Properties of Zirconia, Lithium Disilicate Glass Ceramics, and VITA ENAMIC® Hybrid Ceramic Dental Materials Following Ultra-Short Femtosecond (30 fs) Laser Irradiation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Laser Irradiation
2.3. Sample Analysis
3. Results
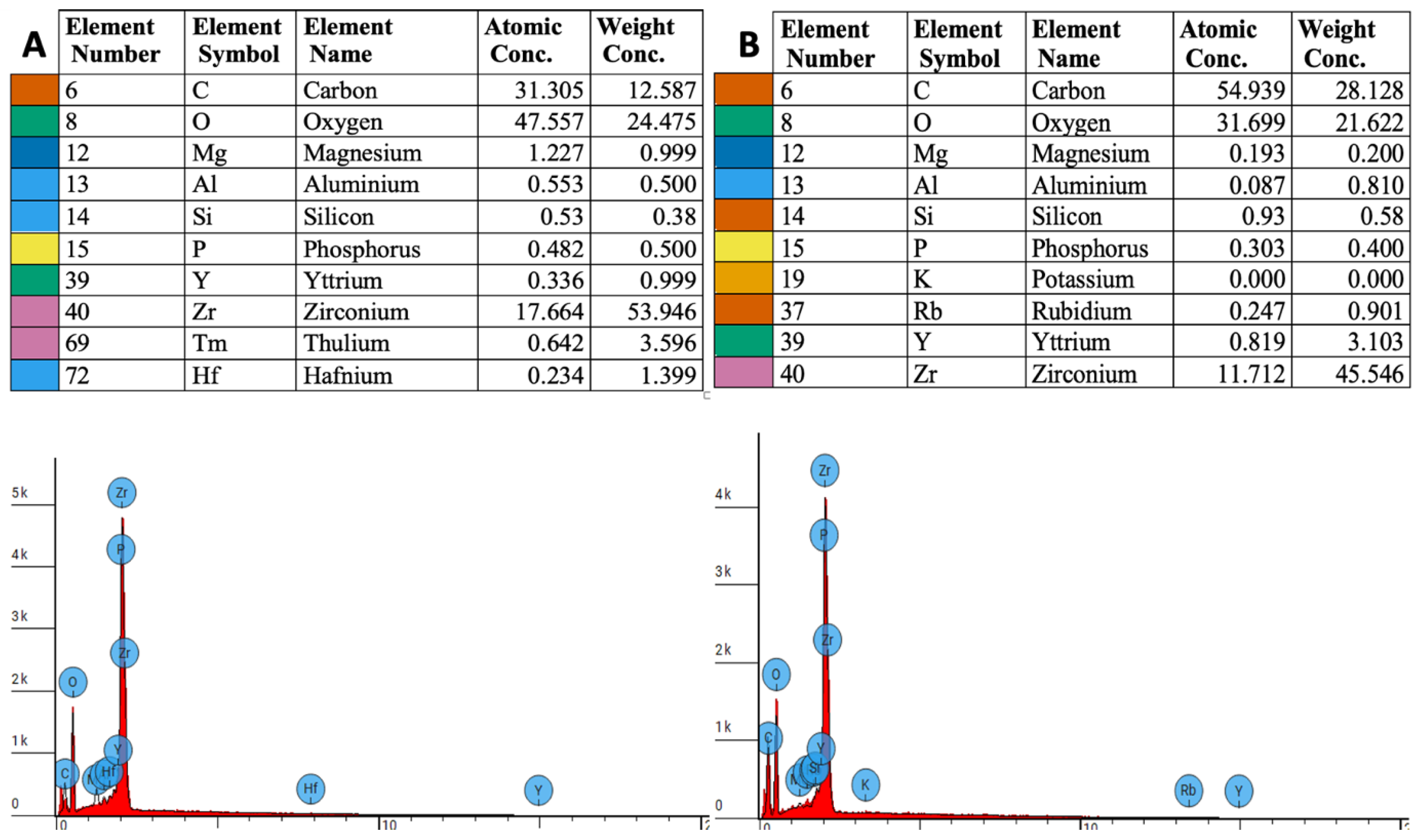
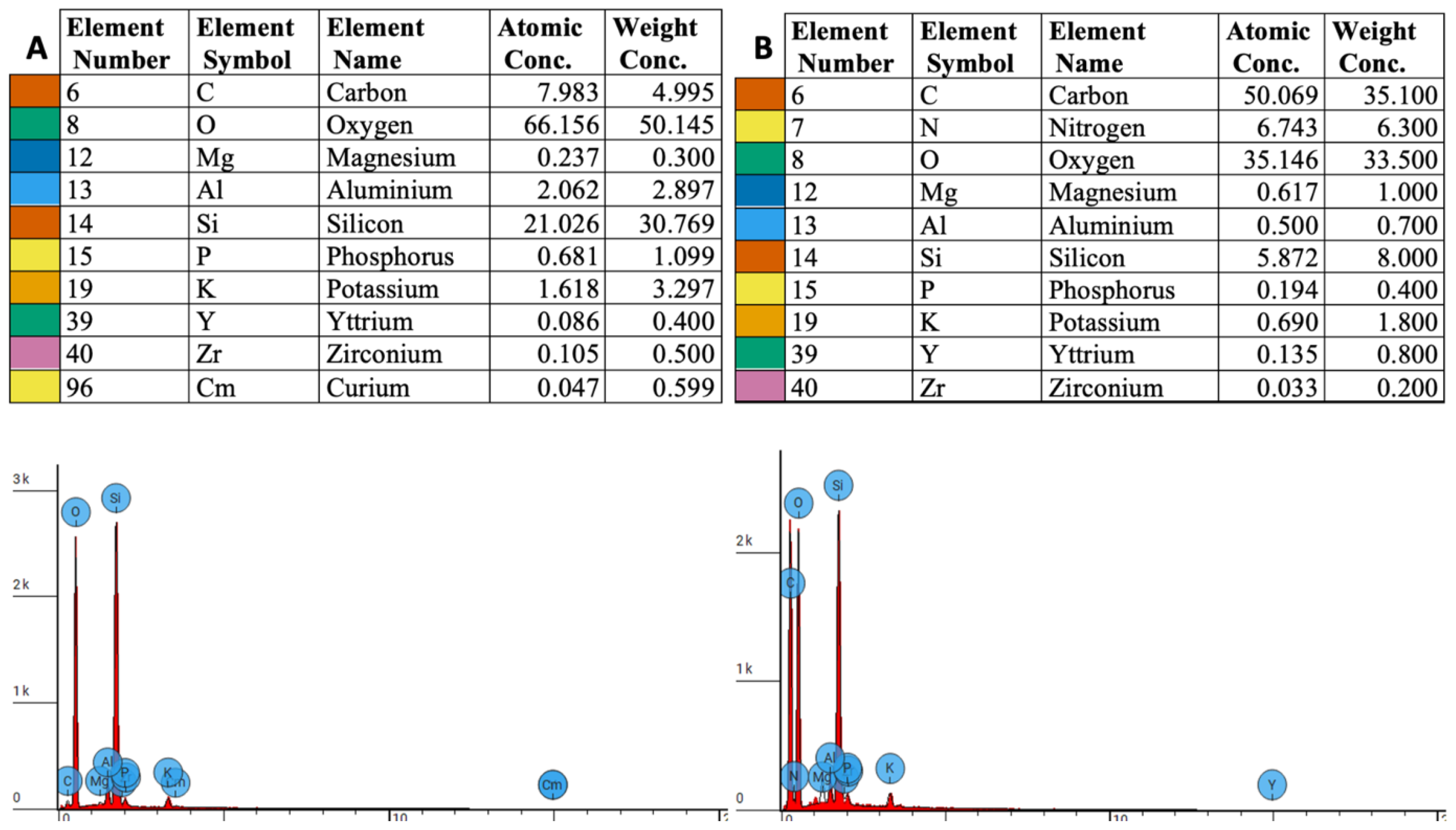
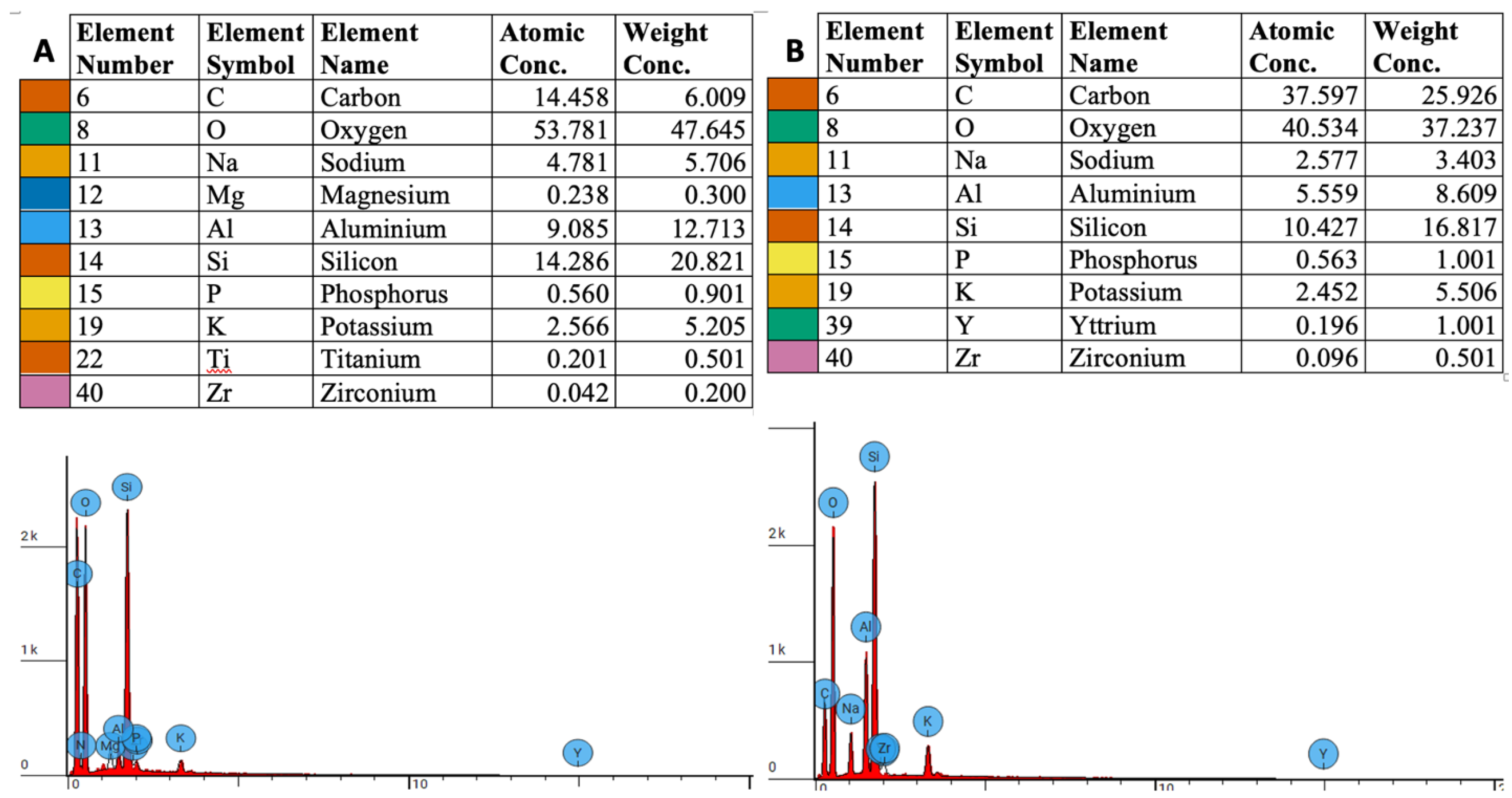
3.1. EDS Measurements
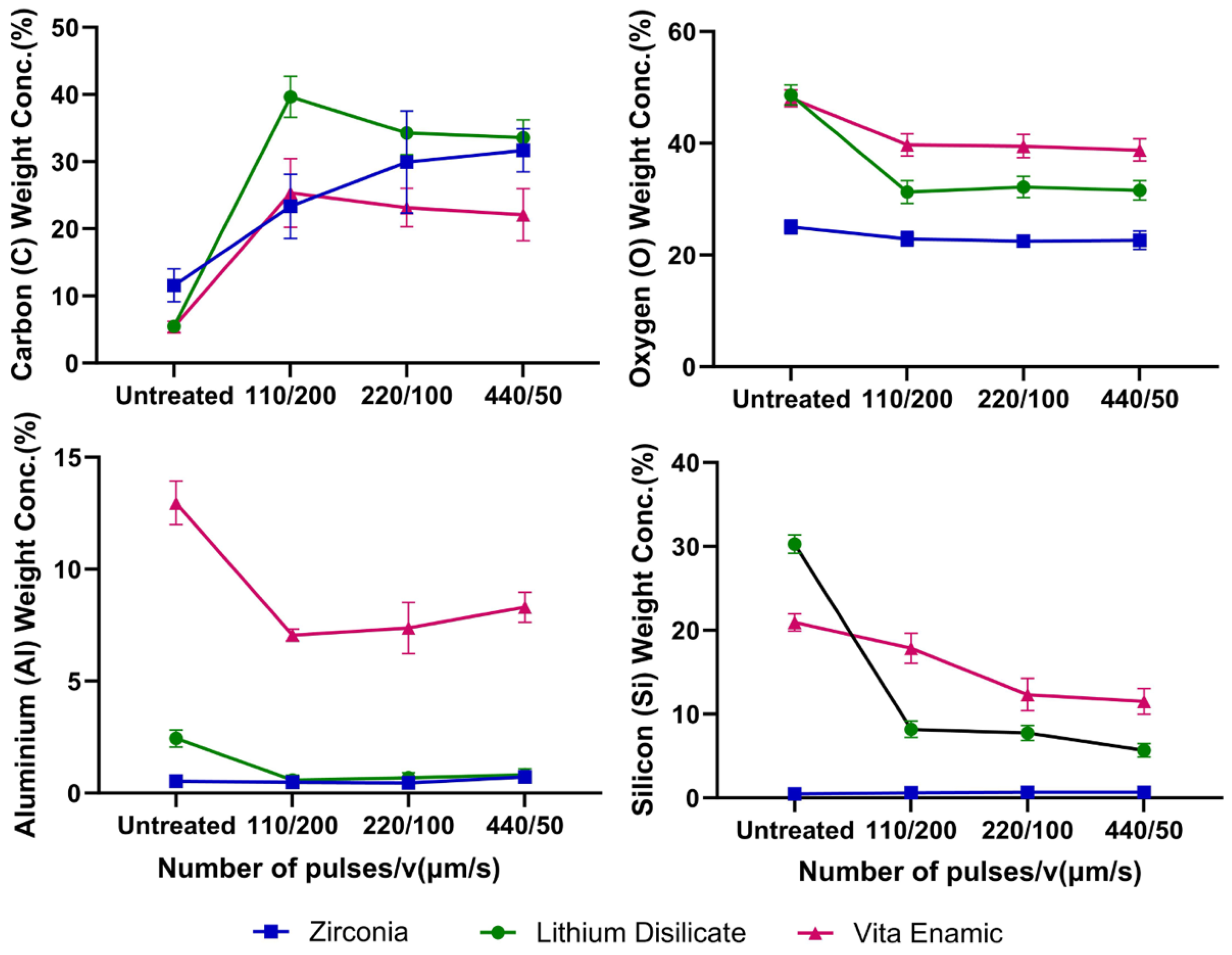
3.2. Elemental Weight Concentration Analysis
3.3. Atomic Concentration Analysis
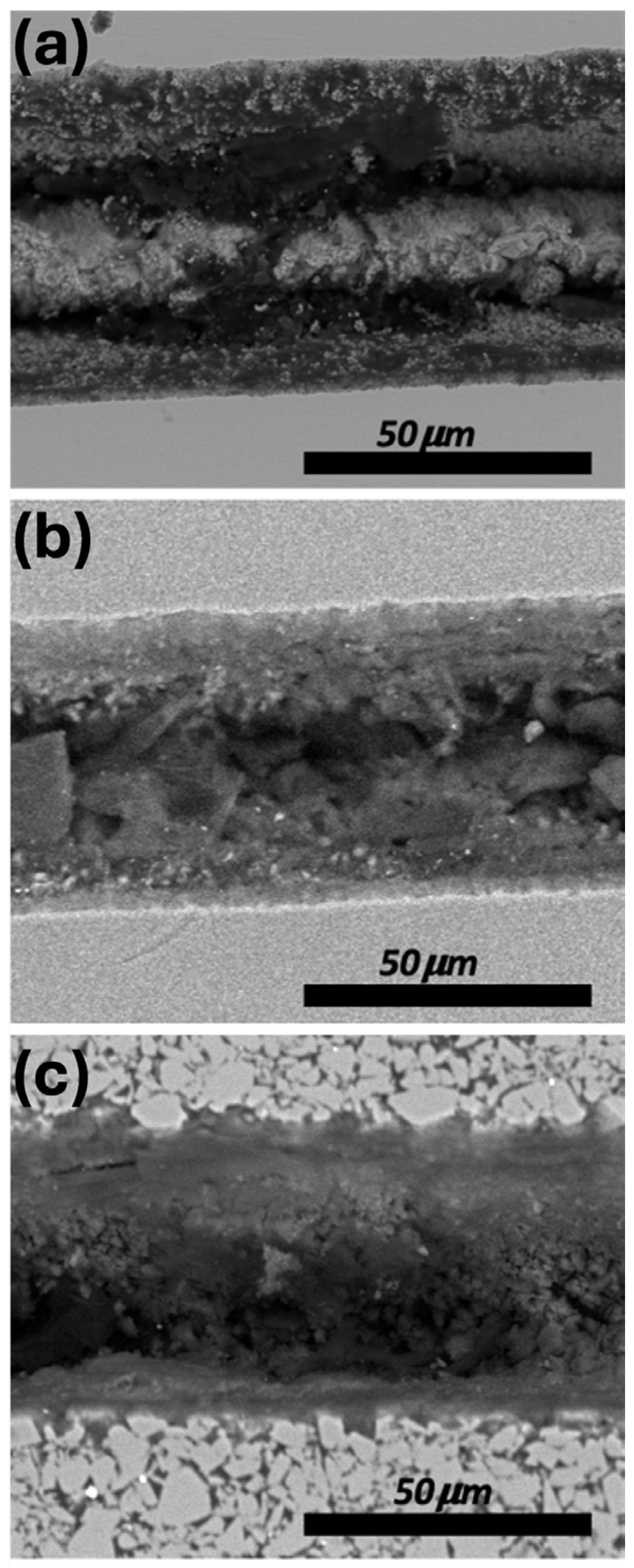
3.4. Morphological Changes of the Processed Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, L.H.D.; Lima, E.D.; Miranda, R.B.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31 (Suppl. S1), e58. [Google Scholar] [CrossRef] [PubMed]
- Conejo, J.; Nueesch, R.; Vonderheide, M.; Blatz, M.B. Clinical performance of all-ceramic dental restorations. Curr. Oral Health Rep. 2017, 4, 112–123. [Google Scholar] [CrossRef]
- Punj, S.; Singh, J.; Singh, K. Ceramic biomaterials: Properties, state of the art and future prospectives. Ceram. Int. 2021, 47, 28059–28074. [Google Scholar] [CrossRef]
- Sjögren, G.; Molin, M.; Van Dijken, J.W. A 10-year prospective evaluation of CAD/CAM-manufactured (Cerec) ceramic inlays cemented with a chemically cured or dual-cured resin composite. Int. J. Prosthodont. 2004, 17, 241–246. [Google Scholar] [PubMed]
- Gonzaga, C.C.; Cesar, P.F.; Okada, C.Y.; Fredericci, C.; Beneduce Neto, F.; Yoshimura, H.N. Mechanical properties and porosity of dental glass-ceramics hot-pressed at different temperatures. Mater. Res. 2008, 11, 301–306. [Google Scholar] [CrossRef]
- Strasding, M.; Sebestyén-Hüvös, E.; Studer, S.; Lehner, C.; Jung, R.E.; Sailer, I. Long-term outcomes of all-ceramic inlays and onlays after a mean observation time of 11 years. Quintessence Int. 2020, 51, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Mavriqi, L.; Valente, F.; Murmura, G.; Sinjari, B.; Macrì, M.; Trubiani, O.; Caputi, S.; Traini, T. Lithium disilicate and zirconia reinforced lithium silicate glass-ceramics for CAD/CAM dental restorations: Biocompatibility, mechanical and microstructural properties after crystallization. J. Dent. 2022, 119, 104054. [Google Scholar] [CrossRef]
- Andrade, J.; Stona, D.; Bittencourt, H.; Borges, G.; Burnett, L.; Spohr, A.M. Effect of different computer-aided design/computer-aided manufacturing (CAD/CAM) materials and thicknesses on the fracture resistance of occlusal veneers. Oper. Dent. 2018, 43, 539–548. [Google Scholar] [CrossRef]
- Leung, B.T.; Tsoi, J.K.; Matinlinna, J.P.; Pow, E.H. Comparison of mechanical properties of three machinable ceramics with an experimental fluorophlogopite glass ceramic. J. Prosthet. Dent. 2015, 114, 440–446. [Google Scholar] [CrossRef]
- Blatz, M.B.; Conejo, J.; Alammar, A.; Ayub, J. Current protocols for resin-bonded dental ceramics. Dent. Clin. N. Am. 2022, 66, 603–625. [Google Scholar] [CrossRef]
- Phark, J.H.; Duarte, S. Microstructural considerations for novel lithium disilicate glass ceramics: A review. J. Esthet. Restor. Dent. 2022, 34, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Matinlinna, J.P.; Pow, E.H.N. Insights into porcelain to zirconia bonding. J. Adhes. Sci. Technol. 2012, 26, 1249–1265. [Google Scholar] [CrossRef]
- Zaia, W.L.S.; de Figueiredo, J.L.G.; Leite, L.M.; Satake, A.; Medeiros, I.S. Bond strenght of zirconia submitted to different surface treatments. Pesqui. Bras. Odontopediatria Clínica Integr. 2015, 15, 387–398. [Google Scholar] [CrossRef]
- Han, J.; Zhang, F.; Van Meerbeek, B.; Vleugels, J.; Braem, A.; Castagne, S. Laser surface texturing of zirconia-based ceramics for dental applications: A review. Mater. Sci. Eng. C 2021, 123, 112034. [Google Scholar] [CrossRef]
- Tzanakakis, E.G.C.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The use of lasers in dental materials: A review. Materials 2021, 14, 3370. [Google Scholar] [CrossRef]
- Lawson, N.C.; Bansal, R.; Burgess, J.O. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent. Mat. 2016, 32, e275–e283. [Google Scholar] [CrossRef]
- Ergun Kunt, G.; Duran, I. Effects of laser treatments on surface roughness of zirconium oxide ceramics. BMC Oral Health 2018, 18, 222. [Google Scholar] [CrossRef]
- Dede, D.Ö.; Yenisey, M.; Rona, N.; Öngöz Dede, F. Effects of laser treatment on the bond strength of differently sintered zirconia ceramics. Photomed. Laser Surg. 2016, 34, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; García-González, S.; Rieux, J.; Jiménez-Piqué, E. Nanosecond pulsed laser surface modification of yttria doped zirconia for solid oxide fuel cell applications: Damage and microstructural changes. J. Eur. Ceram. Soc. 2023, 43, 3396–3403. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photonics 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Ali, B.; Litvinyuk, I.V.; Rybachuk, M. Femtosecond laser micromachining of diamond: Current research status, applications and challenges. Carbon 2021, 179, 209–226. [Google Scholar] [CrossRef]
- Ali, B.; Xu, H.; Sang, R.T.; Litvinyuk, I.V.; Rybachuk, M. Optimised diamond to graphite conversion via a metastable sp1-bonded carbon chain formation under an ultra-short femtosecond (30 fs) laser irradiation. Carbon 2023, 204, 575–586. [Google Scholar] [CrossRef]
- Lagunov, V.L.; Rybachuk, M.; Itthagarun, A.; Walsh, L.J.; George, R. Modification of dental enamel, dentin by an ultra-fast femtosecond laser irradiation: A systematic review. Opt. Laser Technol. 2022, 155, 108439. [Google Scholar] [CrossRef]
- Yamamuro, Y.; Shimoyama, T.; Yan, J. Microscale surface patterning of zirconia by femtosecond pulsed laser irradiation. Int. J. Precis. Eng. Manuf. Green Technol. 2022, 9, 619–632. [Google Scholar] [CrossRef]
- Feng, D.; Shen, H. Hole quality control in underwater drilling of yttria-stabilized zirconia using a picosecond laser. Opt. Laser Technol. 2019, 113, 141–149. [Google Scholar] [CrossRef]
- Li, J.; Ji, L.; Hu, Y.; Bao, Y. Precise micromachining of yttria-tetragonal zirconia polycrystal ceramic using 532 nm nanosecond laser. Ceram. Int. 2016, 42, 4377–4385. [Google Scholar] [CrossRef]
- Panova, N.K.; Nikolova, K.T.; Dikova, T.D. Application of lasers and laser processing technologies in modern dentistry: A review. J. Chem. Technol. Metall. 2023, 58, 1116–1127. [Google Scholar] [CrossRef]
- Schliephake, H.; Hefti, T.; Schlottig, F.; Gédet, P.; Staedt, H. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J. Clin. Periodontol. 2010, 37, 818–828. [Google Scholar] [CrossRef]
- Saulacic, N.; Erdösi, R.; Bosshardt, D.D.; Gruber, R.; Buser, D. Acid and alkaline etching of sandblasted zirconia implants: A histomorphometric study in miniature pigs. Clin. Implant. Dent. Relat. Res. 2014, 16, 313–322. [Google Scholar] [CrossRef]
- Aivazi, M.; hossein Fathi, M.; Nejatidanesh, F.; Mortazavi, V.; HashemiBeni, B.; Matinlinna, J.P.; Savabi, O. The evaluation of prepared microgroove pattern by femtosecond laser on alumina-zirconia nano-composite for endosseous dental implant application. Lasers Med. Sci. 2016, 31, 1837–1843. [Google Scholar] [CrossRef]
- Hu, P.; Yao, L.; Zhang, M.; Nie, Z.; Ji, E.; Lue, Q.; He, Z. Femtosecond laser micro-milling dental glass ceramics: An experimental analysis and COMSOL finite element simulation. Ceram. Int. 2020, 46, 22146–22153. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Carvalho, A.; Trifonov, A.; Nathala, C.; Monteiro, F.; Buchvarov, I. Effect of surface modification by femtosecond laser on zirconia based ceramics for screening of cell-surface interaction. Appl. Surf. Sci. 2020, 513, 145914. [Google Scholar] [CrossRef]
- Han, J.; Malek, O.; Vleugels, J.; Braem, A.; Castagne, S. Ultrashort pulsed laser ablation of zirconia-alumina composites for implant applications. J. Mater. Process. Technol. 2022, 299, 117335. [Google Scholar] [CrossRef]
- Delgado-Ruíz, R.; Calvo-Guirado, J.; Moreno, P.; Guardia, J.; Gomez-Moreno, G.; Mate-Sánchez, J.; Ramirez-Fernández, P.; Chiva, F. Femtosecond laser microstructuring of zirconia dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, A.; Takada, H.; Yoshitomi, D.; Torizuka, K.; Kobayashi, Y. Ultrafast laser processing of ceramics: Comprehensive survey of laser parameters. J. Laser Appl. 2021, 33, 012009. [Google Scholar] [CrossRef]
- VITA YZ® SOLUTIONS Technical and Scientific Documentation. 2022. VITA Zahnfabrik H. Rauter GmbH & Co., Bad Säckingen, Germany. Available online: https://www.vita-zahnfabrik.com/VITA_YZ_en,27568,175081.html (accessed on 21 August 2024).
- VITA AMBRIA® PRESS SOLUTIONS Technical and Scientific Documentation. 2020. VITA Zahnfabrik H. Rauter GmbH & Co., Bad Säckingen, Germany. Available online: https://www.vita-zahnfabrik.com/en/VITA-AMBRIA-Lithium-disilicate-press-ceramic-102005.html (accessed on 21 August 2024).
- VITA ENAMIC® HYBRID CERAMIC. 2024. VITA Zahnfabrik H. Rauter GmbH & Co., Bad Säckingen, Germany. Available online: https://www.vita-zahnfabrik.com/index.php (accessed on 21 August 2024).
- ISO 6872:2024; Dentistry—Ceramic Materials. ISO: Geneva, Switzerland, 2024. Available online: https://www.iso.org/standard/81718.html (accessed on 21 August 2024).
- ISO 12836:2015; Dentistry—Digitizing Devices for CAD/CAM Systems for Indirect Dental Restorations—Test Methods for Assessing Accuracy. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/68414.html (accessed on 21 August 2024).
- Karson, M. Handbook of Methods of Applied Statistics. Volume I: Techniques of Computation Descriptive Methods, and Statistical Inference; John Wiley: New York, NY, USA, 1967. [Google Scholar]
- El Gamal, A.; Fornaini, C.; Rocca, J.P.; Muhammad, O.H.; Medioni, E.; Cucinotta, A.; Brulat-Bouchard, N. The effect of CO2 and Nd: YAP lasers on CAD/CAM Ceramics: SEM, EDS and thermal studies. Laser Ther. 2016, 25, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.-P.; Fornaini, C.; Brulat-Bouchard, N.; Seif, S.B.; Darque-Ceretti, E. CO2 and Nd: YAP laser interaction with lithium disilicate and Zirconia dental ceramics: A preliminary study. Opt. Laser Technol. 2014, 57, 216–223. [Google Scholar] [CrossRef]
- Henriques, B.; Hammes, N.; Souza, J.C.; Özcan, M.; Mesquita-Guimarães, J.; Silva, F.S.; Fredel, M.C.; Volpato, C.M.; Carvalho, Ó. Influence of ns-Nd: YAG laser surface treatment on the tensile bond strength of zirconia to resin-matrix cements. Ceram. Int. 2020, 46, 27822–27831. [Google Scholar] [CrossRef]
- Kim, S.H.; Balasubramani, T.; Sohn, I.-B.; Noh, Y.-C.; Lee, J.; Lee, J.B.; Jeong, S. Precision microfabrication of AlN and Al2O3 ceramics by femtosecond laser ablation. In Proceedings Volume 6879, Photon Processing in Microelectronics and Photonics VII, 68791O; SPIE: Bellingham, WA, USA, 2008; pp. 270–276. [Google Scholar] [CrossRef]
- Allahkarami, M.; Hanan, J.C. Mapping the tetragonal to monoclinic phase transformation in zirconia core dental crowns. Dent. Mater. 2011, 27, 1279–1284. [Google Scholar] [CrossRef]
- Noda, M.; Okuda, Y.; Tsuruki, J.; Minesaki, Y.; Takenouchi, Y.; Ban, S. Surface damages of zirconia by Nd: YAG dental laser irradiation. Dent. Mater. J. 2010, 29, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Harai, T.; Mizutani, M.; Shishido, S.; Nakamura, K.; Ohmori, H.; Konno, T.J.; Kuriyagawa, T. Low-temperature degradation of yttria-stabilized zirconia treated with pulsed laser and annealing techniques. Precis. Eng. 2023, 80, 45–56. [Google Scholar] [CrossRef]
- Ackerl, N.; Wegener, K. Ablation characteristics of alumina and zirconia ceramics on ultra-short pulsed laser machining. J. Laser Micro Nanoeng. 2019, 14, 168–172. [Google Scholar] [CrossRef]
- Carvalho, A.; Grenho, L.; Fernandes, M.H.; Daskalova, A.; Trifonov, A.; Buchvarov, I.; Monteiro, F.J. Femtosecond laser microstructuring of alumina toughened zirconia for surface functionalization of dental implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar] [CrossRef]
- Zhou, H.B.; Li, C.; Zhou, Z.K.; Cao, R.Y.; Chen, Y.; Zhang, S.S.; Wang, G.; Xiao, S.; Li, Z.; Xiao, P. Femtosecond laser-induced periodic surface microstructure on dental zirconia ceramic. Mater. Lett. 2018, 229, 74–77. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Wang, Y. Effect of femtosecond laser ablate ultra-fine microgrooves on surface properties of dental zirconia materials. J. Mech. Behav. Biomed. Mater. 2022, 134, 105361. [Google Scholar] [CrossRef]
- Munro, T.; Miller, C.M.; Antunes, E.; Sharma, D. Interactions of osteoprogenitor cells with a novel zirconia implant surface. J. Funct. Biomater. 2020, 11, 50. [Google Scholar] [CrossRef]
- Pessková, V.; Kubies, D.; Hulejová, H.; Himmlová, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Gruner, W.; Stolle, S.; Wetzig, K. Formation of COx species during the carbothermal reduction of oxides of Zr, Si, Ti, Cr, W, and Mo. Int. J. Refract. Met. Hard Mater. 2000, 18, 137–145. [Google Scholar] [CrossRef]
- Gasparrini, C.; Chater, R.J.; Horlait, D.; Vandeperre, L.; Lee, W.E. Zirconium carbide oxidation: Kinetics and oxygen diffusion through the intermediate layer. J. Am. Ceram. Soc. 2018, 101, 2638–2652. [Google Scholar] [CrossRef]
- Liu, G.; Cheng, L.; Li, K.; Chen, Z.; Xiong, X.; Luan, X. Damage behavior of atomic oxygen on zirconium carbide coating modified carbon/carbon composite. Ceram. Int. 2020, 46, 3324–3331. [Google Scholar] [CrossRef]
- Kucheryavaya, A.; Lenčéš, Z.; Šajgalík, P.; Harmuth, H. Zirconium oxycarbides and oxycarbonitrides: A review. Int. J. Appl. Ceram. Technol. 2023, 20, 541–562. [Google Scholar] [CrossRef]
- David, J.; Trolliard, G.; Gendre, M.; Maitre, A. TEM study of the reaction mechanisms involved in the carbothermal reduction of zirconia. J. Eur. Ceram. Soc. 2013, 33, 165–179. [Google Scholar] [CrossRef]
- Sarkar, S.K. Solubility of Oxygen in Zirconium Carbide. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1969. Available online: https://www.proquest.com/dissertations-theses/solubility-oxygen-zirconium-carbide/docview/302441320/se-2?accountid=14723 (accessed on 21 August 2024).
- Sievers, M.R.; Armentrout, P.B. Oxidation of CO and reduction of CO2 by gas phase Zr+, ZrO+, and ZrO2+. Int. J. Mass Spectrom. 1999, 185–187, 117–129. [Google Scholar] [CrossRef]
- Liu, H.-L.; Man, Z.-Y.; Liu, J.-X.; Wang, X.-G.; Zhang, G.-J. Solid solution and densification behavior of zirconium oxycarbide (ZrCxOy) ceramics via doping ZrO2 and Zr in ZrC. J. Alloys Compd. 2017, 729, 492–497. [Google Scholar] [CrossRef]
- Yamamuro, Y.; Shimoyama, T.; Yan, J. Generation of nanopore structures in yttria-stabilized zirconia by femtosecond pulsed laser irradiation. J. Mater. Res. Technol. 2023, 23, 1155–1176. [Google Scholar] [CrossRef]
- Kaligar, A.B.; Kumar, H.A.; Ali, A.; Abuzaid, W.; Egilmez, M.; Alkhader, M.; Abed, F.; Alnaser, A.S. Femtosecond laser-based additive manufacturing: Current status and perspectives. Quantum Beam Sci. 2022, 6, 5. [Google Scholar] [CrossRef]
- Ali, B.; Xu, H.; Chetty, D.; Sang, R.T.; Litvinyuk, I.V.; Rybachuk, M. Laser-Induced Graphitization of Diamond Under 30 fs Laser Pulse Irradiation. J. Phys. Chem. Lett. 2022, 13, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
| Power mW | Fluence J/cm2 | Number of Pulses (n) | Scanning Speed μm/s |
|---|---|---|---|
| 76 | 20 | 110 | 50 |
| 76 | 20 | 220 | 100 |
| 76 | 20 | 440 | 200 |
| Pulse Number/ Scanning Speed | Carbon | Oxygen | Aluminum | Silicon |
|---|---|---|---|---|
| Zirconia | ||||
| 100/200 | 23.3 (4.8) b | 22.9 (1.3) a | 0.5 (0.1) a | 0.6 (0.2) a |
| 220/100 | 29.9 (7.6) c | 22.5 (0.9) a | 0.5 (0.2) a | 0.7 (0.7) a |
| 440/50 | 31.7 (3.2) c | 22.7 (1.6) a | 0.7 (0.2) a | 0.7 (0.7) a |
| Untreated | 11.6 (2.4) a | 25.0 (1.2) b | 0.5 (0.1) a | 0.5 (0.5) a |
| Lithium disilicate | ||||
| 100/200 | 39.7 (3.0) c | 31.3 (2.0) a | 0.6 (0.2) a | 8.2 (1.0) b |
| 220/100 | 34.3 (3.2) b | 32.2 (1.9) a | 0.7 (0.2) a | 7.7 (0.9) a |
| 440/50 | 33.5 (2.7) b | 31.6 (1.7) a | 0.8 (0.3) a | 5.7 (0.8) a |
| Untreated | 5.5 (0.6) a | 48.7 (1.8) b | 2.4 (0.4) b | 30.3 (1.1) c |
| VITA ENAMIC® | ||||
| 100/200 | 25.3 (5.1) b | 39.7 (2.0) a | 7.1 (0.2) a | 17.9 (0.1) c |
| 220/100 | 23.2 (2.8) b | 39.5 (2.1) a | 7.4 (0.1) a | 12.3 (0.1) b |
| 440/50 | 22.1 (3.9) b | 38.8 (2.0) a | 8.3 (0.1) a | 11.5 (0.1) a |
| Untreated | 5.4 (0.8) a | 48.1 (1.4) b | 13.0 (0.1) b | 20.9 (0.2) d |
| Pulse Number/ Scanning Speed | Carbon | Oxygen | Aluminum | Silicon |
|---|---|---|---|---|
| Zirconia | ||||
| 100/200 | 41.1 (2.2) ** | 31.9 (2.1) *** | 0.2 (0.1) ** | NA |
| 220/100 | 46.9 (2.7) *** | 31.7 (0.8) *** | 0.2 (0.1) ** | NA |
| 440/50 | 54.9 (3.5) **** | 31.7 (2.6) *** | 0.1 (0.1) ** | NA |
| Untreated | 31.3 (4.2) | 47.6 (4.9) | 0.6 (0.2) | NA |
| Lithium disilicate | ||||
| 100/200 | 40.6 (1.8) **** | 32.3 (2.2) **** | 0.6 (0.3) ** | 8.3 (1.5) **** |
| 220/100 | 42.6 (2.0) **** | 33.2 (2.1) **** | 0.6 (0.3) ** | 7.0 (1.4) **** |
| 440/50 | 50.1 (4.0) **** | 35.2 (1.9) **** | 0.5 (0.3) ** | 5.9 (1.4) **** |
| Untreated | 8.0 (2.0) | 66.2 (4.2) | 2.1 (0.7) | 21.0 (2.1) |
| VITA ENAMIC® | ||||
| 100/200 | 37.0 (3.3) **** | 42.7 (2.6) ** | 6.3 (1.7) * | 13.5 (1.7) NS |
| 220/100 | 38.8 (2.0) **** | 41.5 (3.3) **** | 5.5 (1.4) ** | 12.1 (1.8) NS |
| 440/50 | 37.6 (4.3) **** | 40.9 (2.2) ** | 5.6 (1.1) ** | 10.4 (1.4) * |
| Untreated | 14.5 (2.4) | 52.5 (5.1) | 9.1 (1.8) | 14.3 (2.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagunov, V.L.; Ali, B.; Walsh, L.J.; Cameron, A.B.; Litvinyuk, I.V.; Rybachuk, M.; George, R. Properties of Zirconia, Lithium Disilicate Glass Ceramics, and VITA ENAMIC® Hybrid Ceramic Dental Materials Following Ultra-Short Femtosecond (30 fs) Laser Irradiation. Appl. Sci. 2024, 14, 7641. https://doi.org/10.3390/app14177641
Lagunov VL, Ali B, Walsh LJ, Cameron AB, Litvinyuk IV, Rybachuk M, George R. Properties of Zirconia, Lithium Disilicate Glass Ceramics, and VITA ENAMIC® Hybrid Ceramic Dental Materials Following Ultra-Short Femtosecond (30 fs) Laser Irradiation. Applied Sciences. 2024; 14(17):7641. https://doi.org/10.3390/app14177641
Chicago/Turabian StyleLagunov, Victor L., Bakhtiar Ali, Laurence J. Walsh, Andrew B. Cameron, Igor V. Litvinyuk, Maksym Rybachuk, and Roy George. 2024. "Properties of Zirconia, Lithium Disilicate Glass Ceramics, and VITA ENAMIC® Hybrid Ceramic Dental Materials Following Ultra-Short Femtosecond (30 fs) Laser Irradiation" Applied Sciences 14, no. 17: 7641. https://doi.org/10.3390/app14177641
APA StyleLagunov, V. L., Ali, B., Walsh, L. J., Cameron, A. B., Litvinyuk, I. V., Rybachuk, M., & George, R. (2024). Properties of Zirconia, Lithium Disilicate Glass Ceramics, and VITA ENAMIC® Hybrid Ceramic Dental Materials Following Ultra-Short Femtosecond (30 fs) Laser Irradiation. Applied Sciences, 14(17), 7641. https://doi.org/10.3390/app14177641







