Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Preparation of Liposomal Vitamin C in Powder Form
2.3. Verification of the Process of Obtaining Liposomal Powder Formulation
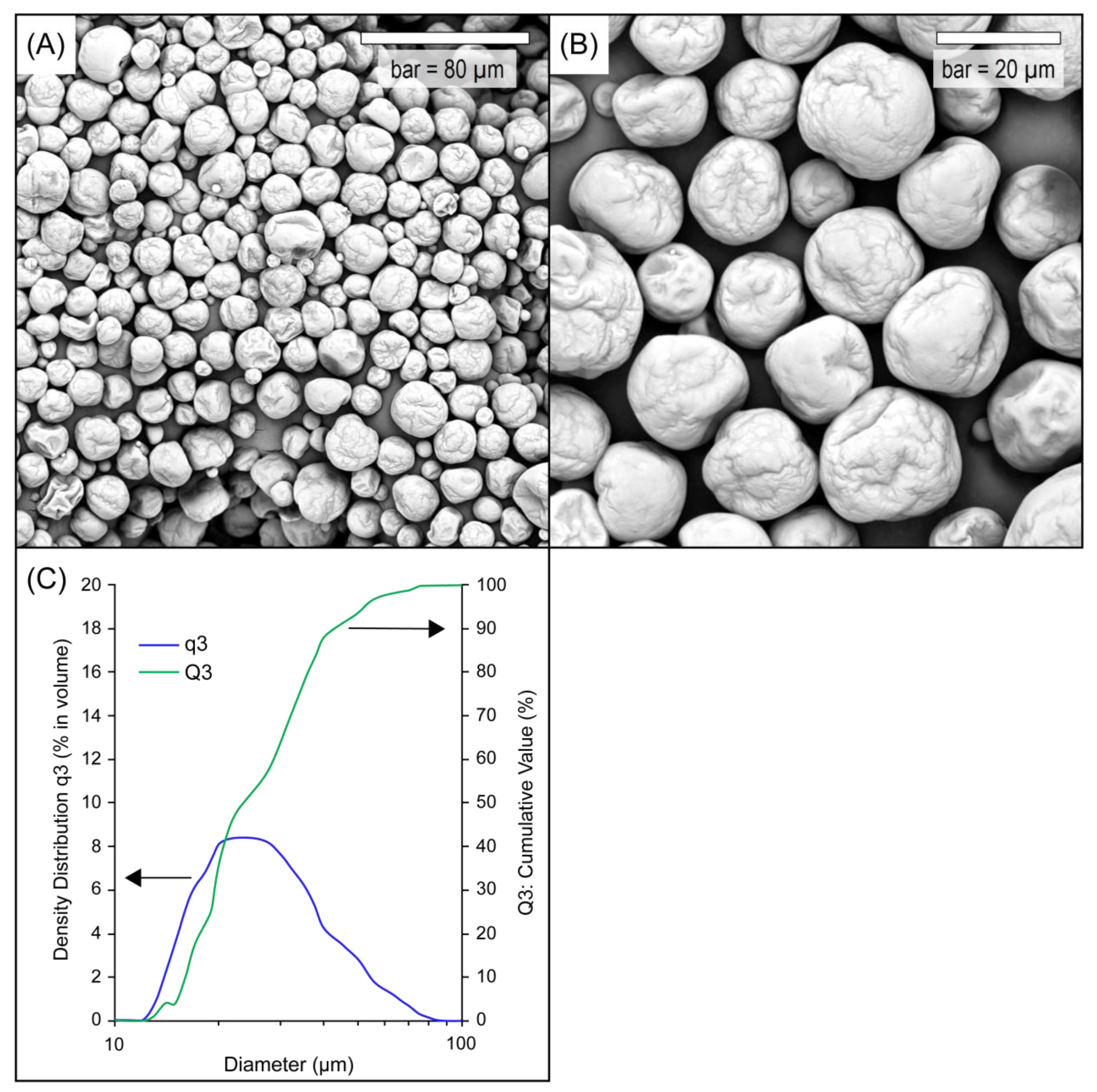
2.3.1. Scanning Electron Microscopy (SEM)
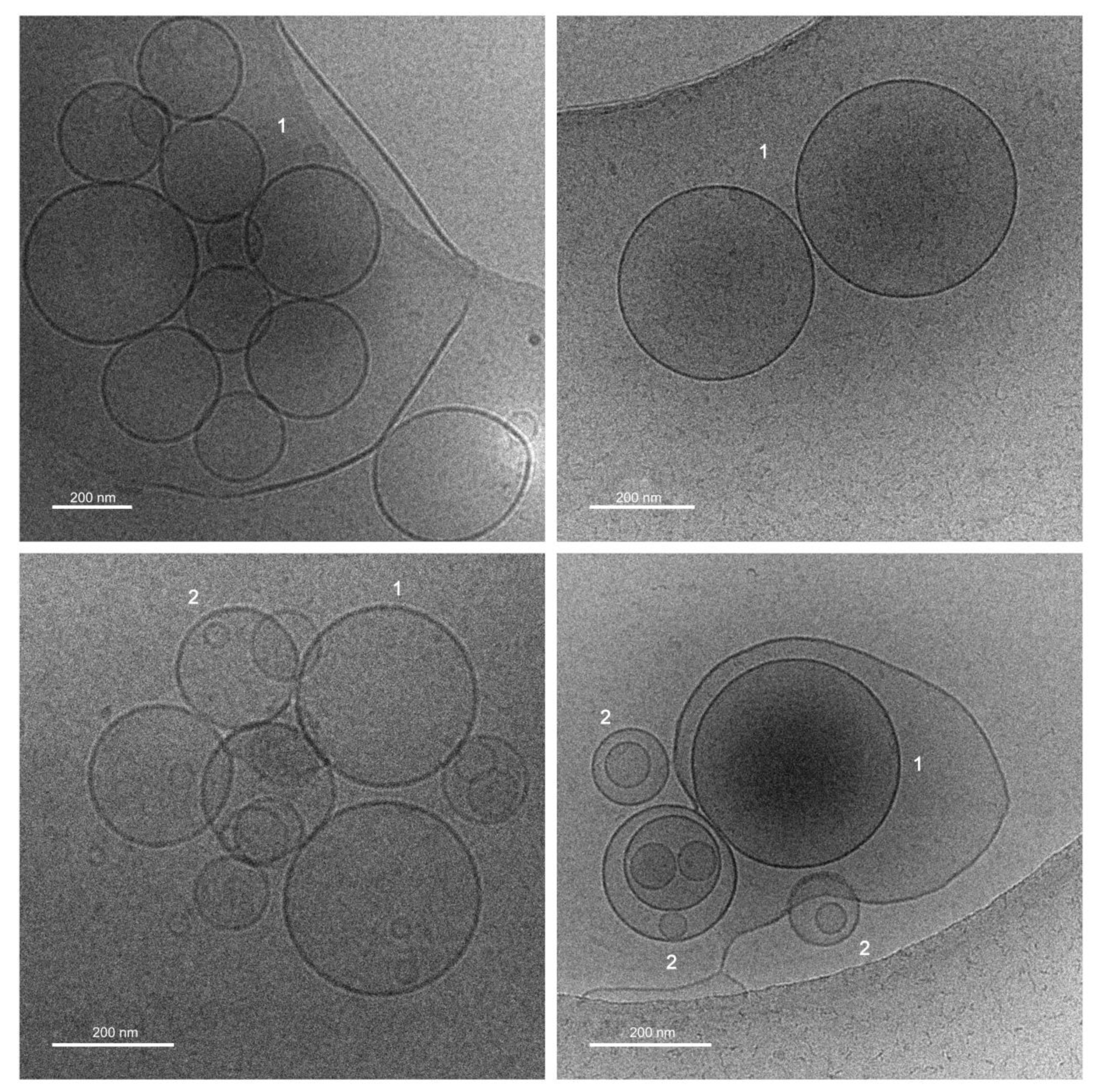
2.3.2. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
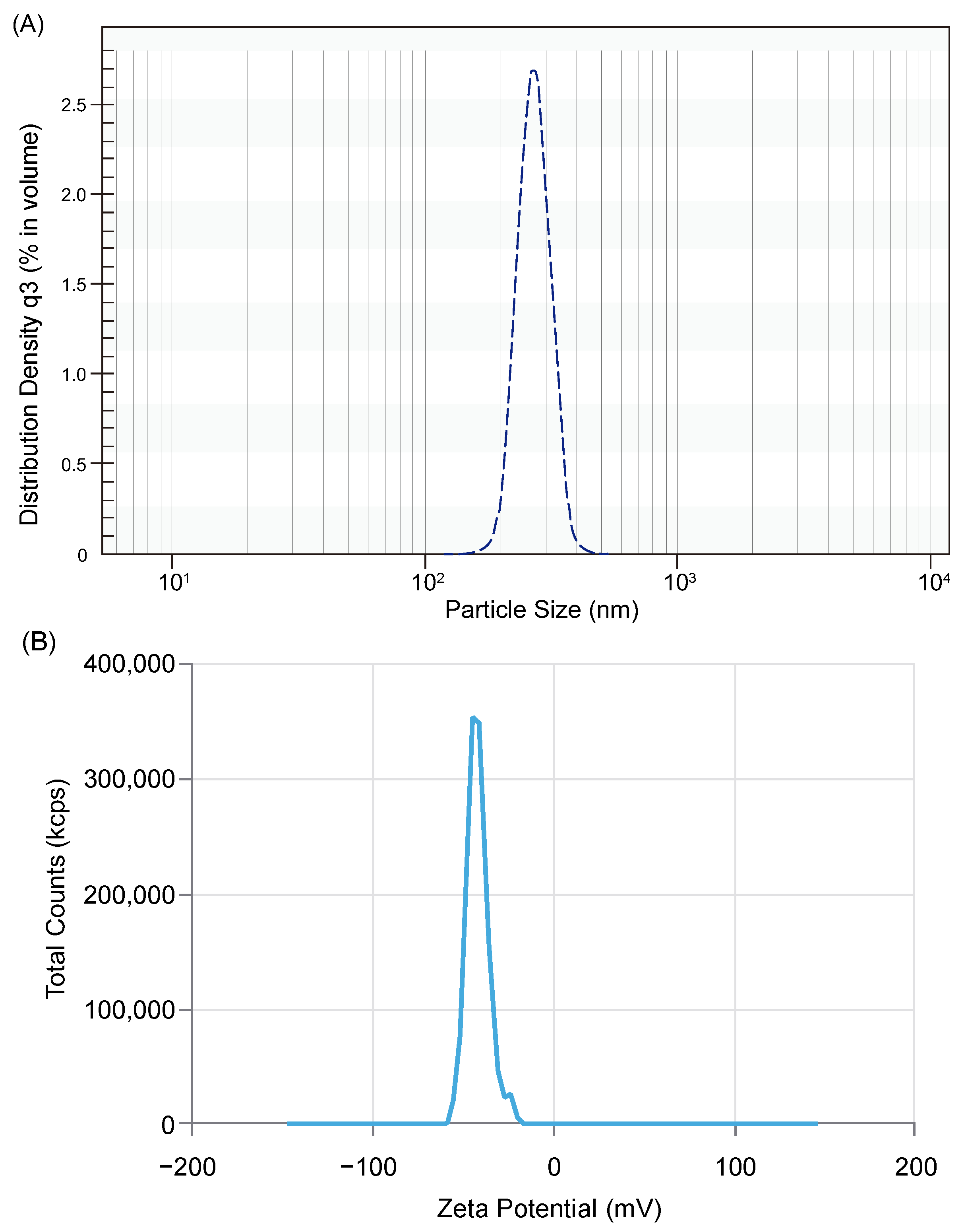
2.3.3. Measurement of Particle Size Distribution (PSD) of the Liposomal Powder and Liposomes
2.3.4. Measurement of Zeta Potential
2.4. In Vitro Bioavailability Study
2.4.1. Cell Culture
2.4.2. Permeability across Human Intestinal Epithelial Cells Monolayer (Caco-2 Cells)
2.4.3. Evaluation of Vitamin C Concentration
Preparation of Buffer and Wash Solutions
Method Optimization
UPLC Analysis
2.4.4. Bioavailability Expression
2.5. Clinical Study
2.5.1. Ethical Approval
2.5.2. Trial Registration
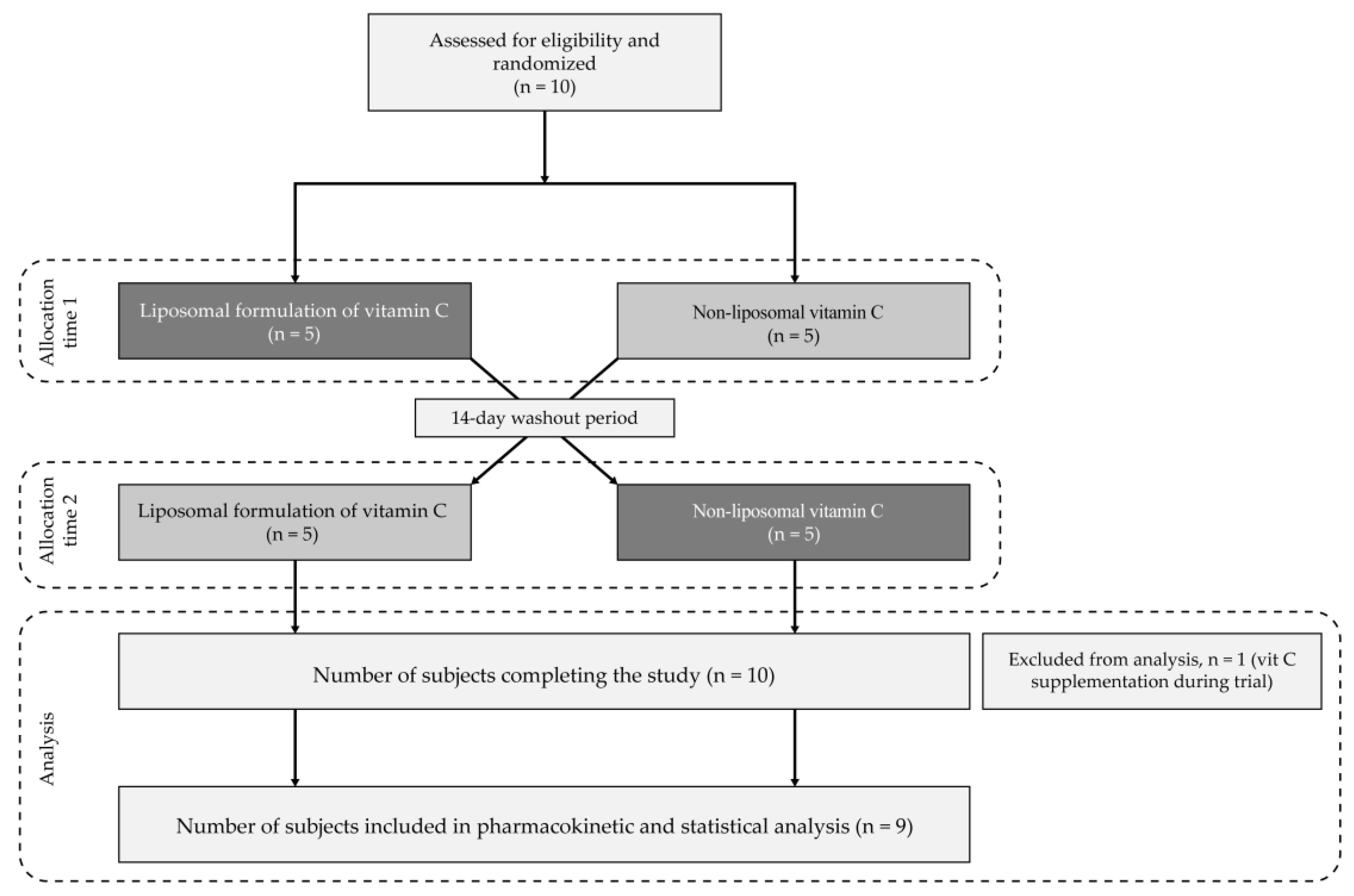
2.5.3. Study Design
2.5.4. Primary and Secondary Endpoints
2.5.5. Methodology
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties of Liposomal Vitamin C in Powder Form
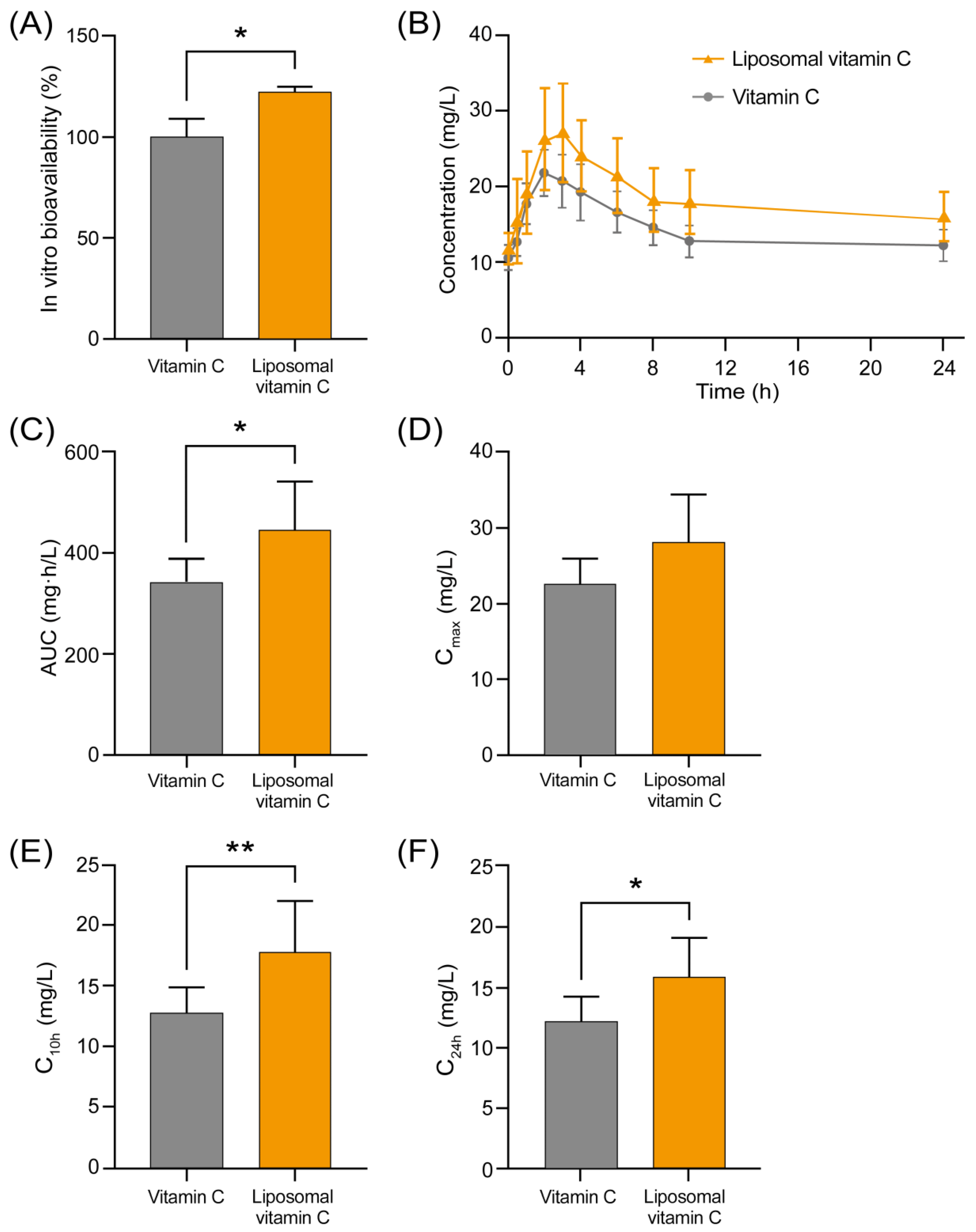
3.2. Comparison of Bioavailability of Non-Liposomal and Liposomal Ascorbic Acid in an In Vitro Cell Model
3.3. Clinical Trial Results
3.3.1. Study Population
3.3.2. Results of Analysis of Pharmacokinetic Properties of the Tested Formulations
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Maurya, V.K.; Shakya, A.; McClements, D.J.; Srinivasan, R.; Bashir, K.; Ramesh, T.; Lee, J.; Sathiyamoorthi, E. Vitamin C fortification: Need and recent trends in encapsulation technologies. Front. Nutr. 2023, 10, 1229243. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Rucker, R.B. Vitamin C. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Daniel Lane, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 530–534. [Google Scholar] [CrossRef]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. 5. Vitamin C. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225480/ (accessed on 20 August 2024).
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://iris.who.int/handle/10665/42716 (accessed on 20 August 2024).
- Blomhoff, R.; Andersen, R.; Arnesen, E.K.; Christensen, J.J.; Eneroth, H.; Erkkola, M.; Gudanaviciene, I.; Halldorsson, T.I.; Høyer-Lund, A.; Lemming, E.W.; et al. Nordic Nutrition Recommendations 2023; Nordic Council of Ministers: Copenhagen, Denmark, 2023. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Ahuja, J.K.C.; Showell, B.; Somanchi, M.; Nickle, M.; Nguyen, Q.A. Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 28; Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA: Beltsville, ML, USA, 2015. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Effect of Alternative Preservation Steps and Storage on Vitamin C Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Barco Leme, A.C.; Fisberg, R.M.; Veroneze de Mello, A.; Sales, C.H.; Ferrari, G.; Haines, J.; Rigotti, A.; Gómez, G.; Kovalskys, I.; Cortés Sanabria, L.Y.; et al. Food Sources of Shortfall Nutrients among Latin Americans: Results from the Latin American Study of Health and Nutrition (ELANS). Int. J. Environ. Res. Public Health 2021, 18, 4967. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, A.; Okada, C.; Okada, E.; Tajima, R.; Takimoto, H. Consumption of meals prepared away from home is associated with inadequacy of dietary fiber, vitamin C and mineral intake among Japanese adults: Analysis from the 2015 National Health and Nutrition Survey. Nutr. J. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Lachat, C.; Nago, E.; Verstraeten, R.; Roberfroid, D.; Van Camp, J.; Kolsteren, P. Eating out of home and its association with dietary intake: A systematic review of the evidence. Obes. Rev. 2012, 13, 329–346. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; Lacey, S.; Arsenic, T.; McCarthy, A.L. The nutritional status and dietary intake of free-living seniors: A cross-sectional study. Clin. Nutr. ESPEN 2021, 43, 478–486. [Google Scholar] [CrossRef]
- Hiatt, A.N.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Deliquescence Behavior and Chemical Stability of Vitamin C Forms (Ascorbic Acid, Sodium Ascorbate, and Calcium Ascorbate) and Blends. Int. J. Food Prop. 2011, 14, 1330–1348. [Google Scholar] [CrossRef]
- Sablani, S.S.; Al-Belushi, K.; Al-Marhubi, I.; Al-Belushi, R. Evaluating Stability of Vitamin C in Fortified Formula Using Water Activity and Glass Transition. Int. J. Food Prop. 2007, 10, 61–71. [Google Scholar] [CrossRef]
- Dickinson, A.; Blatman, J.; El-Dash, N.; Franco, J.C. Consumer usage and reasons for using dietary supplements: Report of a series of surveys. J. Am. Coll. Nutr. 2014, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Ball, L.; Desbrow, B.; Alsharairi, N.; Ahmed, F. Consumption and reasons for use of dietary supplements in an Australian university population. Nutrition 2016, 32, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Wiltgren, A.R.; Booth, A.O.; Kaur, G.; Cicerale, S.; Lacy, K.E.; Thorpe, M.G.; Keast, R.S.; Riddell, L.J. Micronutrient supplement use and diet quality in university students. Nutrients 2015, 7, 1094–1107. [Google Scholar] [CrossRef]
- Günalan, E.; Çavak, B.Y.; Turhan, S.; Cebioğlu, İ.K.; Domínguez, R.; Sánchez-Oliver, A.J. Dietary Supplement Use of Turkish Footballers: Differences by Sex and Competition Level. Nutrients 2022, 14, 3863. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Nováková, L.; Solichová, D.; Pavlovicová, S.; Solich, P. Hydrophilic interaction liquid chromatography method for the determination of ascorbic acid. J. Sep. Sci. 2008, 31, 1634–1644. [Google Scholar] [CrossRef]
- Lee, S.H.; Labuza, T.P. Destruction of ascorbic acid as a function of water activity. J. Food Sci. 1975, 40, 370–373. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, C.M. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Akbıyık, T.; Sönmezoğlu, İ.; Güçlü, K.; Tor, İ.; Apak, R. Protection of Ascorbic Acid from Copper(II)−Catalyzed Oxidative Degradation in the Presence of Fruit Acids: Citric, Oxalic, Tartaric, Malic, Malonic, and Fumaric Acids. Int. J. Food Prop. 2012, 15, 398–411. [Google Scholar] [CrossRef]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S.M. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2022, 62, 3301–3322. [Google Scholar] [CrossRef]
- Kirby, C.J.; Whittle, C.J.; Rigby, N.; Coxon, D.T.; Law, B.A. Stabilization of ascorbic acid by microencapsulation in liposomes. Int. J. Food Sci. Technol. 1991, 26, 437–449. [Google Scholar] [CrossRef]
- Farhang, B.; Kakuda, Y.; Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci. Technol. 2012, 92, 353–366. [Google Scholar] [CrossRef]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: Properties and bioavailability. J. Liposome Res. 2020, 30, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Balakrishnan, P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J. Liposome Res. 2021, 31, 356–364. [Google Scholar] [CrossRef]
- Romano, E.; Palladino, R.; Cannavale, M.; Lamparelli, E.P.; Maglione, B. Enhanced Stability of Oral Vitamin C Delivery: A Novel Large-Scale Method for Liposomes Production and Encapsulation through Dynamic High-Pressure Microfluidization. Nanomaterials 2024, 14, 516. [Google Scholar] [CrossRef]
- Favarin, F.R.; Forrati, É.M.; Bassoto, V.A.; da Silva Gündel, S.; Velho, M.C.; Ledur, C.M.; Verdi, C.M.; Lemos, J.G.; Sagrillo, M.R.; Fagan, S.B.; et al. Ascorbic acid and ascorbyl palmitate-loaded liposomes: Development, characterization, stability evaluation, in vitro security profile, antimicrobial and antioxidant activities. Food Chem. 2024, 460, 140569. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Han, S.; Zha, X.; Xia, J. Preparation of vitamin C liposomes by rapid expansion of supercritical solution process: Experiments and optimization. J. Drug. Deliv. Sci. Technol. 2019, 51, 1–6. [Google Scholar] [CrossRef]
- Sharma, V.K.; Agrawal, M.K. A historical perspective of liposomes-a bio nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Dhayalan, M.; Wang, W.; Riyaz, S.U.M.; Dinesh, R.A.; Shanmugam, J.; Irudayaraj, S.S.; Stalin, A.; Giri, J.; Mallik, S.; Hu, R. Advances in functional lipid nanoparticles: From drug delivery platforms to clinical applications. 3 Biotech 2024, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opin. Drug Deliv. 2011, 8, 375–388. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Donaghey, R.; Giacobbo, V.; Su, Y.; Perrie, Y. Scalable solvent-free production of liposomes. J. Pharm. Pharmacol. 2020, 72, 1328–1340. [Google Scholar] [CrossRef]
- ISO 13318-2:2007; Determination of Particle Size Distribution by Centrifugal Liquid Sedimentation Methods-Part 2: Photocentrifuge Method. International Organization for Standardization: Geneva, Switzerland, 2007.
- Kovács, A.; Berkó, S.; Csányi, E.; Csóka, I. Development of nanostructured lipid carriers containing salicylic acid for dermal use based on the Quality by Design method. Eur. J. Pharm. Sci. 2017, 99, 246–257. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics. 2018, 10, 139. [Google Scholar] [CrossRef]
- Jacob, J.; Sukumaran, N.P.; Jude, S. Fiber-Reinforced-Phospholipid Vehicle-Based Delivery of l-Ascorbic Acid: Development, Characterization, ADMET Profiling, and Efficacy by a Randomized, Single-Dose, Crossover Oral Bioavailability Study. ACS Omega 2021, 6, 5560–5568. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Giuliano, C.B.; Cvjetan, N.; Ayache, J.; Walde, P. Multivesicular Vesicles: Preparation and Applications. ChemSystemsChem 2021, 3, e2000049. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Temoporfin-loaded invasomes: Development, characterization and in vitro skin penetration studies. J. Control. Release 2008, 127, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Wessman, P.; Edwards, K.; Mahlin, D. Structural effects caused by spray- and freeze-drying of liposomes and bilayer disks. J. Pharm. Sci. 2010, 99, 2032–2048. [Google Scholar] [CrossRef]
- Egelhaaf, S.; Wehrli, E.; Müller, M.; Adrian, M.; Schurtenberger, P. Determination of the size distribution of lecithin liposomes: A comparative study using freeze fracture, cryoelectron microscopy and dynamic light scattering. J. Microsc. 1996, 184, 214–228. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and other vesicular systems: Structural characteristics, methods of preparation, and use in nanomedicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 1–52. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Ting, Y.; Zhao, Q.; Xia, C.; Huang, Q. Using in vitro and in vivo models to evaluate the oral bioavailability of nutraceuticals. J. Agric. Food Chem. 2015, 63, 1332–1338. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Chai, G.H.; Xu, Y.; Chen, S.Q.; Cheng, B.; Hu, F.Q.; You, J.; Du, Y.Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. ACS Appl. Mater. Interfaces 2016, 9, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1860, 556–565. [Google Scholar] [CrossRef]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial Transport of Curcumin in Caco-2 Cells Is significantly Enhanced by Micellar Solubilisation. Plant Foods Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. Guidance for Industry. FDA. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/liposome-drug-products-chemistry-manufacturing-and-controls-human-pharmacokinetics-and (accessed on 8 December 2023).
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Olson, J.A.; Schwartz, J.A.; Hahka, D.; Nguyen, N.; Bunch, T.; Jensen, G.M.; Adler-Moore, J.P. Toxicity and efficacy differences between liposomal amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice. Med. Mycol. 2015, 53, 107–118. [Google Scholar] [CrossRef]
- Yung, S.; Mayersohn, M.; Robinson, J.B. Ascorbic acid absorption in humans: A comparison among several dosage forms. J. Pharm. Sci. 1982, 71, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Naidoo, D.; Lui, K.; Koh, T.H.H.G.; Watson, D.; Whitehall, J. Antenatal supplementation of antioxidant vitamins to reduce the oxidative stress at delivery—A pilot study. Early Hum. Dev. 2002, 67, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.C.; Kelly, S.; Basdeo, S.A.; Kinsella, K.; Mulready, K.J.; Mills, K.H.; Tubridy, N.; Walsh, C.; Brady, J.J.; Hutchinson, M.; et al. A pilot study of the immunological effects of high-dose vitamin D in healthy volunteers. Mult. Scler. J. 2012, 18, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Quantiles * | |
|---|---|
| Harmonic mean ** | 267.7 nm |
| D10 | ≤223 nm |
| D16 | ≤224 nm |
| D50 (SD ***) | ≤262 nm (49.8 nm) |
| D84 | ≤334 nm |
| D90 | ≤354 nm |
| Span | 0.500 |
| Parameter | Mean | SD | Min | Max | % CV |
|---|---|---|---|---|---|
| Age (years) | 36.8 | 11.2 | 22 | 59 | 30.5 |
| Height (cm) | 172.0 | 10.3 | 160 | 186 | 6.0 |
| Weight (kg) | 83.2 | 11.9 | 58 | 98 | 14.3 |
| BMI (kg/m2) | 28.3 | 5.2 | 21.3 | 38.2 | 18.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmuda, P.; Khaidakov, B.; Krasowska, M.; Czapska, K.; Dobkowski, M.; Guzowski, J.; Kowalczyk, P.; Lemke, K.; Folwarski, M.; Foryś, A.; et al. Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial. Appl. Sci. 2024, 14, 7718. https://doi.org/10.3390/app14177718
Żmuda P, Khaidakov B, Krasowska M, Czapska K, Dobkowski M, Guzowski J, Kowalczyk P, Lemke K, Folwarski M, Foryś A, et al. Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial. Applied Sciences. 2024; 14(17):7718. https://doi.org/10.3390/app14177718
Chicago/Turabian StyleŻmuda, Przemysław, Barbara Khaidakov, Maria Krasowska, Katarzyna Czapska, Michał Dobkowski, Julian Guzowski, Paulina Kowalczyk, Krzysztof Lemke, Marcin Folwarski, Aleksander Foryś, and et al. 2024. "Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial" Applied Sciences 14, no. 17: 7718. https://doi.org/10.3390/app14177718
APA StyleŻmuda, P., Khaidakov, B., Krasowska, M., Czapska, K., Dobkowski, M., Guzowski, J., Kowalczyk, P., Lemke, K., Folwarski, M., Foryś, A., Domian, E., & Postuła, M. (2024). Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial. Applied Sciences, 14(17), 7718. https://doi.org/10.3390/app14177718







