Bridging Thermochemical Technology and Ecology: Research Progress on Utilization of Factsage Software for Environmental Applications
Abstract
1. Introduction
2. Function of Factsage Software
2.1. Equilib Module
2.2. Viscosity Module
2.3. EpH Module
2.4. Reaction Module
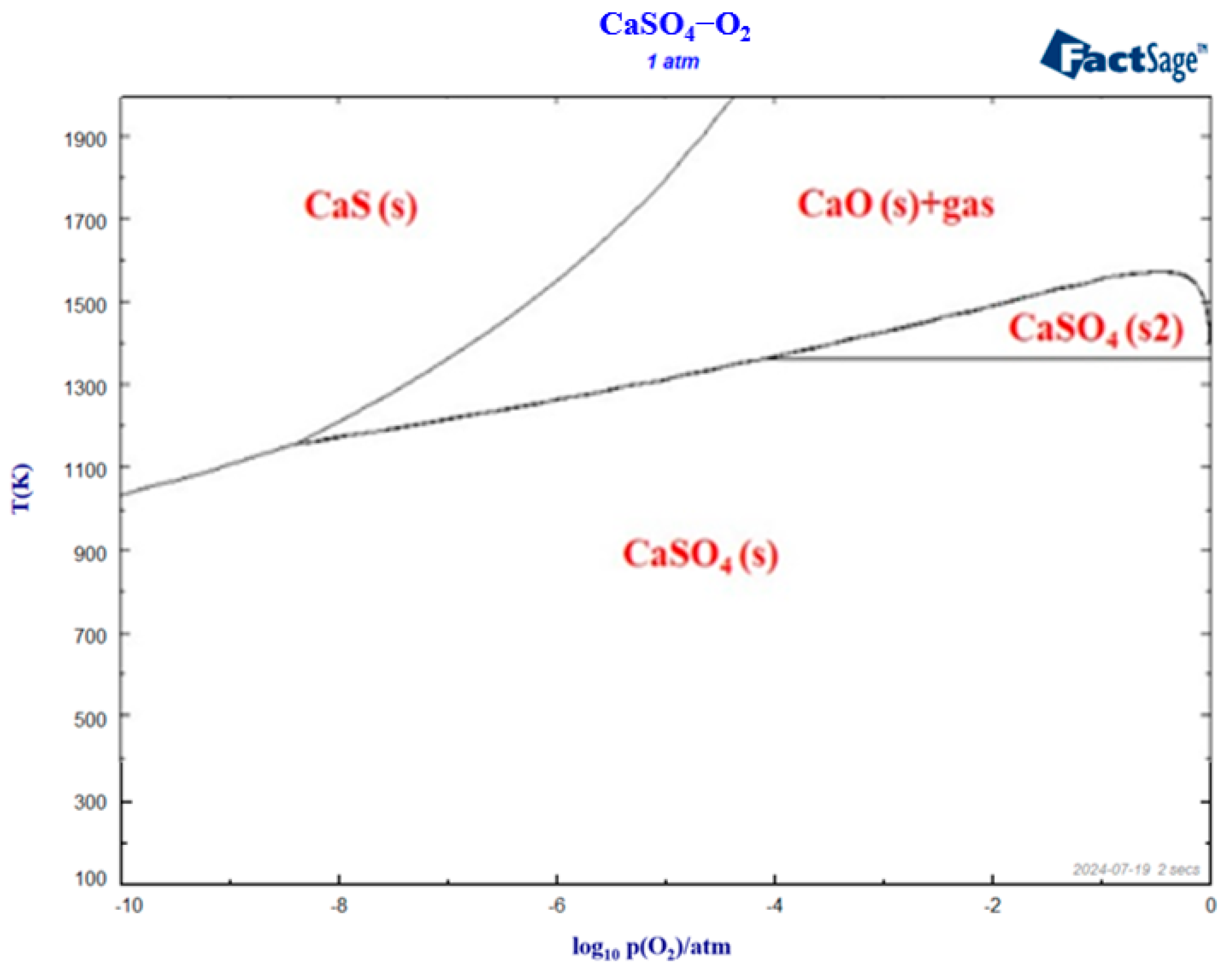
2.5. Phase Diagram Module
3. Factsage in the Environmental Sector
3.1. Applications in the Field of Air Pollution
3.2. Applications in the Field of Water Pollution
3.3. Application in the Field of Solid Waste
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Wang, Y.; Liu, X.; Wu, H. The Role of Environmental Monitoring in Environmental Impact Assessment. Leather Manuf. Environ. Technol. 2022, 3, 175–176+179. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, M.Y.; Ji, P. Firm dynamics decomposition of China’s industrial pollution emission technological progress, resource allocation and selection effects. J. Quant. Technol. Econ. 2022, 39, 153–172. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Z.; Zhou, Z. Environmental issues in China: Monitoring, assessment and management. Ecol. Indic. 2015, 51, 1–2. [Google Scholar] [CrossRef]
- Sui, T. Cultivation of Innovative Talents in Environmental Engineering Based on Multidisciplinary Cross-Integration. West. China Qual. Educ. 2020, 6, 165–167. [Google Scholar] [CrossRef]
- Diwekar, U. Green process design, industrial ecology, and sustainability: A systems analysis perspective. Resour. Conserv. Recycl. 2005, 44, 215–235. [Google Scholar] [CrossRef]
- Zha, L. Study on Mechanism and Aapplication of Calcium-Rish and Iron-Rish Ceramics from Steel Slag. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2017. [Google Scholar]
- Zhu, F.; Liu, X.; Han, M.; Takaoka, M.; Oshita, K.; Dong, Y. Effect of heating program and atmosphere on the evolution of crystal washed fly ash during calcination. China Environ. Sci. 2019, 39, 4212–4220. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bao, S.; Chen, T.; Han, J. Calculation of mineral phase and liquid phase formation temperature during roasting of vanadium-bearing stone coal using FactSage software. Int. J. Miner. Process. 2013, 124, 150–153. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, B. Phase equilibrium studies of titanomagnetite and ilmenite smelting slags. Int. J. Miner. Met. Mater. 2022, 29, 2162–2171. [Google Scholar] [CrossRef]
- Diwekar, U.M. Greener by Design. Environ. Sci. Technol. 2003, 37, 5432–5444. [Google Scholar] [CrossRef]
- Han, X.; Cao, Y.; Jing, D.; Wu, C.; Ma, L.; Wei, J.; Chen, Y.; Jiang, L. Application of Factsage inSteel Slag Treatment. Multipurp. Util. Miner. Resour. 2019, 3, 102–107. [Google Scholar]
- Jung, I.H.; Van Ende, M.A. Computational thermodynamic calculations: FactSage from CALPHAD thermodynamic database to virtual process simulation. Met. Mater. Trans. B 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mah-foud, R.B.; Melancon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Cao, Z.; Song, X.; Qiao, Z. Thermodunamic Modeling Software FactSage and Its Appliacation. Chin. J. Rare Met. 2008, 32, 216–219. [Google Scholar] [CrossRef]
- Jung, I.H.; Kang, D.H.; Park, W.J.; Kim, N.J.; Ahn, S.H. Applications of thermodynamic calculations to Mg alloy design: Mg–Sn based alloy development. Int. J. Mater. Res. 2007, 98, 807–815. [Google Scholar] [CrossRef]
- Sang, L.; Lv, X.; Wu, Y. NaNO3-KNO3-KCl/K2CO3 with the elevated working temperature for CSP application: Phase diagram calculation and machine learning. Sol. Energy 2023, 252, 322–329. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, G.; Xiang, Y.; Long, F.; Dong, C. Thermodynamic Study of the Corrosion of Refractories by Sodium Carbonate. Materials 2018, 11, 2197. [Google Scholar] [CrossRef]
- Villada, C.; Ding, W.; Bonk, A.; Bauer, T. Simulation-assisted determination of the minimum melting temperature composition of MgCl2–KCl–NaCl salt mixture for next-generation molten salt thermal energy storage. Front. Energy Res. 2022, 10, 181. [Google Scholar] [CrossRef]
- Konar, B.; Kim, D.G.; Jung, I.H. Critical thermodynamic optimization of the Li2O-Al2O3-SiO2 system and its application for the thermodynamic analysis of the glass-ceramics. J. Eur. Ceram. Soc. 2018, 38, 3881–3904. [Google Scholar] [CrossRef]
- Jung, I.-H.; Zhu, Z.; Kim, J.; Wang, J.; Chartrand, P.; Pelton, A. Recent progress on the factsage thermodynamic database for new Mg alloy development. Jom 2017, 69, 1052–1059. [Google Scholar] [CrossRef]
- Jankovský, O.; Sedmidubský, D.; Vítek, J.; Šimek, P.; Sofer, Z. Phase diagram of the Sr–Co–O system. J. Eur. Ceram. Soc. 2015, 35, 935–940. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, J.; Xu, J.; Shi, Z. Anode reaction mechanisms of Na|NaCl-CaCl2|Zn liquid metal battery. J. Energy Chem. 2022, 72, 81–87. [Google Scholar] [CrossRef]
- Bernd, M.G.S.; Bragança, S.R.; Heck, N.; Filho, L.C.d.S. Synthesis of carbon nanostructures by the pyrolysis of wood sawdust in a tubular reactor. J. Mater. Res. Technol. 2017, 6, 171–177. [Google Scholar] [CrossRef]
- Gheribi, A.E.; Audet, C.; Le Digabel, S.; Bélisle, E.; Bale, C.; Pelton, A. Calculating optimal conditions for alloy and process design using thermodynamic and property databases, the FactSage software and the Mesh Adaptive Direct Search algorithm. Calphad 2012, 36, 135–143. [Google Scholar] [CrossRef]
- Eriksson, G.; Thompson, W.T. A procedure to estimate equilibrium concentrations in multicomponent systems amd related applications. Calphad 1989, 13, 389–400. [Google Scholar] [CrossRef]
- Hanxu, L.I.; Yoshihiko, N.; Zhongbing, D.; Zhang, M. Application of the FactSage to predict the ash melting behavior in reducing conditions. Chin. J. Chem. Eng. 2006, 14, 784–789. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, E.B.P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.H.; Melançon, J.; Pelton, A.D.; Petersen, S.; et al. Recent developments in Factsage thermochemical software and databases. In Celebrating the Megascale: Proceedings of the Extraction and Processing Division Symposium on Pyrometallurgy in Honor of David GC Robertson; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 141–148. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Pelton, A.D. Thermodynamic database development—Modeling and phase diagram calculations in oxide systems. Rare Met. 2006, 25, 473–480. [Google Scholar] [CrossRef]
- Bielefeldt, W.V.; Vilela, A.C.F. Thermodynamic study of non-metallic inclusion formation in SAE 1141 steel. MatÉRia 2010, 15, 275–282. [Google Scholar] [CrossRef]
- Muñoz, V.; Camelli, S.; Martinez, A.G.T. Slag corrosion of alumina-magnesia-carbon refractory bricks: Experimental data and thermodynamic simulation. Ceram. Int. 2017, 43, 4562–4569. [Google Scholar] [CrossRef]
- Hidayat, T.; Shishin, D.; Decterov, S.A.; Hayes, P.C.; Jak, E. High-temperature experimental and thermodynamic modelling research on the pyrometallurgical processing of copper. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2017; Volume 1805, p. 040004. [Google Scholar] [CrossRef]
- Wu, L.; Wang, H.; Dong, K. Effect of sulfur content on copper recovery in the reduction smelting process. Metals 2022, 12, 857. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.Y.; Wang, X.R.; Zhan, F.D.; He, Y.M.; Liu, H.P.; Li, T.G.; Li, B.; Ning, P. Migration and transformation behavior of harmful elements of lightweight ceramsite preparation from yellow phosphorus slag. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 199, p. 042033. [Google Scholar]
- Ranjan, N.; Kumar, S.; Mahajani, S.M. Autothermal downdraft oxy-steam co-gasification of biomass and plastic wastes for hydrogen-rich syngas production: An Aspen plus modelling. Int. J. Hydrog. Energy 2024, 81, 1045–1061. [Google Scholar] [CrossRef]
- Zalazar-Garcia, D.; Fernandez, A.; Rodriguez-Ortiz, L.; Torres, E.; Reyes-Urrutia, A.; Echegaray, M.; Rodriguez, R.; Mazza, G. Exergo-ecological analysis and life cycle assessment of agro-wastes using a combined simulation approach based on Cape-Open to Cape-Open (COCO) and SimaPro free-software. Renew. Energy 2022, 201, 60–71. [Google Scholar] [CrossRef]
- Li, T.; Yang, C.; Tantikhajorngosol, P.; Sema, T.; Tontiwachwuthikul, P. Studies on advanced configurations of post-combustion CO2 capture process applied to cement plant flue gases. Carbon Capture Sci. Technol. 2022, 4, 100064. [Google Scholar] [CrossRef]
- Yan, X.; Shi, L.; Cai, R. Improvement of nitrogen utilization and soil properties by addition of a mineral soil conditioner: Mechanism and performance. Environ. Sci. Pollut. Res. 2018, 25, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, G.; Königsberger, E. FactSage and ChemApp: Two tools for the prediction of multiphase chemical equilibria in solutions. Pure Appl. Chem. 2008, 80, 1293–1302. [Google Scholar] [CrossRef]
- Mu, L. Study on Kinetics of Pyrolysis and Combustion and Ash Deposits Formation Mcchanisms for the Refining and Chemicals Wastewater. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2012. [Google Scholar]
- Prithiv, T.S.; Thirumurugan, G.; Madan, M.; Kamaraj, A. Thermodynamic Assessment of Steelmaking Practices for the Production of Re-sulfur Steels. Trans. Indian Inst. Met. 2020, 73, 1595–1603. [Google Scholar] [CrossRef]
- Ma, D.G.; Chen, W.Q. Influence of Fe2O3 on molten slag properties in slag splashing on nickel smelting converter. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 402, pp. 249–252. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.; Zhao, Z.; Evans, T.; Zhao, B. Effects of Minor Elements to the Liquidus Temperatures of Blast Furnace Slags. ISIJ Int. 2016, 56, 2156–2160. [Google Scholar] [CrossRef]
- Zhu, M.; Bai, H.; Liu, D. Preparation and properties of ceramsite from stainless steel pickling sludge and clay. J. Wuhan Univ. Sci. Technol. 2016, 39, 185–189. [Google Scholar]
- Liang, W.; Wang, G.; Zhang, J.; Ning, X.; Li, Y.; Jiang, C. Effect of CaO on melting characteristics of high calcium bituminous coal. Iron Steel 2019, 54, 25–29. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Ma, M.; Yu, B.; Zhao, C.; Fang, Y. Prediction of ash flow temperature based on liquid phase mass fraction by FactSage. J. Energy Inst. 2020, 93, 2228–2231. [Google Scholar] [CrossRef]
- Nowak-Woźny, D.; Ferens, W.; Wach, J. Using dissipation factor method in testing the ash sintering process of cereal pellet and coal fuels. Energy 2022, 250, 123718. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Ludwig, M.; Śnieżek, E.; Kalęba, A.; Drożdż, P.; Szczerba, J. Corrosion study of novel Cr-free alumina-spinel refractory material dedicated to the copper industry. J. Eur. Ceram. Soc. 2022, 42, 7311–7327. [Google Scholar] [CrossRef]
- Mishra, V.; Bhowmick, T.; Chakravarty, S.; Varma, A.K.; Sharma, M. Influence of coal quality on combustion behaviour and mineral phases transformations. Fuel 2016, 186, 443–455. [Google Scholar] [CrossRef]
- Wu, Q.F.; Bao, Y.P.; Lin, L. Phases of converter slag and their cooling orecipitation. J. Wuhan Univ. Sci. Technol. 2014, 37, 411–414. [Google Scholar]
- Li, F.; Yu, B.; Wang, G.; Fan, H.; Wang, T.; Guo, M.; Fang, Y. Investigation on improve ash fusion temperature (AFT) of low-AFT coal by biomass addition. Fuel Process. Technol. 2019, 191, 11–19. [Google Scholar] [CrossRef]
- Shevchenko, M.; Ilyushechkin, A.; Abdeyazdan, H.; Jak, E. Integrated experimental phase equilibria study and thermodynamic modeling of the PbO–SnO–SnO2–SiO2 system in air and in equilibrium with Pb–Sn metal. J. Alloy. Compd. 2021, 888, 161402. [Google Scholar] [CrossRef]
- Li, T.; Gu, Z.; Lu, N.; Wu, L.; Chang, S.U. Calculation of heat balance in the process of biomass ash modification to molten steel slag. Mod. Transp. Metall. Mater. 2022, 2, 64–67+73. [Google Scholar]
- Kim, W.Y.; Yang, X.; Yan, L.; Pelton, A.D.; Decterov, S.A. Modeling the viscosity of silicate melts containing zinc oxide. Calphad 2011, 35, 542–550. [Google Scholar] [CrossRef]
- Billen, P.; Mazzotti, M.; Pandelaers, L.; Oo, N.Y.; Zhao, W.; Liu, Z.; Redus, J.; Loya, I.D.; Bartoli, I.; Farnam, Y.; et al. Melt ceramics from coal ash: Constitutive product design using thermal and flow properties. Resour. Conserv. Recycl. 2018, 132, 168–177. [Google Scholar] [CrossRef]
- Wang, S.; Wen, Z.; Dou, R.; Xiao, Y.; Guan, Y.; Liu, X. Experimental Measurement and Model Prediction Analysis of Metallurgical Slag Viscosity. Hot Work. Technol. 2023, 1–4. [Google Scholar] [CrossRef]
- Mostaghel, S.; Matsushita, T.; Samuelsson, C.; Björkman, B.; Seetharaman, S. Influence of alumina on physical properties of an industrial zinc-copper smelting slag: Part 1—Viscosity. Miner. Process. Extr. Metall. 2013, 122, 42–48. [Google Scholar] [CrossRef]
- Melo, G.M.S.; da Silva, C.A.; Peixoto, J.J.M.; Oliveira, J.R.; Silva, C.V.; Silva, I.A. Influence of synthetic slag added during the tapping on inclusion features of a vacuum degassed LCAK steel grade. REM-Int. Eng. J. 2020, 73, 513–521. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, Q.; Qin, J. Study on the Effect of Basicity on the Densification Behavior of Sinter. Trans. Indian Inst. Met. 2022, 75, 1545–1553. [Google Scholar] [CrossRef]
- Kumar, R.; Rommel, S.; Jiang, C.; Jordan, E.H. Effect of CMAS viscosity on the infiltration depth in thermal barrier coatings of different microstructures. Surf. Coat. Technol. 2022, 432, 128039. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Tian, C.; Fan, H.; Xu, M.; Fang, Y. Predict coal ash fusion temperature based on liquid-phase content and its variation trend using FactSage software. J. Therm. Anal. Calorim. 2022, 147, 8895–8899. [Google Scholar] [CrossRef]
- Rocha, V.C.; Silva, M.L.; Bielefeldt, W.V.; Vilela, A.C.F. Assessment of viscosity calculation for calcium-silicate based slags using computational thermodynamics. REM-Int. Eng. J. 2018, 71, 243–252. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.; Shao, J.; Jia, X. Viscosity calculation of ladle slag with high alumine. Henan Metall. 2014, 22, 10–12+52. [Google Scholar]
- Ko, H.; Kim, M.; Park, S.M.; Lim, H.M. High-temperature viscosity analysis of aluminosilicate melts and the comparison to empirical models. J. Korean Ceram. Soc. 2020, 58, 160–168. [Google Scholar] [CrossRef]
- Webster, R.I.; Opila, E.J. Viscosity of CaO-MgO-Al2O3-SiO2 (CMAS) melts: Experimental measurements and comparison to model calculations. J. Non-Cryst. Solids 2022, 584, 121508. [Google Scholar] [CrossRef]
- Liu, S. A System Design Scheme for Computer Drawing E-pH Diagram. Estate Sci. Trib. 2015, 14, 56–57. [Google Scholar]
- Nikolaychuk, P.A. The revised Pourbaix diagram for silicon. Silicon 2014, 6, 109–116. [Google Scholar] [CrossRef]
- Nave, M.I.; Kornev, K.G. Complexity of products of tungsten corrosion: Comparison of the 3D Pourbaix diagrams with the experimental data. Met. Mater. Trans. A 2016, 48, 1414–1424. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, D.N.; Zhou, Y.Q.; Na, X.; Zhang, W.; Han, Y. Effect of cl-concentration on pitting corrosion property of maraging hardened stainless steel based on Pourbaix diagram. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2019; Volume 940, pp. 59–64. [Google Scholar] [CrossRef]
- Sasaki, K.; Takasaki, F.; Noda, Z.; Hayashi, S.; Shiratori, Y.; Ito, K. Alternative electrocatalyst support materials for polymer electrolyte fuel cells. Ecs Trans. 2010, 33, 473–482. [Google Scholar] [CrossRef]
- Channei, D.; Phanichphant, S.; Nakaruk, A.; Mofarah, S.S.; Koshy, P.; Sorrell, C.C. Aqueous and surface chemistries of photocatalytic Fe-doped CeO2 nanoparticles. Catalysts 2017, 7, 45. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Li, X.; Jiang, Z.; Yang, S.; Cui, Z.; Zhang, D.; Terryn, H.; Li, X. New insights into the mechanism of localised corrosion induced by TiN-containing inclusions in high strength low alloy steel. J. Mater. Sci. Technol. 2022, 124, 141–149. [Google Scholar] [CrossRef]
- Na, X.; Zou, D.; Yang, H.; Shen, C. Research on Electrochemical Properties of Aging Cr22Ni5Mo3 Stainless Steel Based on E-pH Diagram of Fe-Cr-CI-H2O System. Mater. Prot. 2018, 51, 18–23+79. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Zeng, Y.; Wang, Z. Toxicity assessment and geochemical model of chromium leaching from AOD slag. Chemosphere 2016, 144, 2052–2057. [Google Scholar] [CrossRef]
- Perez-Labra, M.; Romero-Serrano, A.; Salinas-Rodriguez, E.; Avila-Davila, E.O.; Reyes-Perez, M. Synthesis, thermodynamic, and kinetics of rubidium jarosite decomposition in calcium hydroxide solutions. Met. Mater. Trans. B 2012, 43, 773–780. [Google Scholar] [CrossRef]
- Sadeghi, N.; Alamdari, E.K. A new approach for monitoring and controlling the extraction of gold by tri-butyl phosphate from chloride media. Miner. Eng. 2016, 85, 34–37. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Revilla, R.I.; Sun, T.; Zhao, J.; Zhang, D.; Yang, S.; Liu, Z.; Cheng, X.; Terryn, H.; et al. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corros. Sci. 2020, 179, 109150. [Google Scholar] [CrossRef]
- Yan, X.; Ma, L.; Zhu, B.; Zheng, D.; Lian, Y. Reaction Mechanism Process Analysis with Phosphogypsum Decomposition in Multiatmosphere Control. Ind. Eng. Chem. Res. 2014, 53, 19453–19459. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Wang, Y.; Huang, Y.; Yuxin, S.; Dai, Q.; Liu, H.; Ma, L. Phosphogypsum Resource Utilization Based on Thermodynamic Analysis. Chem. Eng. Technol. 2022, 45, 776–790. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, B.; Ma, L.; Liu, H. Investigation of Al2O3 and Fe2O3 transmission and transformation during the decomposition of phosphogypsum. Chin. J. Chem. Eng. 2018, 27, 1125–1131. [Google Scholar] [CrossRef]
- Jian, S.; Sun, M.; He, G.; Zhi, Z.; Ma, B.; Xiao, H. Emission of Harmful Gases From High-Temperature Combustion of Bio-Materials. J. Ecol. Rural. Environ. 2016, 32, 842–846. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, L.; Wang, R.; Yang, J.; Dai, Q. Decomposing properties of phosphogypsum with iron addition under two-step cycle multi-atmosphere control in fluidised bed. Waste Manag. Res. 2018, 36, 183–193. [Google Scholar] [CrossRef]
- Song, W.; Zhou, J.; Wang, B.; Li, S.; Cheng, R. Production of SO2 Gas: New and Efficient Utilization of Flue Gas Desulfurization Gypsum and Pyrite Resources. Ind. Eng. Chem. Res. 2019, 58, 20450–20460. [Google Scholar] [CrossRef]
- Dai, Z.; Lu, J.; Kong, G. Progress in Study on CALPHAD Approach. Mater. Rep. 2006, 94–97. [Google Scholar]
- Xu, T.; Wang, P.; Li, L.; Van Der Omer, B.; Jef, V. Research and Progress on Computational Phase Diagram of Oxide Systems Containing Zirconia. J. Inorg. Mater. 2002, 399–406. [Google Scholar]
- Schupsky, J.P.; Saar, O.; Wu, G.; Dohrn, M.; Müller, M. Viscosity and crystal morphology data of anorthite bearing synthetic coal slag systems. Fuel 2020, 280, 118663. [Google Scholar] [CrossRef]
- Yang, G.; Ren, Q.; Xu, J.; Lyu, Q. Co-melting properties and mineral transformation behavior of mixtures by MSWI fly ash and coal ash. J. Energy Inst. 2021, 96, 148–157. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Friedmann, D.; Kuhn, T.; Friedrich, B. “Zero-Waste”: A sustainable approach on pyrometallurgical processing of manganese nodule slags. Minerals 2018, 8, 544. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Y.; Ma, H.; Dai, W.; Cang, D. Influence of Al2O3 content on microstructure and properties of different binary basicity slag glass ceramics. Adv. Appl. Ceram. 2014, 113, 394–403. [Google Scholar] [CrossRef]
- Mittal, A.; Albertsson, G.J.; Gupta, G.S.; Seetharaman, S.; Subramanian, S. Some Thermodynamic Aspects of the Oxides of Chromium. Met. Mater. Trans. B 2014, 45, 338–344. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Chi, G. Prediction on the Ash Melting Flowing Temperature by FactSage. Boil. Technol. 2018, 49, 17–21. [Google Scholar]
- Liang, W.; Wang, G.-W.; Ning, X.-J.; Zhang, J.-L.; Li, Y.-J.; Jiang, C.-H. Effect of CaO mineral change on coal ash melting characteristics. J. Energy Inst. 2019, 93, 642–648. [Google Scholar] [CrossRef]
- Guo, X.; Su, H.; Wu, Y.; Wu, W. Thermodynamic Analysis of Phase Diagrams Simulated by FactSage in Process of Rare Earth OxidesProduction by Spray Pyrolysis Method. J. Rare Earths 2020, 38, 788–797. [Google Scholar]
- Yu, H.; Shen, Q.; Liu, D.; Chen, D.; Ban, Q.; Hao, Z. Using Factsage to calculate the effect of CaO slagging constituent on the phase structure of magnesia chromium bricks. Min. Metall. 2019, 28, 47–49+76. [Google Scholar]
- Zhu, S.; Li, H.; Li, S.; Zhang, C. Study on application of Factsage thermodynamic calcuation in zinc hydrometallurgy. China Nonferrous Metall. 2022, 51, 43–51. [Google Scholar] [CrossRef]
- Wei, F.R.; Zhang, Y.L.; Wei, W.J.; Yang, X.G. Chemical Composition of Dust from Stainless Steel Smelting and Existing Forms of Cr and Ni. Chin. J. Process Eng. 2011, 11, 786–793. [Google Scholar]
- Fan, Y.; Yang, Y.; Xiao, Y.; Zhao, Z.; Lei, Y. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: Thermodynamic evaluation and experimental study. Hydrometallurgy 2013, 139, 95–99. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Mineralogical Assessment of the Solid Phase Obtained on Leaching of Brazilian Red Mud. In Light Metals 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 21–25. [Google Scholar] [CrossRef]
- Attah-Kyei, D.; Akdogan, G.; Dorfling, C.; Zietsman, J.; Lindberg, D.; Tesfaye, F.; Reynolds, Q. Investigation of waste PCB leach residue as a reducing agent in smelting processes. Miner. Eng. 2020, 156, 106489. [Google Scholar] [CrossRef]
- Schwitalla, D.; Reinmöller, M.; Forman, C.; Wolfersdorf, C.; Gootz, M.; Bai, J.; Guhl, S.; Neuroth, M.; Meyer, B. Ash and slag properties for co-gasification of sewage sludge and coal: An experimentally validated modeling approach. Fuel Process. Technol. 2018, 175, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, J.; Sun, S.; Huang, S.; Kuo, J.; Chen, N. Combined effects of FeCl3 and CaO conditioning on SO2, HCl and heavy metals emissions during the DDSS incineration. Chem. Eng. J. 2016, 299, 449–458. [Google Scholar] [CrossRef]
- Meng, X.; De Jong, W.; Verkooijen, A. Thermodynamic analysis and kinetics model of H2S sorption using different sorbents. Environ. Prog. Sustain. Energy 2009, 28, 360–371. [Google Scholar] [CrossRef]
- Dinda, S.K.; Chattopadhyay, K. Numerical Modeling of Volatile Organic Compounds (VOC) Emissions during Preheating of Magnesia-Carbon Bricks in a Basic Oxygen Furnace. Metals 2020, 10, 1277. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, H.; Fan, X.; Wang, Z. Predicting Gaseous Pollution of Sintered Brick Preparation from Yellow Phosphorus Slag. Pol. J. Environ. Stud. 2019, 28, 1719–1725. [Google Scholar] [CrossRef]
- Tang, H. The Research of CO2 Carbonation Fixation by Using Steel Plant Slag. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Zhao, S. Study of Mechanism of CO2 Mineralization Capture by Phosphogypsum Decomposition Slag. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2018. [Google Scholar]
- Wang, Y.; Chai, Z.; Wang, X. Thermodynamic Study on the Characteristics of SO Reduction by Coal Gasification in Circulating Fluidized Bed. Contemp. Chem. Ind. 2022, 51, 2061–2065+2071. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, S.; Li, J.; Xia, X.; Li, L.; Zhao, X.; Ma, C. Experimental and thermodynamic study on SO2 reduction to elemental sulfur by activated coke and pyrolysis gas: Influence of the reaction atmosphere. Int. J. Hydrog. Energy 2020, 45, 20120–20131. [Google Scholar] [CrossRef]
- Brandaleze, E.; Bazán, V.; Orozco, I.; Valentini, M.; Gomez, G. Application of thermal analysis to the rhenium recovery process from copper and molybdenum sulphides minerals. J. Therm. Anal. Calorim. 2018, 133, 435–441. [Google Scholar] [CrossRef]
- Liao, Y.F.; Yang, G.; Ma, X.Q. Study on NO Emission during Co-combustion of PVC and Biomass. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 347, pp. 2423–2427. [Google Scholar] [CrossRef]
- Kalicka, Z.; Jerzak, W.; Cebula, E.K. The Effect of Combustion of Natural Gas With 21–29% O2/CO2/N2 Mixtures on Emission of Carbon Monoxide. Arch. Environ. Prot. 2013, 39, 93–103. [Google Scholar] [CrossRef]
- Yilmaz, C.; Wendelstorf, J.; Turek, T. Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, X.; Peng, D.; Zhu, X. Development of a fly ash derived Li4SiO4-based sorbent for CO2 capture at high temperatures. Thermochim. Acta 2018, 669, 80–87. [Google Scholar] [CrossRef]
- Lee, J.; Feng, B. A thermodynamic study of the removal of HCl and H2S from syngas. Front. Chem. Sci. Eng. 2012, 6, 67–83. [Google Scholar] [CrossRef]
- Ibezim-Ezeani, M.U.; Ihunwo, O.C. FactSage Modelling of Pb and Ni Speciation in Surface Water from Woji Creek, Rivers State, Nigeria. Adv. Res. 2021, 22, 100–110. [Google Scholar] [CrossRef]
- Luo, X.; Peng, J.; Luo, X.; Peng, J.; Zhang, S.; Zhang, M.; Li, X.; Wei, C.; Deng, Z. The settling behavior of scaling ions in brine purification process by NaOH-flue gas method. Chin. J. Process Eng. 2021, 21, 174–182. [Google Scholar]
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T.A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, L.; Dou, C.; Xiao, H. Characteristics of Cl precipitation by spraying before and after desulfurization wastewater treatment. Electr. Power 2020, 53, 197–202. [Google Scholar]
- Desfitri, E.R.; Sutopo, U.M.; Hayakawa, Y.; Kambara, S. Effect of Additive Material on Controlling Chromium (Cr) Leaching from Coal Fly Ash. Minerals 2020, 10, 563. [Google Scholar] [CrossRef]
- Li, F.; Yu, B.; Fan, H.; Guo, M.; Wang, T.; Huang, J.; Fang, Y. Investigation on regulation mechanism of red mud on the ash fusion characteristics of high ash-fusion-temperature coal. Fuel 2019, 257, 116036. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, J.; Liu, S.; Yuan, Z. Mechanism and clean procedure to extract gold from printed circuit board. Procedia Environ. Sci. 2016, 31, 171–177. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Fabrication and characterization of porous cordierite ceramics prepared from fly ash and natural minerals. Ceram. Int. 2019, 45, 18306–18314. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Dou, Z. A Thermodynamic and Experimental Assessment of the Recovery of Copper, Iron, Zinc, and Lead from Copper Slag. Minerals 2022, 12, 496. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, M.; Wang, N. Calculation on the liquid zone and phase relations of CaO-SiO-FeOx-MgO oxide system. J. Mater. Metall. 2012, 11, 93–97. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zheng, Y. Quantitative chemical composition determination and thermal analysis for typical biomass ashes in China. Asia-Pac. J. Chem. Eng. 2014, 9, 751–758. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Zhang, J.; Li, Y.; Wang, Y. Metallurgical treatment for municipal solid waste incineration fly ash. J. Iron Steel Res. 2020, 32, 601–609. [Google Scholar] [CrossRef]
- Liu, L.; Ai, J.; Yin, Y.; Tian, H.; Song, C.; Liu, C.; Gong, Q.; Yu, J.; Ling, P.; Jiang, Z. Evolution of lron and Sulfur Containing Minerals in the Process of Mixed Combustion of MunicipalSewage Sludge and Coal by Factsage. Environ. Sci. Technol. 2017, 40, 32–37. [Google Scholar]
- Fang, Z.; Man, J.; Liu, F.; Li, Y.; Li, R. Study on the occurrence characteristics of heavy metals in fine particulate matter during sludge incineration. China Environ. Sci. 2020, 40, 3044–3053. [Google Scholar] [CrossRef]
- Kaußen, F.; Friedrich, B. Reductive Smelting of Red Mud for Iron Recovery. Chem. Ing. Tech. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Li, Y.; Duan, D.; Liu, Y. Thermodynamics analysis on the carbochlorination of fly ash. J. Chongqing Univ. 2015, 38, 130–137. [Google Scholar] [CrossRef]
- Khaki, J.V.; Shalchian, H.; Rafsanjani-Abbasi, A.; Alavifard, N. Recovery of iron from a high-sulfur and low-grade iron ore. Thermochim. Acta 2018, 662, 47–54. [Google Scholar] [CrossRef]
- Back, G.-S.; Park, H.S.; Seo, S.M.; Jung, W.-G. Exploring high-strength glass-ceramic materials for upcycling of industrial wastes. Met. Mater. Int. 2015, 21, 1061–1067. [Google Scholar] [CrossRef]
- Ai, X.-B.; Bai, H.; Zhao, L.-H.; Cang, D.-Q.; Tang, Q. Thermodynamic analysis and formula optimization of steel slag-based ceramic materials by FACTsage software. Int. J. Miner. Met. Mater. 2013, 20, 379–385. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, C.; Liu, S.; Zong, Y.; Zhou, Q.; Qin, S. Preparation of Steel Slag Ceramics with Different MgO/Al2O3 Ratios. Appl. Sci. 2019, 9, 4741. [Google Scholar] [CrossRef]
- Liu, J.; Fu, J.; Ning, X.; Sun, S.; Wang, Y.; Xie, W.; Huang, S.; Zhong, S. An experimental and thermodynamic equilibrium investigation of the Pb, Zn, Cr, Cu, Mn and Ni partitioning during sewage sludge incineration. J. Environ. Sci. 2015, 35, 43–54. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Woźny, D.; Wilk, M. Mineral phase transformation of biomass ashes–Experimental and thermochemical calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Ling, P.; Liu, L.; Ai, J.; Yin, Y.; Tian, H.; Xie, F. Mineral migration of alkali metal during the combustion of coal and sewage sludge. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 61, p. 012011. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yang, J.; Ma, L.; Jiang, M.; Li, Y.; Cheng, Y.; Li, Y.; Cao, Z. Preparation of glass-ceramics from phosphogypsum. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 199, p. 042044. [Google Scholar] [CrossRef]
- Miao, Z.; Li, N.; Yan, W. Effect of sintering temperature on the phase composition and microstructure of anorthite–mullite–corundum porous ceramics. Ceram. Int. 2014, 40, 15795–15799. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Friedrich, B. Phase characterization and thermochemical simulation of (landfilled) bauxite residue (“red mud”) in different alkaline processes optimized for aluminum recovery. Hydrometallurgy 2018, 176, 49–61. [Google Scholar] [CrossRef]
- Shishin, D.; Shevchenko, M.; Jak, E. Experimental Study and Thermodynamic Calculations in the CaO–Cu2O–FeO–Fe2O3–SiO2 System for Applications in Novel Copper-Based Processes. J. Sustain. Metall. 2021, 7, 300–313. [Google Scholar] [CrossRef]
- Luo, W.; Hu, G.; Ding, J.; Wu, J.; Ma, W. Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery. Metals 2022, 12, 2183. [Google Scholar] [CrossRef]
- Junior, A.B.B.; Espinosa, D.C.R.; Tenório, J.A.S. The use of computational thermodynamic for yttrium recovery from rare earth elements-bearing residue. J. Rare Earths 2020, 39, 201–207. [Google Scholar] [CrossRef]
- Kumari, A.; Raj, R.; Randhawa, N.; Sahu, S.K. Energy efficient process for recovery of rare earths from spent NdFeB magnet by chlorination roasting and water leaching. Hydrometallurgy 2021, 201, 105581. [Google Scholar] [CrossRef]

| Application Area | Case Descriptions | Reference |
|---|---|---|
| Simulation of ceramic granule preparation | Simulation of the sintering results of ceramic pellets prepared from sludge was conducted, and the optimal sludge addition ratio was determined. | [44] |
| Predicting the melting temperature of coal ash | The Equilib module was employed to forecast the impact of CaO supplementation on the temperature of the liquid phase in high-calcium coals, with a view to elucidating the underlying mechanism of this effect. | [45] |
| The relationship between the flow temperature of coal ash and its liquid-phase temperature has been calculated and determined, with satisfactory results for coals with a low silica-aluminum ratio. | [46] | |
| Study on the properties of steel slag | The Equilib module was employed to ascertain the liquid-phase ratio and viscosity of MgO-FeO-Fe2O3-SiO2 melts, with a view to predicting the impact of Fe2O3 on the sputtering slag guard performance of nickel smelting converters. | [42] |
| Factsage was employed to forecast the impact of disparate trace element concentrations on the temperature of the liquid phase in blast furnace slag. To validate these findings, high-temperature tests were conducted. | [43] |
| Application Area | Case Descriptions | Reference |
|---|---|---|
| Modelling the viscosity of zinc oxide-containing oxide solutes | A viscosity model for oxide melts containing zinc oxide was constructed, validated through experimental and computational means, and subsequently integrated into the Factsage Viscosity module to guarantee the precision of the predicted viscosity values generated by Factsage. | [54] |
| Preparation of ceramic aggregates | Simulation of viscosity changes during the sintering of ceramic raw materials using Viscosity to determine the optimum sintering process for the production of ceramic aggregates. | [55] |
| Predicting desulphurization slag viscosity | The viscosity of desulphurization slag was predicted using various models and compared, and the results showed that Factsage had the best prediction results, with an absolute error value of less than 0.1 Pa-s. | [56] |
| Application Area | Case Descriptions | Reference |
|---|---|---|
| Predicting the corrosion behavior of metallic material | Predicting the corrosion of this metallic material in seawater by plotting the Pour-baix diagram of the Fe-Cr-Cl-H2O system under different conditions. | [73] |
| Predicting the form of heavy metals present in leachate | Pourbaix plots of the Cr-H2O system were drawn to study the forms of chromium present in the argon oxygen decarburization slag leachate to provide a theoretical basis for the prevention and control of heavy metals in water. | [74] |
| Application Area | Case Descriptions | Reference |
|---|---|---|
| Exploring the performance of soil amendments | The Reaction module was used to calculate the chemical reactions between the soil amendment and the soil to determine that these reactions were feasible | [38] |
| Predicting pollutant emissions from biomass combustion | The Reaction module was used to simulate the combustion process of biomass, such as straw, to investigate the emission of harmful gases HF and HCl under different conditions. | [81] |
| Module Function | Case Descriptions | Reference |
|---|---|---|
| Prediction of eutectic properties | Ternary phase diagram of SiO2-CaO-Al2O3 to predict the melt flow temperature of coal ash. | [91] |
| The effect of CaO content on the melting temperature of coal ash was analyzed by plotting SiO2-CaO-Fe2O3-Al2O3 quaternary phase diagrams with different CaO contents. | [92] | |
| Analysis of reaction products | Ternary phase diagram of RECl3-O2-H2O to calculate the effect of temperature and carrier gas composition on the yield of light rare earth oxides prepared by pyrolysis. | [93] |
| MgO-Cr2O3-CaO ternary phase diagram to analyze the phase changes in the preparation of Magnesium chrome brick refractories and to determine that CaO as a slagging agent has little effect on the refractoriness of the material. | [94] | |
| Mapping areas of strength | The dominant zone of the Zn-S-O system was mapped at different temperatures to study the variation of the stability zone range of each phase with temperature in the oxidative roasting of Zn sulfide. | [95] |
| Mapping the dominant region of the Cr-Fe-C-O system in order to analyze the thermodynamic presence of Cr in stainless steel under different conditions. | [96] |
| Case Descriptions | Related Modules | Reference |
|---|---|---|
| Simulated the emissions of HCl and SO2 pollutants during sludge incineration and validated the accuracy of the thermodynamic model predictions through experiments. | Equilib | [101] |
| Calculated the chemical equilibrium states of H2S adsorption on different adsorbents and studied the main factors affecting H2S adsorption efficiency. | Equilib | [102] |
| Established a mathematical model to predict VOC* emission rates and analyzed the effects of different parameters on VOC* emission rates using Factsage. | Reaction | [103] |
| Predicted gas pollutants generated during the production of sintered bricks using yellow phosphorus slag and determined the reaction conditions that minimize pollutant emissions. | Equilib Reaction | [104] |
| Calculated the thermodynamic properties of wet and dry carbonation reactions of steel slag to determine the feasibility of carbon sequestration using steel slag. | Reaction | [105] |
| Simulated and analyzed the thermal decomposition of phosphogypsum and the process of capturing carbon dioxide with the decomposition residue. Used phase diagrams to analyze the reaction mechanism. | Equilib Phase Diagram | [106] |
| Utilized circulating fluidized bed gasification technology to convert solid carbon into CO and H2, and other reducing gases. Simulated the reduction products of SO2 in this highly reducing atmosphere. | Equilib | [107] |
| Utilized CO and H2 to elemental sulfur, and analyzed the thermodynamic properties of the reaction. | Equilib | [108] |
| Case Descriptions | Related Modules | Reference |
|---|---|---|
| Simulated and predicted the ionic forms of lead and nickel in surface water, explored the impact of different environmental conditions on the forms of these heavy metal ions. | EpH | [115] |
| Simulated the settling behavior of various ions in brine to determine the optimal conditions for the deposition of scale-forming ions. | EpH | [116] |
| Investigated the selective capture of heavy metals Cr and As from solution using metal polymers. | EpH | [117] |
| Simulated the chloride release characteristics after tail flue injection before and after pretreatment of desulfurization wastewater. | Equilib | [118] |
| Case Descriptions | Related Modules | Reference |
|---|---|---|
| Used phase diagrams to analyze the sintering mechanism for the preparation of porous ceramics from fly ash. | Phase Diagram | [122] |
| Calculated the reduction thermodynamics of copper slag and determined the process conditions for recovering metal elements from copper slag. | Phase Diagram | [123] |
| Studied the thermodynamic properties of the CaO-SiO2-FeOx-MgO quaternary oxide system to provide a basis for the slagging treatment of incineration bottom ash. | Phase Diagram | [124] |
| Used various analytical tools to study the chemical and mineral compositions of ash from burning corn stalks, wheat straw, and poplar wood at different temperatures. | Reaction Phase Diagram | [125] |
| Used phase diagrams to analyze the melting behavior and mechanisms of co-melting for municipal solid waste incineration fly ash and fly ash. | Phase Diagram | [87] |
| Used a metallurgical shaft furnace reactor to process municipal solid waste incineration fly ash and analyzed the physical phase changes during the melting process of the fly ash. | Equilib Viscosity | [126] |
| Performed thermodynamic calculations and simulations on the evolution of iron and sulfur compounds during the co-combustion of coal and sludge. | Equilib | [127] |
| Studied and analyzed the changes in the chemical forms of heavy metals during sludge incineration. | Equilib | [128] |
| Studied the reduction smelting process of red mud and calculated the composition of the resulting metal and slag phases. | Equilib Viscosity | [129] |
| Performed a thermodynamic analysis of the carbothermic chlorination process for extracting aluminum from fly ash to determine its feasibility. | Phase Diagram Equilib | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, H.; Lv, P.; Ma, H. Bridging Thermochemical Technology and Ecology: Research Progress on Utilization of Factsage Software for Environmental Applications. Appl. Sci. 2024, 14, 7784. https://doi.org/10.3390/app14177784
Li H, Wang H, Lv P, Ma H. Bridging Thermochemical Technology and Ecology: Research Progress on Utilization of Factsage Software for Environmental Applications. Applied Sciences. 2024; 14(17):7784. https://doi.org/10.3390/app14177784
Chicago/Turabian StyleLi, Hao, Hao Wang, Pin Lv, and Hongzhi Ma. 2024. "Bridging Thermochemical Technology and Ecology: Research Progress on Utilization of Factsage Software for Environmental Applications" Applied Sciences 14, no. 17: 7784. https://doi.org/10.3390/app14177784
APA StyleLi, H., Wang, H., Lv, P., & Ma, H. (2024). Bridging Thermochemical Technology and Ecology: Research Progress on Utilization of Factsage Software for Environmental Applications. Applied Sciences, 14(17), 7784. https://doi.org/10.3390/app14177784








