Approaching Breakthrough: Resource-Efficient Micropollutant Removal with MBR-GAC Configuration
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Syvab’s Wastewater Treatment Plant (WWTP) Himmerfjärdsverket
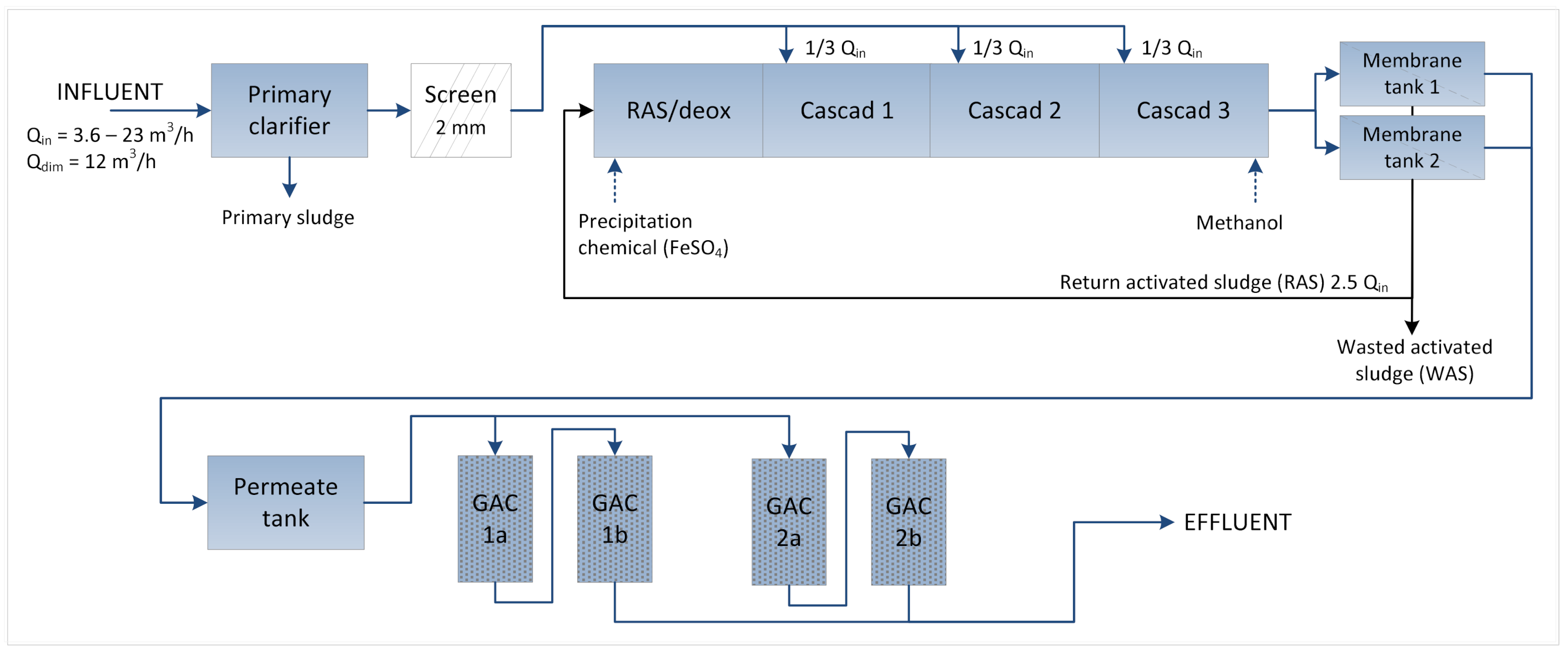
2.2. Large-Scale Membrane BioReactor and Activated Carbon Filtration Pilot (MBR-GAC)
2.3. Sampling and Analysis
3. Results
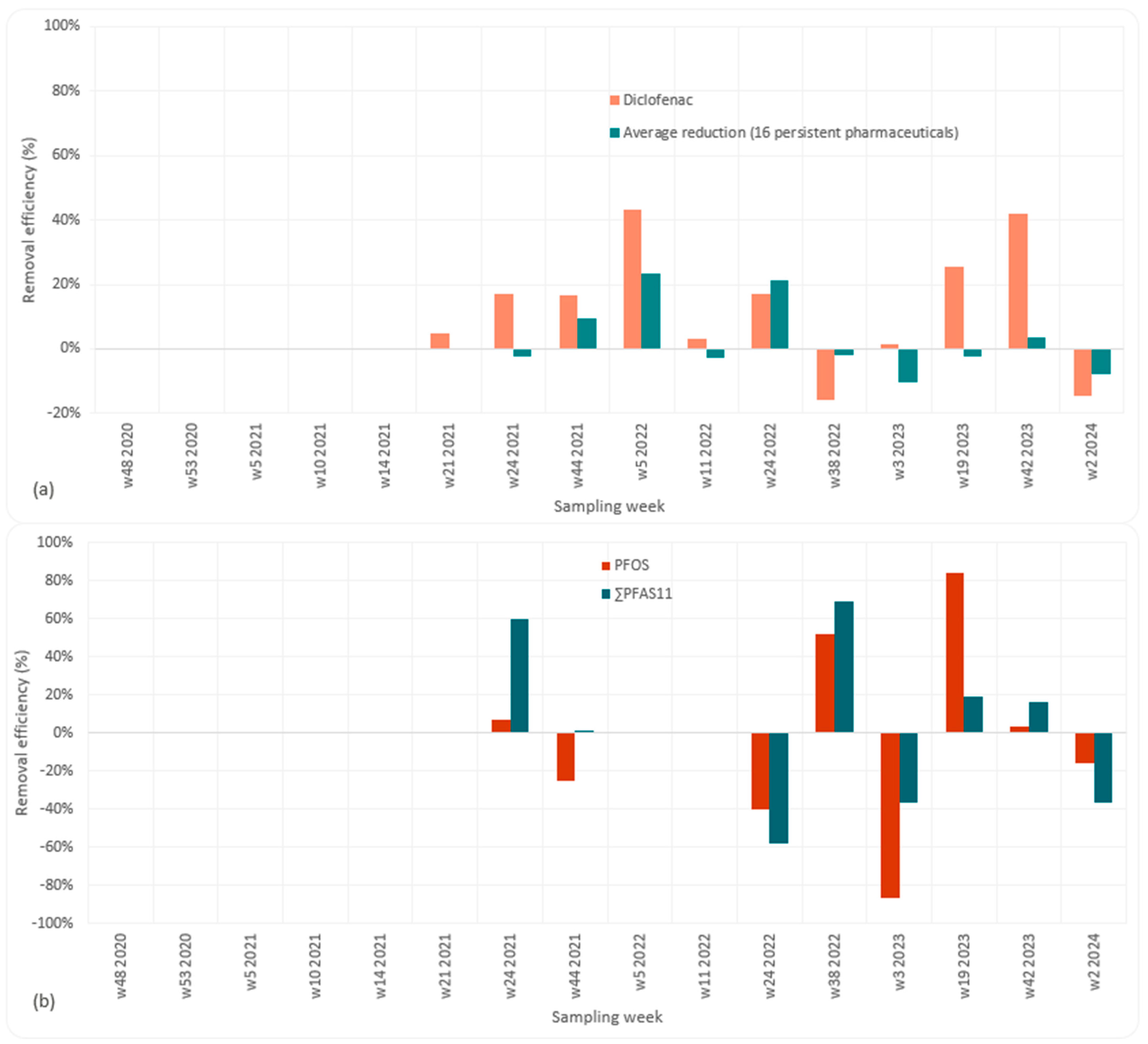
3.1. Removal Efficiency for Pharmaceuticals
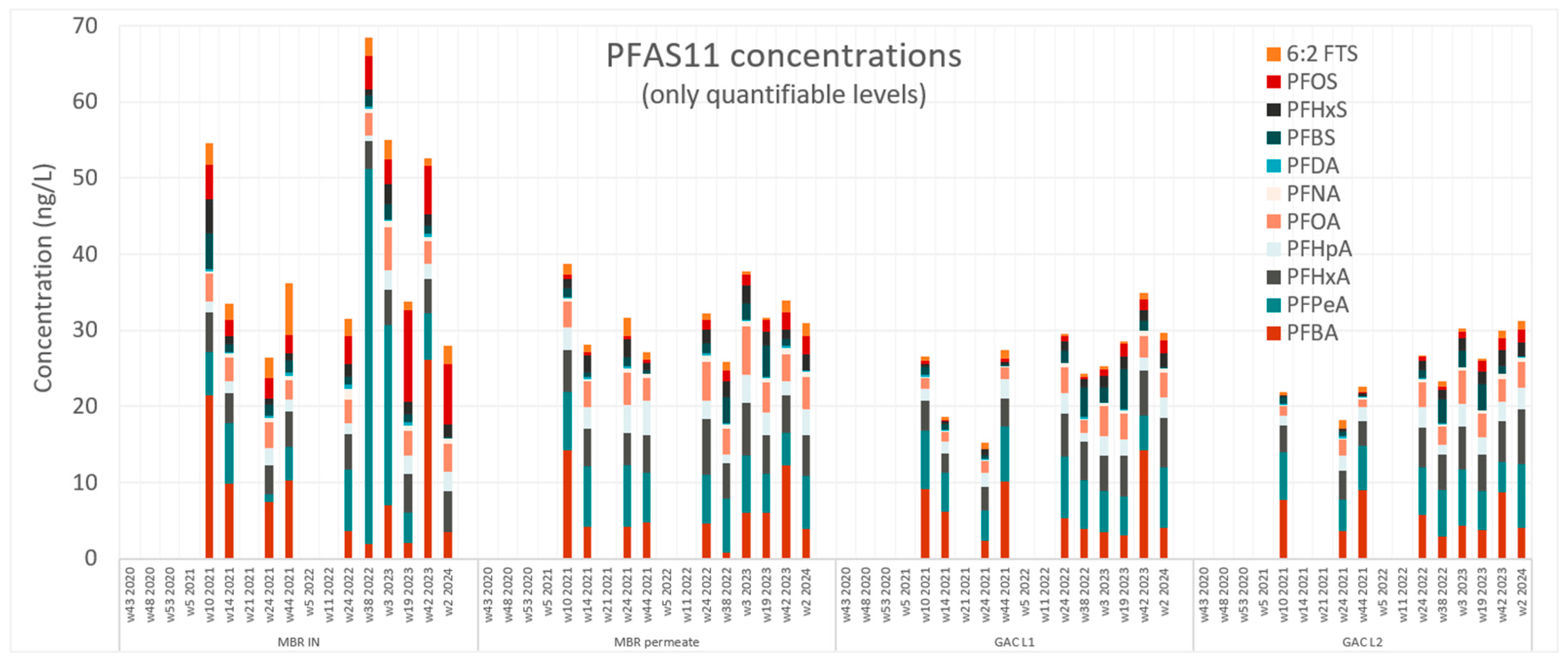
3.2. Removal Efficiency for PFASs
3.3. Full-Scale Implications
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Substance | Average Recovery | Average LOD (ng/mL) | Average LOQ (ng/mL) |
|---|---|---|---|
| Pharmaceuticals | |||
| Atenolol 1 | 109% | 1 | 4 |
| Carbamazepine 1 | 106% | 1 | 3 |
| Ciprofloxacin 1,2 | 104% | 6 | 20 |
| Citalopram 1 | 79% | 1 | 4 |
| Clarithromycin 1,2 | 106% | 1 | 4 |
| Diclofenac 1 | 107% | 3 | 10 |
| Erythromycin 2 | 93% | 1 | 4 |
| Fluconazole 1 | 110% | 1 | 3 |
| Furosemide 1 | 67% | 5 | 17 |
| Ibuprofen | 87% | 10 | 34 |
| Ketoconazole | 43% | 6 | 19 |
| Losartan 1 | 104% | 3 | 8 |
| Methotrexate | 106% | 6 | 21 |
| Metoprolol 1 | 107% | 1 | 3 |
| Naproxen | 127% | 5 | 14 |
| Oxazepam 1 | 131% | 2 | 6 |
| Paracetamol | 144% | 6 | 20 |
| Propranolol 1 | 91% | 1 | 3 |
| Sertraline | 51% | 2 | 5 |
| Sulfamethoxazole 1,2 | 108% | 2 | 5 |
| Tramadol 1 | 50% | 3 | 9 |
| Trimethoprim 1,2 | 108% | 1 | 3 |
| Venlafaxine 1 | 61% | 1 | 4 |
| Zolpidem | 104% | 1 | 3 |
| Per- and polyfluoroalkyl substances (PFAS) | |||
| Perfluorobutane sulfonic acid (PFBS) | N/A | 0.05 | 0.15 |
| Perfluoropentanoic acid (PFPeA) | N/A | 0.06 | 0.17 |
| Perfluorohexane sulfonic acid (PFHxS) | N/A | 0.06 | 0.17 |
| Perfluorohexanoic acid (PFHxA) | N/A | 0.08 | 0.24 |
| Perfluoroheptanoic acid (PFHpA) | N/A | 0.08 | 0.26 |
| Perfluorooctane sulfonic acid (PFOS) | N/A | 0.06 | 0.17 |
| Perfluorooctanoic acid (PFOA) | N/A | 0.07 | 0.22 |
| Perfluorononanoic acid (PFNA) | N/A | 0.14 | 0.46 |
| ∑PFAS11 | |||
| Category | Substance |
|---|---|
| Category 1—substances that can be very easily removed with advanced purification | Amisulpride |
| Carbamazepine | |
| Citalopram | |
| Clarithromycin | |
| Diclofenac | |
| Hydrochlorothiazide | |
| Metoprolol | |
| Venlafaxine | |
| Category 2—substances that are easy to remove with advanced purification | Benzotriazole |
| Candesartan | |
| Irbesartan | |
| Mixture of 4- and 6-methylbenzotriazole |

References
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef]
- Schäfer, C.E.; Hooper, J.L.; Strom, L.E.; Abusallout, I.; Dickenson, E.R.V.; Thompson, K.A.; Mohan, G.R.; Drennan, D.; Wu, K.; Guelfo, J.L. Occurrence of quantifiable and semi-quantifiable poly- and perfluoroalkyl substances in united states wastewater treatment plants. Water Res. 2023, 233, 119724. [Google Scholar] [CrossRef]
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Kaj, L.; Brorström-Lundén, E. Results from the Swedish National Screening Programme 2010, Subreport 3. Pharmaceuticals; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2011; 54p. [Google Scholar]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in south Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Meyer, C.; Patrick, M.; Kosfeld, V.; Rüdel, H.; Koschorreck, J.; Hollender, J. Comprehensive screening of polar emerging organic contaminants including PFASs and evaluation of the trophic transfer behavior in a freshwater food web. Water Res. 2022, 218, 118514. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Sundin, A.M.; Linderholm, L.; Hedlund, B.; Bly Joyce, K.; Klingspor, K. Advanced Treatment of Pharmaceutical Substances and Other Hazardous Substances in Wastewater: Needs, Technologies and Consequences; Report 6766; Swedish Environmental Protection Agency: Stockholm, Sweden, 2017. [Google Scholar]
- Formas. Swedish Municipal Wastewater and Its Impact on Aquatic Organisms—A Systematic Review; Report F1:2022; The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas): Borås, Sweden, 2022; ISBN 978-91-540-6162-4. [Google Scholar]
- EU. Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast); Interinstitutional File 2022/0345(COD); Council of the European Union: Brussels, Belgium, 2024; Available online: https://data.consilium.europa.eu/doc/document/ST-7108-2024-INIT/en/pdf (accessed on 1 May 2024).
- EC. Proposal for a Directive Amending the Water Framework Directive, the Groundwater Directive, and the Environmental Quality Standards Directive; EU Commission: Luxembourg, 26 October 2022; Available online: https://environment.ec.europa.eu/publications/proposal-amending-water-directives_en (accessed on 1 May 2024).
- Abegglen, C.; Siegrist, H. Mikroverunreinigungen aus Kommunalem Abwasser. Verfahren zur Weitergehenden Elimination auf Kläranlagen (Micropollutants from Municipal Wastewater. Methods for Further Elimination at Sewage Treatment Plants); Umwelt-Wissen Nr. 1214: 210 S; Bundesamt für Umwelt: Bern, Switzerland, 2012. [Google Scholar]
- Arge Spurenstoffe NRW. Elimination von Arzneimitteln und Organischen Spurenstoffen: Entwicklung von Konzeptionen und Innovativen, Kostengünstigen Reinigungsverfahren Abschlussbericht zur Phase 2 (Elimination of Pharmaceuticals and Organic Trace Substances: Development of Concepts and Innovative, Cost-Effective Treatment Processes—Final Report Phase 2); Teilprojekt 6; Arge Spurenstoffe NRW: Bochum, Germany, 2013. [Google Scholar]
- Magdeburg, A.; Stalter, D.; Schlüsener, M.; Ternes, T.; Oehlmann, J. Evaluating the efficiency of advanced wastewater treatment: Target analysis of organic contaminants and (geno-)toxicity assessment tell a different story. Water Res. 2014, 50, 35–47. [Google Scholar] [CrossRef]
- Maus, C.; Herbst, H.; Ante, S.; Becker, H.-P.; Glathe, W.; Bärgers, A.; Türk, J. Guidance on the interpretation and design of ozonation plants for micropollutants elimination. Korresp. Abwasser Abfall 2014, 61, 998–1006. (In German) [Google Scholar]
- Micropoll. VSA—Platform Process Engineering Micropollutants. VSA. Available online: https://micropoll.ch/en/home/ (accessed on 1 May 2024).
- Eschauzier, C.; Beerendonk, E.; Scholte-Veenendaal, P.; De Voogt, P. Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ. Sci. Technol. 2012, 46, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Angelotti, B.; Brooks, M.; Dowbiggin, B.; Evans, P.J.; Devins, B.; Wang, Z.-W. A pilot-scale investigation of disinfection by-product precursors and trace organic removal mechanisms in ozone-biologically activated carbon treatment for potable reuse. Chemosphere 2018, 210, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, J.; Yu, G.; Deng, S.; Wang, B. Stability of 6:2 fluorotelomer sulfonate in advanced oxidation processes: Degradation kinetics and pathway. Environ. Sci. Pollut. Res. 2014, 21, 4634–4642. [Google Scholar] [CrossRef] [PubMed]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar]
- Iglesias, R.; Simón, P.; Moragas, L.; Arce, A.; Rodriguez-Roda, I. Cost comparison of full-scale water reclamation technologies with an emphasis on membrane bioreactors. Water Sci. Technol. 2017, 75, 2562–2570. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- The MBR Site. Available online: https://www.thembrsite.com/ (accessed on 2 February 2024).
- Andersson, S.L.; Baresel, C.; Andersson, S.; Westling, K.; Eriksson, M.; Munoz, A.C.; Persson, G.; Narongin-Fujikawa, M.; Johansson, K.; Rydberg, T. Chemical-Saving Potential for Membrane Bioreactor (MBR) Processes Based on Long-Term Pilot Trials. Membranes 2024, 14, 126. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Nghiem, L.D.; Nguyen, L.N. Membrane Bioreactor for Wastewater Treatment: Current Status, Novel Configurations and Cost Analysis. In Cost-Efficient Wastewater Treatment Technologies, The Handbook of Environmental Chemistry; Nasr, M., Negm, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 147–167. [Google Scholar]
- Pathak, N.; Tran, V.H.; Merenda, A.; Johir, M.A.H.; Phuntsho, S.; Shon, H. Removal of Organic Micro-Pollutants by Conventional Membrane Bioreactors and High-Retention Membrane Bioreactors. Appl. Sci. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by a membrane bioreactor–granular activated carbon (MBR–GAC) system. Bioresour. Technol. 2012, 113, 169–173. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Grillini, V.; Mutavdžić Pavlović, D.; Verlicchi, P. Activated carbon coupled with advanced biological wastewater treatment: A review of the enhancement in micropollutant removal. Sci. Total Environ. 2021, 790, 148050. [Google Scholar] [CrossRef]
- Liu, W.; Song, X.; Na, Z.; Li, G.; Luo, W. Strategies to enhance micropollutant removal from wastewater by membrane bioreactors: Recent advances and future perspectives. Bioresour. Technol. 2022, 344, 126322. [Google Scholar] [CrossRef] [PubMed]
- Takman, M.; Svahn, O.; Paul, C.; Cimbritz, M.; Blomqvist, S.; Struckmann Poulsen, J.; Lund Nielsen, J.; Davidsson, Å. Assessing the potential of a membrane bioreactor and granular activated carbon process for wastewater reuse—A full-scale WWTP operated over one year in Scania, Sweden. Sci. Total Environ. 2023, 895, 165185. [Google Scholar] [CrossRef] [PubMed]
- Syvab. Pre-Study Quaternary Treatment at Syvab—Principle Design GAC Filter; Report 613T1356758-025; Syvab: Grödinge, Sweden, 2019. (In Swedish) [Google Scholar]
- Lemström, H.; Roberts, R.; Grim, J.; Baresel, C.; Malovanyy, A. Pre-Study Quaternary Treatment at Syvab—Construction of a Pilot Plant with Granular Activated Carbon in Combination with Membrane BioReactor (MBR-GAC); SYVAB: Grödinge, Sweden, 2021. [Google Scholar]
- Baresel, C.; Ek, M.; Ejhed, H.; Allard, A.S.; Magnér, J.; Dahlgren, L.; Westling, K.; Wahlberg, C.; Fortkamp, U.; Söhr, S.; et al. Sustainable Treatment Systems for Removal of Pharmaceutical Residues and Other Priority Persistent Substances. Water Sci. Technol. 2019, 79, 537–543. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fång, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- Ek, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar] [CrossRef]
- Edefell, E.; Svahn, O.; Falås, P.; Bengtsson, E.; Axelsson, M.; Ullman, R.; Cimbritz, M. Digging deep into a GAC filter—Temporal and spatial profiling of adsorbed organic micropollutants. Water Res. 2022, 218, 118477. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Baldi, M.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Rada, E.C.; Torretta, V. Foams in Wastewater Treatment Plants: From Causes to Control Methods. Appl. Sci. 2020, 10, 2716. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baresel, C.; Salem, M.; Roberts, R.; Malovanyy, A.; Lemström, H.; Esfahani, B. Approaching Breakthrough: Resource-Efficient Micropollutant Removal with MBR-GAC Configuration. Appl. Sci. 2024, 14, 7759. https://doi.org/10.3390/app14177759
Baresel C, Salem M, Roberts R, Malovanyy A, Lemström H, Esfahani B. Approaching Breakthrough: Resource-Efficient Micropollutant Removal with MBR-GAC Configuration. Applied Sciences. 2024; 14(17):7759. https://doi.org/10.3390/app14177759
Chicago/Turabian StyleBaresel, Christian, Marion Salem, Ross Roberts, Andriy Malovanyy, Heidi Lemström, and Bahare Esfahani. 2024. "Approaching Breakthrough: Resource-Efficient Micropollutant Removal with MBR-GAC Configuration" Applied Sciences 14, no. 17: 7759. https://doi.org/10.3390/app14177759






