Photocatalytic Degradation of Selected Non-Opioid Analgesics Driven by Solar Light Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Photocatalysts
2.2. Irradiation Experiments
3. Results
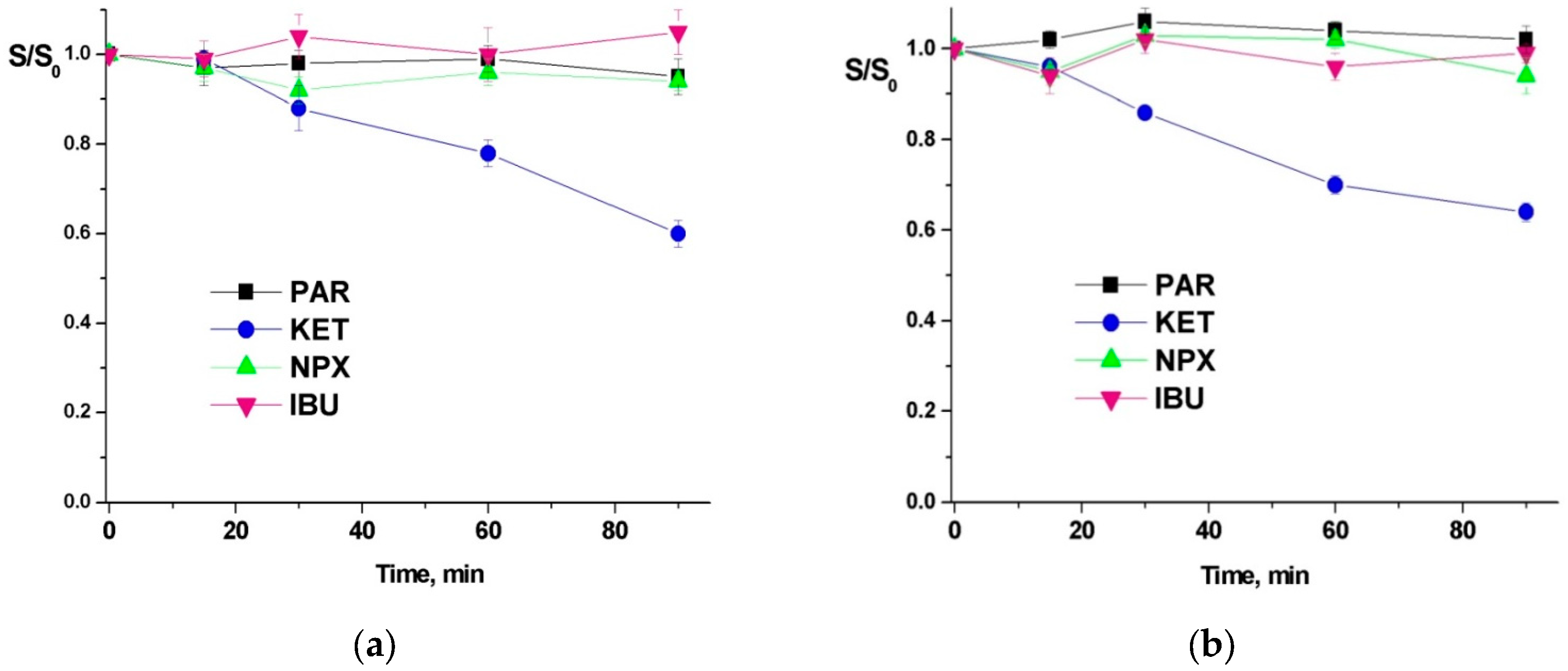
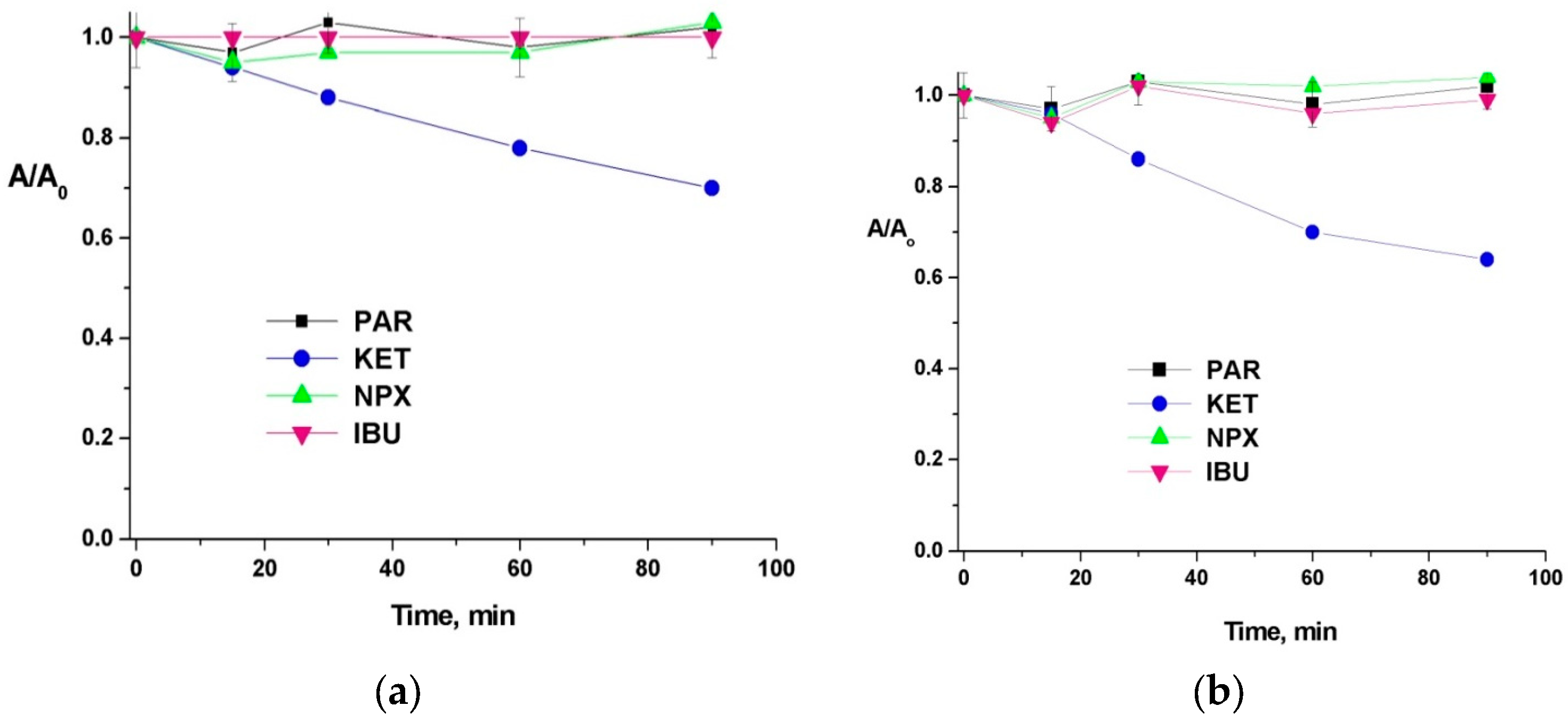
3.1. UV Photodegradation
3.2. Photocatalysis and Photoelectrocatalysis—Systems with Tungsten Oxide and Iron Oxide
3.2.1. Application of WO3 and Fe2O3 Used Individually
3.2.2. Application of Hybrid WO3/Fe2O3 Photocatalyst
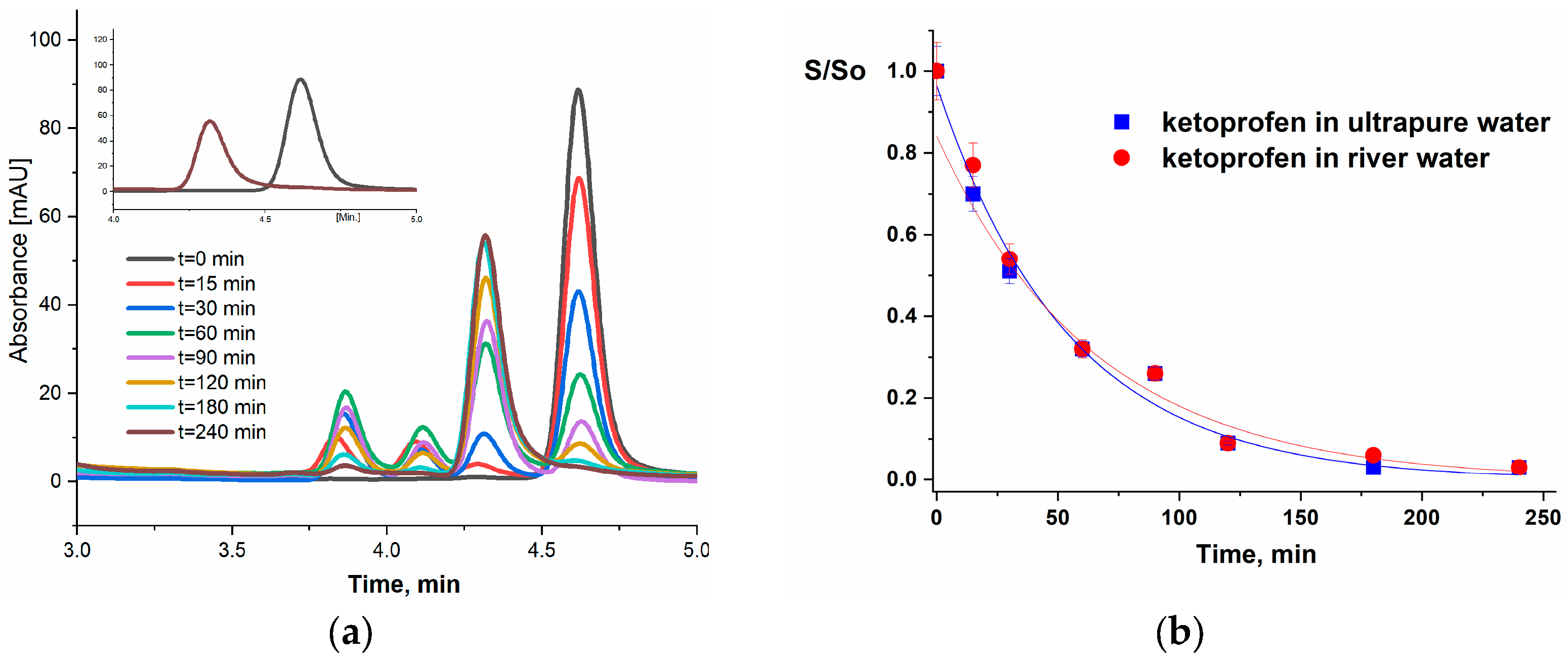
3.3. Effect of the Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Ok, J.S.; Kim, K.H.; Kwon, E.F.; Tsang, Y.F. Occurrences and removal of pharmaceutical and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Morales-Paredes, C.A.; Rodríguez-Díaz, J.M.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci. Total Environ. 2022, 2021, 152691. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Bokhari, A.; Karimian, M.; Zahra, M.M.A.; Sillanpää, M.; Panchal, H.; Alrubaie, A.J.; Rezakhani, Y. A comprehensive review of various approaches for treatment of tertiary wastewater with emerging contaminants: What do we know? Environ. Monit. Assess. 2022, 194, 884. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Almeida, C.M.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical compounds in aquatic environments—Occurrence, fate and bioremediation perspective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; Mac-Rae, J.I.; Barron, L.P. A review of the pharmaceutical exposome in aquatic fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef]
- Nyirenda, J.; Mwanza, A.; Lengwe, C. Assessing the biodegradability of common pharmaceutical products (PPs) on the Zambian market. Heliyon 2020, 6, e05286. [Google Scholar] [CrossRef]
- Fu, J.; Lee, W.N.; Coleman, C.; Nowack, K.; Carter, J.; Huang, C.H. Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment. Sci. Total Environ. 2019, 664, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated carbon for pharmaceutical removal at point-of-entry. Processes 2021, 9, 1091. [Google Scholar] [CrossRef]
- Cao, Y.; Nakhjiri, A.T.; Ghadiri, M. Numerical investigation of ibuprofen removal from pharmaceutical wastewater using adsorption process. Sci. Rep. 2021, 11, 24478. [Google Scholar] [CrossRef]
- Narayanan, M.; El-Sheekh, M.; Ma, Y.; Pugazhendhi, A.; Natarajan, D.; Kandasamy, G.; Raja, R.; Kumar, R.M.S.; Kumarasamy, S.; Sathiyan, G.; et al. Current status of microbes involved in the degradation of pharmaceutical and personal care products (PPCPs) pollutants in the aquatic ecosystem. Environ. Pollut. 2022, 300, 118922. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, A.; Gozlan, I.; Avisar, D. Pharmaceutical transformation products fordem by ozonation—Does degradation occur? Molecules 2023, 28, 1227. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Hu, X.; Ye, Y.; Dong, W.; Huang, Y.; Zhu, M. Quantitative evaluation of structure-activity relationships in heterogeneous photocatalytic oxidation towards organic contaminants. Appl. Catal. B 2022, 309, 121238. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Drwal, K.; Miecznikowski, K.; Krasnodębska-Ostręga, B. Photoactive materials for decomposition of organic matter prior to water analysis—A review containing original research. Catalysts 2022, 12, 616. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Akhavan, O. Nanomaterials for photocatalytic degradation of analgesic, mucolytic and anti-biotic/Viral/inflammatory drugs widely used in controlling SARS-CoV-2. Catalysts 2022, 12, 667. [Google Scholar] [CrossRef]
- Fu, K.; Pan, Y.; Ding, C.; Shi, J.; Deng, H. Reduced graphene oxide/ZnIn2S4 nanocomposite photocatalyst with enhanced photocatalytic performance for the degradation of naproxen under visible light irradiation. Catalysts 2020, 10, 710. [Google Scholar] [CrossRef]
- Santos, E.N.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Binazadeh, M.; Rasouli, J.; Sabbaghi, S.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. An Overview of Photocatalytic Membrane Degradation Development. Materials 2023, 16, 3526. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Li, Z.; Chen, M.; Zheng, J.; Wang, X. Recent advances in photocatalytic membranes for pharmaceuticals and personal care products removal from water and wastewater. Chem. Eng. J. 2023, 475, 146036. [Google Scholar] [CrossRef]
- Dalida, M.L.P.; Amer, K.M.S.; Su, C.C.; Lu, M.C. Photocatalytic degradation of acetaminophen in modified TiO2 under visible irradiation. Environ. Sci. Pollut. Res. 2014, 21, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Rodriguez, J.J.; Belver, C. C-modified TiO2 using lignin as carbon precursor for the solar photocatalytic degradation of acetaminophen. Chem. Eng. J. 2019, 358, 1574–1582. [Google Scholar] [CrossRef]
- Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic treatment of paracetamol using TiO2 nanotubes: Effect of pH. Processes 2019, 7, 319. [Google Scholar] [CrossRef]
- Chaker, H.; Fourmentin, S.; Chérif-Aouali, L. Efficient photocatalytic degradation of ibuprofen under visible light irradiation using silver and cerium co-doped mesoporous TiO2. ChemistrySelect 2020, 5, 11787–11796. [Google Scholar] [CrossRef]
- Khalaf, S.; Shoqeir, J.H.; Lelario, F.; Sabino, A.; Bufo, S.A.; Karaman, R.; Scrano, L. TiO2 and active coated glass photodegra- dation of ibuprofen. Catalysts 2020, 10, 560. [Google Scholar] [CrossRef]
- Fakhri, A.; Behrouz, S. Photocatalytic properties of tungsten trioxide (WO3) nanoparticles for degradation of Lidocaine under visible and sunlight irradiation. Sol. Energy 2015, 112, 163–168. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.N.; Son, J.; Oh, J. Tungsten Trioxide (WO3)-assisted Photocatalytic Degradation of Amoxicillin by Simulated Solar Irradiation. Sci. Rep. 2019, 9, 9349. [Google Scholar] [CrossRef]
- Biaduń, E.; Gajewska, S.; Miecznikowski, K.; Krasnodębska-Ostręga, B. Application of hierarchical nanostructured WO3 and Fe2O3 composites for degradation of surfactants in water samples. Catalyst 2019, 9, 1039. [Google Scholar] [CrossRef]
- Biaduń, E.; Nowak, N.; Kowalska, J.; Miecznikowski, K.; Krasnodębska-Ostręga, B. Organic matter decomposition before arsenic speciation analysis of water samples—“Soft decomposition” using nano-photocatalysts. Chemosphere 2018, 207, 481–488. [Google Scholar] [CrossRef]
- Biaduń, E.; Miecznikowski, K.; Sadowska, M.; Kużelewska, A.; Drwal, K.; Krasnodębska-Ostręga, B. Simplification of organic matter before voltammetric determination of Tl(I) and Tl(III) in water using nanostructured photocatalyst and solar light. Anal. Chim. Acta 2019, 1076, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Ni, H.; Chen, R.; Zhang, B.; Zhan, W.; Li, Y. Hydrothermal synthesis of WO3/Fe2O3 nanosheet arrays on iron foil for photocatalytic degradation of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 10481–10487. [Google Scholar] [CrossRef]
- Kiamouche, S.; Messaadia, L.; Lahmar, H.; Rekhila, G.; Trari, M.; Benamir, M. Enhanced photocatalytic degradation of Ponceau S Red dye on the novel hetero-system Fe2O3/WO3 under solar light irradiation. Reac. Kinet. Mech. Cat. 2022, 135, 3411–3426. [Google Scholar] [CrossRef]
- Miecznikowski, K.; Ramírez, A.; Fiechter, S.; Bogdanoff, P.; Szaniawska, E.; Wadas, A.; Kuesza, P.J. Development of Hybrid Tungsten Oxide Photoanodes Admixed with Borododecatungstate-Polyanion Modified-Hematite: Enhancement of Water Oxidation upon Irradiation with Visible Light. Electrochim. Acta 2015, 179, 379–385. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically Oriented Mesoporous WO3 Films: Synthesis, Characterization, and Applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef]
- Sivula, K.; Formal, F.L.; Grätzel, M. WO3−Fe2O3 Photoanodes for Water Splitting: A Host Scaffold, Guest Absorber Approach. Chem. Mater. 2009, 21, 2862–2867. [Google Scholar] [CrossRef]
- Sivula, K.; LeFormal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- van de Krol, R. Principles of photoelectrochemical cells. In Photoelectrochemical Hydrogen Production; van de Krol, R., Gratzel, M., Eds.; Springer Science: New York, NY, USA, 2012; pp. 13–68. [Google Scholar]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Enhanced photocatalytic activity of ZnO-graphene oxide nanocomposite by electron scavenging. Catalysts 2021, 11, 187. [Google Scholar] [CrossRef]
- Ma, H.Y.; Zhao, L.; Guo, L.H.; Zhang, H.; Chen, F.J.; Yu, W.C. Roles of reactive oxygen species (ROS) in the photocatalytic degradation of pentachlorophenol and its main toxic intermediates by TiO2/UV. J. Hazard. Mater. 2019, 369, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C–TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef]
- Trujillano, R.; Rives, V.; García, I. Photocatalytic Degradation of Paracetamol in Aqueous Medium Using TiO2 Prepared by the Sol-Gel Method. Molecules 2022, 27, 2904. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Pereira, V.J.; Carvalho, G.; Soeiro, R.; Gaffney, C.; Almeida, V.; Vale Cardoso, V.; Ferreira, E.; Benoliel, M.J.; Ternes, T.A.; et al. Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater. J. Hazard. Mater. 2013, 244–245, 516–527. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Viljoen, E.L. WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water—A review. J. Water Process. Eng. 2021, 40, 101930. [Google Scholar] [CrossRef]
- Senthil, R.A.; Priya, A.; Theerthagiri, J.; Selvi, A.; Nithyadharseni, P.; Madhavan, L. Facile synthesis of α-Fe2O3/WO3 composite with an enhanced photocatalytic and photo-electrochemical performance. Ionics 2018, 24, 3673–3684. [Google Scholar] [CrossRef]
- Reis, R.Y.N.; Goulart, L.A.; Mascaro, L.H.; Alves, A. A critical view of the contributions of photoelectrochemical technology to pharmaceutical degradation. J. Environ. Chem. Eng. 2022, 10, 107859. [Google Scholar] [CrossRef]
- Cristino, V.; Longobucco, G.; Marchetti, N.; Caramori, S.; Bignozzi, C.A.; Martucci, A.; Molinari, A.; Boaretto, R.; Stevanin, C.; Argazzi, R.; et al. Photoelectrochemical degradation of pharmaceuticals at β25 modified WO3 interfaces. Catal. Today 2020, 340, 302–310. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt–TiO2–Nb2O5 heterojunction as effective photocatalyst for the degradation of diclofenac and ketoprofen. Mater. Sci. Semicond. Process 2020, 107, 104839. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Aatik, A.E.; Aliste, M.; Navarro, G.; Fenoll, J.; Navarr, S. Removal of Contaminants of Emerging Concern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water Air Soil Pollut. 2023, 234, 55. [Google Scholar] [CrossRef]
- Friedmann, D.A. General Overview of Heterogeneous Photocatalysis as a Remediation Technology for Wastewaters Containing Pharmaceutical Compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors, International. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Yu, J.C. Photocatalytic degradation of ibuprofen on S-doped BiOBr. Chemosphere 2021, 278, 13037. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Fatahi, N.; Jafari, Z.; Jafari, F.; Jafari Motlagh, R. The visible-light photocatalytic degradation of ibuprofen by the CuS-Fe3O4/RGO catalyst. Inorg. Chem. Commun. 2023, 158, 111597. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pobozy, E.; Kaczmarek, S.; Miecznikowski, K.; Pyrzynska, K.; Biesaga, M. Photocatalytic Degradation of Selected Non-Opioid Analgesics Driven by Solar Light Exposure. Appl. Sci. 2024, 14, 7768. https://doi.org/10.3390/app14177768
Pobozy E, Kaczmarek S, Miecznikowski K, Pyrzynska K, Biesaga M. Photocatalytic Degradation of Selected Non-Opioid Analgesics Driven by Solar Light Exposure. Applied Sciences. 2024; 14(17):7768. https://doi.org/10.3390/app14177768
Chicago/Turabian StylePobozy, Ewa, Sylwia Kaczmarek, Krzysztof Miecznikowski, Krystyna Pyrzynska, and Magdalena Biesaga. 2024. "Photocatalytic Degradation of Selected Non-Opioid Analgesics Driven by Solar Light Exposure" Applied Sciences 14, no. 17: 7768. https://doi.org/10.3390/app14177768





