Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Biomass and Nanoparticles
2.1.1. Preparation of Biomass
2.1.2. FT-IR Analysis
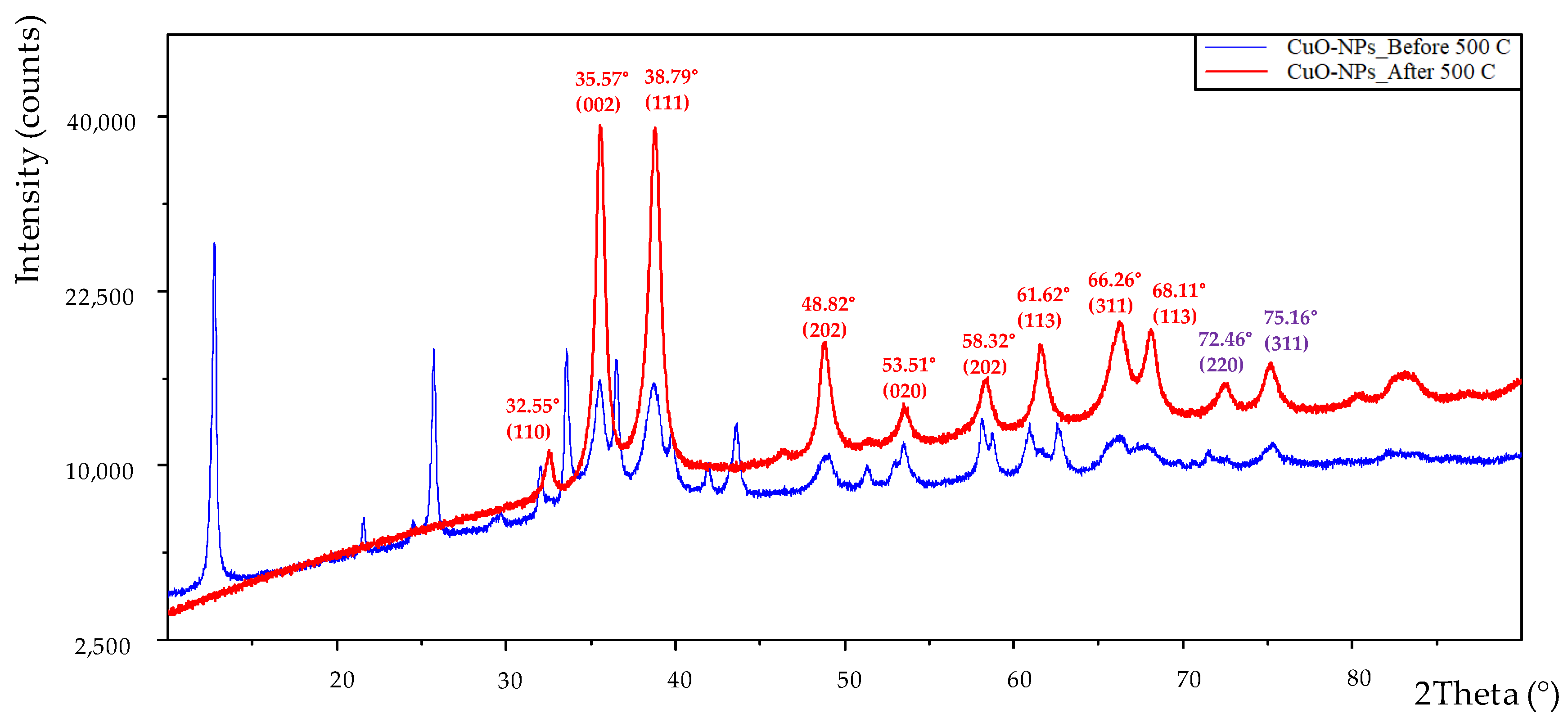
2.1.3. XRD Analysis
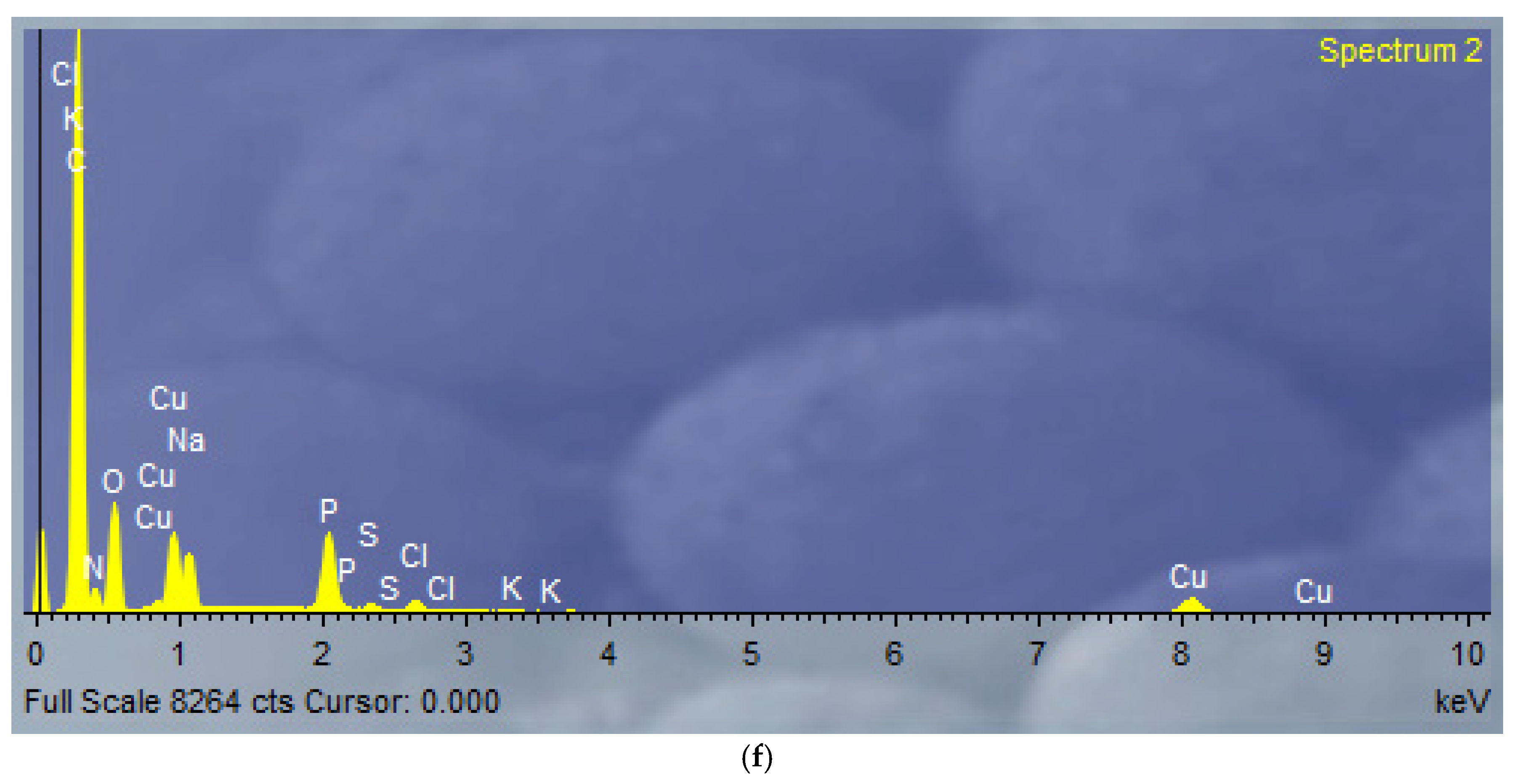
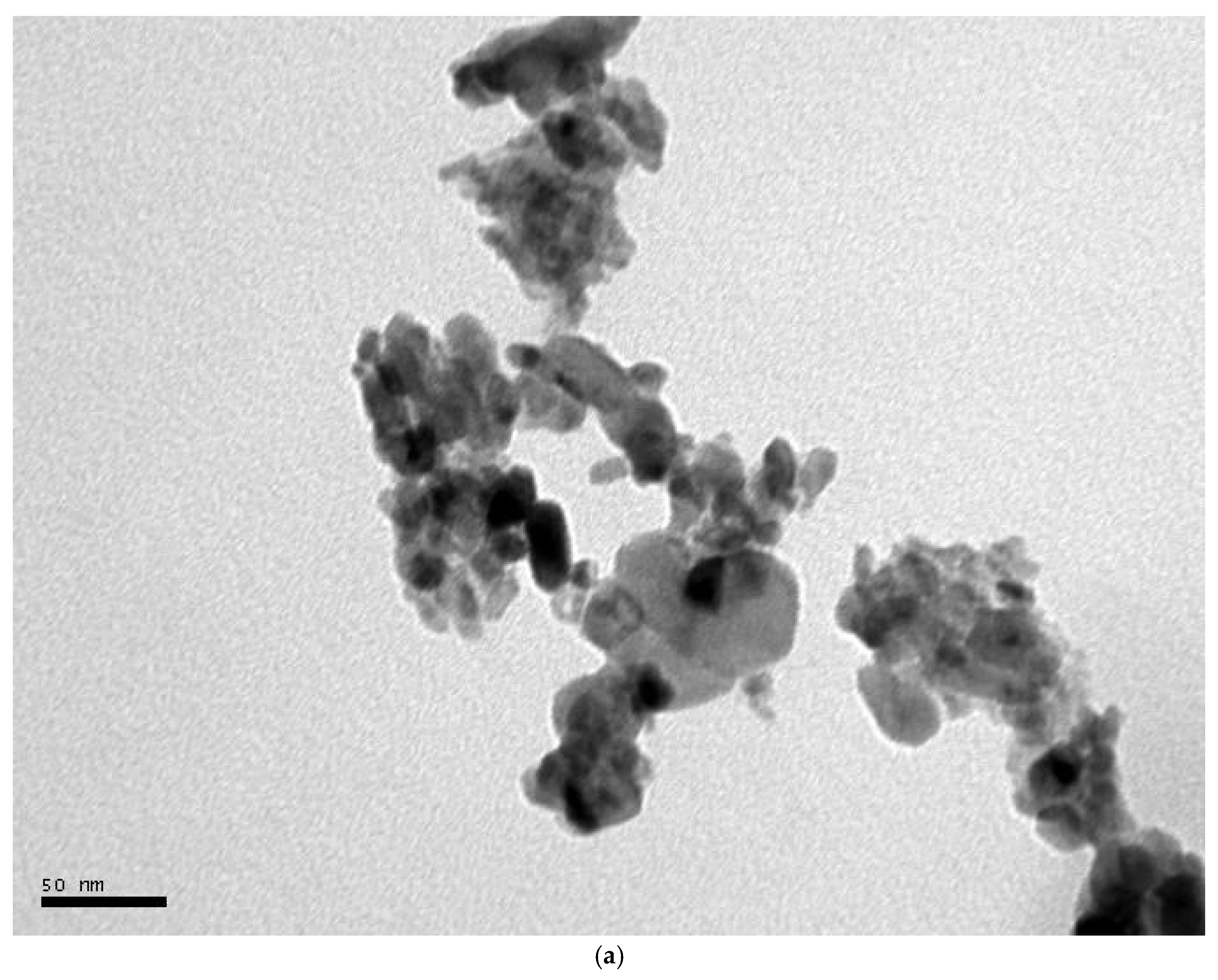
2.1.4. FE-SEM-EDX and TEM Analysis
2.2. Biosorption Assays
2.3. Obtaining Cell Extract and Synthesis of Nanoparticles
2.4. Determination of Biocidal Effect
3. Results and Discussion
3.1. FT-IR Analysis
3.2. XRD Analysis
3.3. FE-SEM-EDX and TEM Analysis
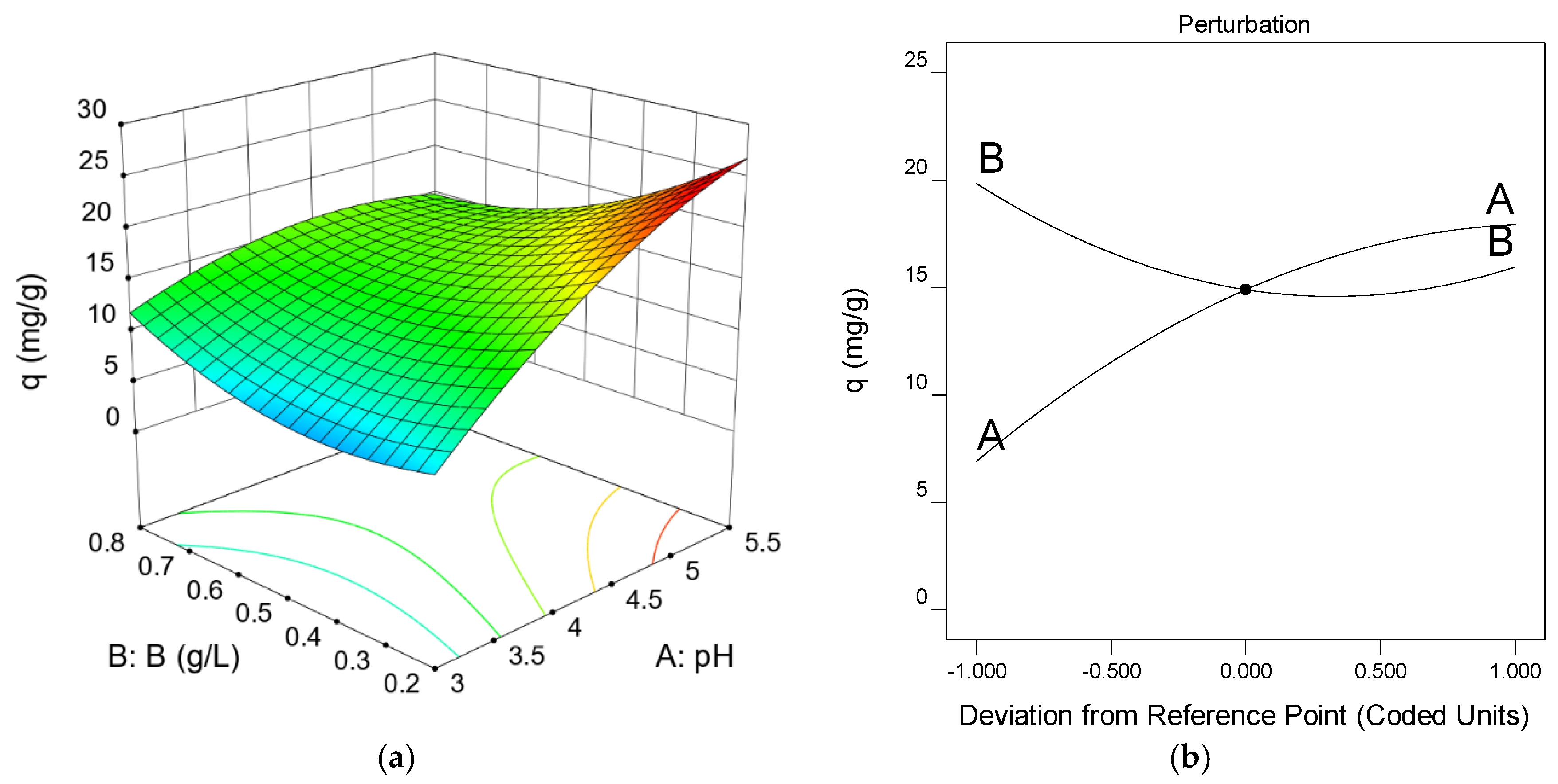
3.4. Experiment Design: Optimal Operating Conditions
3.5. Biosorption Isotherms
3.6. Characterization of Nanoparticles and Biocidal Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, X.; Zhang, Z.; Chen, H.; Zhang, X.; Zhang, X.; Tan, C.; Bai, X.; Gong, Y.; Li, H. The investigation of the binding ability between sodium dodecyl sulfate and Cu (II) in urban stormwater runoff. J. Environ. Manag. 2024, 350, 119671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wang, Z.; Yan, X.; Yu, Y.; Liu, L.; Gao, Y.; Zhang, H.; Yang, X. Characteristics and pollution risks of Cu, Ni, Cd, Pb, Hg and As in farmland soil near coal mines. Soil Environ. Health 2023, 1, 100035. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, Y.; Moon, H.B.; Ra, K. Characteristics of metal pollution and multi-isotopic signatures for C, Cu, Zn, and Pb in coastal sediments from special management areas in Korea. Mar. Pollut. Bull. 2023, 188, 114642. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Cora, C.; Soto-Gómez, D.; Arias-Estévez, M.; Baath, E.; Fernández-Calviño, D. Bacterial community tolerance to Cu in soils with geochemical baseline concentrations (GBCs) of heavy metals: Importance for pollution induced community tolerance (PICT) determinations using the leucine incorporation method. Soil Biol Biochem. 2021, 155, 108157. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Liu, Y.; Ban, Y.; Li, K.; Li, X.; Zhang, X.; Xu, Z. Removal of sulfamethoxazole and Cu, Cd compound pollution by arbuscular mycorrhizal fungi enhanced vertical flow constructed wetlands. Environ. Res. 2024, 245, 117982. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Z.; Chen, H.; Zhang, X.; Tan, C.; Bai, X.; Gong, Y.; Qu, Y.; Li, H.; Zhang, Z. Investigation the existence and mechanism of Cu(II)-sulfamethoxazole co-pollution by road-deposited sediments in stormwater runoff. Sci. Total Environ. 2024, 924, 171634. [Google Scholar] [CrossRef]
- Assefa, E.T.; Shumi, G.; Gendo, K.M.; Kenasa, G.; Roba, N. Review on green synthesis, characterization, and antibacterial activity of CuO nanoparticles using biomolecules of plant extract. Results Chem. 2024, 8, 101606. [Google Scholar] [CrossRef]
- Tijani, N.A.; Hokello, J.; Awojobi, K.A.; Marnadu, R.; Shkir, R.; Ahmad, Z.; Afolabi, A.O.; Adewinbi, S.A.; Adebayo, I.A. Recent advances in Mushroom-mediated nanoparticles: A critical review of mushroom biology, nanoparticles synthesis, types, characteristics and applications. J. Drug Deliv. Technol. 2024, 96, 105695. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ. Sci. 2022, 34, 102092. [Google Scholar] [CrossRef]
- Devaraji, M.; Thanikachalam, P.V.; Elumalai, K. The potential of copper oxide nanoparticles in nanomedicine: A comprehensive review. Biotechnol. Notes 2024, 5, 80–89. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Moya, M.; Castro, E. Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1. Nanomaterials 2023, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Castro, E. Biosorption mechanisms of Ag(I) and the synthesis of nanoparticles by the biomass from Botryosphaeria rhodina MAMB-05. J. Hazard. Mater. 2021, 420, 126598. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Adsorption in solutions. Phys. Chem. 1906, 57, 384–410. [Google Scholar]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–549. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1039. [Google Scholar] [CrossRef]
- Baglari, S.; Wary, R.R.; Kalita, P.; Baruah, M.B. Green synthesis of CuO nanoparticles using citrus maxima peel aqueous extract. Mater Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Jayashima, H.M.; Chandrappa, K.G.; Sanualla, P.F.; Dileepkumar, V.G. Green synthesis of CuO nanoparticles: A promising material for photocatalysis and electrochemical sensor. Sens. Int. 2024, 5, 100254. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, k.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. J. Chem. Eng. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Vishwakarma, M.C.; Joshi, H.K.; Tiwari, P.; Bhandari, N.S.; Joshi, S.K. Thermodynamic, kinetic, and equilibrium studies of Cu(II), Cd(II), Ni(II), and Pb(II) ion biosorption onto treated Ageratum conyzoid biomass. Int. J. Biol. Macromol. 2024, 274, 133001. [Google Scholar] [CrossRef]
- Mir, D.H.; Rather, M.A. Kinetic and thermodynamic investigations of copper (II) biosorption by green algae Chara vulgaris obtained from the waters of Dal Lake in Srinagar (India). J. Water Process. Eng. 2024, 58, 104850. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Mechanism of heavy metal ion biosorption by microalgal cells: A mathematic approach. J. Hazard. Mater. 2024, 463, 132875. [Google Scholar] [CrossRef]
- Gu, S.; Su, Y.; Lan, C.Q. Effect of phosphate in medium on cell growth and Cu(II) biosorption by green alga Neochloris oleoabundans. Chem. Eng. Res. Des. 2022, 185, 186–197. [Google Scholar] [CrossRef]
- Fathollahi, A.; Coupe, S.J.; El-Sheikh, A.H.; Nnadi, E.O. Cu(II) biosorption by living biofilms: Isothermal, chemical, physical and biological evaluation. J. Environ. Manag. 2021, 282, 111950. [Google Scholar] [CrossRef] [PubMed]
- Sedefoglu, N.; Er, S.; Veryer, K.; Zalaoglu, Y.; Bozok, F. Green synthesized CuO nanoparticles using macrofungi extracts: Characterization, nanofertilizer and antibacterial effects. Mater. Chem. Phys. 2023, 309, 128393. [Google Scholar] [CrossRef]
- Akar, T.; Tunali, S. Biosorption performance of Botrytis cinerea fungal by-products for removal of Cd(II) and Cu(II) ions from aqueous solutions. Miner Eng. 2005, 18, 1099–1109. [Google Scholar] [CrossRef]
- Nascimento Júnior, W.J.; Silva, M.G.C.; Vieira, M.G.A. Competitive fixed-bed biosorption of Ag(I) and Cu(II) ions on Sargassum filipendula seaweed waste. J. Water Process. Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Do Nascimento, J.M.; De Oliveira, J.D.; Rizzo, A.C.L.; Leite, S.G.F. Biosorption Cu (II) by the yeast Saccharomyces cerevisiae. Biotechnol. Rep. 2019, 21, e00315. [Google Scholar] [CrossRef]
- Verma, A.; Shalu; Singh, A.; Bishnoi, N.R.; Gupta, A. Biosorption of Cu (II) using free and immobilized biomass of Penicillium citrinum. Ecol. Eng. 2013, 61, 486–490. [Google Scholar] [CrossRef]
- Özdemir, S.; Kilinc, E.; Poli, A.; Nicolaus, B.; Güven, K. Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii sub.sp. decanicus and Geobacillus thermoleovorans sub.sp. stromboliensis: Equilibrium, kinetic and thermodynamic studies. J. Chem. Eng. 2009, 152, 195–206. [Google Scholar] [CrossRef]
- Kumar, A.; Mukhia, S.; Kumar, R. Production, characterisation, and application of exopolysaccharide extracted from a glacier bacterium Mucilaginibacter sp. ERMR7:07. Process Biochem. 2022, 113, 27–36. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.R.; Yang, Y.; Yin, H.; Shengming, J.; Jiang, T. Potentiality of phosphorus−accumulating organisms biomasses in biosorption of Cd(II), Pb(II), Cu(II) and Zn(II) from aqueous solutions: Behaviors and mechanisms. Chemosphere 2022, 303, 135095. [Google Scholar] [CrossRef]
- Manimaran, K.; Yanto, D.H.I.; Camaraj, C.; Selvaraj, K.; Pandiaraj, S.; Elgorban, A.M.; Vignesh, S.; Kim, H. Eco-friendly approaches of mycosynthesized copper oxide nanoparticles (CuONPs) using Pleurotus citrinopileatus mushroom extracts and their biological applications. Environ. Res. 2023, 232, 116319. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef]
- Amin, F.; Fozia; Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. eCAM Volume 2021, 2021, 5589703. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Mobarak, M.B.; Uddin, M.N.; Quddus, M.S.; Naim, M.R.; Pinky, N.S. Biogenic synthesis of copper oxide (CuO) NPs exploiting Averrhoa carambola leaf extract and its potential antibacterial activity. Mater. Chem. Phys. 2023, 305, 127979. [Google Scholar] [CrossRef]
- Singh, D.; Jain, D.; Rajpurohit, D.; Jat, G.; Kushwaha, H.S.; Singh, A.; Mohanty, S.R.; Al-Sadoon, M.-K.; Zaman, W.; Upadhyay, S.K. Bacteria assisted green synthesis of copper oxide nanoparticles and their potential applications as antimicrobial agents and plant growth stimulants. Front. Chem. 2023, 11, 1154128. [Google Scholar] [CrossRef]
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Díaz-Gómez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sánchez-Sanhueza, G. Antibacterial and cytotoxic evaluation of copper and zinc oxide nanoparticles as a potential disinfectant material of connections in implant provisional abutments: An in-vitro study. Arch. Oral Biol. 2021, 122, 105031. [Google Scholar] [CrossRef]





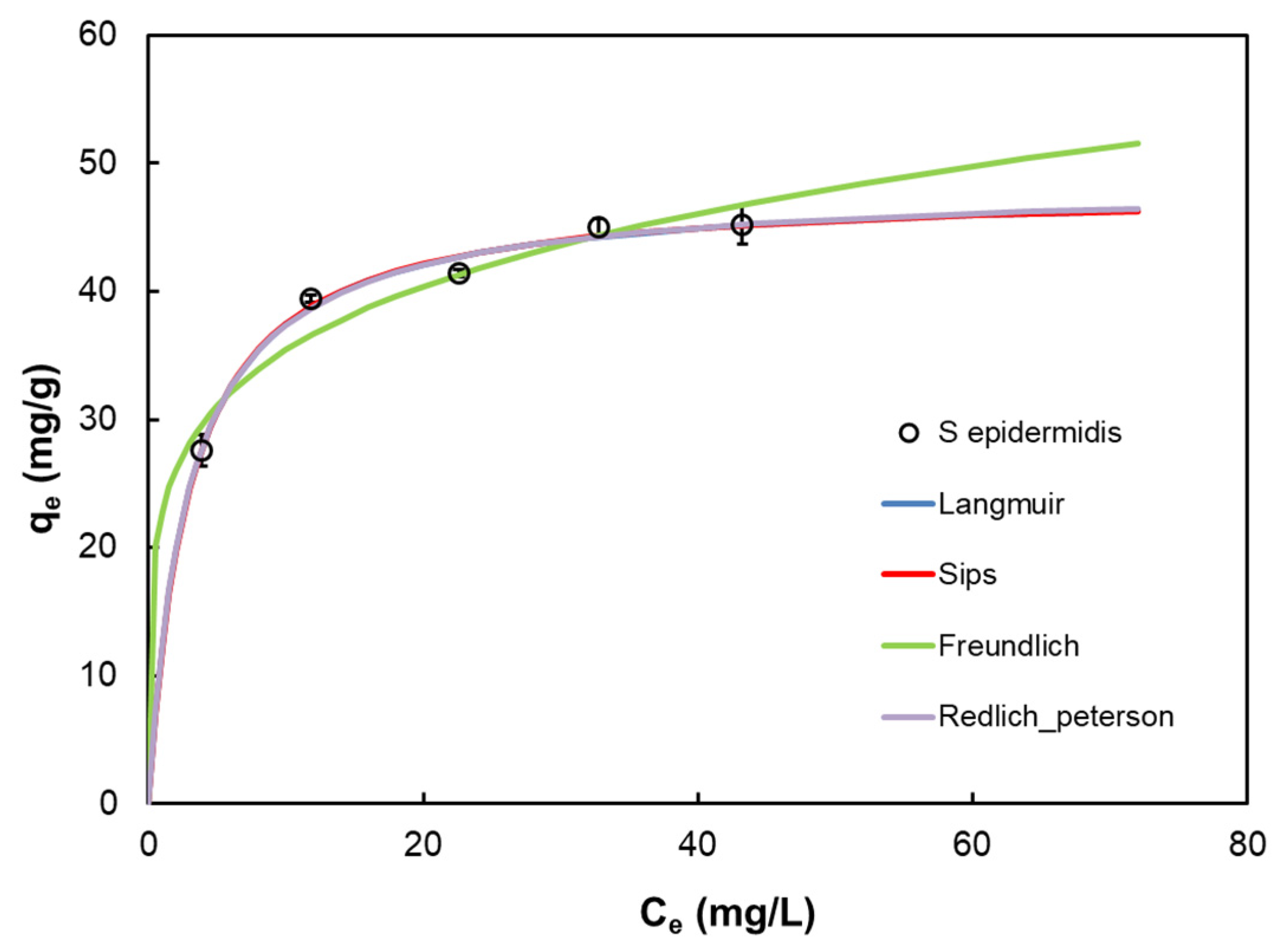

| Model | Equation | Parameter | |
|---|---|---|---|
| Langmuir [13] | qm | 48.14 | |
| b | 0.349 | ||
| R2 | 0.987 | ||
| Σ(q − qcal)2 | 0.876 | ||
| Freundlich [14] | KF | 22.884 | |
| n | 5.27 | ||
| R2 | 0.928 | ||
| Σ(q − qcal)2 | 4.984 | ||
| Sips [15] | Ks | 16.530 | |
| as | 0.344 | ||
| n | 0.987 | ||
| R2 | 0.987 | ||
| Σ(q − qcal)2 | 1.313 | ||
| Redlich–Peterson [16] | KRP | 17.149 | |
| aRP | 0.363 | ||
| β | 0.995 | ||
| R2 | 0.987 | ||
| Σ(q − qcal)2 | 1.310 | ||
| pH | B, g/L | q, mg/g |
|---|---|---|
| 3.0 | 0.20 | 7.05 |
| 3.0 | 0.76 | 10.93 |
| 4.3 | 0.50 | 16.22 |
| 4.3 | 0.50 | 14.74 |
| 4.3 | 0.85 | 15.91 |
| 2.5 | 0.50 | 2.96 |
| 5.5 | 0.20 | 42.28 * |
| 4.3 | 0.50 | 14.44 |
| 4.3 | 0.50 | 15.06 |
| 4.3 | 0.08 | 24.50 |
| 6.0 | 0.50 | 17.00 |
| 4.3 | 0.50 | 15.24 |
| 5.5 | 0.76 | 16.14 |
| Type of Biomass | Biomass | qm (mg/g) * | Reference |
|---|---|---|---|
| Plant | Ageratum conyzoid | 51.57 | [21] |
| Marine macroalgae | Sargassum filipendula | 40.8 | [28] |
| Yeast | Saccharomyces cerevisiae | 4.73 | [29] |
| Filamentous fungus | Penicillium citrinum | 22.7 | [30] |
| Bacteria | Geobacillus toebii Geobacillus thermoleovorans | 41.5 48.5 | [31] |
| Bacterial extract | Mucilaginibacter sp. ERMR7:07 | 8.36 | [32] |
| Bacteria | Ochrobactrum cicero Stenotrophomonas maltophilia Pseudomonas putida | 50.56 39.26 38.97 | [33] |
| Bacteria | S. epidermidis CECT 4183 | 48.14 | This work |
| Bacteria | CuO-NPs | CuO-NPs + PVA (10%) * |
|---|---|---|
| B. cereus | 1000–2000 | 62.5–125 |
| S. epidermidis | 125–250 | 62.5–125 |
| E. coli | 250–500 | 62.5–125 |
| P. fluorescens | 500–1000 | 250–500 |
| R. mucilaginosa 1S1 | - | 500–1000 |
| Type of Synthesis | Starting Biomass/ Chemical Method | Size (nm) | Microorganisms Tested | CMI (µg/mL) | Reference |
|---|---|---|---|---|---|
| Biological synthesis | Gum karaya | 4.8-1.6 | E. coli S. aureus | 103.5 120.4 | [35] |
| Biological synthesis | Aerva javanica | 15–23 | E. coli S. aureus | 128 | [36] |
| Biological synthesis | Penicillium chrysogenum | 15.5–59.7 | S. aureus B. subtilis P. aeruginosa E. coli | 1000 1500 2000 3000 | [37] |
| Biological synthesis | Averrhoa carambola | 80–100 | E. coli P. aeruginosa S. typhimurium B. megaterium S. aureus | 50 | [38] |
| Biological synthesis | Bacteria ZTB29 | 15–30 | Xanthomonas sp. Alternaria sp. | 200 | [39] |
| Chemical synthesis | DARC-AC * | 40–50 | S. sanguinis P. gingivalis P. melaninogenica S. mutans | 125–625 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.J.; Espínola, F.; Moya, M.; Martín, C.; Ruiz, E. Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect. Appl. Sci. 2024, 14, 7623. https://doi.org/10.3390/app14177623
Muñoz AJ, Espínola F, Moya M, Martín C, Ruiz E. Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect. Applied Sciences. 2024; 14(17):7623. https://doi.org/10.3390/app14177623
Chicago/Turabian StyleMuñoz, Antonio J., Francisco Espínola, Manuel Moya, Celia Martín, and Encarnación Ruiz. 2024. "Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect" Applied Sciences 14, no. 17: 7623. https://doi.org/10.3390/app14177623







