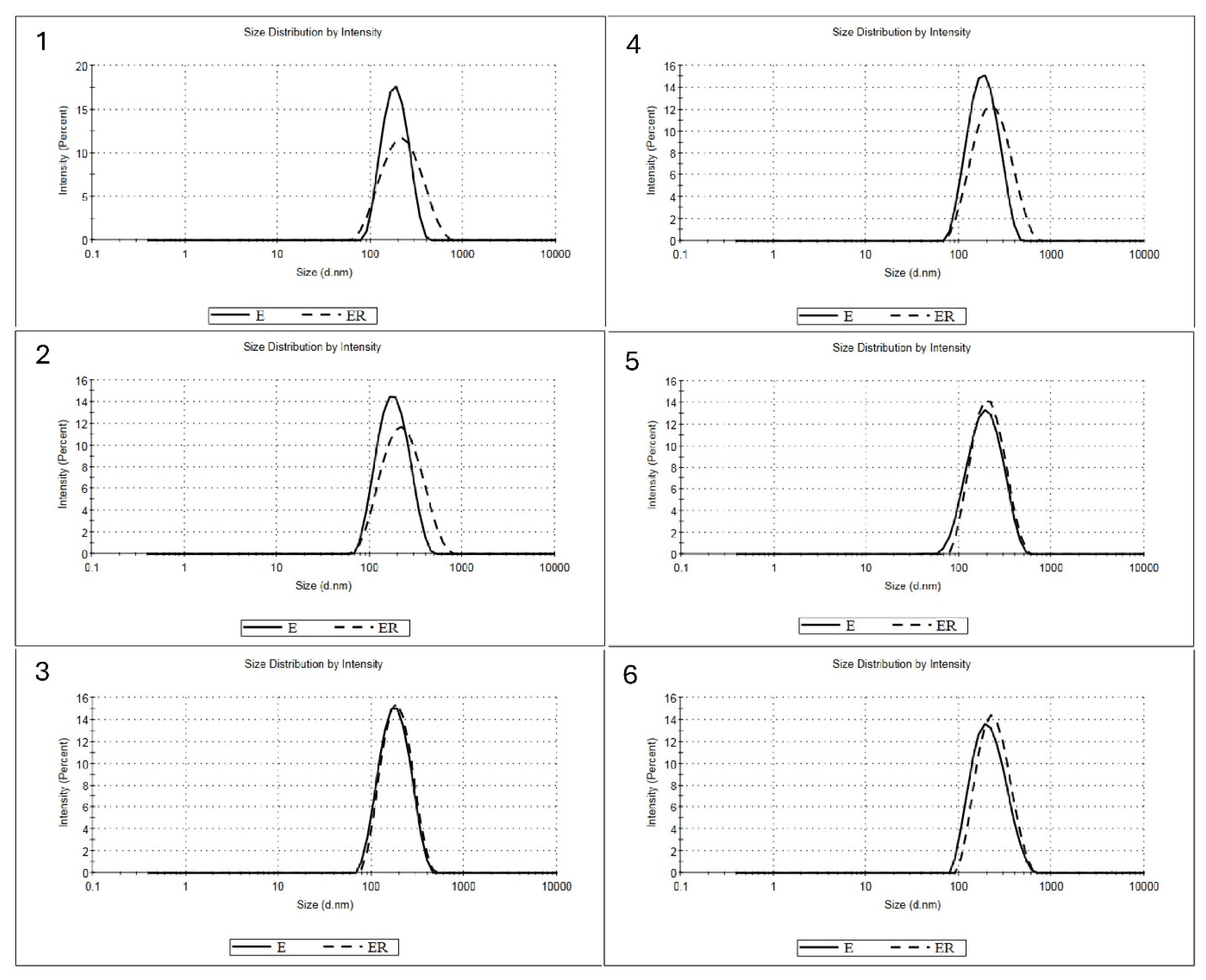
2.4.1. Droplet Size Distribution, Mean Particle Diameter (Z-Average) and Polydispersity Index (PDI) Analyses
2.4.2. Emulsion Viscosity Analysis
2.5. Characterization of Powders
2.5.1. Surface Oil Content Analysis
2.5.2. Encapsulation Efficiency Analysis
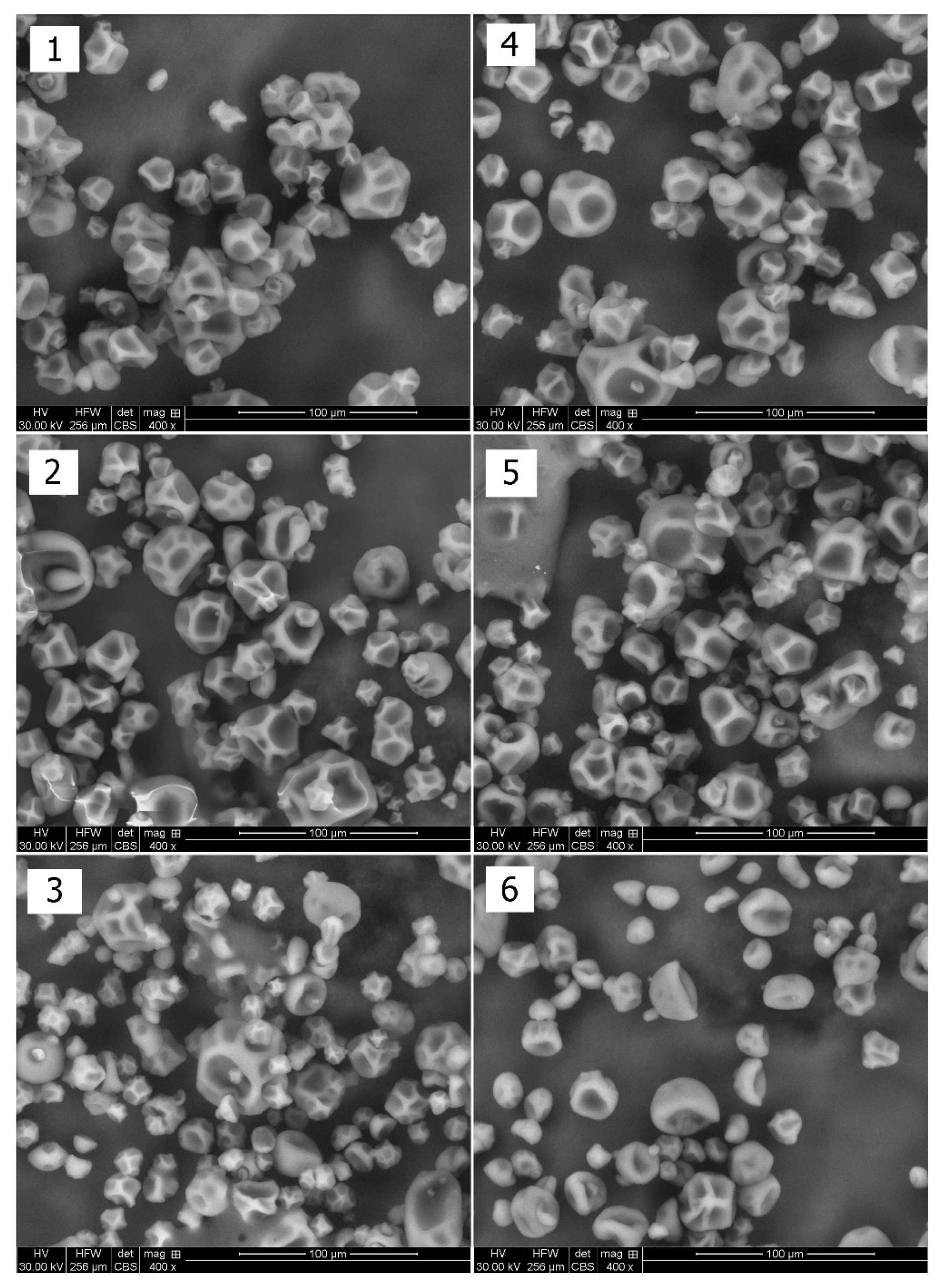
2.5.3. Powder Morphology Analysis
2.6. Characterization of Juices
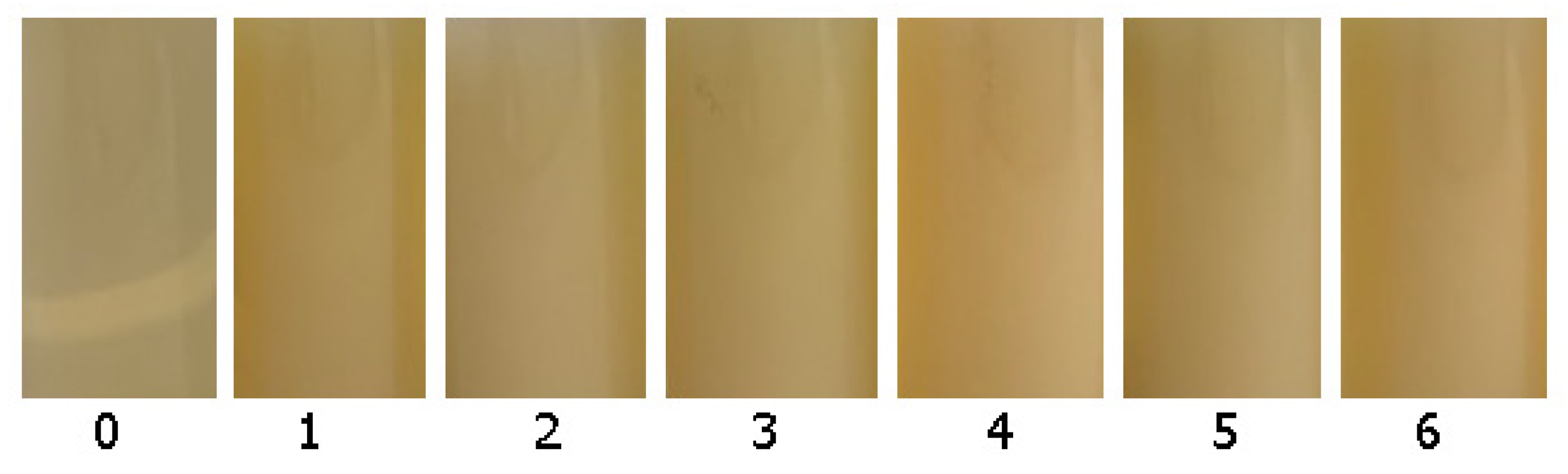
2.6.1. Colour Analysis
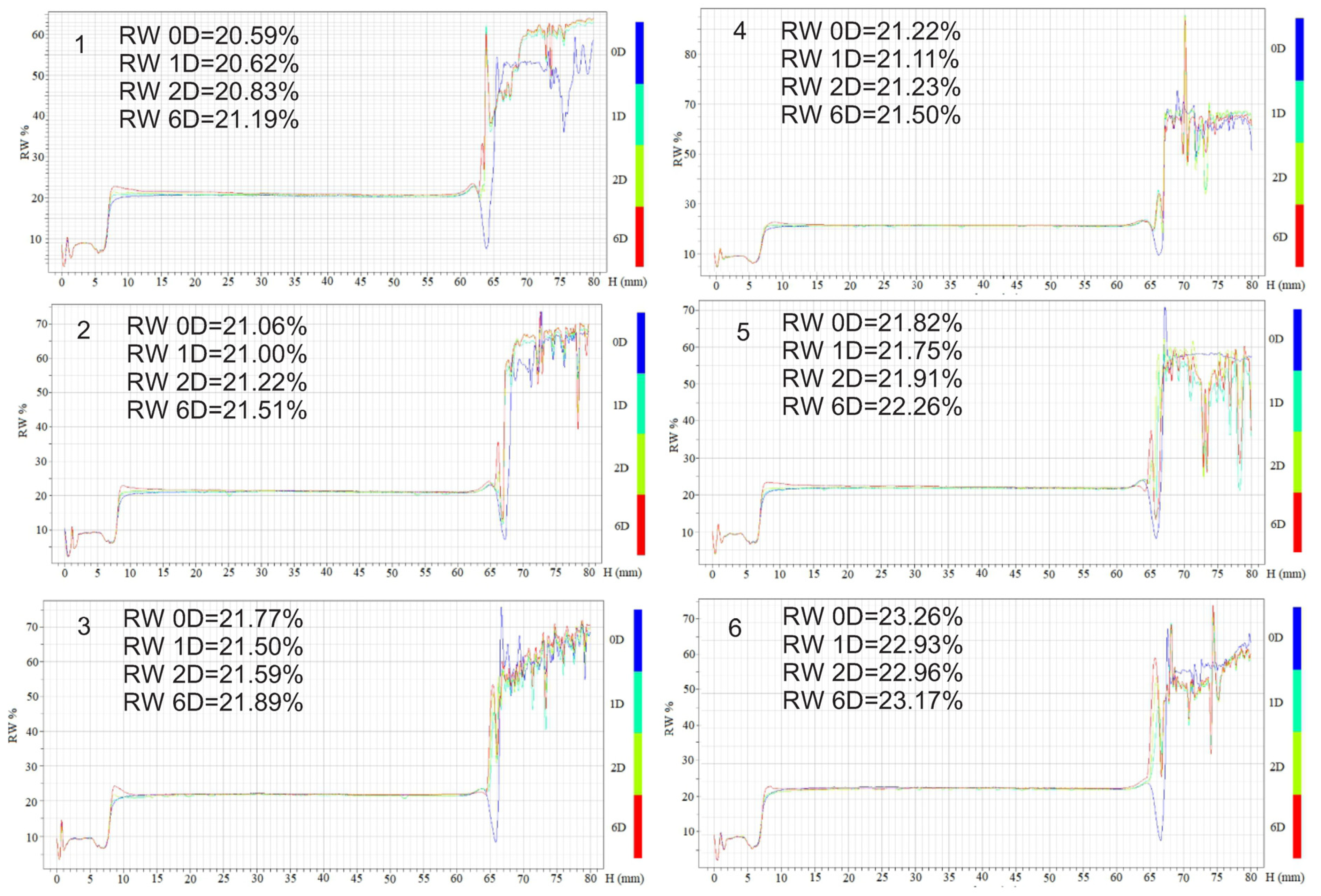
2.6.2. Physical Stability Analysis
2.6.3. Juice Volatile Compounds Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Prepared Linseed Oil Emulsions
3.2. Physical Properties of Linseed Oil Powders
3.2.1. Encapsulation Efficiency and Surface Oil Content
3.2.2. Powder Morphology
3.3. Physical Properties of Juice with Linseed Oil Powders
3.3.1. Colour
3.3.2. Physical Stability
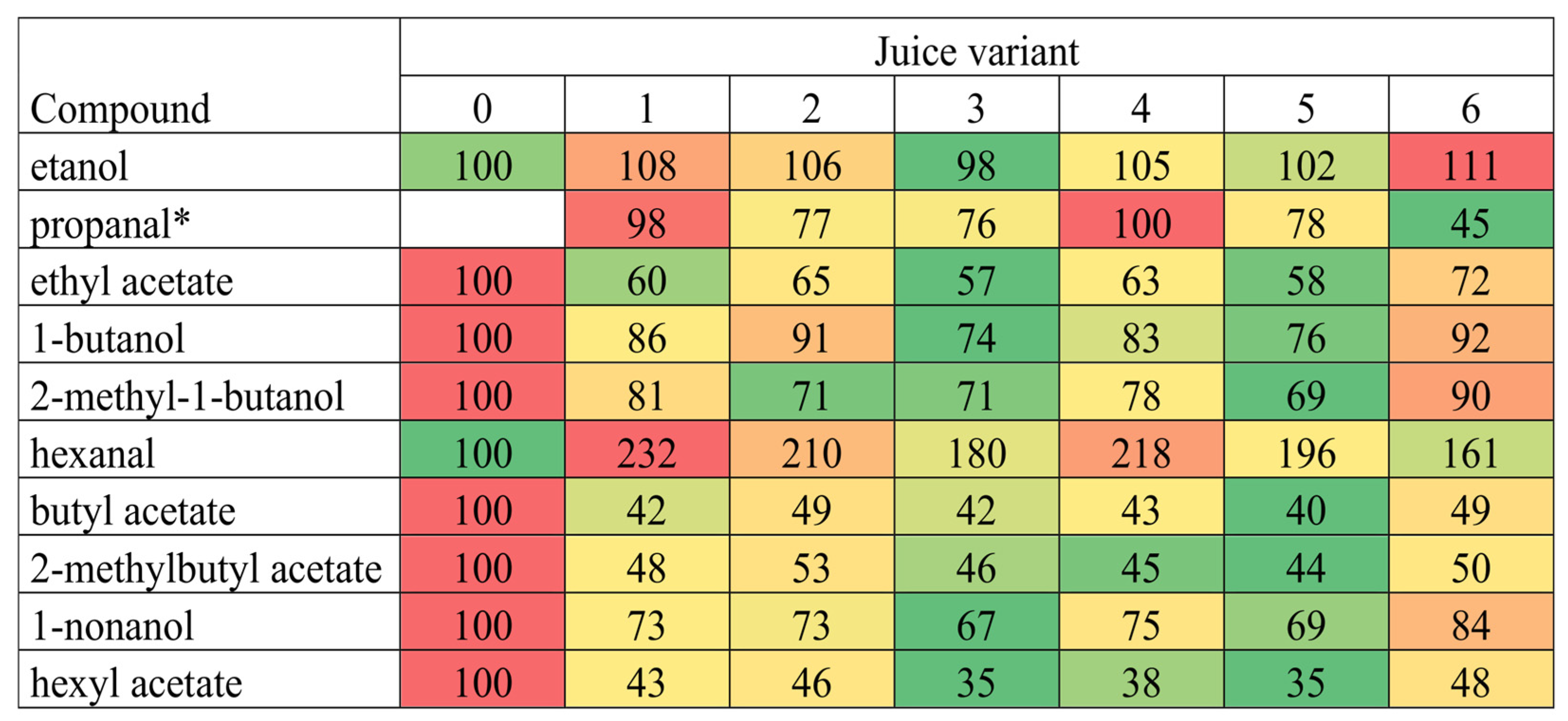
3.4. Volatile Compounds of Apple Juice with Linseed Oil Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silska, G.; Walkowiak, M. Comparative analysis of fatty acid composition in 84 accessions of flax (Linum usitatissimum L.). J. Pre-Clin. Clin. Res. 2019, 13, 118–129. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia seeds (Salvia hispanica L.): An overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum usitatissimum L.): A potential candidate for phytoremediation? Biological and economical points of view. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Focus on: Chia Seeds. Available online: https://www.unicorningredients.com/news/focus-on-chia-seeds/ (accessed on 1 July 2024).
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Elimam, D.M.; Afifi, S.M. Outgoing and potential trends of the omega-3 rich linseed oil quality characteristics and rancidity management: A comprehensive review for maximizing its food and nutraceutical applications. Trends Food Sci. Technol. 2021, 114, 292–309. [Google Scholar] [CrossRef]
- National Institutes of Health Omega-3 fatty Acids: Fact Sheet for Consumers. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer/ (accessed on 1 July 2024).
- Chytilová, M.; Mudroňová, D.; Nemcová, R.; Gancarčíková, S.; Buleca, V.; Koščová, J.; Tkáčiková, Ľ. Anti-inflammatory and immunoregulatory effects of flax-seed oil and Lactobacillus plantarum—BiocenolTM LP96 in gnotobiotic pigs challenged with enterotoxigenic Escherichia coli. Res. Vet. Sci. 2013, 95, 103–109. [Google Scholar] [CrossRef]
- Al-Mathkhury, H.J.F.; Al-Dhamin, A.S.; Al-Taie, K.L. Antibacterial and antibiofilm activity of flaxseed oil. Iraqi. J. Sci. 2016, 57, 1086–1095. [Google Scholar]
- Nam, T.-G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Damerau, A.; Banaszczyk, P.; Tańska, M.; Konopka, I.Z.; Piłat, B.; Dajnowiec, F.; Linderborg, K.M. Native and pregelatinized potato and rice starches and maltodextrin as encapsulating agents for linseed oil ethyl esters—Comparison of emulsion and powder properties. J. Food Eng. 2024, 364, 111799. [Google Scholar] [CrossRef]
- Dantas, A.; Piella-Rifà, M.; Costa, D.P.; Felipe, X.; Gou, P. Innovations in spray drying technology for liquid food processing: Design, mechanisms, and potential for application. Appl. Food Res. 2024, 4, 100382. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2023, 63, 6983–7015. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Han, H.; Zhang, H.; Li, E.; Li, C.; Wu, P. Structural and functional properties of OSA-starches made with wide-ranging hydrolysis approaches. Food Hydrocoll. 2019, 90, 132–145. [Google Scholar] [CrossRef]
- Anggriawan, R.; Maksum, A.; Nurhayati, N. Production and application of octenyl succinic-modified starch as fat replacer: A review of established and recent research. Preprints 2020, 2020050283. [Google Scholar]
- Romero-Hernandez, H.A.; Sánchez-Rivera, M.M.; Alvarez-Ramirez, J.; Yee-Madeira, H.; Yañez-Fernandez, J.; Bello-Pérez, L.A. Avocado oil encapsulation with OSA-esterified taro starch as wall material: Physicochemical and morphology characteristics. LWT 2021, 138, 110629. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schmidt-Hansberg, B.; Kind, M.; Schwarz, K. Process engineering parameters and type of n-octenylsuccinate-derivatised starch affect oxidative stability of microencapsulated long chain polyunsaturated fatty acids. J. Food. Eng. 2009, 95, 386–392. [Google Scholar] [CrossRef]
- NUTRIOSE® Health Benefits. Digestive Health. Available online: https://www.roquette.com/media-center/resources/nutriose-peer-review-articles-digestive-health (accessed on 1 July 2024).
- Lefranc-Millot, C. NUTRIOSE® 06: A useful soluble dietary fibre for added nutritional value. Nutr. Bull. 2008, 33, 234–239. [Google Scholar] [CrossRef]
- Guerin-Deremaux, L.; Ringard, F.; Desailly, F.; Wils, D. Effects of a soluble dietary fibre NUTRIOSE® on colonic fermentation and excretion rates in rats. Nutr. Res. Pract. 2010, 4, 470. [Google Scholar] [CrossRef]
- Weenink, G.R.J.; Vermeer, W.H.; Kupecz, A.F.; Granules. World Intellectual Property Organization Patent. 2017. Available online: https://patents.google.com/patent/WO2017171553A1/en (accessed on 26 August 2024).
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef]
- Abed, S.M.; Ali, A.H.; Noman, A.; Niazi, S.; Ammar, A.F.; Bakry, A.M. Inulin as prebiotics and its applications in food industry and human health; a review. Int. J. Agric. Innov. Res. 2016, 5, 88–97. [Google Scholar]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Caloric value of inulin and oligofructose. J. Nutr. 1999, 129, 1436S–1437S. [Google Scholar] [CrossRef]
- Molet-Rodríguez, A.; Turmo-Ibarz, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Incorporation of antimicrobial nanoemulsions into complex foods: A case study in an apple juice-based beverage. LWT 2021, 141, 110926. [Google Scholar] [CrossRef]
- Lončarić, A.; Flanjak, I.; Kovač, T.; Tomac, I.; Skoko, A.-M.G.; Babojelić, M.S.; Fruk, G.; Zrinušić, S.Z.; Čiček, D.; Babić, J.; et al. Unveiling apple diversity: The quality of juice produced from old vs. commercial apple cultivars. Plants 2023, 12, 3733. [Google Scholar] [CrossRef]
- Potanin, A.; Marron, G. Rheological characterization of yield-stress fluids with Brookfield viscometer. Appl. Rheol. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Konopka, I.Z.; Tańska, M.; Brandt, W.; Piłat, B. Effect of maltodextrin replacement by selected native starches and disaccharides on physicochemical properties of pumpkin oil capsules prepared by spray-drying. Appl. Sci. 2021, 12, 33. [Google Scholar] [CrossRef]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Khan, M.A.; Majeed, H.; Safdar, W.; Shamoon, M.; Shoaib, M.; Haider, J.; Zhong, F. Influence of OSA-starch on the physico chemical characteristics of flax seed oil-eugenol nanoemulsions. Food Hydrocoll. 2017, 66, 365–377. [Google Scholar] [CrossRef]
- Domian, E.; Cenkier, J.; Górska, A.; Brynda-Kopytowska, A. Effect of oil content and drying method on bulk properties and stability of powdered emulsions with OSA starch and linseed oil. LWT 2018, 88, 95–102. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Al-Maqtari, Q.A.; Al-Adeeb, A.; Cui, H.; Lin, L. Stabilization of the oil-in-water emulsions of citrus reticulata essential oil by different combinations of gum arabic/maltodextrin/whey protein. J. Food Process. Preserv. 2022, 46, e16976. [Google Scholar] [CrossRef]
- Jan, Y.; Al-Keridis, L.A.; Malik, M.; Haq, A.; Ahmad, S.; Kaur, J.; Adnan, M.; Alshammari, N.; Ashraf, S.A.; Panda, B.P. Preparation, modelling, characterization and release profile of vitamin D3 nanoemulsion. LWT 2022, 169, 113980. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Domagała, J.; Kasprzak, M.M. Critical review of techniques for food emulsion characterization. Appl. Sci. 2024, 14, 1069. [Google Scholar] [CrossRef]
- van Aken, G.A.; Vingerhoeds, M.H.; de Wijk, R.A. Textural perception of liquid emulsions: Role of oil content, oil viscosity and emulsion viscosity. Food Hydrocoll. 2011, 25, 789–796. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Bello-Perez, L.A. Starch as an emulsions stability: The case of octenyl succinic anhydride (OSA) starch. Curr. Opin. Food Sci. 2017, 13, 78–83. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tańska, M.; Banaszczyk, P.; Dąbrowski, G.; Czaplicki, S.; Wachowicz, M.; Konopka, I.Z. Influence of selected compositions of wall materials and drying techniques used for encapsulation of linseed oil and its ethyl esters. Appl. Sci. 2024, 14, 1372. [Google Scholar] [CrossRef]
- Karim, N.; Liu, S.; Rashwan, A.K.; Xie, J.; Mo, J.; Osman, A.I.; Rooney, D.W.; Chen, W. Green synthesis of nanolipo-fibersomes using Nutriose® FB 06 for delphinidin-3-O-sambubioside delivery: Characterization, physicochemical properties, and application. Int. J. Biol. Macromol. 2023, 247, 125839. [Google Scholar] [CrossRef]
- Sotelo-Bautista, M.; Bello-Perez, L.A.; Gonzalez-Soto, R.A.; Yañez-Fernandez, J.; Alvarez-Ramirez, J. OSA-maltodextrin as wall material for encapsulation of essential avocado oil by spray drying. J. Dispers. Sci. Technol 2020, 41, 235–242. [Google Scholar] [CrossRef]
- Granados-Vallejo, M.; Espinosa-Andrews, H.; Guatemala-Morales, G.M.; Esquivel-Solis, H.; Arriola-Guevara, E. Oxidative stability of green coffee oil (Coffea arabica) microencapsulated by spray drying. Processes 2019, 7, 734. [Google Scholar] [CrossRef]
- He, H.; Hong, Y.; Gu, Z.; Liu, G.; Cheng, L.; Li, Z. Improved stability and controlled release of CLA with spray-dried microcapsules of OSA-modified starch and xanthan gum. Carbohydr. Polym. 2016, 147, 243–250. [Google Scholar] [CrossRef]
- Yousefi, M.; Khanniri, E.; Khorshidian, N.; Sohrabvandi, S.; Mortazavian, A.M. Development of probiotic apple juice using encapsulated probiotics in xanthan-chitosan based hydrogels. Appl. Food Biotech. 2023, 10, 205–213. [Google Scholar]
- Zhang, L.; Han, C.; Liu, M.; Yang, H.; Zhang, F.; Liu, B.; Meng, X. The formation, stability of DHA/EPA nanoemulsion prepared by emulsion phase inversion method and its application in apple juice. Food Res. Int. 2020, 133, 109132. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of volatile and sensory profiles of apple juices to trace fruit origins and investigation of the relationship between the aroma properties and volatile constituents. LWT 2020, 124, 109203. [Google Scholar] [CrossRef]
- Chemical Precursors to Life Found in Space. Available online: https://news.softpedia.com/news/Chemical-Precursors-to-Life-Found-in-Space-32259.shtml (accessed on 5 July 2024).
- Azarbad, M.H.; Jeleń, H. Determination of hexanal—An indicator of lipid oxidation by static headspace gas chromatography (SHS-GC) in fat-rich food matrices. Food Anal. Methods 2015, 8, 1727–1733. [Google Scholar] [CrossRef]
- Glibowski, P.; Bukowska, A. The effect of pH, temperature and heating time on inulin chemical stability. Acta Sci. Pol. Technol. Aliment. 2011, 10, 189–196. [Google Scholar]
- Walker, S.; Prescott, J. The influence of solution viscosity and different viscosifying agents on apple juice flavor. J Sens. Stud. 2000, 15, 285–307. [Google Scholar] [CrossRef]

| Sample | Water | Linseed Oil | SDF N | SDF I | OSA-Starch |
|---|---|---|---|---|---|
| 1 | 70 | 10 | 5 | - | 15 |
| 2 | 70 | 10 | 10 | - | 10 |
| 3 | 70 | 10 | 15 | - | 5 |
| 4 | 70 | 10 | - | 5 | 15 |
| 5 | 70 | 10 | - | 10 | 10 |
| 6 | 70 | 10 | - | 15 | 5 |
| Mean Particle Diameter [nm] | Polydispersity Index | Viscosity [mPa·s] | |
|---|---|---|---|
| Fresh | |||
| 1 | 175.02 ± 1.5 c | 0.12 ± 0.02 b | 269.51 ± 11.8 f |
| 2 | 167.70± 1.9 a | 0.11 ± 0.01 a | 152.97 ± 13.0 d |
| 3 | 166.60 ± 1.7 a | 0.10 ± 0.01 a | 58.67 ± 0.8 b |
| 4 | 170.34 ± 1.7 b | 0.14 ± 0.01 bc | 242.20 ± 13.2 e |
| 5 | 177.38 ± 2.3 d | 0.14 ± 0.01 c | 85.20 ± 1.8 c |
| 6 | 197.31 ± 2.5 e | 0.13 ± 0.01 bc | 42.80 ± 1.0 a |
| Reconstituted | |||
| 1 | 199.24 ± 2.7 c | 0.21 ± 0.02 e | |
| 2 | 199.68 ± 2.0 c | 0.19 ± 0.01 cd | |
| 3 | 174.28 ± 1.4 a | 0.12 ± 0.01 a | |
| 4 | 204.59 ± 2.1 e | 0.19 ± 0.01 d | |
| 5 | 195.02 ± 1.5 b | 0.18 ± 0.01 bc | |
| 6 | 223.19 ± 1.4 d | 0.17 ± 0.02 b | |
| Sample | Surface Oil [%] | Encapsulation Efficiency [%] |
|---|---|---|
| 1 | 0.41 ± 0.03 a | 98.76 ± 1.8 b |
| 2 | 0.42 ± 0.01 a | 98.73 ± 1.3 b |
| 3 | 0.54 ± 0.06 b | 98.36 ± 1.5 a |
| 4 | 0.55 ± 0.03 b | 98.33 ± 1.0 a |
| 5 | 0.44 ± 0.02 a | 98.67 ± 2.1 b |
| 6 | 0.57 ± 0.02 b | 98.27 ± 2.0 a |
| Sample No. | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| Pure | 95.15 ± 0.4 c | −2.10 ± 0.15 d | 10.09 ± 1.23 c | 10.31 | - |
| 1 | 88.59 ± 0.6 a | −3.22 ± 0.10 a | 7.94 ± 0.29 ab | 8.57 | 6.99 |
| 2 | 88.39 ± 0.4 a | −3.27 ± 0.05 a | 7.80 ± 0.58 ab | 8.46 | 7.23 |
| 3 | 88.36 ± 0.5 a | −3.06 ± 0.02 b | 8.69 ± 0.81 b | 9.21 | 7.00 |
| 4 | 88.92 ± 0.0 a | −3.03 ± 0.00 b | 8.59 ± 0.00 b | 9.11 | 6.48 |
| 5 | 88.13 ± 0.8 a | −3.09 ± 0.04 b | 6.96 ± 0.98 ab | 7.62 | 7.75 |
| 6 | 89.78 ± 1.2 b | −2.75 ± 0.11 c | 8.05 ± 0.92 b | 8.51 | 5.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogrodowska, D.; Tańska, M.; Banaszczyk, P.; Czaplicki, S.; Piłat, B.; Konopka, I. Selected Soluble Dietary Fibres as Replacers of Octenyl Succinic Anhydride (OSA) Starch in Spray-Drying Production of Linseed Oil Powders Applied to Apple Juice. Appl. Sci. 2024, 14, 7611. https://doi.org/10.3390/app14177611
Ogrodowska D, Tańska M, Banaszczyk P, Czaplicki S, Piłat B, Konopka I. Selected Soluble Dietary Fibres as Replacers of Octenyl Succinic Anhydride (OSA) Starch in Spray-Drying Production of Linseed Oil Powders Applied to Apple Juice. Applied Sciences. 2024; 14(17):7611. https://doi.org/10.3390/app14177611
Chicago/Turabian StyleOgrodowska, Dorota, Małgorzata Tańska, Paweł Banaszczyk, Sylwester Czaplicki, Beata Piłat, and Iwona Konopka. 2024. "Selected Soluble Dietary Fibres as Replacers of Octenyl Succinic Anhydride (OSA) Starch in Spray-Drying Production of Linseed Oil Powders Applied to Apple Juice" Applied Sciences 14, no. 17: 7611. https://doi.org/10.3390/app14177611






