Free Ammonia Strategy for Nitrite-Oxidizing Bacteria (NOB) Suppression in Mainstream Nitritation Start-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Set-Up and Operational Condition
2.2. Activity Measurement of AOB and NOB
2.3. Chemical Analysis Methods
2.4. Microbial Analysis Methods
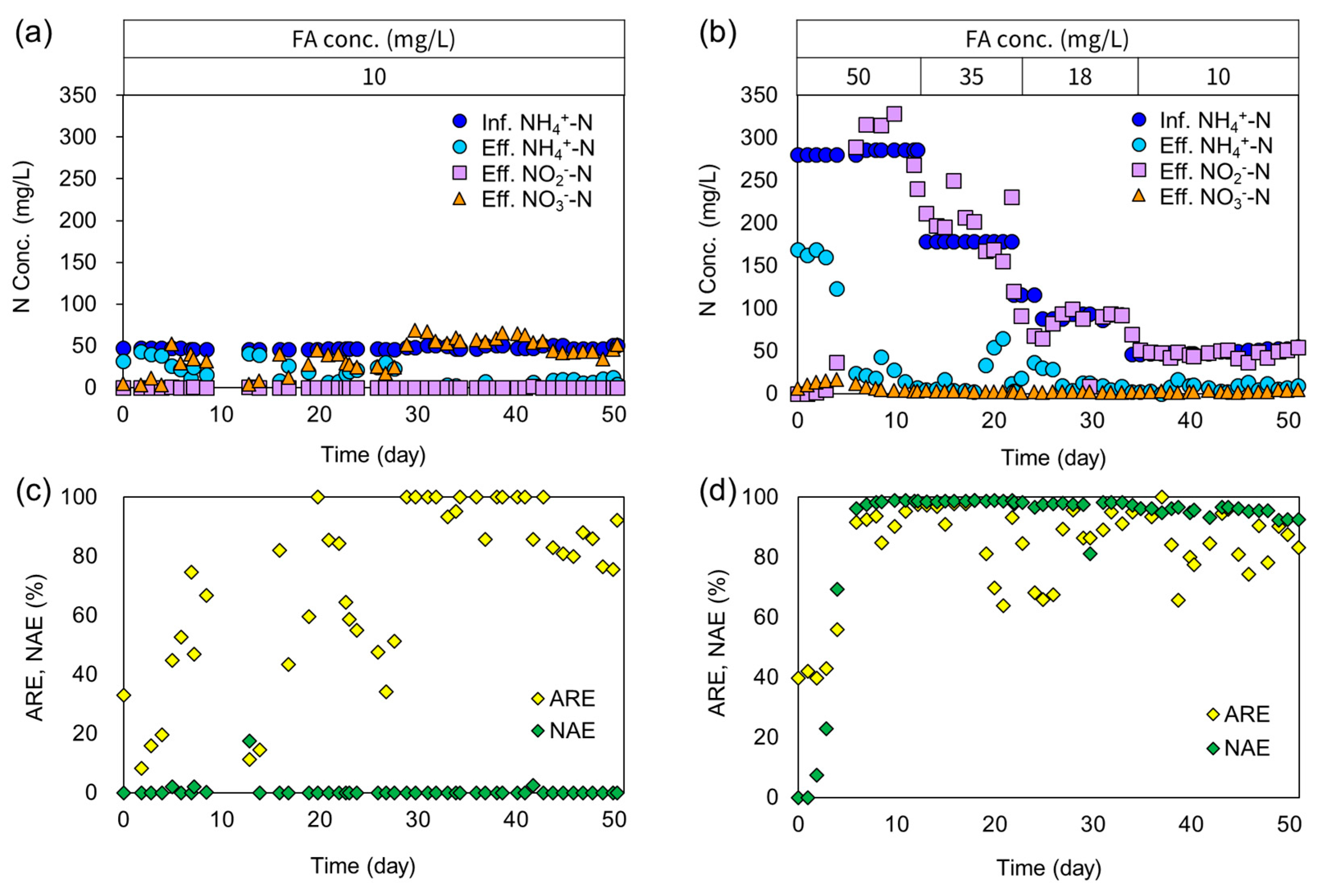
3. Results and Discussion
3.1. Overall Performance of PN1 and PN2
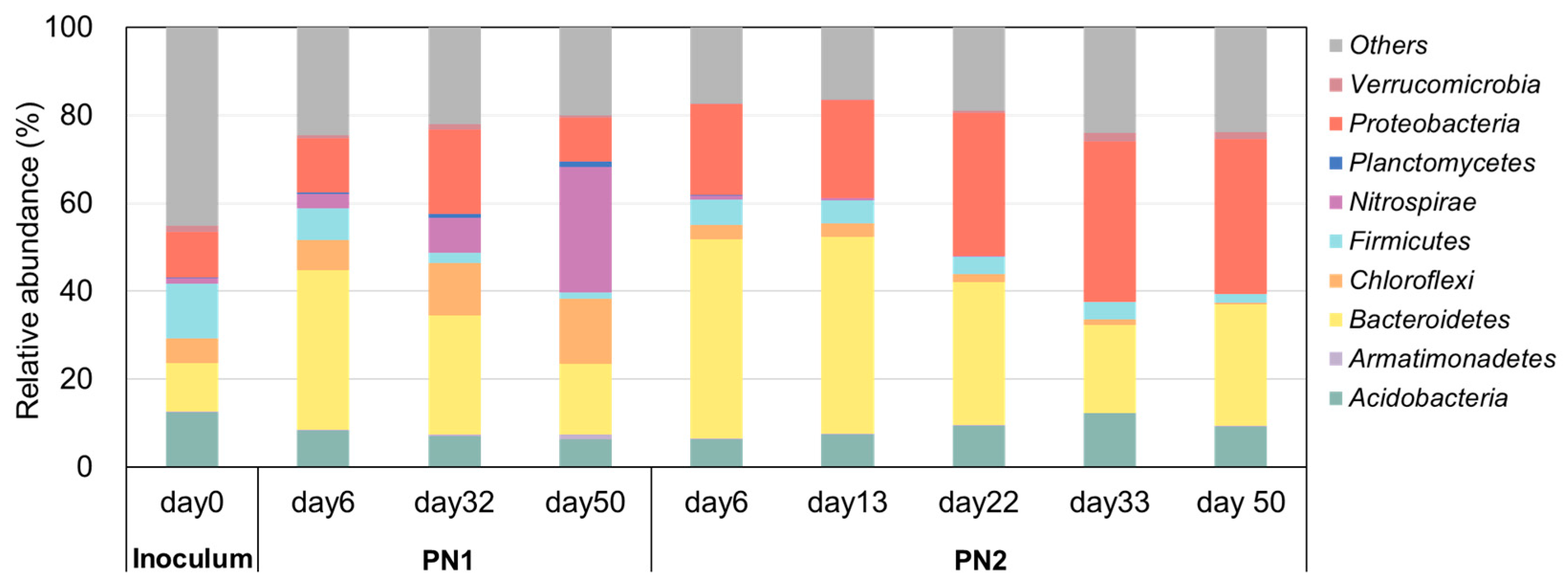
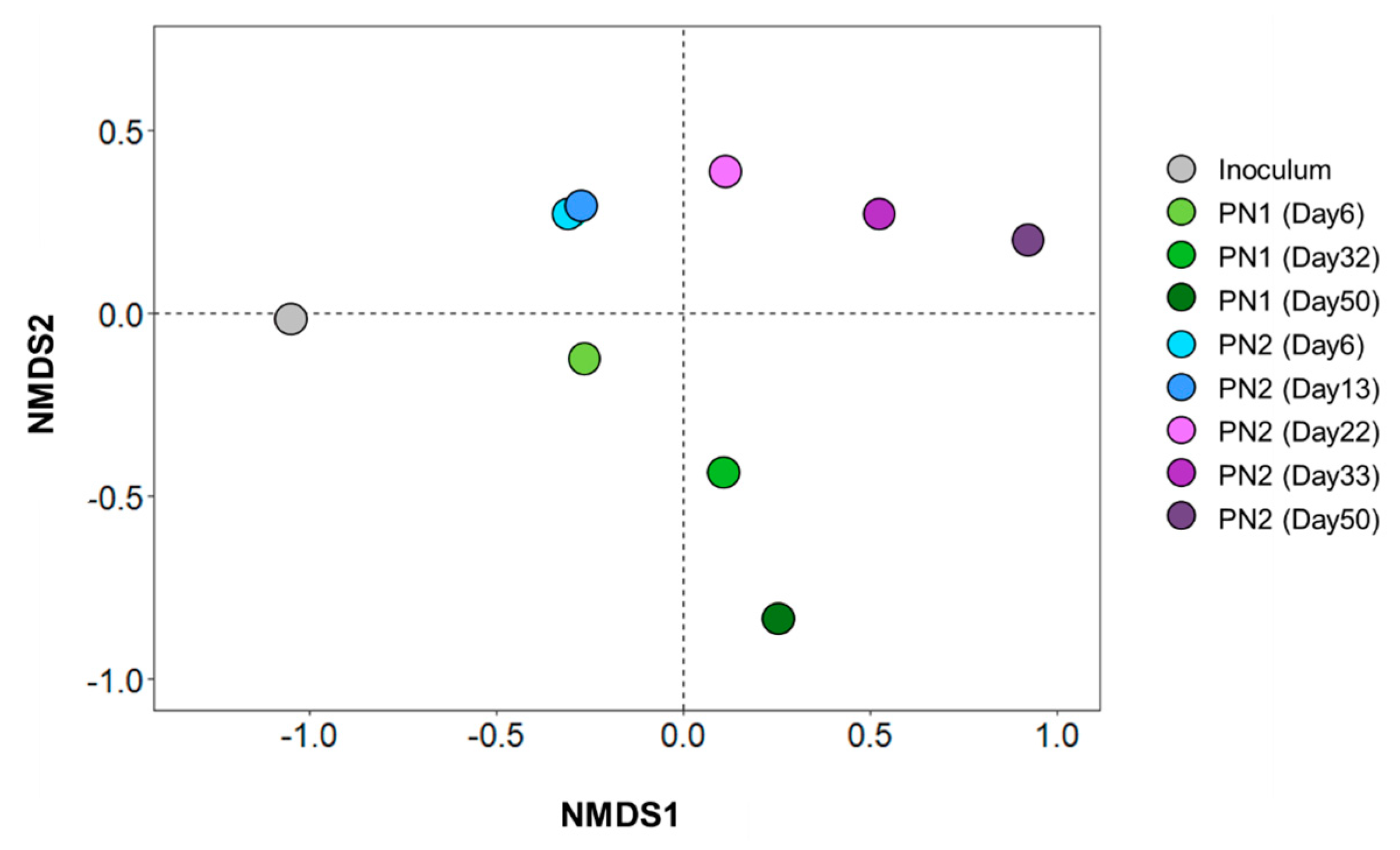
3.2. Changes in Microbial Communities According to FA Concentration
3.3. Changes in Dominant Nitrifying Bacteria According to FA Concentration
3.4. Implications for the Start-Up of Nitritation Processes in Mainstream WWTPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagga, U.; Bachner, J.; Strotmann, U. Inhibition of Nitrification in Laboratory Tests and Model Wastewater Treatment Plants. Chemosphere 2006, 65, 1–8. [Google Scholar] [CrossRef]
- Jetten, M.S.; Strous, M.; Van De Pas-Schoonen, K.T.; Schalk, J.; Van Dongen, U.G.; Van De Graaf, A.A.; Kuenen, J.G. The anaerobic oxidation of ammonium. FEMS Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; van Niftrik, L.; Keltjens, J.T.; den Camp, H.J.O.; Jetten, M.S. Anammox-growth physiology, cell biology, and metabolism. Adv. Microb. Physiol. 2012, 60, 211–262. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yu, J.; Jeong, S.; Kim, J.; Park, S.; Bae, H.; Rhee, S.K.; Unno, T.; Ni, S.Q.; Lee, T. Differences in the effects of calcium and magnesium ions on the anammox granular properties to alleviate salinity stress. Appl. Sci. 2021, 12, 19. [Google Scholar] [CrossRef]
- Yu, J.; Kim, Y.; Kim, J.; Jeong, S.; Park, S.; Lee, T. A simple analysis method of specific anammox activity using a respirometer. Appl. Sci. 2022, 12, 1121. [Google Scholar] [CrossRef]
- Kim, J.; Yu, J.; Kwon, T.; Choi, W.; Direstiyani, L.C.; Jeong, S.; Kim, Y.; Park, S.; Bae, H.; Lee, T. The real-time monitoring system strategy for stable long-term operation of pilot-scale single-stage deammonification (SSD) process treating moderate-strength NH4+. J. Water Process Eng. 2022, 48, 102895. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.; Direstiyani, L.C.; Kim, Y.; Yu, J.; Lee, T. Long-term adaptation of two anammox granules with different ratios of Candidatus Brocadia and Candidatus Jettenia under increasing salinity and their application to treat saline wastewater. Sci. Total Environ. 2023, 860, 160494. [Google Scholar] [CrossRef]
- Cao, Y.; van Loosdrecht, M.C.; Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: Status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef]
- Kim, J.; Direstiyani, L.C.; Jeong, S.; Kim, Y.; Park, S.; Yu, J.; Lee, T. Feeding strategy for single-stage deammonification to treat moderate-strength ammonium under low free ammonia conditions. Sci. Total Environ. 2023, 857, 159661. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.; Xia, W.; Ye, M.; Li, Y.Y. Dynamic pulse approach to enhancing mainstream Anammox process stability: Integrating sidestream support and tackling nitrite-oxidizing bacteria challenges. Bioresour. Technol. 2024, 395, 130327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Liu, Y.; Li, W. A review of partial nitrification in biological nitrogen removal processes: From development to application. Biodegradation 2021, 32, 229–249. [Google Scholar] [CrossRef]
- Trinh, H.P.; Lee, S.H.; Jeong, G.; Yoon, H.; Park, H.D. Recent developments of the mainstream anammox processes: Challenges and opportunities. J. Environ. Chem. Eng. 2021, 9, 105583. [Google Scholar] [CrossRef]
- Xu, G.; Zhou, Y.; Yang, Q.; Lee, Z.M.P.; Gu, J.; Lay, W.; Cao, Y.; Liu, Y. The challenges of mainstream deammonification process for municipal used water treatment. Appl. Microbiol. Biotechnol. 2015, 99, 2485–2490. [Google Scholar] [CrossRef]
- Kao, C.; Li, J.; Gao, R.; Li, W.; Li, X.; Zhang, Q.; Peng, Y. Advanced nitrogen removal from real municipal wastewater by multiple coupling nitritation, denitritation and endogenous denitritation with anammox in a single suspended sludge bioreactor. Water Res. 2022, 221, 118749. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Di, F.; Zhang, J.; Chen, M.; Wei, Y. A single-stage membrane aerated biofilm reactor achieving the combination of partial nitritation/anammox and enhanced biological phosphorus removal. J. Water Process Eng. 2024, 58, 104933. [Google Scholar] [CrossRef]
- Klaus, S.; Baumler, R.; Rutherford, B.; Thesing, G.; Zhao, H.; Bott, C. Startup of a partial nitritation-anammox MBBR and the implementation of pH-based aeration control. Water Environ. Res. 2017, 89, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, X.; Li, B.; Bai, X.; Peng, Y. Nitritation and denitrifying phosphorus removal via nitrite pathway from domestic wastewater in a continuous MUCT process. Bioresour. Technol. 2013, 143, 187–195. [Google Scholar] [CrossRef]
- Laureni, M.; Weissbrodt, D.G.; Szivák, I.; Robin, O.; Nielsen, J.L.; Morgenroth, E.; Joss, A. Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Water Res. 2015, 80, 325–336. [Google Scholar] [CrossRef]
- Ma, B.; Bao, P.; Wei, Y.; Zhu, G.; Yuan, Z.; Peng, Y. Suppressing nitrite-oxidizing bacteria growth to achieve nitrogen removal from domestic wastewater via anammox using intermittent aeration with low dissolved oxygen. Sci. Rep. 2015, 5, 13048. [Google Scholar] [CrossRef]
- Yeshi, C.; Hong, K.B.; Van Loosdrecht, M.C.; Daigger, G.T.; Yi, P.H.; Wah, Y.L.; Chye, C.S.; Ghani, Y.A. Mainstream partial nitritation and anammox in a 200,000 m3/day activated sludge process in Singapore: Scale-down by using laboratory fed-batch reactor. Water Sci. Technol. 2016, 74, 48–56. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. Available online: https://www.jstor.org/stable/25038971 (accessed on 1 August 2024).
- Duan, H.; Ye, L.; Lu, X.; Yuan, Z. Overcoming nitrite oxidizing bacteria adaptation through alternating sludge treatment with free nitrous acid and free ammonia. Environ. Sci. Technol. 2019, 53, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Silva, B.M.; Jonassen, K.R.; Bakke, I.; Østgaard, K.; Vadstein, O. Nitrification at different salinities: Biofilm community composition and physiological plasticity. Water Res. 2016, 95, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hüpeden, J.; Wemheuer, B.; Indenbirken, D.; Schulz, C.; Spieck, E. Taxonomic and functional profiling of nitrifying biofilms in freshwater, brackish and marine RAS biofilters. Aquac. Eng. 2020, 90, 102094. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, Y.; Wang, Q.; Zhang, Z.; Wu, C.; Gao, M.; Liu, F. The summary of nitritation process in mainstream wastewater treatment. Sustainability 2022, 14, 16453. [Google Scholar] [CrossRef]
- Strotmann, U.J.; Windecker, G. Kinetics of Ammonium Removal with Suspended and Immobilized Nitrifying Bacteria in Different Reactor Systems. Chemosphere 1997, 35, 2939–2952. [Google Scholar] [CrossRef]
- APHA; AWWA; AEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Wang, B.; Wang, Z.; Wang, S.; Qiao, X.; Gong, X.; Gong, Q.; Liu, W.; Peng, Y. Recovering Partial Nitritation in a PN/A System During Mainstream Wastewater Treatment by Reviving AOB Activity After Thoroughly Inhibiting AOB and NOB with Free Nitrous Acid. Environ. Int. 2020, 139, 105684. [Google Scholar] [CrossRef]
- Le, L.T.; Lee, S.; Bui, X.T.; Jahng, D. Suppression of nitrite-oxidizing bacteria under the combined conditions of high free ammonia and low dissolved oxygen concentrations for mainstream partial nitritation. Environ. Technol. Innov. 2020, 20, 101135. [Google Scholar] [CrossRef]
- Blackburne, R.; Vadivelu, V.M.; Yuan, Z.; Keller, J. Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Water Res. 2007, 41, 3033–3042. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Gao, D. Effects of three storage conditions on the long-term storage and short-term reactivation performances of anammox granular sludge. Int. Biodeterior. Biodegrad. 2021, 164, 105310. [Google Scholar] [CrossRef]
- Dacewicz, E.; Lenart-Boroń, A. Waste Polyurethane Foams as Biomass Carriers in the Treatment Process of Domestic Sewage with Increased Ammonium Nitrogen Content. Materials 2023, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Weisburg, W.G.; Paster, B.J.; Hahn, C.M.; Tanner, R.S.; Krieg, N.R.; Stackebrandt, E. The phylogeny of purple bacteria: The beta subdivision. Syst. Appl. Microbiol. 1984, 5, 327–336. [Google Scholar] [CrossRef]
- Wang, X.; Wen, X.; Criddle, C.; Wells, G.; Zhang, J.; Zhao, Y. Community analysis of ammonia-oxidizing bacteria in activated sludge of eight wastewater treatment systems. J. Environ. Sci. 2010, 22, 627–634. [Google Scholar] [CrossRef] [PubMed]

| Operational Condition | PN1 | PN2 |
|---|---|---|
| Influent NH4+-N (mg/L) | 50 | 300, 200, 100, 50 |
| Influent FA (mg/L) | 10 | 50, 35, 18, 10 |
| HRT (h) | 5–7.5 | 5–10 |
| DO (mg/L) | <0.5 | |
| pH | 8.5 | |
| Primer | Sequence (5’-3’) | PCR Conditions |
|---|---|---|
| V3F | CCTACGGGAGGCAGCAG |
|
| V4R | GGACTACHVGGGTWTCTAAT |
| Reactor | Sample Name | FA (mg/L) | ASVs | Chao1 | Shannon | Good’s Coverage |
|---|---|---|---|---|---|---|
| Inoculum | 0 | 1299 | 1308 | 8.66 | 0.99 | |
| PN1 | Day 6 | 10 | 1018 | 1024 | 7.46 | 0.99 |
| Day 33 | 10 | 942 | 853 | 7.06 | 0.99 | |
| Day 50 | 10 | 826 | 836 | 6.74 | 0.99 | |
| PN2 | Day 6 | 50 | 763 | 764 | 6.41 | 0.99 |
| Day 13 | 50 | 763 | 764 | 6.21 | 0.99 | |
| Day 22 | 35 | 651 | 652 | 6.37 | 0.99 | |
| Day 33 | 18 | 625 | 625 | 6.72 | 0.99 | |
| Day 50 | 10 | 550 | 552 | 6.52 | 0.99 | |
| Inoculum | PN1 | PN2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | 0 | 6 | 33 | 50 | 6 | 13 | 22 | 33 | 50 |
| Nitrosomonas | 0.00 | 0.24 | 6.45 | 2.82 | 3.04 | 3.64 | 14.75 | 21.78 | 23.82 |
| Nitrospira | 1.29 | 3.23 | 7.94 | 28.48 | 0.87 | 0.58 | 0.19 | 0.09 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Park, S.; Kim, H.; Yoon, S.; Park, S.; Kim, D.; Kim, J.; Kim, Y.; Yu, J.; Lee, T. Free Ammonia Strategy for Nitrite-Oxidizing Bacteria (NOB) Suppression in Mainstream Nitritation Start-Up. Appl. Sci. 2024, 14, 7801. https://doi.org/10.3390/app14177801
Jeong S, Park S, Kim H, Yoon S, Park S, Kim D, Kim J, Kim Y, Yu J, Lee T. Free Ammonia Strategy for Nitrite-Oxidizing Bacteria (NOB) Suppression in Mainstream Nitritation Start-Up. Applied Sciences. 2024; 14(17):7801. https://doi.org/10.3390/app14177801
Chicago/Turabian StyleJeong, Soyeon, Seongjae Park, Hojun Kim, Seongwon Yoon, Sewon Park, Doheung Kim, Jeongmi Kim, Yeonju Kim, Jaecheul Yu, and Taeho Lee. 2024. "Free Ammonia Strategy for Nitrite-Oxidizing Bacteria (NOB) Suppression in Mainstream Nitritation Start-Up" Applied Sciences 14, no. 17: 7801. https://doi.org/10.3390/app14177801
APA StyleJeong, S., Park, S., Kim, H., Yoon, S., Park, S., Kim, D., Kim, J., Kim, Y., Yu, J., & Lee, T. (2024). Free Ammonia Strategy for Nitrite-Oxidizing Bacteria (NOB) Suppression in Mainstream Nitritation Start-Up. Applied Sciences, 14(17), 7801. https://doi.org/10.3390/app14177801






