Abstract
A random copolymer (PTBM), utilized as deep ultra-violet (DUV) photoresist, was prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization with tert-butyl methacrylate (tBMA), methyl methacrylate (MMA), triphenylsulfonium p-styrenesulfonate (TPS-SS), and functional poly (sesquicarbonylsiloxanes) (POSS-MA) as the monomer components, and 4-cyano-4-[(dodecylsulfanylthiocarbonyl) sulfanyl]pentanoic acid (CDSPA) as the RAFT reagent. Fourier transform infrared spectroscopy (FT-IR) and proton nuclear magnetic resonance (1H NMR) proved successful synthesis. Ultraviolet absorption spectroscopy (UV) analysis verified the transparency of the polymer in the DUV band. RAFT polymerization kinetics showed that the polymerization rate conformed to the first-order kinetic relationship, and the polymerization process exhibited a typical controlled free radical polymerization behavior. Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and static thermo-mechanical analysis (TMA) showed that the incorporation of POSS groups improved the thermal properties of the copolymer. According to scanning electron microscopy (SEM) images, the copolymerization of photoacid monomers (TPS-SS) resulted in photoresist copolymers exhibiting good resistance to acid diffusion and low roughness.
1. Introduction
Photoresists are extensively applied in microelectronics, encompassing semiconductor devices, printed circuit boards, color filters, and micro-electromechanical systems [1,2,3,4,5]. In the early 1980s, Ito and Willson introduced chemical amplification resist (CAR) [6,7,8], which has become a fundamental component in advanced photolithography imaging systems due to its exceptional sensitivity and resolution. CARs typically comprise a polymer matrix with protected functional groups, the photoacid generator (PAG), and supplementary additives. The matrix resin serves as the principal constituent of the photoresist, contributing to etch resistance and film-forming properties. Simultaneously, the acid-sensitive functional groups within the matrix play a pivotal role in modifying the solubility of photoresists, thereby influencing photoresist performance. The chemical amplification mechanism involves the decomposition of the photoacid generator and proton release upon light exposure [9]. This acid catalyzes a chemical reaction with the acid-labile groups within the photoresist. Following the completion of this catalytic reaction, the proton is released once more, sustaining a chain reaction [10]. Owing to the dissolution inhibition attributed to the protected functional groups, the unexposed photoresist remains insoluble in alkaline aqueous solutions. The exposed portion is converted to an alkali-soluble form after post-exposure baking (PEB) to produce a positive image [11,12].
Line width roughness (LWR) and line edge roughness (LER) play vital roles in evaluating photolithography patterns. LWR is significantly influenced by the prevalent acid diffusion occurring within the photoresist during chemical amplification [13,14]. This diffusion arises from the erratic movement of excess protons formed after the chain reactions. Historically, most commercially available photoresists have been developed by physically combining small molecular photoacid generators [15]. The elevated temperature during post-exposure bake (PEB) exacerbates the erratic movement of these small molecular photoacid, leading to increased acid diffusion. Addressing acid diffusion has become a critical concern in chemical amplification photoresists with high resolution. Meanwhile, variations in the composition of photoresist resin have notable impacts on LER, primarily due to the uneven distribution of chain lengths, resulting from the conventional free radical polymerization used in synthesis of photoresist resins. In addition, the conventional free radical polymerization contributes to drawbacks of the photoresist, such as uncontrollable molecular weight and poor batch-to-batch reproducibility, which negatively affect photolithography processes [16,17,18]. Currently, by regulating the equilibrium between dormant and active free radicals [19], various Reversible Deactivation Radical Polymerization (RDRP) methods [20,21] were developed, including Atom Transfer Radical Polymerization (ATRP) [22,23,24], Nitroxide-Mediated Polymerization (NMP) [25], and Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization [26,27]. These techniques have rapidly evolved into potent tools for synthesizing macromolecules with precise structures, low polydispersity, and controlled average molecular weight [28,29]. Preparation of photoresist resins by controlled free radical polymerization has become a new research direction.
Thermal performance of photoresist significantly influences the quality and resolution of photoresist. The increased temperatures, even as high as 100–120 °C during pre-baking and post-exposure bake steps in photolithography, may cause deformation or collapse of photoresist patterns. Li3 improved thermal performance of photoresist through the introduce of adamantyl groups to reach 120 °C for Tg and 190 °C for Td, while Zheng [30] enhanced the heat resistance of photoresist by introducing cedryl alcohol ester groups to reach 128 °C for Tg and 200 °C for Td. Presently, cage-type polyhedral oligomeric silsesquioxane (POSS) is a novel organic-inorganic hybrid material. Its unique nanoscale size effects and effective polymer modification have attracted considerable interest, finding applications in nanohybrid materials, medical materials and catalysts [31,32]. The inorganic structure present in the cage-type POSS has a high modulus, stiffness and spatial site resistance, which maintains the strength of the base polymers and also increases the glass transition temperature of the polymers. The introduction of POSS moieties into photoresist polymers shows potential as a novel method to improve the heat resistance of photoresist resins.
In this study, two novel monomers including triphenylsulfonium p-styrenesulfonate (TPS-SS) and polyhedral oligomeric silsesquioxane-based methyl acrylate (POSS-MA),were polymerized through RAFT polymerization to synthesize the photoresist copolymer (denoted as PTBM) for DUV lithography. In this context, tBMA and MMA monomers can optimize the performance of the photoresist by allowing the formation of fine patterns at a high resolution, while also providing excellent mechanical properties and thermal stability. The POSS-MA monomer reduces acid diffusion and simultaneously enhances the thermal resistance of polymeric matrix in the photoresist and its adhesion to silicon substrates. The PAG-based monomer, TPP-SS, chemically bonded into the polymer backbone after RAFT polymerization, increases the loading of photoacid and improves the uniform distribution of PAG in polymer matrix, effectively reducing acid diffusion. The research work highlighted the controllable nature of RAFT polymerization and the reproducibility of photoresist by examining polymerization kinetics. Furthermore, the impact of incorporating POSS-MA on the thermal properties of the copolymer matrix was evaluated using thermal gravimetric analyzer (TGA), differential scanning calorimetry (DSC) and thermomechanical analyzer (TMA) analyses. Ultimately, scanning electron microscopy (SEM) validation confirmed that integrating photoacid units into the main chain of copolymers effectively minimized the acid diffusion effect. Thus, the POSS/photoacid included copolymers provide valuable guidance to the rational design of DUV and EUV photoresists.
2. Experimental Section/Methods
2.1. Materials
Tert-butyl methacrylate (tBMA) and methyl methacrylate (MMA) were purchased from Shanghai Meryer Reagent Company, and purified by alkaline alumina column to remove the polymerization inhibitor. Sodium p-styrenesulfonate, sodium p-toluenesulfonate, triphenylsulfonium chloride, methacryloyl chloride, Aminopropyllsobutyl POSS, and 2.38% tetramethyl ammonium hydroxide (TMAH) were procured from Shanghai Titan Technology Co. (Shanghai, China) 2,2′-Azobis(2-methylpropionitrile) (AIBN) was used after purification by ethanol recrystallization twice. 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid (CDSPA) was used as a RAFT reagent according to the previous literature [33]. Ethyl lactate, tetrahydrofuran, acetonitrile, dichloromethane, petroleum ether, methanol, and acetone were procured from Shanghai Boer Reagent Co. (Shanghai, China), and deionized water was prepared in the laboratory.
2.2. Synthesis of Photoacid Generators TPS-SS
The monomer TPS-SS was synthesized by the electrostatic interaction between sodium p-styrenesulfonate and triphenylsulfonium chloride [15]. Sodium p-styrenesulfonate (3.093 g, 15 mmol) and triphenylsulfonium chloride (2.988 g, 10 mmol) were dissolved in 50 mL of deionized water. The mixed solution was then transferred to a Schlenk flask and stirred in the dark for 6 h. After the reaction, the mixed solution was extracted with 50 mL of dichloromethane twice. The organic phases were combined and washed with a saturated NaCl solution. The organic phase obtained was then concentrated by rotary evaporation to remove the solvent and was subsequently dried in a vacuum drying oven, resulting in the formation of a white solid, TPS-SS, with a yield of 70%. 1H NMR (400 MHz, CDCl3): δ ppm: 7.79 (d, 2H), 7.75–7.55 (m, 15H), 7.24 (d, 2H), 6.61 (q, 1H), 5.65 (d, 1H), 5.16 (d, 1H).
In addition, the small molecule photoacid triphenylsulfonium p-toluenesulfonate (TPS-ST) was synthesized to be used as a control experiment. The synthesis of TPS-ST followed a similar procedure to that of TPS-SS, except that sodium p-styrenesulfonate was replaced with sodium p-toluenesulfonate. 1H NMR (400 MHz, CDCl3): δ ppm: 7.81–7.62 (m, 17H), 7.08 (d, 2H), 2.30 (s, 3H).
2.3. Synthesis of POSS-MA
Aminopropylisobutyl POSS (4.373 g, 5 mmol) and triethylamine (0.557 g, 5.5 mmol) were dissolved in 50 mL of anhydrous dichloromethane. Simultaneously, methacryloyl chloride (0.575 g, 5.5 mmol) were diluted in 15 mL of anhydrous dichloromethane and transferred into a dropping funnel under atmospheric pressure. A nitrogen atmosphere protection was set up, and it was cooled to 0 °C in an ice-water bath. After the addition, the reaction mixture was stirred at room temperature for 12 h [31,32]. The organic phase was washed three times with saturated sodium bicarbonate solution, and the solvent was removed by rotary evaporation to obtain a crude product (ca. 4.0 g). Subsequently, purification was carried out using a gel chromatography column (PE:DCM = 1:1) with a yield of 80%. 1H NMR (400 MHz, CDCl3): δ ppm: 5.66 (s, 1H), 5.31 (s, 1H), 3.31 (t, 2H), 1.96 (s, 3H), 1.90–1.79 (m, 9H), 0.95 (d, 42H), 0.60 (t, 16H).
2.4. Synthesis of the Copolymer PTBM by RAFT Polymerization
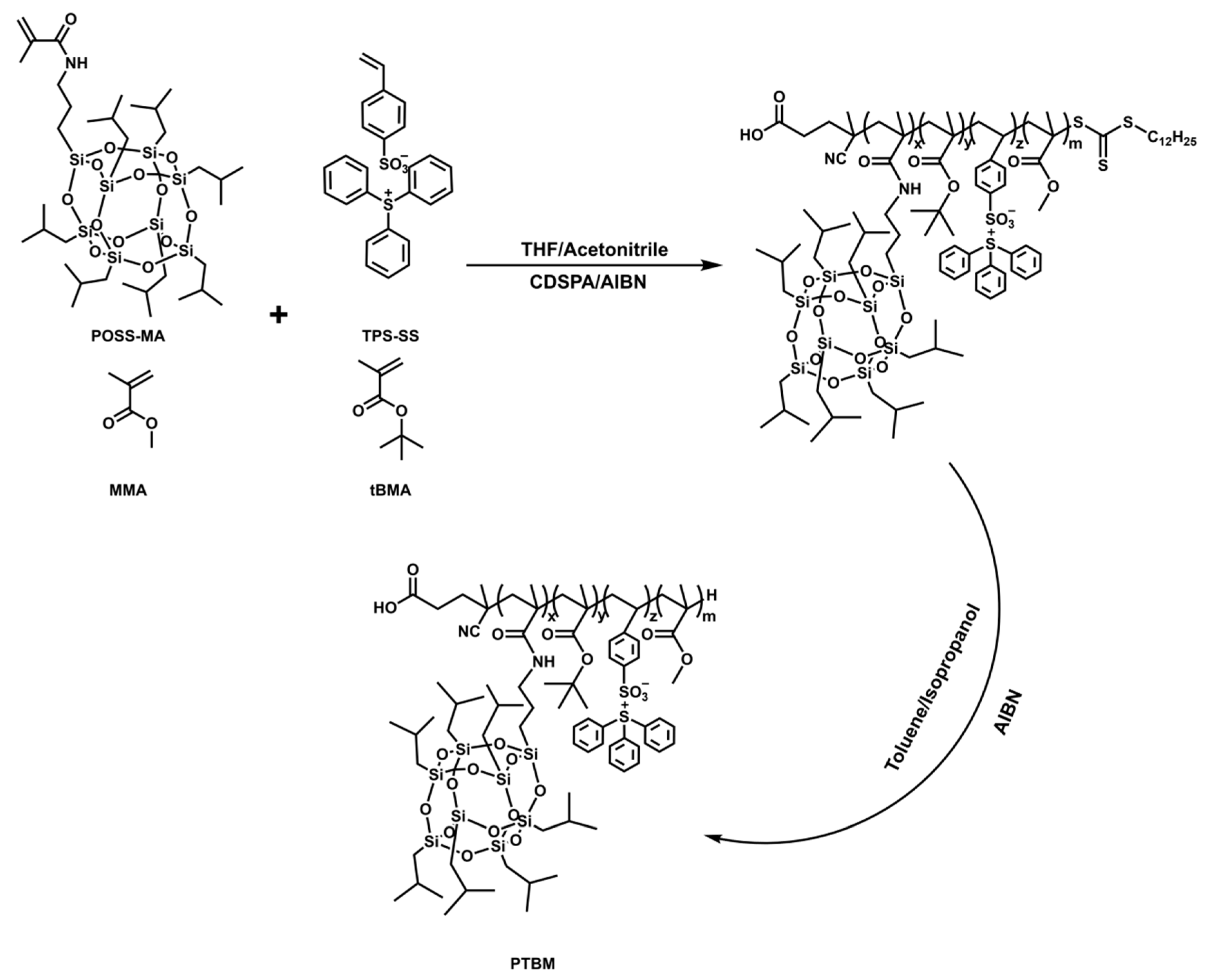
The copolymer PTBM was synthesized by copolymerization of tBMA, MMA, TPS-SS and POSS-MA, using CDSPA as the chain transfer agent and AIBN as the initiator [29]. (Scheme 1). For example, the typical procedure of copolymer PTBM-4 was as following: tBMA (5.68 g, 40 mmol), MMA (2.00 g, 20 mmol), TPS-SS (0.72 g, 1.6 mmol), and POSS-MA (1.51 g, 1.6 mmol) were dissolved in a 10 mL mixed solution of tetrahydrofuran and acetonitrile (1:1), followed by the addition of CDSPA (0.0806 g, 0.2 mmol) and AIBN (0.0164 g, 0.1 mmol). After degassing for 30 min, the polymerization solution was immerged in a 65 °C oil bath. Samples at certain time points were withdrawn using a long needle for GPC and 1H NMR analysis. The copolymers were precipitated with excess methanol. By adjusting the ratio between different monomers, the corresponding polymers were recommended as polymers PTBM-0, PTBM-1, PTBM-2, PTBM-3, and PBM, whose detailed compositions are shown in Table 1. Considering the possible absorption interference of the thiocarbonate groups, the terminal thiocarbonate groups of polymers were removed according to the previous literature (Scheme 1) [34]. The polymers and excess AIBN were redissolved in the mixture solution (toluene:isopropanol = 1:1). After degassing by nitrogen, the reaction was carried out in an oil bath at 85 °C for 2.5 h. The final product was obtained after precipitation by petroleum ether and drying.
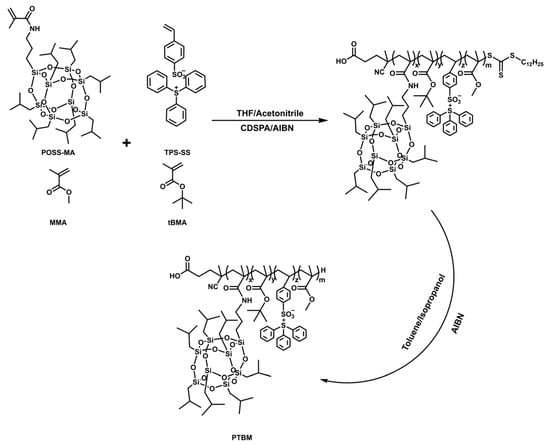
Scheme 1.
Synthetic route of the copolymer PTBM.

Table 1.
RAFT polymerization of PTBM.
2.5. Photoresist Formulation and Exposure Testing
The synthesized copolymers were dissolved in ethyl lactat (10 wt%), and TPS-ST (7 wt% of PBM) were additionally added to PBM. The solution was filtered through a 0.2 μm organic microporous membrane filter to obtain photoresist. The photoresist was spin-coated onto 2 cm × 2 cm quartz plate and 2-inch wafer, respectively, and then prebaked at 100 °C for 1 min. The quartz plate was used to test the FT-IR before and after exposure to a UV point source at 254 nm. After the wafers were exposed through the mask (Lmask = 100 μm), post-bake was performed using the same conditions as pre-bake. After post-baking, the patterns were developed by immerged the wafers in 2.38% TMAH solution and analyzed by SEM.
2.6. Characterization
All 1H NMR spectra were recorded on a Bruker AV400 Spectrophotometer at 400 MHz in CDCl3 with tetramethylsilane (TMS) as an internal reference. FT-IR spectra was acquired on an FTLA2000-104 spectrophotometer in the wavelength range of 4500–500 cm−1. Absorption spectra were recorded on a Shimadzu UV-2550 UV spectrophotometer using a quartz cuvette with 1 cm beam path length. The number average weight (Mn) and polydispersity index (Mw/Mn) were measured on a Waters 1515 gel permeation chromatography (GPC) system using THF as the eluent and the flow rate was 1 mL min−1. GPC data were calibrated to polystyrene standards. Thermal gravimetric analyzer (TGA) (Mettler Toledo of Switzerland) was used to measure the thermal decomposition temperature (Td), samples (5–10 mg) were heated from 25 to 650 °C with a heating rate of 20 °C/min in nitrogen flow. Differential scanning calorimetry (DSC) was performed on a DSC822e (Mettler Toledo, Zurich, Switzerland), the temperature range was from 25 to 200 °C with a heating rate of 10 °C/min in nitrogen flow. Thermomechanical analyzer (TMA) was performed on a TMA/SDTA840 (Mettler Toledo of Switzerland), the temperature range was from 25 to 100 °C with a heating rate of 5 °C/min in nitrogen flow. Scanning electron microscopy (SEM, S-3400, Hitachi, Tokyo, Japan) was performed with an electron voltage of 15 kV.
3. Results and Discussion
3.1. RAFT Polymerization Kinetics
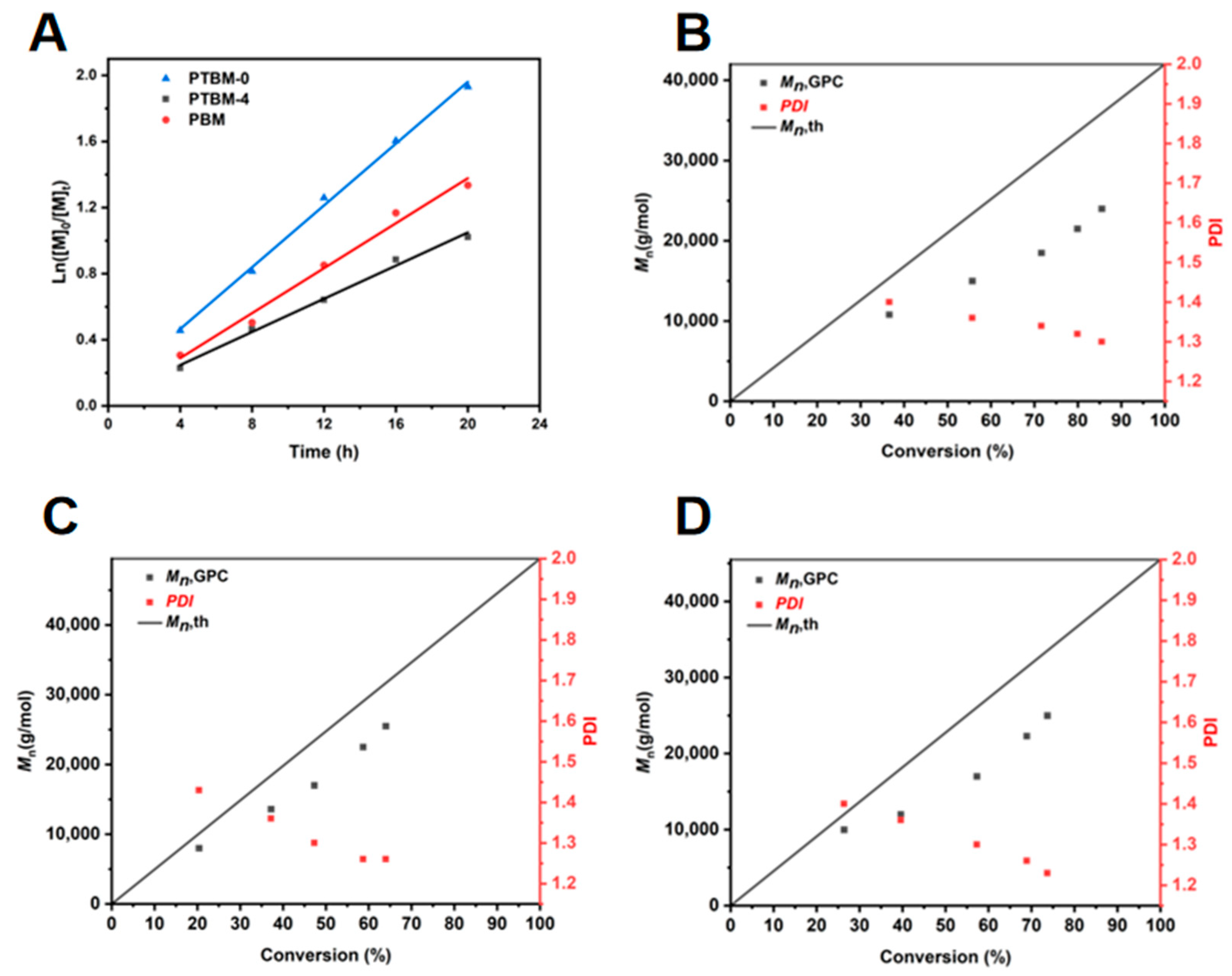
It is well known that the chain transfer agent CDSPA provides excellent control over the RAFT polymerization of methacrylate monomers [35]. To verify the applicability of the new functional monomers, POSS-MA and TPS-SS, a polymerization kinetics study, was conducted. The Ln ([M]0/[M]t) versus time (t) plots for PTBM-0, PTBM-4 and PBM are displayed in Figure 1A. It can be seen that Ln ([M]0/[M]t) exhibited a linear relationship with the polymerization time, and the monomer conversion increased with increasing polymerization time. All plots were in accordance with first-order kinetics. The rate constants (kp) were as follows: kPTBM-0 = 0.094 h−1, kPTBM-4 = 0.050 h−1, and kPBM = 0.068 h−1. A comparison between PTBM-0 and PTBM-4 revealed that the incorporation of POSS-based monomers led to a reduction in the overall polymerization rate. This is presumably because of the bulky spatial hindrance of the POSS unit’s structure, leading to a decreased collision probability among free radicals and a subsequent reduction in the conversion rate between dormant and active chains. A comparison between PTBM-4 and PBM showed that the addition of TPS-SS units also resulted in a decrease in the polymerization rate, possibly due to the suppression of free radical propagation by the ion pairs in TPS-SS units.
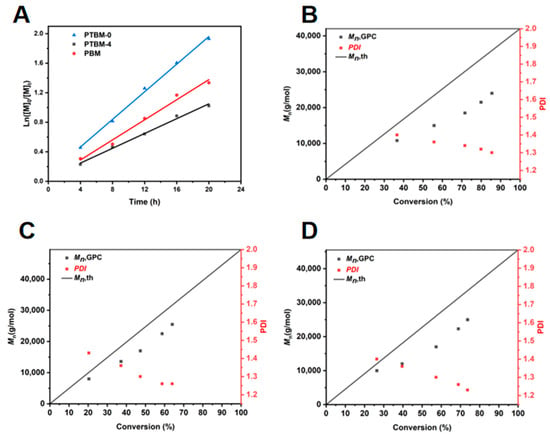
Figure 1.
(A) Polymerization kinetic curves of PTBM-0, PTBM-4 and PBM. Dependence of the molecular weight and polydispersity (Mw/Mn) on the conversion for (B) PTBM-0, (C) PTBM-4, and (D) PBM.
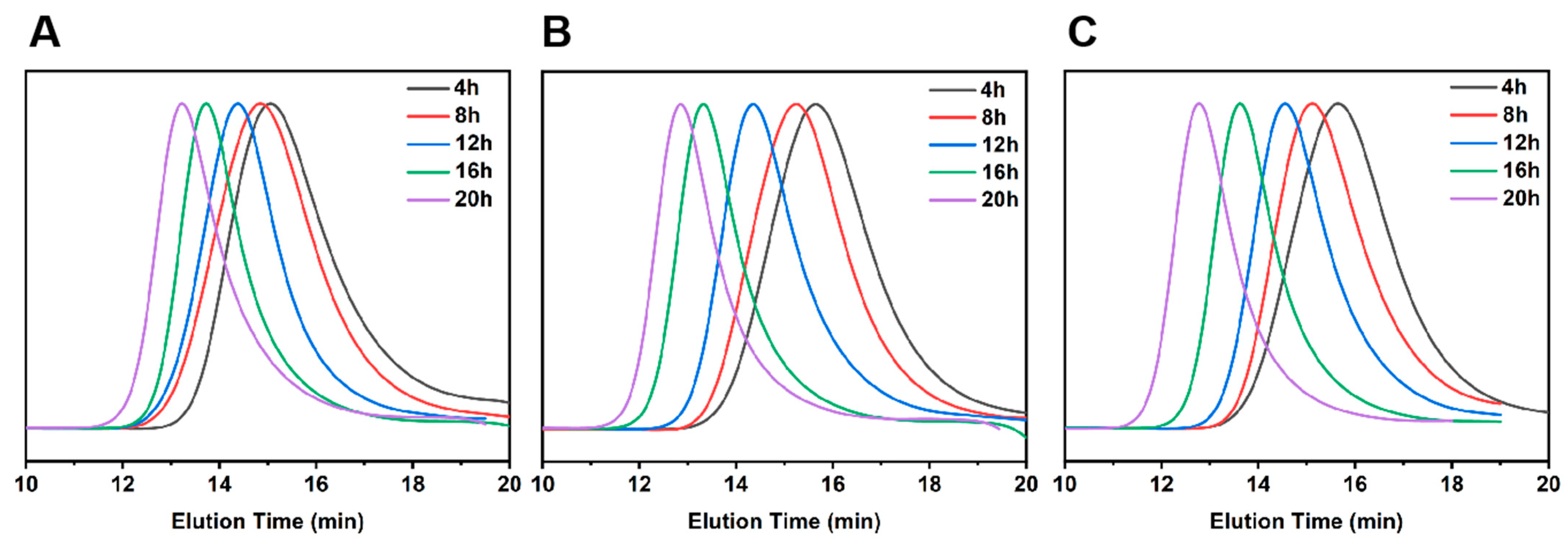
The plots of the Mn and PDI of copolymers PTBM-0, PTBM-4 and PBM with conversions were shown in the Figure 1B,D, respectively. As the polymerization progresses, the Mn of all groups increased linearly with conversions, and the PDI gradually decreased with the increasing conversions, ultimately falling within the range of 1.2–1.3. Meanwhile, the GPC curves (Figure 2A–C) shift to the left in a regular manner while maintaining a uniform distribution of symmetric peaks, indicating that the polymerization process was well controlled. The practical Mn (GPC) value of the copolymers exhibited some negative deviations from the theoretical values, which may be due to chain transfer and termination of polymerization reactions involving active chains with solvents or other molecules [36]. On the other hand, the coupling termination of propagating radicals resulted in the formation of low molecular weight polymers. As shown in Figure 1B,D, it can be observed that PTBM-4 and PBM, which were synthesized from POSS-based monomer and others, possessed Mn (GPC) values closer to the theoretical molecular weight and narrower molecular weight distributions, indicating that the spatial hindrance of the POSS structure can reduce the coupling termination of propagating radicals. The use of polystyrene as a GPC standard calibration sample may also contribute to deviations from theoretical values [37]. Overall, all groups exhibited the characteristics of controlled radical polymerization, demonstrating the feasibility of preparing photolithography resist polymers through controlled polymerization.
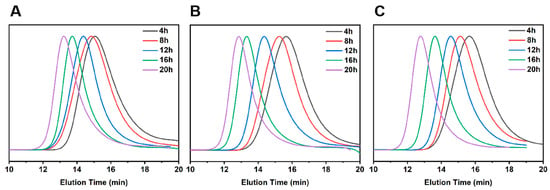
Figure 2.
GPC traces of (A) PTBM-0, (B) PTBM-4, and (C) PBM corresponding to different polymerization time points.
3.2. RAFT Agent Removal
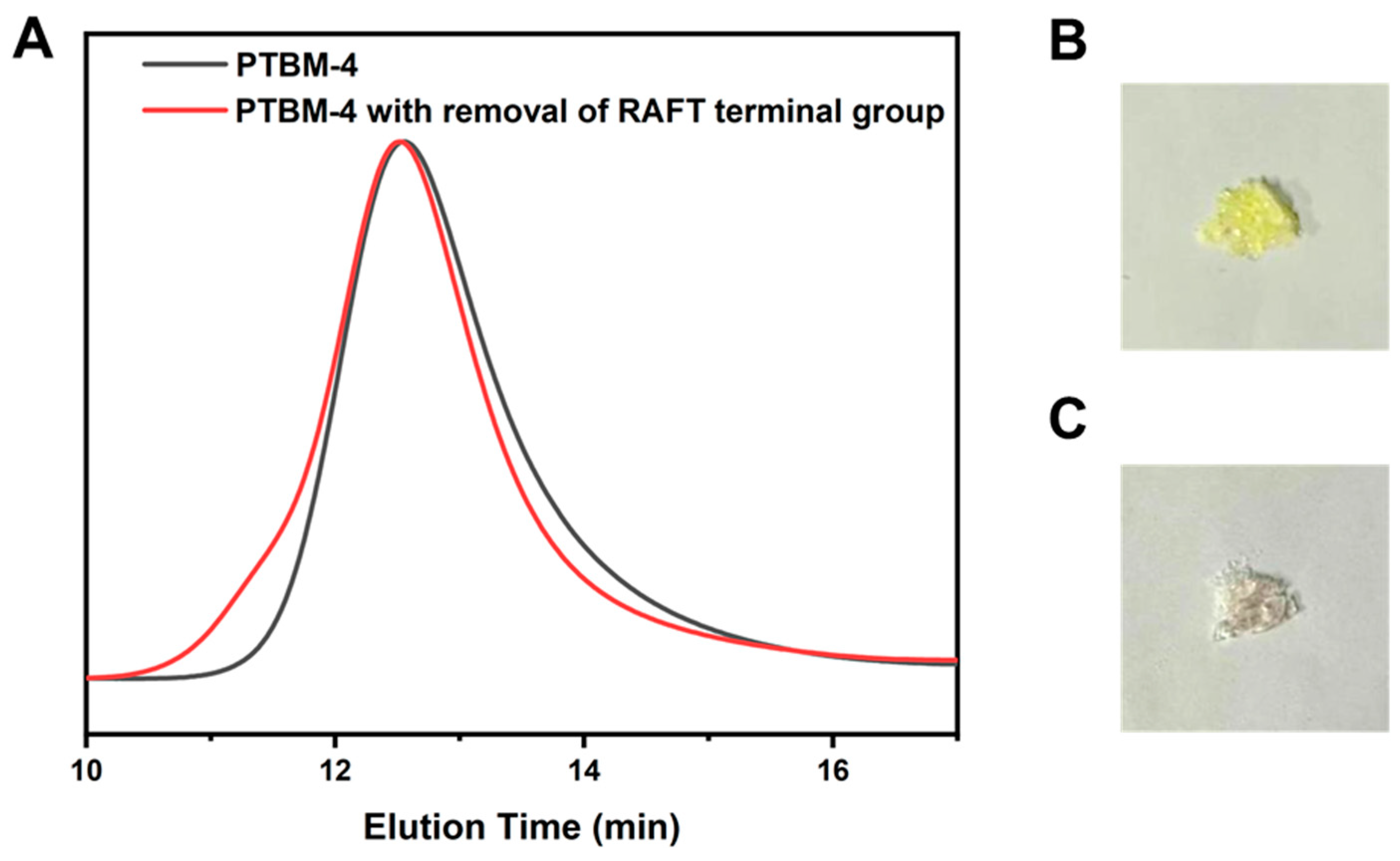
Since the chain transfer agent was inevitably employed in RAFT polymerization to prepare the copolymers, the residual trithiocarbonate terminal groups in the copolymers are present on the copolymer chains after polymerizations. However, the presence of sulfur residue is detrimental to photolithography [38]. On one hand, the trithiocarbonate terminal groups confer color to the polymeric product, which impacts the UV transparency of photoresist. On the other hand, trithiocarbonate terminal groups may thermally decompose during pre-baking and post-baking stages, generating harmful substances that cause irreversible damage to the photolithography equipment. The results of radical-induced-reduction of PTBM-4 in toluene/isopropanol (1:1) as solvent and with AIBN as initiator are illustrated in Figure 3. As shown in Figure 3B,C, the photographs of PTBM-4 before and after the removal of RAFT terminal groups illustrated that the copolymer changed from a pale-yellow color to colorless, indicating the effective removal of trithiocarbonate end groups. In addition, the results of the elemental analyses of PTBM-4 and the product after removal of RAFT terminal group were displayed in Table 2. After the radical-induced-reduction of PTBM-4, the S content significantly decreased compared to that of PTBM-4. According to the calculation of the decreased S content and the average S content of TPS-SS repeating units in PTBM-4, most of the RAFT terminal group was removed, which more clearly verified the success of the removal of the RAFT terminal group. As shown in Figure 3A, it is observed that there is no bimodal molecular weight distribution nor formation of high molecular weight shoulders. It suggested that the toluene/isopropanol mixed solvent was suitable as a hydrogen donor for PTBM-4 propagating radical. Furthermore, a high radical flux also increases the likelihood that termination will involve reactions with a small radical rather than another propagating radical. Even so, there is a small increase in the molecular weight of the polymer, but no significant change in the molecular weight distribution. Polymers with RAFT terminal groups, when exposed to initiators with a high concentration and being heated, the polymers generated propagation radicals (Pn). Theoretically, these propagation radicals can undergo chain transfer to a hydrogen donor (H-X, in this study using toluene/isopropanol (1:1)) with a higher chain transfer constant, forming Pn-H. However, in practice, side reactions are unavoidable. Propagation radicals can undergo disproportionation termination or bimolecular termination to produce Pn-X or Pn-Pn, resulting in a small number of polymers with doubled molecular weight, leading to an increase in the molecular weight [27]. Although bimolecular termination meets the requirement of the RAFT terminal group removal, a more effective and optimized reaction condition needs to be explored for disproportionation termination.
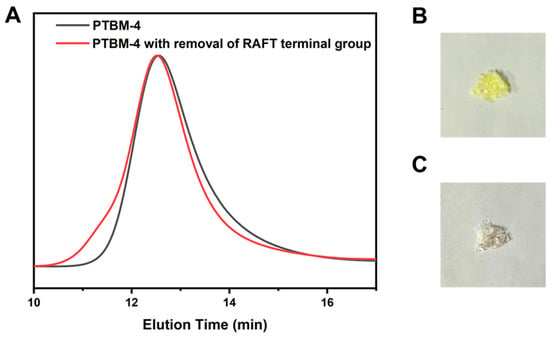
Figure 3.
(A) GPC curves of PTBM-4 and after removal of RAFT terminal group of PTBM-4. (B) Photographs of copolymers with RAFT terminal group, and (C) after removal of RAFT terminal group.

Table 2.
Elemental analyses of PTBM-4 before and after removal of RAFT terminal group.
3.3. 1H NMR, FT-IR and UV-Vis Analysis of Polymers
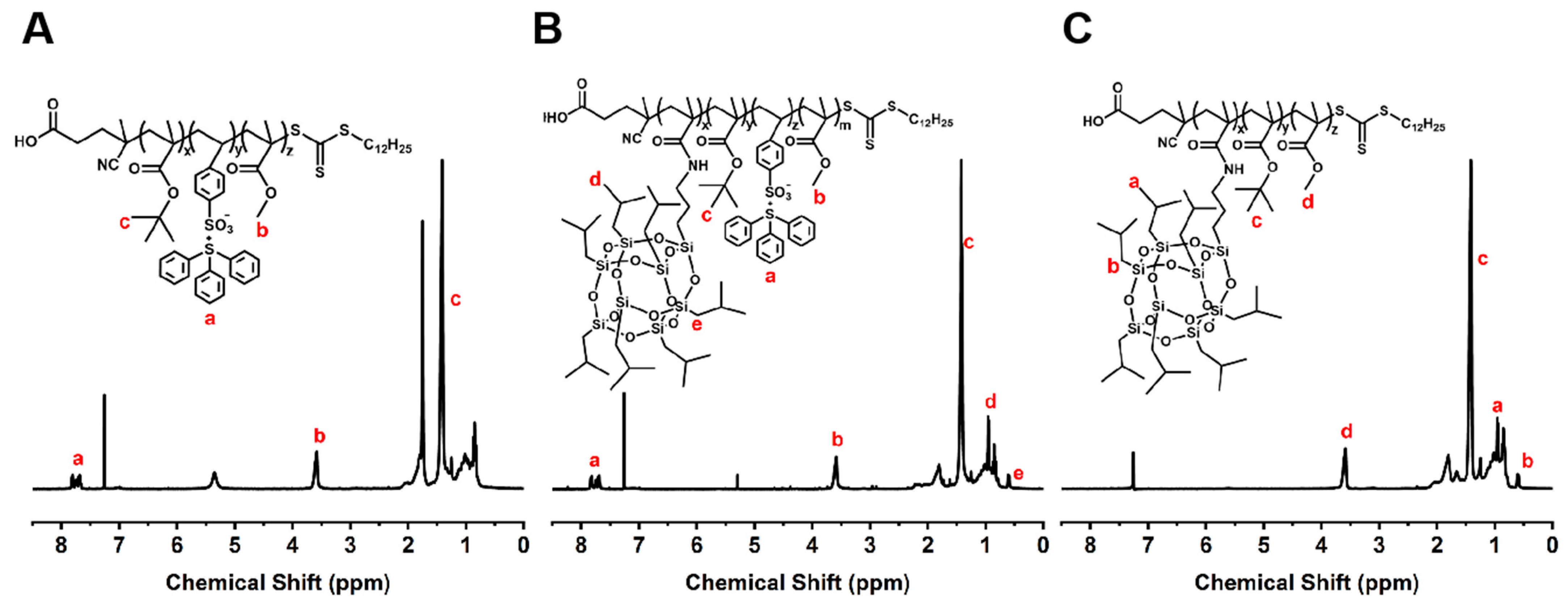
The 1H NMR spectra of PTBM-0, PTBM-4, and PBM are shown in Figure 4. The signal peaks at 7.65–7.87 ppm originated from the hydrogen atoms on the phenyl rings in TPS-SS, the single peak at 3.61 ppm was attributed to the hydrogen of “-O-CH3” units in MMA, the prominent signal peaks at 1.4–1.5 ppm corresponded to the tert-butyl hydrogen in tBMA, and the peaks at 0.96–0.99 ppm and 0.60–0.64 ppm were associated with the hydrogens of isopropyl and “Si-CH2-” units in POSS-MA, respectively. The remaining peaks primarily arose from the hydrogens in the main chains of polymers. This analysis data confirmed the successful synthesis of the polymers. The actual proportions of repeat units in the copolymers calculated by 1H NMR are shown in Table 3.
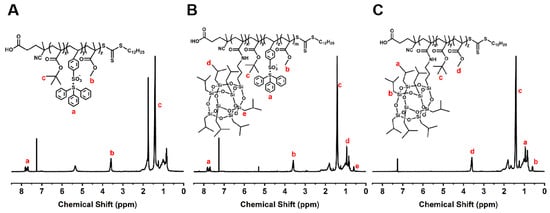
Figure 4.
1H NMR spectra of (A) PTBM-0, (B) PTBM-4, and (C) PBM.

Table 3.
The comparison of feed ratio and practical composition of copolymers.
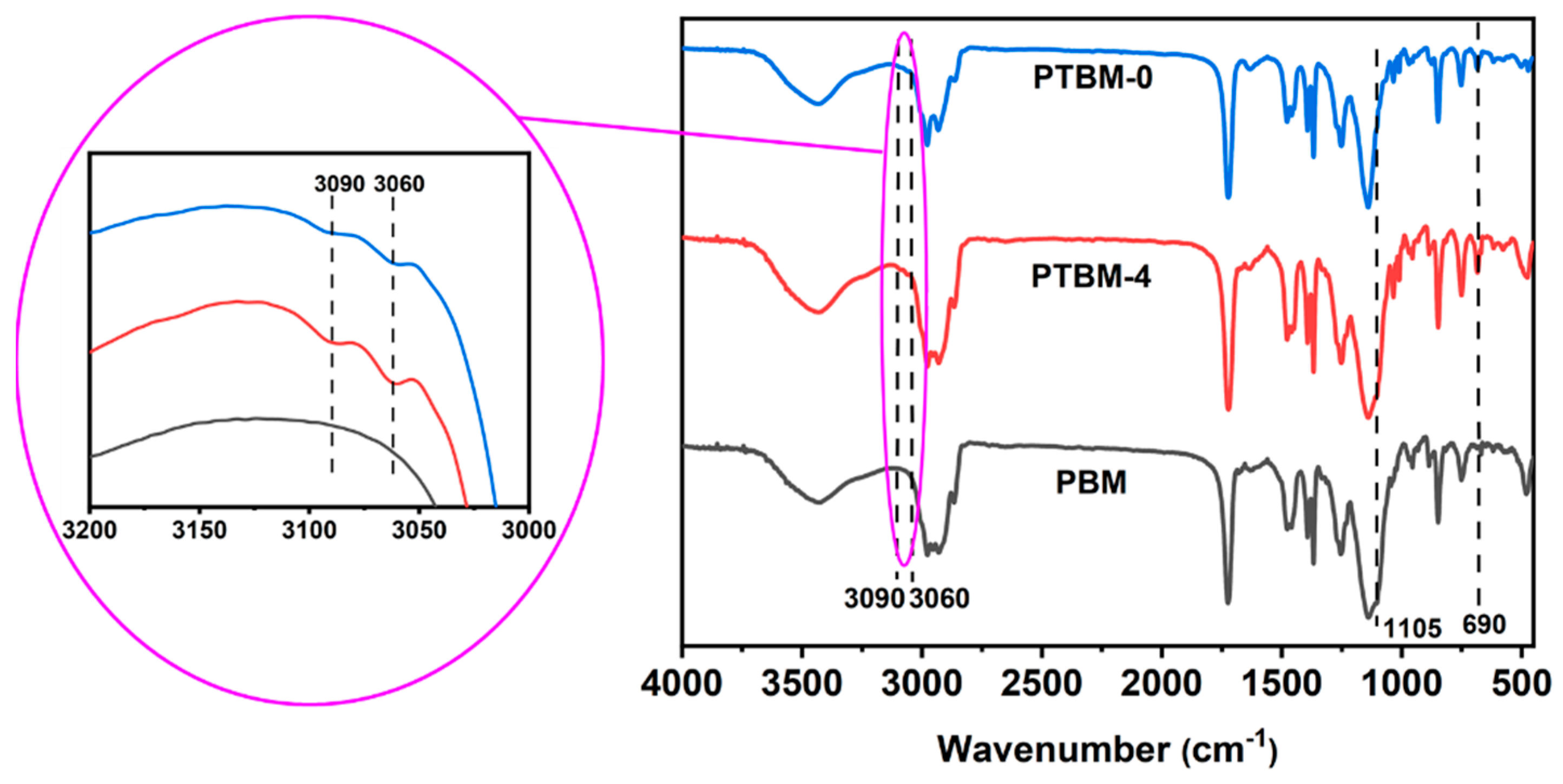
The FT-IR spectra of PTBM-0, PTBM-4, and PBM are shown in Figure 5. The split double peaks near 1380 cm−1 were attributed to the in-plane bending vibration of tert-butyl methyl (CH3) groups. The absorption peak at 1140 cm−1 was attributed to the stretching vibration of the -C-O-C- groups in the tBMA and MMA units, and the peak at 1722 cm−1 was caused by the stretching vibration of the C=O groups. The absorption peaks at 2977 cm−1, 2930 cm−1, and 2865 cm−1 were ascribed to the stretching vibration of C-H in methyl, methylene, and methine groups, respectively. The absorption peak at 1105 cm−1 corresponds to the stretching vibration of the Si-O-Si groups in the POSS-MA unit. As shown in the enlarged image on the left side in Figure 5, The absorption peaks around 3000–3100 cm−1 and near 690 cm−1 were attributed to the stretching vibration and out-of-plane bending vibration of the C-H groups on the phenyl rings of the TPS-SS unit, respectively. The absence of a significant C=C double bond absorption peak in the 1630–1690 cm−1 range indicates that the polymerization was quite completely, with most of the C=C double bonds being eliminated. Thus, it confirmed the successful synthesis of the copolymers.
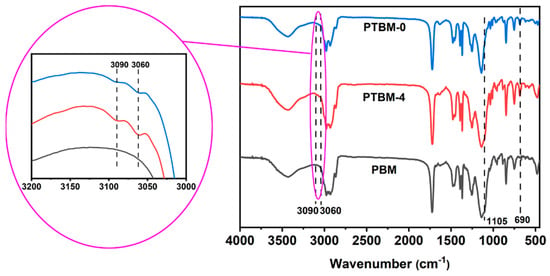
Figure 5.
FT-IR curves of PTBM-0, PTBM-4, and PBM.
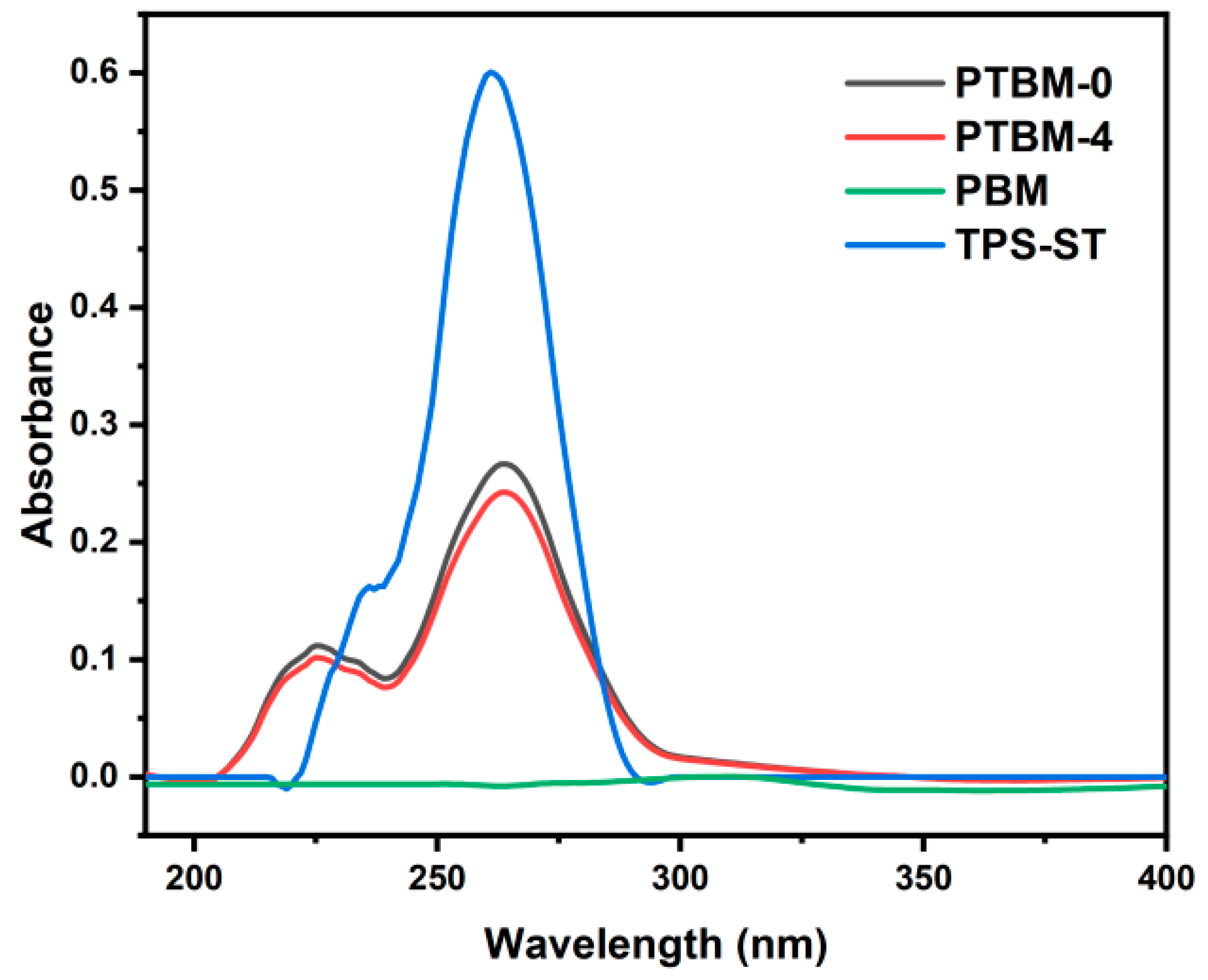
The UV–vis spectra of copolymer solutions and TPS-ST solutions are shown in Figure 6. A comparison between PTBM-0 and PTBM-4 revealed that the addition of POSS units did not change their ultraviolet absorption. PBM, which did not contain the photoacid TPS-SS units, exhibited almost no ultraviolet absorption, while PTBM-0 and PTBM-4, with the addition of TPS-SS units, possessed maximum absorption wavelengths identical to that of TPS-ST. It indicated that the ultraviolet absorption of the polymers was solely provided by the photoacid units, and the other components were entirely transparent in the deep ultraviolet (DUV) range. Therefore, the copolymer PTBM meets the exposure requirements of DUV photoresist.
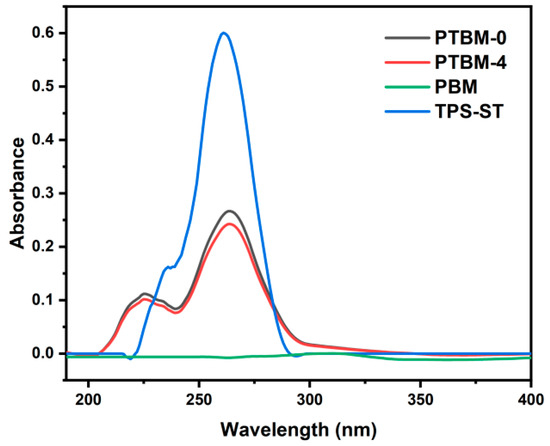
Figure 6.
UV–vis absorption curves of PTBM-0, PTBM-4, PBM, and TPS-ST (dissolved in ethyl lactate, 0.1 mg/mL).
3.4. Thermal Properties of Polymers
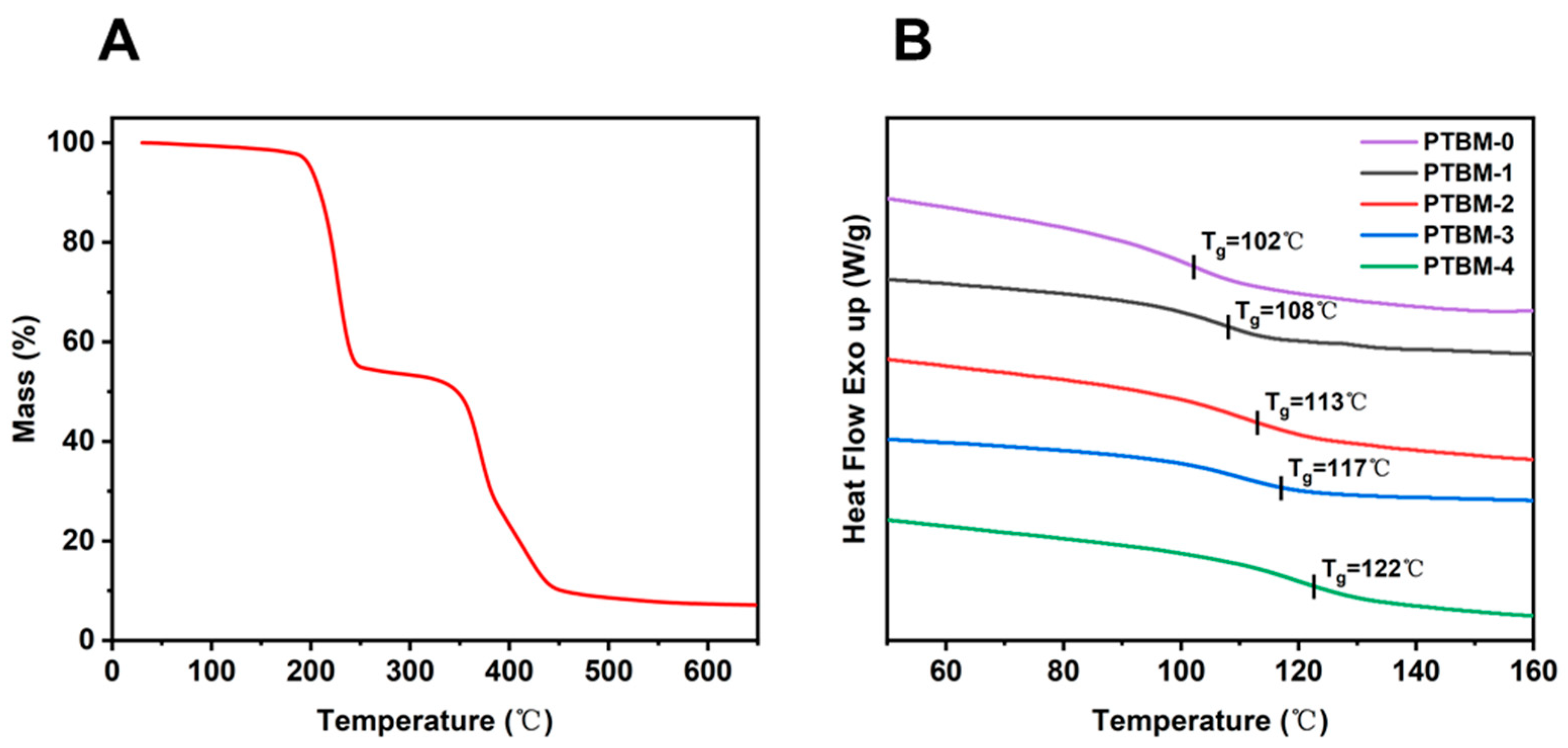
The thermal performance of photoresist polymers plays a critical role in the lithography process. Firstly, the thermal decomposition temperature of the copolymer PTBM is 200.1 °C at 5% heat loss by TGA analysis (Figure 7A), which meets the temperature requirement in the photolithography process. Then the glass transition temperature (Tg) of copolymer PTBM was analyzed by DSC. The DSC curves of PTBM samples with different contents of POSS unit were shown in Figure 7B, revealing the glass transition temperature (Tg) of the samples. It can be observed that with the increase of POSS units, the Tg of the copolymers gradually increased. The Tg values of PTBM-0, PTBM-1, PTBM-2, PTBM-3, PTBM-4 are 102 °C, 108 °C, 113 °C, 117 °C and 122 °C, respectively. It was speculated that the increase of Tg was attributed to the rigid cage-like Si-O-Si structure in the POSS units, as well as the significant steric hindrance of the POSS units, which hindered the mobility of polymer chain segments. Furthermore, the comparison of the thermal stability of PTBM with other photoresist polymers reported indicate the promising potential of PTBM in photolithography. For example, Kim et al. [39] constructed a poly (glycidyl methacrylate)-based copolymer with Tg of 75 °C. Li et al. [3] proposed a methacrylate-based quaternary copolymer with Tg of 105 °C. Li and co-workers [40] constructed an acrylate-based photoresist copolymer, by introducing p-chlorostyrene, with Tg of 101.5 °C. In addition, IBM developed a p-hydroxystyrene-based copolymer with Tg at 85–110 °C [11]. Compared with these copolymers, PTBM-4 possesses a higher Tg of 122 °C, which has the advantage of thermal stability. Chemically amplification photoresists require sufficient temperature (80–120 °C) during the PEB stage to maintain the energy required for the deprotection reaction, so photoresist resins with high Tg can maintain thermal stability during PEB to avoid pattern collapse.
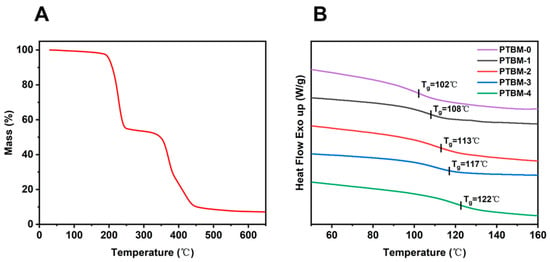
Figure 7.
(A) TGA curve of PTBM-4, (B) DSC curves of PTBM-0, PTBM-1, PTBM-2, PTBM-3, and PTBM-4.
The thermal expansion coefficient α of the copolymer was measured using static thermal mechanical analysis (TMA). The calculation method is as shown in Equation (1):
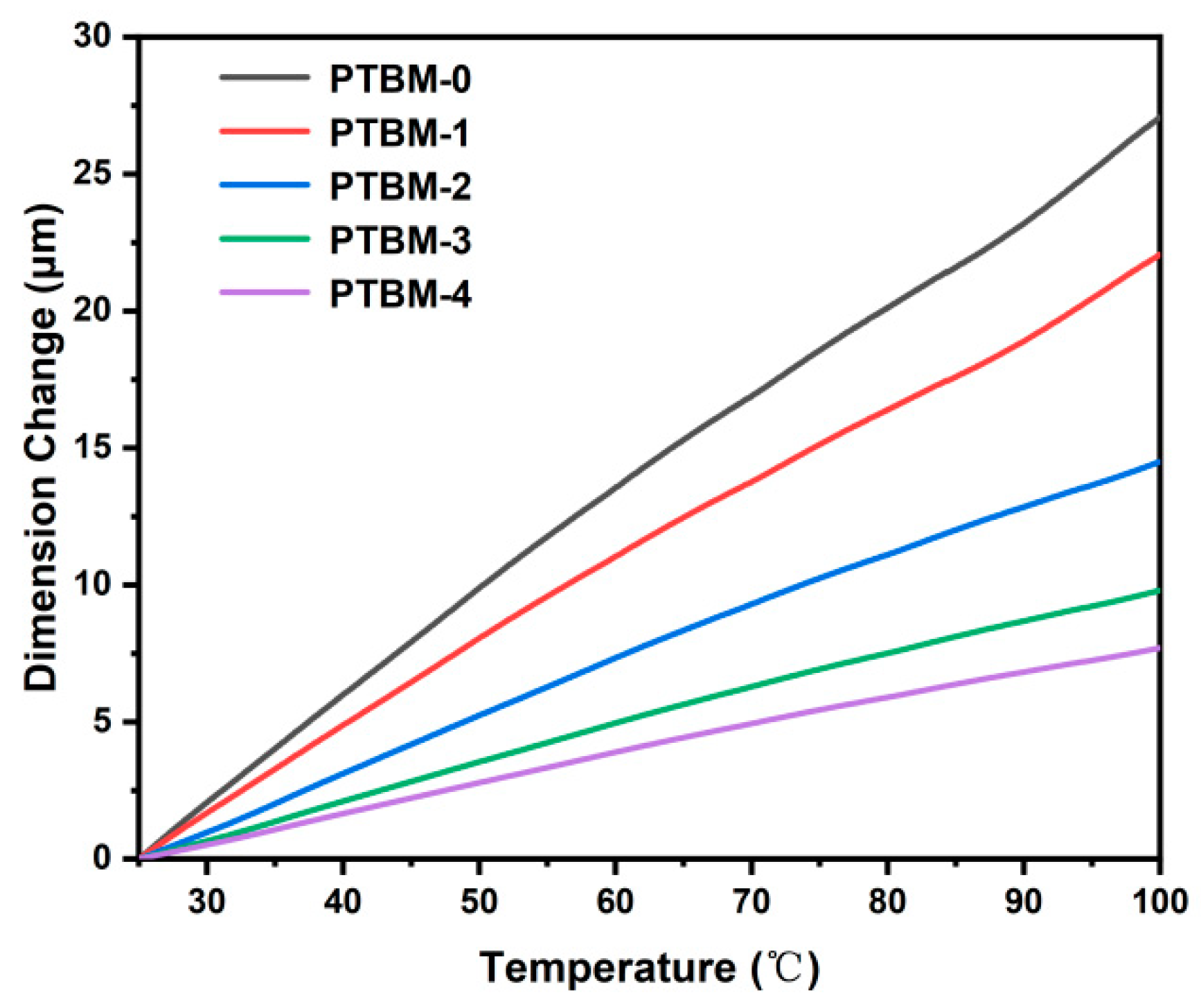
where, ΔL represents the deformation, L is the original size, and ΔT is the temperature change. The calculation yielded thermal expansion coefficients α0 to α4 for the copolymers PTBM-0~PTBM-4 as 1.56 × 10−4, 1.26 × 10−4, 9.17 × 10−5, 5.96 × 10−5, and 4.67 × 10−5 (°C), respectively. The results indicated that with an increase in the content of POSS units, the thermal expansion coefficient of the copolymers decreased (Figure 8). A low thermal expansion coefficient is advantageous for maintaining the original morphology of photolithography patterns during pre-baking and post-baking, ultimately improving the resolution of photoresist. The thermal expansion coefficient of methyl methacrylate-based copolymers commonly used as photoresist resins [41] is typically between 8 × 10−5 and 12 × 10−5 °C−1. However, Ito [11] and co-workers pointed out that the PEB temperature stability is desired to be <5 × 10−5 °C−1. By introducing POSS in the photoresist matrix, the expansion coefficient of the methyl methacrylate-based copolymer was reduced to meet the process requirements.
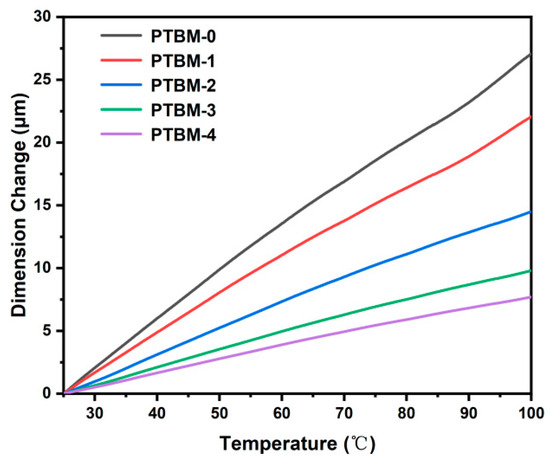
Figure 8.
TMA curves of PTBM-0, PTBM-1, PTBM-2, PTBM-3, and PTBM-4.
3.5. Exposure Testing of Photoresist Copolymer
3.5.1. FT-IR Analysis before and after Exposure
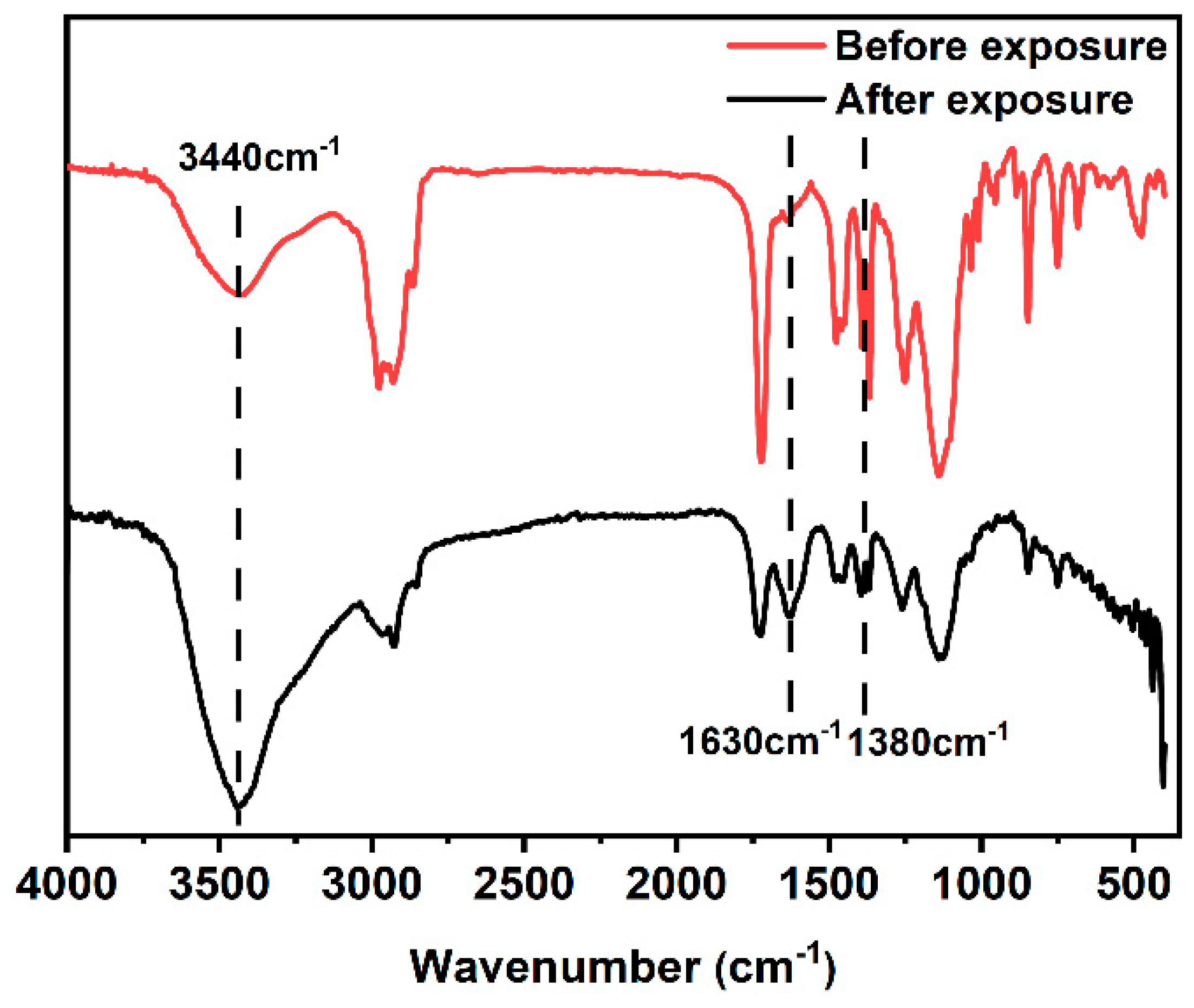
The FT-IR spectra of PTBM-4 before and after exposure were explored and shown in Figure 9. It can be seen that after light exposure, there was a significant increase in the absorption peak near 3440 cm−1, while the split double peak at 1380 cm−1 significantly decreased. This was mainly attributed to the hydrolysis of tert-butyl groups in the tBMA units by H+ generated from the photoacid TPS-SS units, which led to the deprotection of the tert-butyl units and the formation of carboxyl groups. The presence of -OH in the carboxyl groups resulted in an increased absorption peak at 3440 cm−1. A new absorption peak appeared at 1630 cm−1, which was attributed to the stretching vibration of C=O in the formed carboxyl groups. This analysis confirmed the successful acid-catalyzed deprotection reaction of the copolymer and its feasibility as a photoresist.
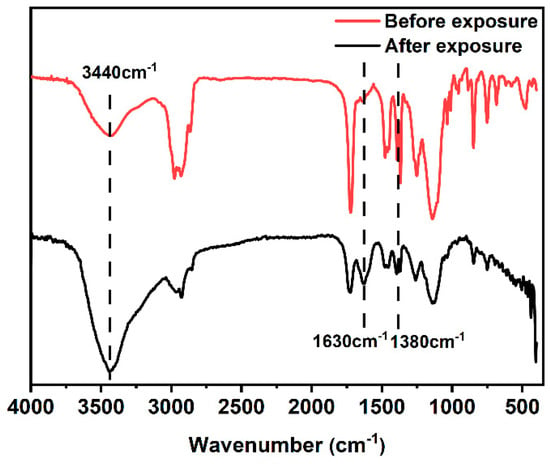
Figure 9.
FT-IR of PTBM-4 before and after exposure.
3.5.2. Lithographic Evaluation
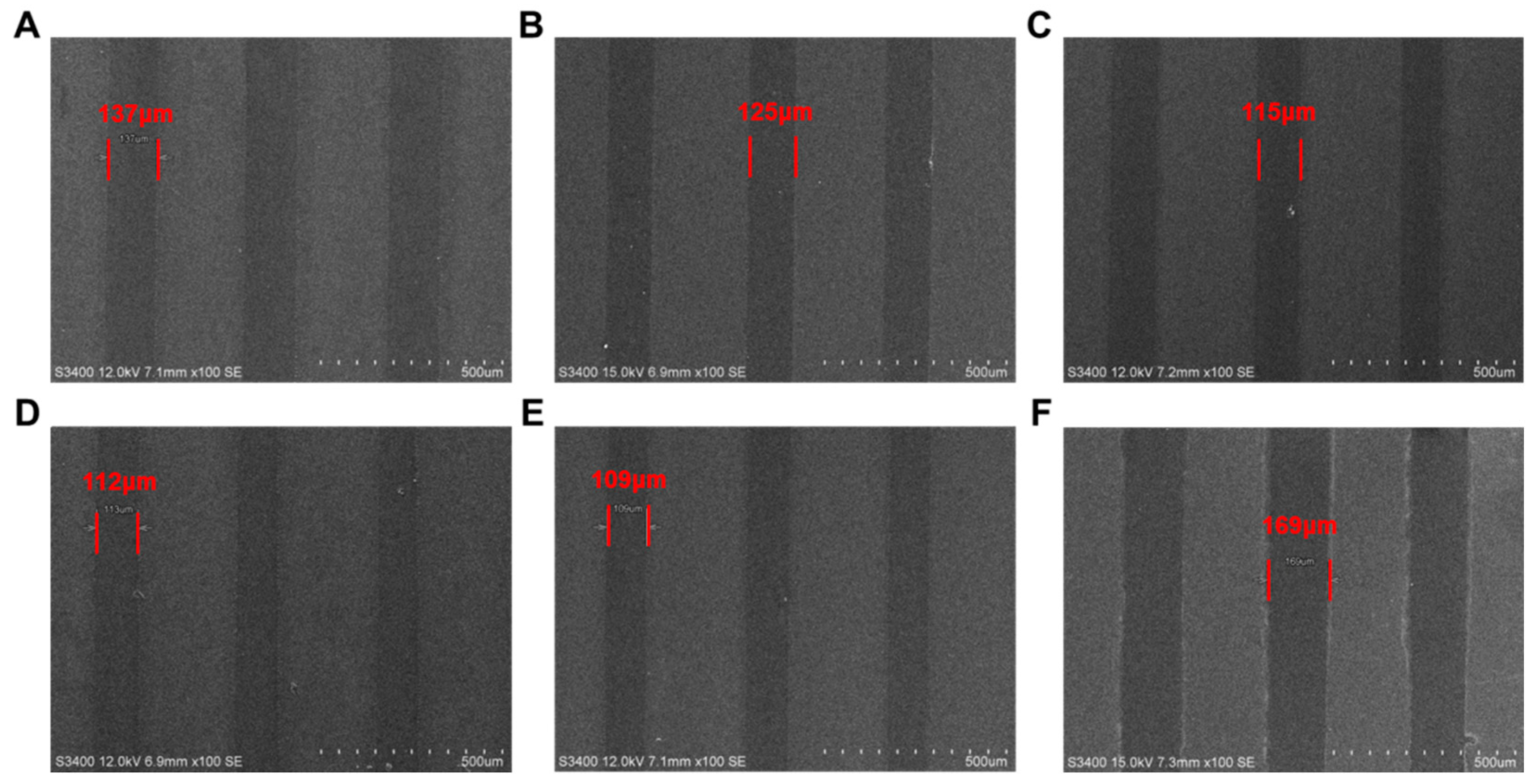
After a series of processing steps including pre-baking (100 °C, 1 min), mask exposure (the exposure light source is a 248 nm UV lamp and the exposure dose is 20 mJ/cm2 and the line width of the exposed portions on the mask is 100 µm), post-baking (100 °C, 1 min), and development (2.38% TMAH, 1 min), the photolithographic patterns of photoresists PTBMs and PBM were analyzed by SEM. The acid diffusion size (S), defined as the difference between the actual line width and the theoretical line width, was calculated. As depicted in Figure 10, the values of SPTBM-0, SPTBM-1, SPTBM-2, SPTBM-3, SPTBM-4, and SPBM were 37 μm, 25 μm, 15 μm, 12 μm, 9 μm, and 69 μm, respectively. The acid diffusion ratio (s), which represents the ratio of S to the mask line width (Lmask), the values of sPTBM-0, sPTBM-1, sPTBM-2, sPTBM-3, sPTBM-4, and sPBM were 37%, 25%, 15%, 12%, 9%, and 69%, respectively.
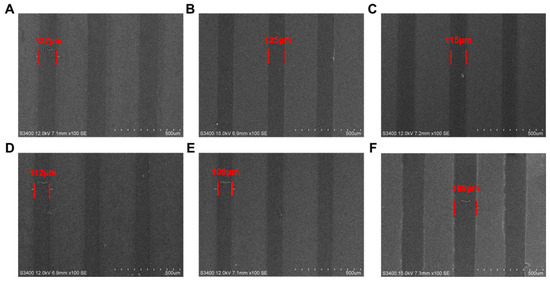
Figure 10.
SEM images of photolithographic patterns of (A) PTBM-0, (B) PTBM-1, (C) PTBM-2, (D) PTBM-3, (E) PTBM-4, and (F) PBM.
Compared the SEM images of patterns of PTBM-4 and PBM (Figure 10E,F), it can be seen that the pattern of PTBM-4 exhibited a lower acid diffusion ratio because of the covalent bonding of photoacid units onto the copolymer backbone, which effectively reduced the diffusion effect of the acid and reduced the line width roughness. From Figure 10A–E, it can be seen that the addition of POSS units not only enhanced the thermal performance of the copolymer but also, due to the steric hindrance effect of the POSS structure, restricted the irregular movement of H+, thereby reducing acid diffusion to a certain extent. It can be observed that there is a significant decrease in the acid diffusion size with the gradual increase of POSS content from PTBM-0 to PTBM-1 and from PTBM-1 to PTBM-2. However, the decrease in acid diffusion size is limited when continuing to increase the POSS content from PTBM-2 to PTBM-3 and PTBM-4, indicating that the impact of POSS on acid diffusion has reached an upper threshold limit.
4. Conclusions
We prepared a series of POSS and photoacid-based copolymers by RAFT polymerization for the application of DUV photoresists. The polymerization process showed typical controlled radical polymerization behavior, allowing the preparation of photoresist resins with PDI < 1.3. The incorporation of POSS units in the polymer backbone enhanced the thermal properties of the photoresist resin with the high Tg and the low coefficient of thermal expansion α. In addition, in contrast to the conventional physical doping, the covalent bonding of photoacid units onto the copolymer effectively reduced the acid diffusion, leading to the low acid diffusion ratio. The reduced acid diffusion ratio of the photoresist matrix can enhance the resolution and processing precision of photoresists, while also optimizing the control of exposure and development processes, ensuring the stability and consistency of the lithography process, particularly increasing the production qualification rate of DUV photoresist.
Author Contributions
Conceptualization, H.Y. and J.T.; Methodology, H.Y. and H.F.; Software, H.Y. and Z.C.; Validation, H.Y., S.L. and Z.C.; Formal analysis, H.Y.; Investigation, H.Y. and H.F.; Resources, J.T.; Data curation, H.Y.; Writing—original draft, H.Y.; Writing—review & editing, H.Y. and J.T.; Visualization, H.Y.; Supervision, J.T.; Project administration, L.Z. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
There are no conflicts of interest to report.
References
- Chang, S.; Yang, J.-H.; Chien, J.-H.; Lee, Y.-D. Synthesis of a novel alkaline-developable photosensitive copolymer based on MMA, MAA, SM, and 2-HEMA-grafted GMA copolymer for an innovative photo-imageable dry-peelable temporary protective plastisol. J. Polym. Res. 2013, 20, 115. [Google Scholar] [CrossRef]
- Singh, V.; Satyanarayana, V.S.V.; Sharma, S.K.; Ghosh, S.; Gonsalves, K.E. Towards novel non-chemically amplified (n-CARS) negative resists for electron beam lithography applications. J. Mater. Chem. C 2014, 2, 2118–2122. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Zheng, X.; Ji, C.; Mu, Q.; Liu, R.; Liu, X. Synthesis of chemically amplified photoresist polymer containing four (Meth)acrylate monomers via RAFT polymerization and its application for KrF lithography. J. Polym. Res. 2016, 23, 102. [Google Scholar] [CrossRef]
- Lee, C.-K.; Hwang, F.-H.; Chen, C.-C.; Chang, C.-L.; Cheng, L.-P. Preparation and characterization of nanosilica-filled color resist. Adv. Polym. Technol. 2012, 31, 163–171. [Google Scholar] [CrossRef]
- Accoto, C.; Qualtieri, A.; Pisanello, F.; Ricciardi, C.; Pirri, C.F.; Vittorio, M.D.; Rizzi, F. Two-Photon Polymerization Lithography and Laser Doppler Vibrometry of a SU-8-Based Suspended Microchannel Resonator. J. Microelectromech. Syst. 2015, 24, 1038–1042. [Google Scholar] [CrossRef]
- Ito, H. Chemical amplification resists: History and development within IBM. IBM J. Res. Dev. 2000, 44, 119–130. [Google Scholar] [CrossRef]
- Ito, H.; Willson, C.G. Chemical amplification in the design of dry developing resist materials. Polym. Eng. Sci. 1983, 23, 1012–1018. [Google Scholar] [CrossRef]
- Ito, H. Chemical amplification resists: Inception, implementation in device manufacture, and new developments. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3863–3870. [Google Scholar] [CrossRef]
- Itani, T.; Hashimoto, S.; Yamana, M.; Samoto, N.; Kasama, K. A study of dissolution characteristics and acid diffusion in chemically amplified DUV resist. Microelectron. Eng. 1998, 41, 363–366. [Google Scholar] [CrossRef]
- Reichmanis, E.; Houlihan, F.M.; Nalamasu, O.; Neenan, T.X. Chemical amplification mechanisms for microlithography. Chem. Mater. 1991, 3, 394–407. [Google Scholar] [CrossRef]
- Ito, H. Chemical Amplification Resists for Microlithography. In Microlithography Molecular Imprinting; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–245. [Google Scholar]
- Przybilla, K.J.; Roeschert, H.; Spiess, W.; Eckes, C.; Chatterjee, S.; Khanna, D.N.; Pawlowski, G.; Dammel, R.R. Progress in DUV resins. Proc. SPIE 1991, 1466, 174–187. [Google Scholar]
- Prabhu, V.M.; Kang, S.; VanderHart, D.L.; Satija, S.K.; Lin, E.K.; Wu, W.-L. Photoresist Latent and Developer Images as Probed by Neutron Reflectivity Methods. Adv. Mater. 2011, 23, 388–408. [Google Scholar] [CrossRef]
- Guo, Y.; Hill, D.J.T.; Whittaker, A.K.; Jack, K.S.; Peng, H. Terpolymerization of Styrenic Photoresist Polymers: Effect of RAFT Polymerization on the Compositional Heterogeneity. Macromolecules 2015, 48, 3438–3448. [Google Scholar] [CrossRef]
- Poliakov, P.; Blomme, P.; Pret, A.V.; Corbalan, M.M.; Gronheid, R.; Verkest, D.; Houdt, J.V.; Dehaene, W. Trades-off between lithography line edge roughness and error-correcting codes requirements for NAND Flash memories. Microelectron. Reliab. 2012, 52, 525–529. [Google Scholar] [CrossRef]
- Gogolides, E.; Constantoudis, V.; Patsis, G.P.; Tserepi, A. A review of line edge roughness and surface nanotexture resulting from patterning processes. Microelectron. Eng. 2006, 83, 1067–1072. [Google Scholar] [CrossRef]
- Patsis, G.P.; Gogolides, E. Effects of model polymer chain architectures of photo-resists on line-edge-roughness: Monte Carlo simulations. J. Phys. Conf. Ser. 2005, 10, 389. [Google Scholar] [CrossRef]
- Kang, S.; Vogt, B.D.; Wu, W.-l.; Prabhu, V.M.; VanderHart, D.L.; Rao, A.; Lin, E.K.; Turnquest, K. Characterization of Compositional Heterogeneity in Chemically Amplified Photoresist Polymer Thin Films with Infrared Spectroscopy. Macromolecules 2007, 40, 1497–1503. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Cha, S.-H.; Lee, W.-K.; Kim, D.-G.; Yun, H.-J.; Kim, M.-S.; Kim, B.-D.; Kim, Y.-H.; Lee, J.-W.; Kim, J.-S.; et al. Synthesis of ArF photoresist polymer composed of three methacrylate monomers via reversible addition-fragmentation chain transfer (RAFT) polymerization. Macromol. Res. 2011, 19, 722–728. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Tazaki, T. A model for living radical polymerization. Die Makromol. Chem. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M. Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters. Die Makromol. Chem. Rapid Commun. 1982, 3, 127–132. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris- (triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wieberger, F.; Forman, D.C.; Neuber, C.; Gröschel, A.H.; Böhm, M.; Müller, A.H.E.; Schmidt, H.-W.; Ober, C.K. Tailored star-shaped statistical teroligomers viaATRP for lithographic applications. J. Mater. Chem. 2012, 22, 73–79. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Wang, C.; Qu, R. Atom transfer radical polymerization using activators regenerated by electron transfer of acrylonitrile in 1-(1-ethoxycarbonylethyl)-3-methylimidazolium hexafluorophospate. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1046–1049. [Google Scholar] [CrossRef]
- Fukukawa, K.-i.; Zhu, L.; Gopalan, P.; Ueda, M.; Yang, S. Synthesis and Characterization of Silicon-Containing Block Copolymers from Nitroxide-Mediated Living Free Radical Polymerization. Macromolecules 2005, 38, 263–270. [Google Scholar] [CrossRef]
- Lai, J.T.; Filla, D.; Shea, R. Functional Polymers from Novel Carboxyl-Terminated Trithiocarbonates as Highly Efficient RAFT Agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Chong, Y.K.; Moad, G.; Rizzardo, E.; Skidmore, M.A.; Thang, S.H. Reversible Addition Fragmentation Chain Transfer Polymerization of Methyl Methacrylate in the Presence of Lewis Acids: An Approach to Stereocontrolled Living Radical Polymerization. Macromolecules 2007, 40, 9262–9271. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X. Reversible addition–fragmentation transfer (RAFT) copolymerization of methyl methacrylate and styrene in miniemulsion. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6248–6258. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, C.; Zeng, Q.; Liu, J.; Liu, R.; Mu, Q.; Liu, X. Synthesis of novel copolymer based on precipitation polymerization and its application in positive-tone photoresist. J. Polym. Res. 2017, 24, 198. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Guo, Y.-D.; Yang, Y.-B.; Yu, Q.; Liu, J.-G.; Wu, B.-H.; Lv, F.-Z. Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers. Nanomaterials 2021, 11, 141. [Google Scholar] [CrossRef]
- Lakshmipriya, S.; Anil Kumar, S.; Nandakumar, K.; Thomas, S. Influence of POSS fillers on the transport properties of natural rubber nanocomposites. Polym. Compos. 2019, 40, 3020–3031. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ye, Y.; Peng, H.; Zhou, X.; Xie, X.; Wang, X.; Wang, F. A One-Step Route to CO2-Based Block Copolymers by Simultaneous ROCOP of CO2/Epoxides and RAFT Polymerization of Vinyl Monomers. Angew. Chem. Int. Ed. 2018, 57, 3593–3597. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio End Group Removal from RAFT-Synthesized Polymers by Radical-Induced Reduction. Macromolecules 2007, 40, 4446–4455. [Google Scholar] [CrossRef]
- Nothling, M.D.; Fu, Q.; Reyhani, A.; Allison-Logan, S.; Jung, K.; Zhu, J.; Kamigaito, M.; Boyer, C.; Qiao, G.G. Progress and Perspectives Beyond Traditional RAFT Polymerization. Adv. Sci. 2020, 7, 2001656. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Babu, R.P.; Dhamodharan, R. Synthesis of block and graft copolymers of styrene by raft polymerization, using dodecyl-based trithiocarbonates as initiators and chain transfer agents. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1066–1078. [Google Scholar] [CrossRef]
- Marić, M.; Seok, J.; Métafiot, A.; Wylie, K. Nitroxide-mediated polymerization of adamantyl-functional methacrylates for 193 nm photoresists. Can. J. Chem. Eng. 2017, 95, 708–716. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wylie, K.; Marić, M. Synthesis of Narrow Molecular Weight Distribution Copolymers for ArF Photoresist Materials by Nitroxide Mediated Polymerization. Macromol. React. Eng. 2017, 11, 1600029. [Google Scholar] [CrossRef]
- Kim, K.; Yu, S.; Kim, S.-W.; Kim, T.; Kim, S.-M.; Kang, S.-Y.; Han, S.M.; Jang, J.-H. Highly transparent poly(glycidyl methacrylate-co-acryloisobutyl POSS) for 100 μm-thick submicron patterns with an aspect ratio over 100. Chem. Commun. 2017, 53, 8172–8175. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Zhao, J.; Xue, H. Synthesis of a PCST-containing acrylic polymers and its application in negative-tone photoresist. Mater. Lett. 2023, 345, 134426. [Google Scholar] [CrossRef]
- Miller, R.L.; Nielsen, L.E. Crystallographic data for various polymers. II. J. Polym. Sci. 1961, 55, 643–656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).