Abstract
Pearl oyster shells are composed of a double layer of calcium carbonate polymorphs: prismatic and nacreous. The nacreous layer is used in functional foods and cosmetics. In an earlier work, we reported that sulfated polysaccharides in nacre extract ameliorated memory impairment induced by a single dose of scopolamine. Here, we investigated whether sulfated polysaccharides suppress amyloid-beta (Aβ) deposition in an Alzheimer’s disease model induced by prolonged administration of scopolamine. Chronic scopolamine administration induces Aβ deposition; however, sulfated polysaccharides suppressed this effect. Additionally, sulfated polysaccharides ameliorated the accumulation of phosphorylated tau, neuroinflammation, and neuronal cell death in the brain, which are common features of patients with Alzheimer’s disease. To further determine the inhibitory mechanisms of Aβ deposition, we assessed the amount of the Aβ-degrading enzyme insulin-degrading enzyme (IDE). In animal experiments, sulfated polysaccharides increased IDE levels in scopolamine-treated mice. To study the effect of sulfated polysaccharides on insulin signaling, which regulates IDE expression, we evaluated the expression levels of phosphorylated Akt and nuclear factor-kB. Sulfated polysaccharides restored the levels of phosphorylated Akt and nuclear factor-kB, which were decreased and increased, respectively, using scopolamine treatment. Overall, our findings suggest that sulfated polysaccharides suppress Aβ deposition by regulating IDE expression.
1. Introduction
Alzheimer’s disease (AD) causes dementia, which is linked to substantial memory loss combined with cognitive deterioration. The main pathological characteristics of AD include significant lesions in the brain, identified by abnormally hyperphosphorylated neurofibrillary tangles within neurons and the extracellular buildup of amyloid-beta (Aβ) plaques. These hallmarks, especially the excessive accumulation of toxic protein Aβ, are commonly observed in patients with AD [1,2,3].
Aβ is formed from the amyloid precursor protein (APP) via hydrolysis by β-secretase (or β-site APP-cleaving enzyme 1 [BACE1]) [4]. Aβ aggregates give rise to soluble oligomers that exist in various forms [5]. The buildup of Aβ is harmful, as it induces neuroinflammation, the formation of hyperphosphorylated tau, neuronal damage, synaptic dysfunction, and neurodegeneration [6]. Aβ levels in the brain are maintained by the dynamic balance between its production from APP and removal by amyloid-degrading enzymes, e.g., insulin-degrading enzyme (IDE) and neprilysin (NEP) [7,8,9,10].
IDE is a zinc metalloprotease that has a major role in insulin degradation. Additionally, IDE also plays a crucial function in Aβ peptide breakdown in AD [11]. Loss-of-function mutations in IDE result in impaired Aβ degradation [12], and IDE knockout animals exhibit increased Aβ plaque formation in the brain [13]. By contrast, IDE overexpression significantly reduces the Aβ levels in Chinese hamster ovary cells [14,15]. Transgenic mice overexpressing IDE exhibit significantly lower Aβ levels and high survival rates [16].
Insulin signaling in the brain regulates various functions and processes, including energy homeostasis, cognitive processes, and neuronal survival [17]. Disruption of this signaling pathway is associated with AD pathogenesis. Insulin signaling and IDE expression are intricately connected via mechanisms regulating insulin levels and Aβ degradation [18,19]. Drugs improving insulin sensitivity, such as metformin, enhance insulin signaling and upregulate IDE expression, thereby decreasing the level of Aβ [20].
Scopolamine is an anticholinergic drug that disrupts cholinergic neurotransmission, leading to cognitive impairment similar to that observed in AD [21]. Chronic administration of scopolamine causes Aβ deposition and tau phosphorylation in mice [22]. These pathological changes mimic those of patients with AD and other AD models [23,24]. Therefore, scopolamine is widely used to establish AD mouse models.
Pearl oysters are harvested in Japan to acquire jewelry pearls. The unwanted shells are disposed of as industrial waste, necessitating their proper recycling. In an earlier report, we showed that nacre extract can enhance memory in several mouse models of memory impairment [25,26,27,28,29,30]. The mouse models were established via intracerebroventricular administration of Aβ, a single injection of scopolamine, and chronic administration of D-galactose. Intraperitoneal treatment with sulfated polysaccharides from pearl oyster shells blocks single-dose scopolamine-induced memory loss [31]. However, the specific effect of sulfated polysaccharides on Aβ accumulation remains unclear. Here, we sought to explore the effects of sulfated polysaccharides on Aβ accumulation using a chronic scopolamine-induced mouse model.
2. Methods
2.1. Materials
Pearl oyster (Pinctada fucata) shells were harvested from Iki Bay (Nagasaki, Japan). The antibodies against IDE (orb135709), phosphorylated tau (p-tau) (orb14829), insulin (orb10922), phosphorylated protein kinase B (p-Akt) (orb304681), NF-kB (orb11118), β-actin (orb378579), and goat anti-rabbit IgG alkaline phosphatase-conjugated secondary antibody (orb1878909) were from Biorbyt (San Francisco, CA, USA).
2.2. Preparation of the Nacre Extract and Sulfated Polysaccharides
First, the prismatic part of the shells was removed, and the nacreous layer finely ground and then decalcified in a 10% acetic acid solution for at least seven days. The decalcified solution was thoroughly dialyzed against demineralized water to completely remove the calcium carbonate. After dialysis, the solution was lyophilized, and the dried powder was suspended again in deionized water. The resulting solution was used as the nacre extract [25,26,27,28,29,30]. Sulfated polysaccharides were purified from the nacre extract as reported in an earlier study [31]. Firstly, nacre extract was adsorbed on a TSKgel DEAE-5PW column (TOSOH, Tokyo, Japan) equilibrated with 20 mM Tris-HCl (pH 7.5). The bound substance was then eluted using a salt gradient of 0–0.5 M NaCl. Fractions eluted with 0.35 M–0.5 M NaCl were collected and further fractionated on a C18 reverse-phase column (ODS80TS; TOSOH, Tokyo, Japan). The bound substance was eluted using a linear gradient of 0–50% acetonitrile. Fractions obtained with 35–50% acetonitrile were pooled, concentrated, and designated as nacre polysaccharides.
2.3. Animals
Male ICR mice (4 weeks old) were purchased from CREA (Tokyo, Japan). The mice were housed in a facility maintained at a constant 24 °C, 50% humidity, with a 12 h light/dark cycle. Five mice per group were caged and acclimated for 2 weeks. Mice were fed 4 g of food per day (AIN-76A-based control diet; Oriental Yeast Co., Ltd., Tokyo, Japan) with free access to water.
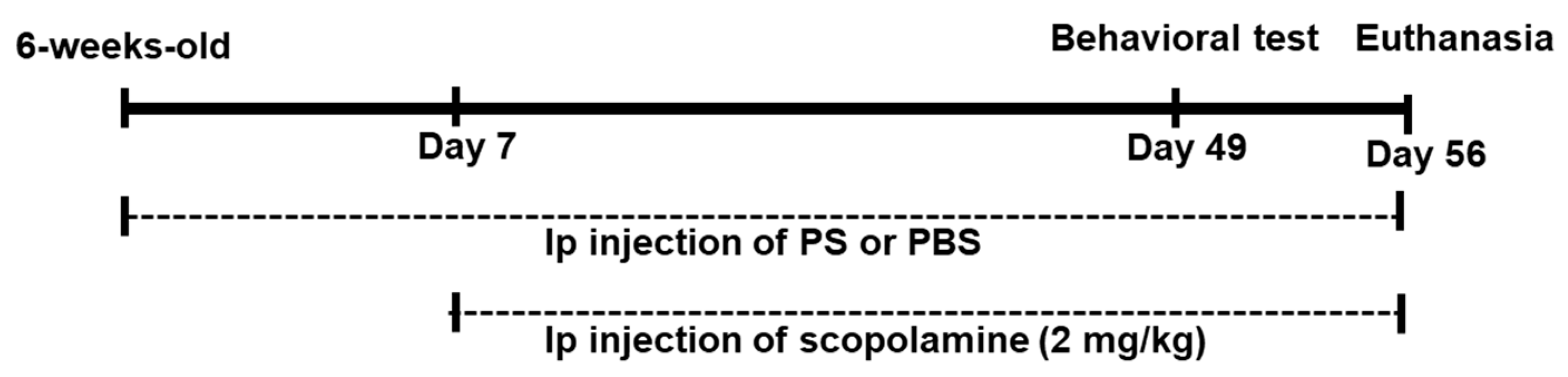
Scopolamine was dissolved in phosphate-buffered saline (PBS). Each mouse was intraperitoneally injected with scopolamine (2 mg/kg) or PBS (control group) daily for two months. Sulfated polysaccharides were intraperitoneally injected daily for seven weeks at doses of 50 µg/kg (PS50 group) or 100 µg/kg (PS100 group) (Figure 1). Doses of sulfated polysaccharides were determined based on the findings of a previous study [31]. Following 49 days of administration, a Y-maze test and a novel object recognition test were carried out. After euthanasia, brain tissue (cerebral cortex including hippocampus) was promptly removed and stored at −80 °C.

Figure 1.
Experimental schedule of chronic scopolamine and sulfated polysaccharides (PS) administration.
All animal studies were performed in accordance with “the Regulations and Guidelines for Animal Care and Use” in the Muroran Institute of Technology (approval number: H29KS01). The Committee on Animal Research and Ethics at the Muroran Institute of Technology approved all experimental protocols.
2.4. Novel Object Recognition Test
The novel object recognition test was carried out as reported earlier [25,26,27,28,29,30,31]. Briefly, mice were separately introduced into an arena (60 cm in diameter) containing two different shaped colored objects and were allowed to explore for five minutes. Twenty-four hours later, one of the previous objects was swapped for a novel object, and the mice were placed in the arena again. Interactions with both objects were recorded for five minutes. The discrimination index was measured as follows:
Discrimination index = ([time spent on the exploration of the novel object]/[time spent on the exploration of the familiar object + the novel object]) × 100
2.5. Y-Maze Test
The Y-maze test was performed as outlined in a previous report [25,26,27,28,29,30,31]. Briefly, each mouse was introduced to the central area of a Y-maze apparatus (arm length: 35 cm, height: 25 cm) and allowed to freely explore the maze for 10 min. An alternation was defined as entering the three different arms consecutively. For each mouse, the number of entries into each arm, as well as the sequence of visits, were recorded. The percentage of spontaneous alternations was determined as follows:
Spontaneous alternation (%) = ([number of correct alternations]/[total number of arm entries − 2]) × 100
2.6. Histochemistry
For histochemical analysis, mice were anesthetized using sevoflurane and transcardially perfused with 4% paraformaldehyde in PBS. Brain tissue samples were collected and embedded in paraffin. Regions containing the dorsal hippocampus (−1.5 to −3.5 mm from bregma) were sectioned into 3 µm thick slices. After deparaffinization, sections were stained with hematoxylin-eosin, Congo red, as well as immunohistochemically. Immunohistochemistry was carried out on the brain sections using specific antibodies and a Polymer-Based 1-Step immunohistochemistry (IHC) system (VitroVivo Biotech, Rockville, MD, USA). Briefly, the deparaffinized sections underwent heat-induced epitope retrieval (10 mM citric acid at 110 °C for 15 min). Slides were washed in PBS-Triton X-100 (0.025%) prior to incubation with normal goat serum at 25 °C for 30 min. Next, slides were treated with primary antibody (100- to 200-fold dilution) and incubated overnight at 4 °C. After rinsing with PBS, slides were incubated in 0.3% H2O2 solution at 25 °C for 10 min. The slides were then washed with PBS and incubated with secondary antibody (goat anti-rabbit IgG antibody, VitroVivo Biotech, Rockville, MD, USA) at 25 °C for 1 h. Following extensive washing with PBS, a 3,3′-diaminobenzidine (DAB) solution was applied to the slides and was allowed to develop. When sufficient staining was achieved, sterile water was added to stop the reaction. Hematoxylin was then applied and stained for 30 s.
For the analysis of brain sections from two or three mice, ten to twenty non-overlapping fields from six different sections were captured using a Nikon TS100 microscope (Tokyo, Japan). Regions stained with 3,3′-diaminobenzidine were quantified with the aid of ImageJ software (version 1.8.0). The DAB-stained area was expressed as the stained area (arbitrary units) relative to the unit area of brain tissue. The number of amyloid plaques per field was counted and quantified.
2.7. RT-qPCR
Total RNA was prepared from brain tissue (cerebral cortex including hippocampus) using an RNAiso Plus Kit (Takara, Shiga, Japan) and was reverse-transcribed as outlined in previous reports [26,27,28,29,30,31], followed by quantitative PCR using iTaq Universal SYBR Green Supermix (Bio-Rad, Tokyo, Japan). Sequences of the oligonucleotide primers used for qRT-PCR were as follows: β -actin, sense 5′-TTGTTACAGGAAGTCCCTTGCC-3′ and anti-sense 5′-ATGCTATCACCTCCCCTGTGTG-3′; IDE, sense 5′-ACTAACCTGGTGGTGAAG-3′ and anti-sense 5′-GGTCTGGTATGGGAAATG-3′; BACE1, sense 5′-TTCCCAAGAAAGTATTTGA-3′ and anti-sense 5′-TGATGCGGAAGGACTGATT-3′; IL6 sense 5′-CCACTGCCTTCCCTACTTCA-3′ and anti-sense 5′-ACTCCAGAAGACCAGAGCAG-3′; TNF-α, sense 5′-GAGCACGGAAAGCATGATCC-3′ and anti-sense 5′-TAGACAGAAGAGCGTGGTGG-3′. With β-actin as the internal standard, the comparative ΔCt method was used to determine the relative expression level of each mRNA.
2.8. Western Blot Analyses
The brain tissue (including the cerebral cortex and hippocampus) was homogenized in deionized water, followed by the addition of 2% sodium dodecyl sulfate (SDS), 2-mercaptoethanol, and bromophenol blue. Upon heating at 100 °C for 5 min, the lysate was resolved using SDS-PAGE [32] and then transferred to polyvinylidene difluoride membranes. After blocking with 5% skimmed milk solution containing PBS and 0.05% Tween 20 (PBS-tween20 solution) for 2 h, the membranes were incubated with antibodies against β-actin, p-tau, and IDE (Biorbyt) overnight at 4 °C. After washing with PBS-tween20 solution, membranes were incubated with alkaline phosphatase-conjugated secondary antibody for 2 h at 25 °C. Membranes were washed with PBS-tween 20 solution, and color development was initiated with the addition of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. The intensity of stained bands was quantified using ImageJ software (version 1.8.0).
2.9. Statistical Analyses
Data of five mice in each group are given as the mean ± standard deviation in behavioral tests and real-time PCR analysis. The results were analyzed using Fisher’s test. p < 0.05 was considered significantly different. All statistical analyses were conducted using the statistics software Bell Curve for Excel v2.15 (Tokyo, Japan).
3. Results
3.1. Effect of Sulfated Polysaccharides on Chronic Scopolamine-Induced Memory Impairment
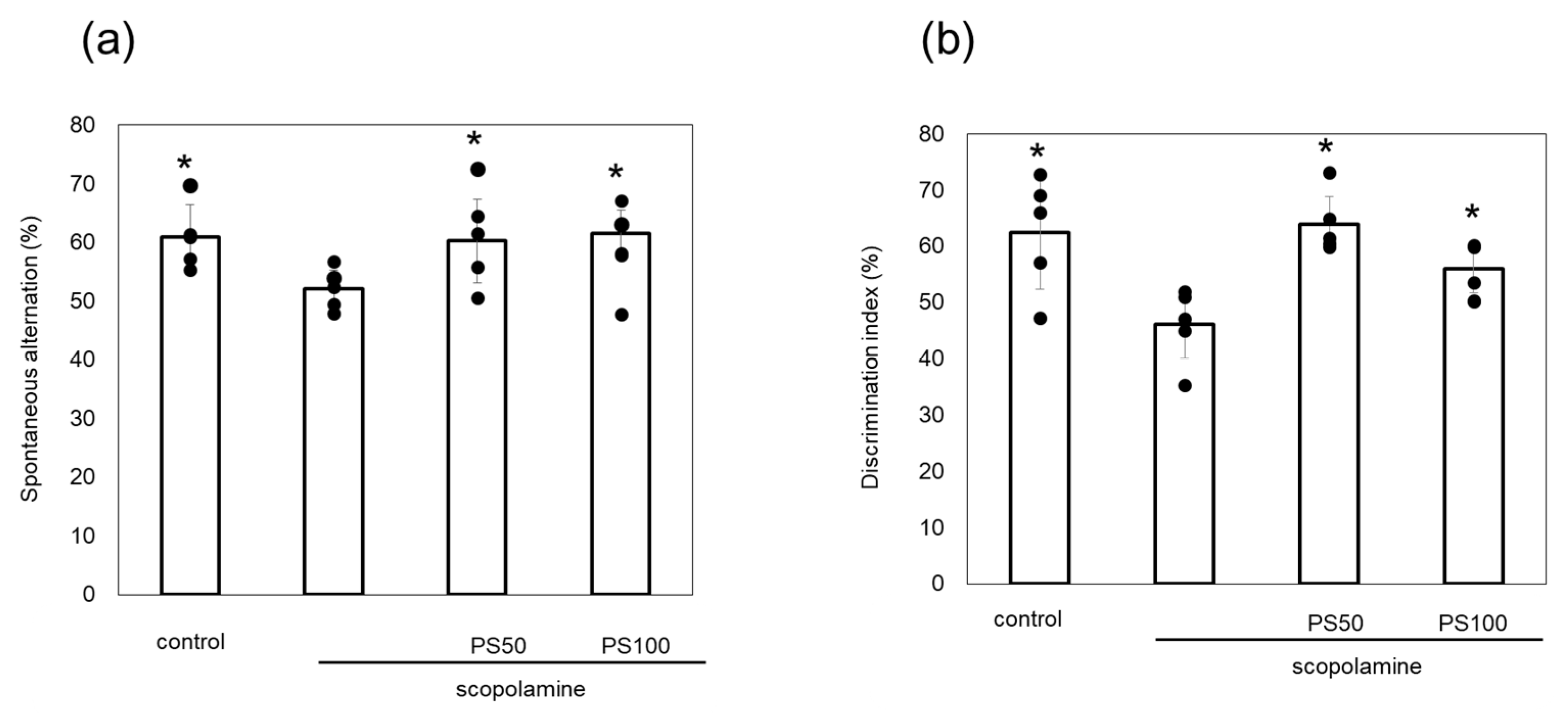
Scopolamine induces memory loss, and its chronic administration leads to Aβ plaque formation and tau phosphorylation in the brain. Here, the effects of sulfated polysaccharides on chronic scopolamine-induced memory impairment were evaluated via Y-maze and novel object recognition tests. Chronic scopolamine administration significantly reduced spontaneous alternation by comparison to control mice. However, treatment with sulfated polysaccharides (50 and 100 µg/kg) reversed this loss of spontaneous alternation (Figure 2a). In the novel object recognition test, which measures recognition memory, chronic scopolamine-treated mice spent less time investigating the novel object than the control mice. However, mice treated with the sulfated polysaccharides exhibited significantly increased relative exploration time (Figure 2b). These observations indicate that sulfated polysaccharides improve chronic scopolamine-induced memory impairment.
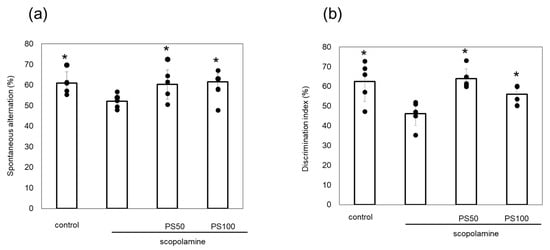
Figure 2.
Effect of sulfated polysaccharides (PS) on memory impairment induced by the chronic administration of scopolamine. (a) Spontaneous alternation behavior in the Y-maze test. (b) Discrimination index measured using the novel object recognition test. PSs were injected at 50 µg/kg (PS50) or 100 µg/kg (PS100). Data of five mice are shown as the mean ± standard deviation (SD). * p < 0.05 vs. scopolamine group.
3.2. Effect of Sulfated Polysaccharides on Aβ Deposition
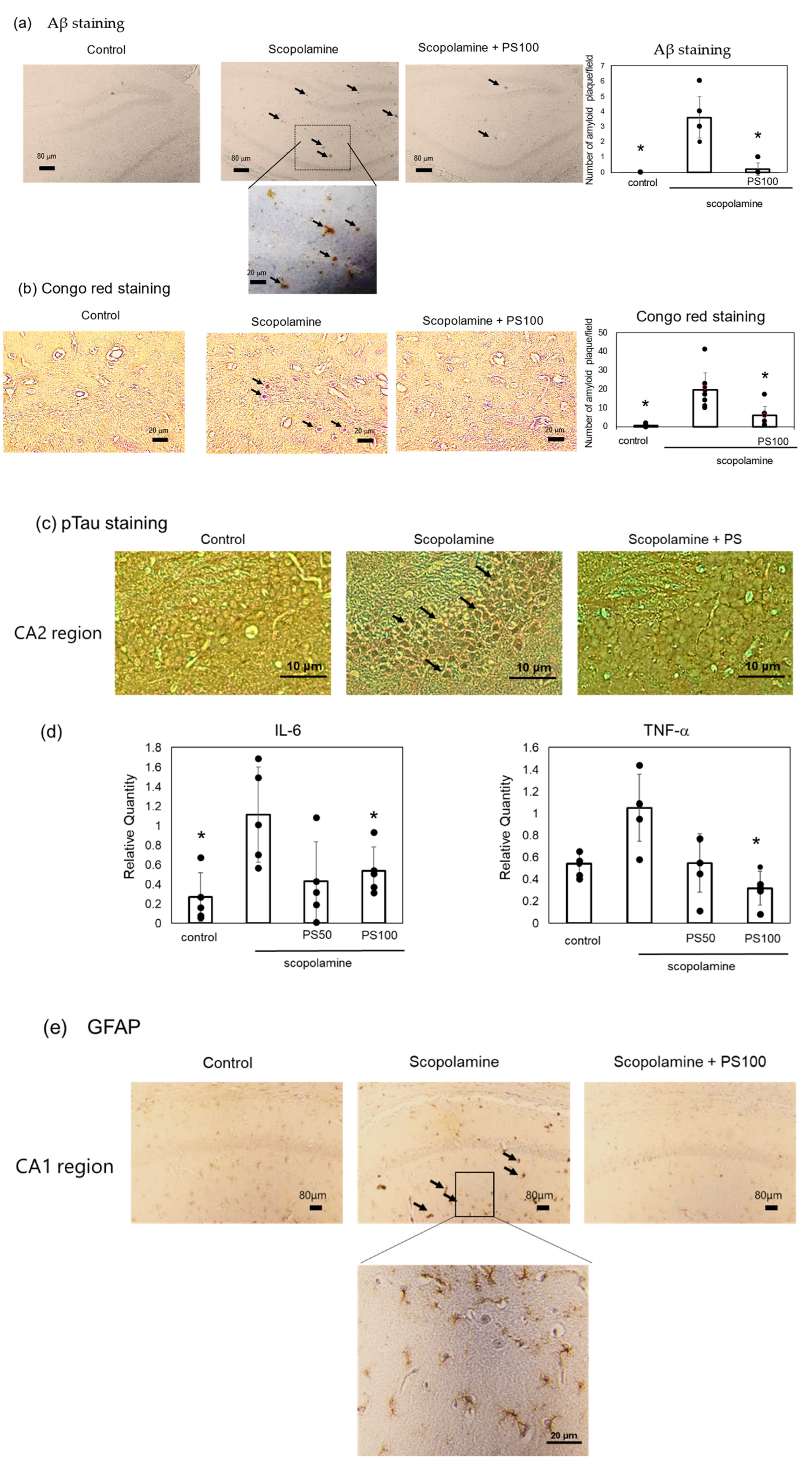
Chronic scopolamine treatment induces Aβ accumulation in the brain [22]. Here, Aβ accumulation was investigated via immunostaining. Sulfated polysaccharide-treated mice exhibited significantly lower Aβ deposition than the controls (Figure 3a). A reduction in Aβ deposition by sulfated polysaccharides was also verified via Congo red staining (Figure 3b). Aβ deposition is associated with high phosphorylated tau levels [33,34]. Immunohistochemistry revealed higher levels of phosphorylated tau (p-tau) in the hippocampus of chronic scopolamine-treated mice than in the hippocampus of controls; however, sulfated polysaccharides significantly suppressed phosphorylated tau expression (Figure 3c). Chronic scopolamine administration and Aβ accumulation trigger an inflammatory response [21]. Therefore, changes in the expression levels of the inflammatory cytokines, interleukin-6 and tumor necrosis factor-α, were evaluated via RT-qPCR (Figure 3d). Chronic scopolamine administration increased the inflammatory cytokine levels, but sulfated polysaccharides reversed this effect. Furthermore, glial fibrillary acidic protein (GFAP) expression, which is increased during inflammation, was also elevated by scopolamine, but was suppressed by the sulfated polysaccharides (Figure 3e). These results indicate that sulfated polysaccharides reduce chronic scopolamine-induced Aβ accumulation.
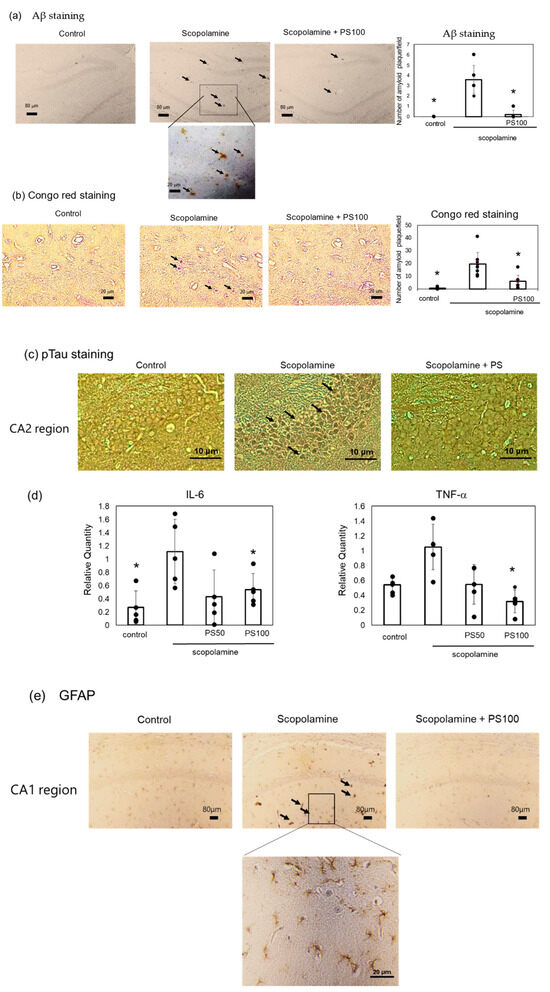
Figure 3.
Effect of sulfated polysaccharide (PS) on amyloid-beta (Aβ) deposition induced by the chronic administration of scopolamine. (a) Aβ deposits detected via immunostaining in the control group, scopolamine group, and scopolamine + PS group. Arrows indicate Aβ deposits in the hippocampus CA1 and DG regions. Scale bar: 20 μm or 80 μm. (b) Aβ deposits detected via Congo red staining. Scale bar: 20 μm. At least five areas per mouse (n = 3 mice) were randomly selected and analyzed, and the number of amyloid plaques per field is represented as the mean ± SD. (c) Phosphorylated tau estimated via immunostaining in the hippocampus CA2 region. Stained areas indicated by arrows. Scale bar: 20 μm. (d) Expression levels of inflammatory cytokines estimated via reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Data of five mice are represented as the mean ± SD. (e) Expression of glial fibrillary acidic protein (GFAP) determined via immunostaining. Arrows indicate stained GFAP in the hippocampus CA1 region. Scale bar: 20 μm. * p < 0.05 vs. scopolamine group.
3.3. Effects of Sulfated Polysaccharides on Hippocampal Neurons
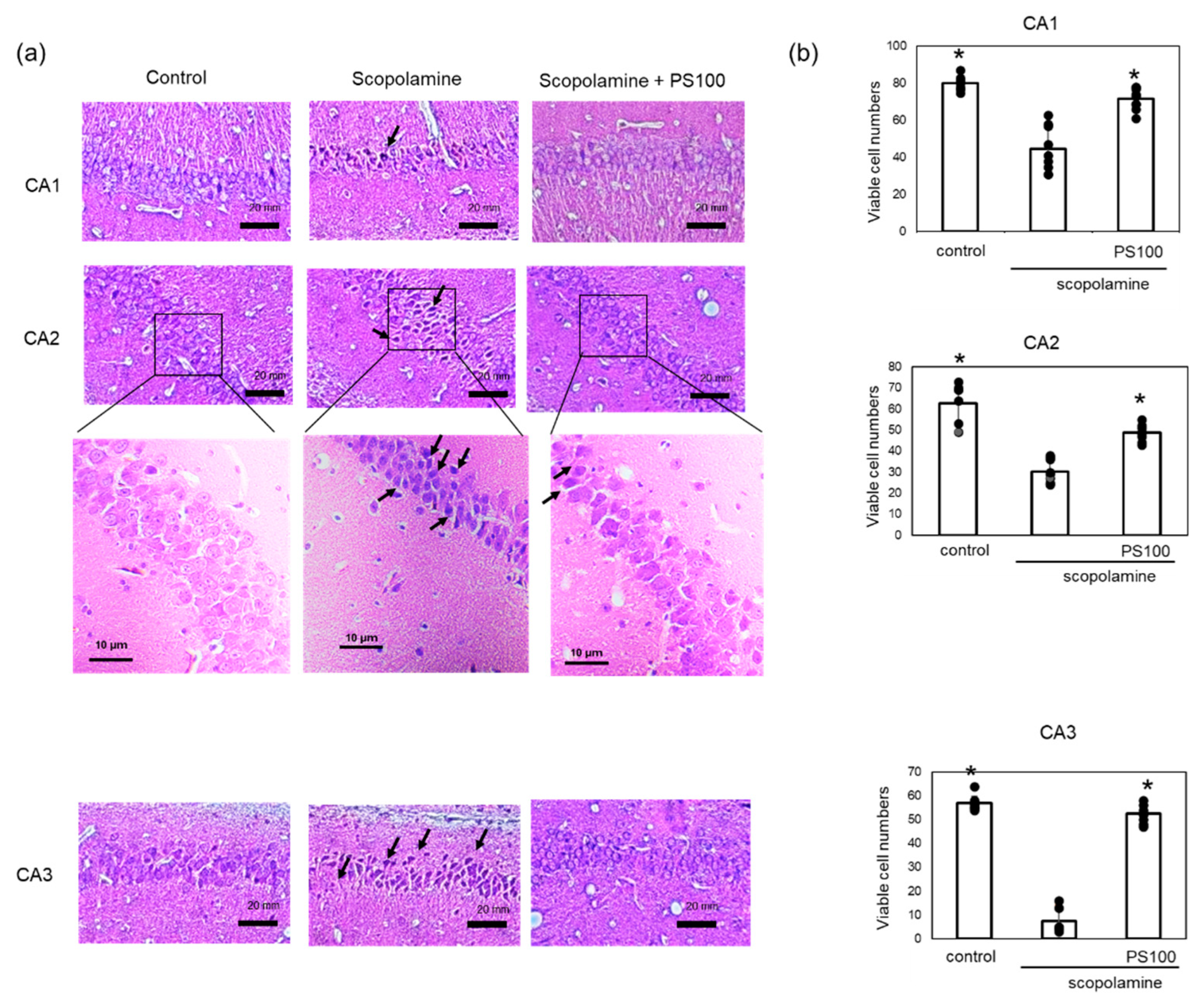
Chronic administration of scopolamine and Aβ accumulation cause damage to the hippocampal tissue. To confirm the effects of sulfated polysaccharides on hippocampal neurons, a histochemical analysis was conducted (Figure 4a,b). Control mice exhibited normal histological features of hippocampal neurons. Compared to that in the controls, chronic scopolamine treatment resulted in neuronal degeneration in the CA1, CA2, and CA3 regions. Specifically, chronic scopolamine treatment reduced the number of viable neurons in the CA3 region by approximately 10%; however, sulfated polysaccharides mitigated neuronal degeneration. These findings indicate that sulfated polysaccharides prevent chronic scopolamine-induced neuronal degeneration.
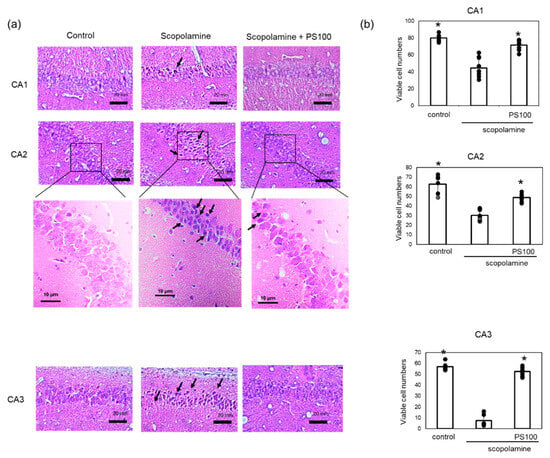
Figure 4.
Effect of sulfated polysaccharide (PS) on neuronal degeneration induced by the chronic administration of scopolamine. (a) Neuronal cells in the hippocampus of mice in the control group (left panel), scopolamine group (middle panel), and scopolamine + PS group (right panel) were stained with hematoxylin and eosin. Degenerated neurons are highlighted with arrows. Scale bars: 20 µm. (b) Numbers of viable neuronal cells were counted in the CA1, CA2, and CA3 regions. Approximately five areas per mouse (n = 2 mice) were randomly selected and analyzed, and the number of viable cells per field is given as the mean ± SD. * p < 0.05 vs. scopolamine group.
3.4. Effects of Sulfated Polysaccharide on the Aβ Metabolism-Associated Enzyme Levels
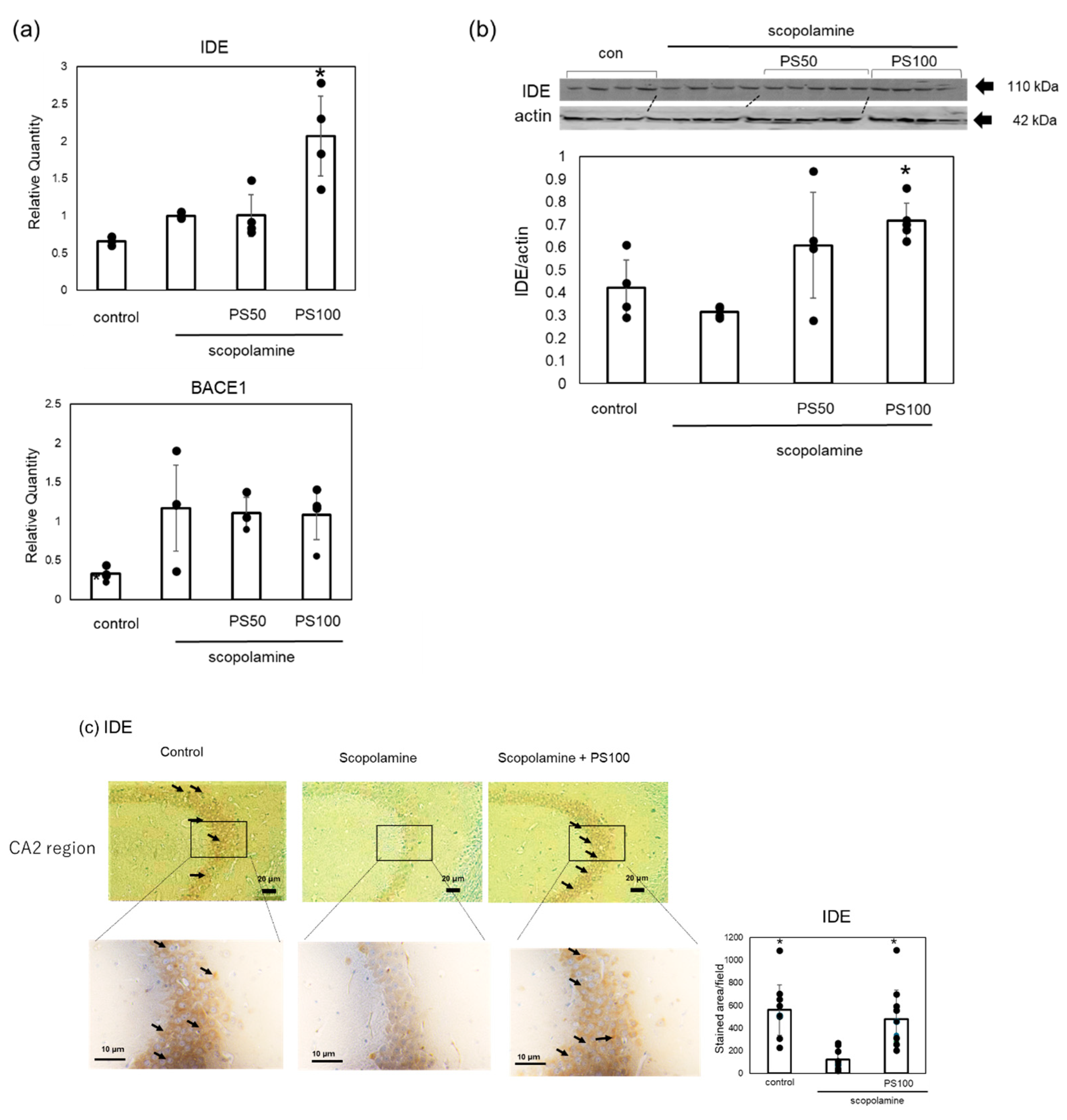
To elucidate the mechanisms by which sulfated polysaccharides inhibit Aβ accumulation, we examined changes in the expression level of IDE, which is involved in Aβ clearance, and BACE1, which is involved in Aβ formation, using RT-qPCR (Figure 5a). Scopolamine treatment increased BACE1 expression, but sulfated polysaccharide treatment did not decrease its expression. By contrast, IDE expression was not reduced by scopolamine treatment, but IDE expression significantly increased with sulfated polysaccharide treatment compared to scopolamine-treated mice. To further confirm that sulfated polysaccharides increased IDE levels, we examined the changes in IDE levels via Western blotting (Figure 5b). Scopolamine slightly decreased IDE levels; however, sulfated polysaccharides reversed this effect. Immunohistochemistry also revealed that scopolamine decreased the IDE levels in the hippocampus; however, sulfated polysaccharides restored the IDE levels (Figure 5c). These results suggest that sulfated polysaccharides inhibit scopolamine-induced Aβ deposition by increasing IDE expression.
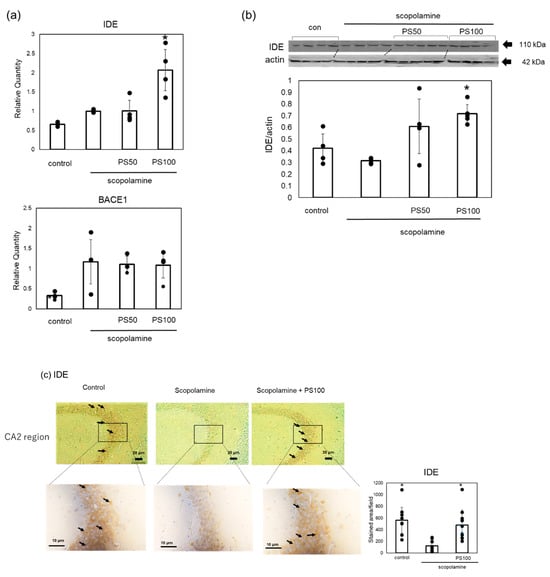
Figure 5.
Effects of sulfated polysaccharide (PS) on the expression levels of insulin-degrading enzyme (IDE) and β-site APP-cleaving enzyme 1 (BACE1) in chronic scopolamine-induced mice. (a) Gene expression levels of BACE1 and IDE quantified using RT-qPCR. (b) Protein expression levels of IDE assessed using Western blot analysis. Western blotting of IDE and β-actin was performed separately, and the IDE expression levels were quantified and normalized to β-actin. (c) Expression levels of IDE in the hippocampus CA2 region determined via immunostaining in the control group, scopolamine group, and scopolamine + PS group. Stained areas are indicated by arrows. At least ten areas per mouse (n = 2 mice) were randomly selected and analyzed, and the results are represented as the mean ± SD. Data of four or five mice are represented as the mean ± SD via real-time PCR and Western blotting. * p < 0.05 vs. scopolamine group.
3.5. Effect of Sulfated Polysaccharides on Insulin Signaling
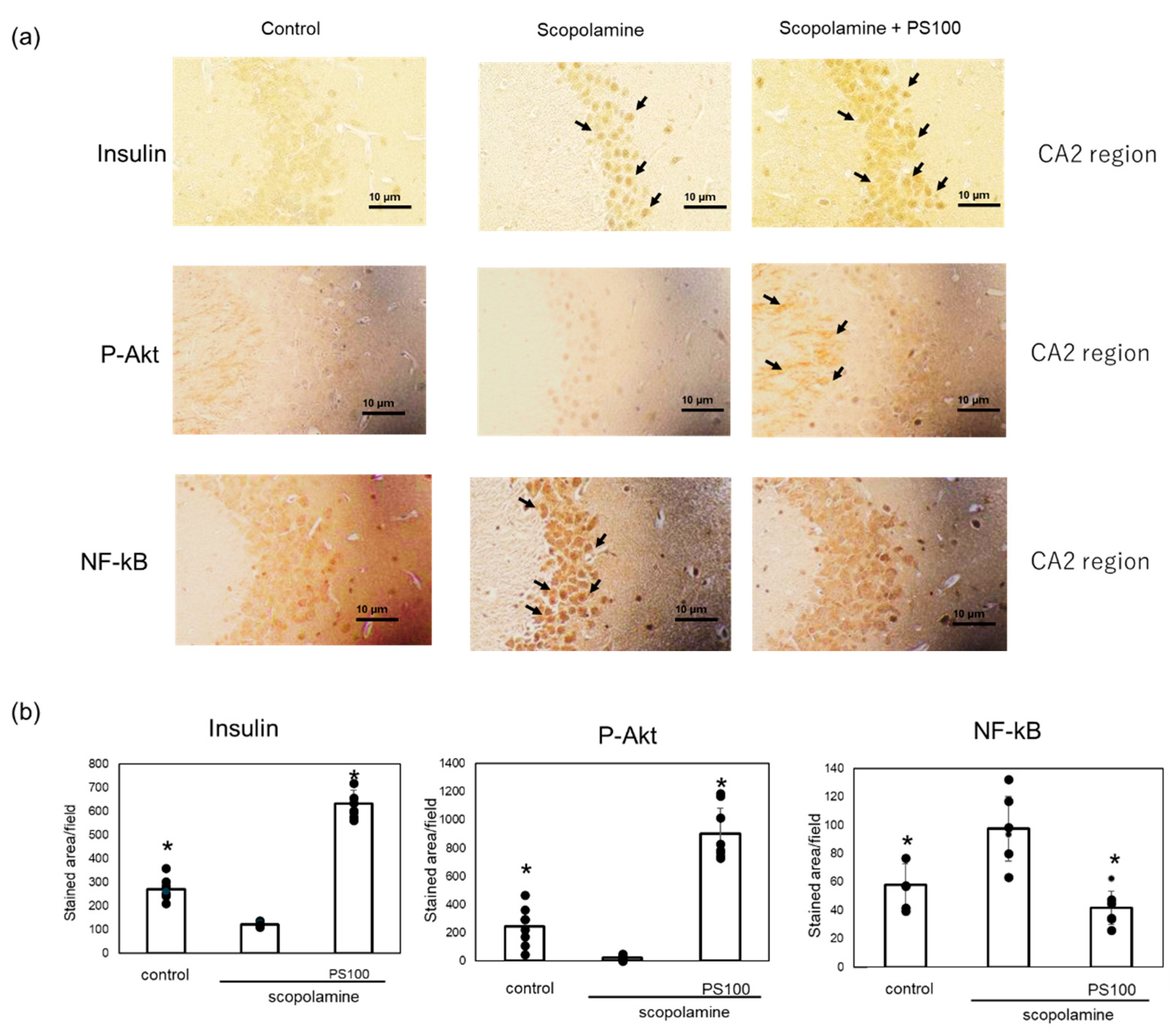
Activation of insulin signaling increases IDE expression in the brain [18]. Therefore, we investigated changes in the expression levels of proteins involved in insulin signaling in the brain via immunostaining. Insulin expression in the hippocampus were higher in the sulfated polysaccharide-treated mice compared with scopolamine-treated mice (Figure 6a). To confirm the activation of insulin signaling, we examined the expression levels of p-Akt, which activates insulin signaling [35], and nuclear factor (NF)-kB, which inhibits insulin signaling [36], via immunostaining. p-Akt was expressed in the plasma and dendritic membranes of neurons in the CA2 region, as reported previously by Wang et al. [37] (Figure 6b). Scopolamine decreased the p-Akt levels, which were restored by the sulfated polysaccharides. In comparison to control mice, chronic administration of scopolamine increased the NF-kB levels in neurons of the CA2 region, whereas sulfated polysaccharides reversed this effect (Figure 6b). These results suggest that sulfated polysaccharides restore insulin signaling, which is suppressed by chronic scopolamine administration.
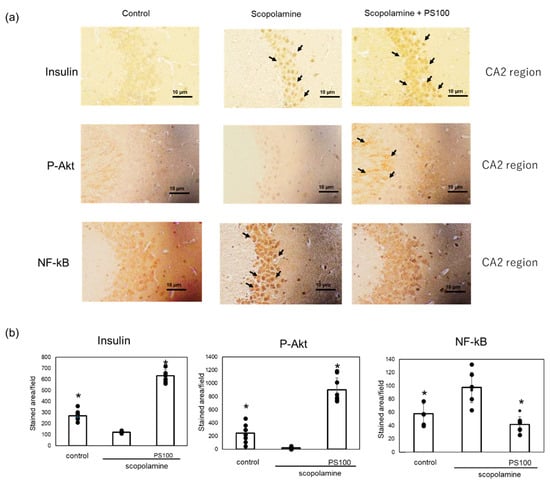
Figure 6.
Effect of sulfated polysaccharide (PS) on insulin signaling in chronic scopolamine-induced mice. (a) Expression levels of insulin, phosphorylated protein kinase B (p-Akt), and nuclear factor (NF)-kB in the hippocampus CA2 region determined via immunostaining in the hippocampus in the control group, scopolamine group, and scopolamine + PS group. Stained areas are indicated by arrows. Scale bar: 10 μm. (b) At least five areas per mouse (n = 2 mice) were randomly selected and analyzed, and the results are represented as the mean ± SD (right panel). * p < 0.05 vs. scopolamine group.
4. Discussion
We previously reported that sulfated polysaccharides in nacre extract mitigate memory impairment induced by a single administration of scopolamine [31]. In this study, we further demonstrated that sulfated polysaccharides alleviate memory impairment following the chronic administration of scopolamine.
Hernández-Rodríguez et al. suggested that chronic administration of scopolamine to rats causes Aβ deposition via increased expression of BACE1, which produces Aβ from APP [22]. Here, we showed that chronic administration of scopolamine increased the expression levels of BACE1 and promoted Aβ deposition in the brain. Although sulfated polysaccharides did not decrease BACE1 expression, it suppressed Aβ deposition. Accumulation of Aβ induces tau phosphorylation, increases expression of inflammatory cytokines, and promotes neuronal degeneration [6]. Chronic administration of scopolamine increases the p-tau, inflammatory cytokine, and GFAP levels in hippocampal neurons. However, sulfated polysaccharides suppressed these effects. These results suggest that sulfated polysaccharides inhibit Aβ deposition.
In this study, sulfated polysaccharides increased the IDE expression levels in a chronic scopolamine administration model. This increase in IDE expression may contribute to the suppression of Aβ deposition. Activation of insulin signaling significantly upregulates IDE expression [18,19]. Conversely, inhibition of insulin signaling pathways delays Aβ degradation by altering NEP and IDE levels in vitro [18]. The expression of p-Akt, which is increased by insulin signaling activation, was decreased, whereas NF-kB expression, which inhibits insulin signaling, was increased in the hippocampal region of chronic scopolamine-treated mice. These observations suggest that the chronic administration of scopolamine suppresses insulin signaling. This finding is consistent with other reports that scopolamine administration decreases p-Akt expression in the brain [38,39]. In contrast, sulfated polysaccharides suppressed the downregulation in p-Akt expression and enhanced expression of NF-kB.
Brain-derived insulin synthesis and functions remain controversial [40,41]. Xuemin et al. showed that exendin-4 improves cognitive function in diabetic mice by augmenting brain insulin synthesis; moreover, a decrease in brain insulin levels leads to increased phosphorylated tau levels [42]. Here, administration of sulfated polysaccharides increased these levels in the hippocampus. These results suggest that sulfated polysaccharides restore insulin signaling by increasing brain-derived insulin expression, leading to high IDE expression.
Administration of sulfated polysaccharide altered gene expression and inhibited Aβ deposition. Polysaccharides typically cannot cross the blood–brain barrier. Recent studies have reported exosome-mediated signal transduction in the brain [43,44]. Exosomes secreted after polysaccharide administration may transmit information to the brain. Polysaccharide metabolites may be also involved in brain signal transduction. However, further studies are necessary to determine the effects of exosomes and metabolites.
Aβ and p-tau are produced by different mechanisms in different types of AD model mice [45]. We previously demonstrated that the nacre extract improves memory impairment in several AD mouse models [25,26,29,31]. Further studies are necessary to determine whether sulfated polysaccharides suppress Aβ deposition and increased p-tau expression in different types of AD model mice.
5. Conclusions
In conclusion, this study showed that sulfated polysaccharides isolated from nacre extract ameliorated memory impairment by suppressing chronic scopolamine-induced Aβ accumulation, phosphorylated tau expression, and inflammatory cytokine expression. Furthermore, activation of insulin signaling and high IDE levels increased the clearance and inhibited the deposition of Aβ.
Author Contributions
Conceptualization, Y.H.; Investigation, M.W. and K.O.; Formal Analysis, M.W. and K.O.; Writing—Original Draft Preparation, Y.H.; Writing—Review and Editing, Y.H., M.W. and K.O. All authors approved the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Animal experiments were approved by the Animal Ethics Committee of the Muroran Institute of Technology (approval number: H30KS01).
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
The authors acknowledge Kaneko Pearl Farming Company Limited for providing the pearl oyster shells used in this study. The authors thank the Department of Molecular Medicine (Akita University) for help with immunohistochemistry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. New pathways identify novel drug targets for the prevention and treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 5383. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. Oligomerization and conformational change turn monomeric β-amyloid and tau proteins toxic: Their role in Alzheimer’s pathogenesis. Molecules 2020, 25, 1659. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Experimental approaches for altering the expression of Abeta-degrading enzymes. J. Neurochem. 2023, 164, 725–763. [Google Scholar] [CrossRef]
- Sikanyika, N.L.; Parkington, H.C.; Smith, A.I.; Kuruppu, S. Powering amyloid beta degrading enzymes: A possible therapy for Alzheimer’s disease. Neurochem. Res. 2019, 44, 1289–1296. [Google Scholar] [CrossRef]
- Żukowska, J.; Moss, S.J.; Subramanian, V.; Acharya, K.R. Molecular basis of selective amyloid-b degrading enzymes in Alzheimer’s disease. FEBS J. 2023, 291, 2999–3029. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Beckett, C.; Belyaev, N.D.; Turner, A.J. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J. Neurochem. 2012, 120 (Suppl. S1), 167–185. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, G.; Zhang, M. Insulin-degrading enzyme: Roles and pathways in ameliorating cognitive impairment associated with Alzheimer’s disease and diabetes. Ageing Res. Rev. 2023, 90, 101999. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Leissring, M.A.; Eckman, E.A.; Bertram, L.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid β-protein. Am. J. Pathol. 2004, 164, 1425–1434. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of Cerebral Aβ in Alzheimer’s disease: Reassessing the Role of Microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.-H.; Tan, L.; Cao, X.; Yu, J.-T.; Tan, L. Clearance of amyloid beta and tau in Alzheimer’s disease: From mechanisms to therapy. Neurotox. Res. 2018, 34, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Leissring, M.A.; Farris, W.; Chang, A.Y.; Walsh, D.M.; Wu, X.; Sun, X.; Frosch, M.P.; Selkoe, D.J. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003, 40, 1087–1093. [Google Scholar] [CrossRef]
- Ohyagi, Y.; Takei, S.I. Insulin signaling as a therapeutic target in Alzheimer’s disease: Efficacy of apomorphine. Neurol. Clin. Neurosci. 2020, 8, 146–154. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ishikuro, R.; Tanida, M.; Suzuki, K.; Ikeda-Matsuo, Y.; Sobue, K. Insulin-signaling Pathway Regulates the Degradation of Amyloid β-protein via Astrocytes. Neurosci 2018, 385, 227–236. [Google Scholar] [CrossRef]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: Implications for Alzheimer’s disease intervention. J. Neurosci. 2004, 24, 11120–11126. [Google Scholar] [CrossRef]
- Inoue, Y.; Masuda, T.; Misumi, Y.; Ando, Y.; Ueda, M. Metformin attenuates vascular pathology by increasing expression of insulin-degrading enzyme in a mixed model of cerebral amyloid angiopathy and type 2 diabetes mellitus. Neurosci. Lett. 2021, 762, 136136. [Google Scholar] [CrossRef]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Chronic administration of scopolamine increased GSK3βP9, beta secretase, amyloid beta, and oxidative stress in the hippocampus of Wistar rats. Mol. Neurobiol. 2020, 57, 3979–3988. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Fuji, T.; Inoue, T.; Hasegawa, Y. Nacre extract prevents scopolamine-induced memory deficits in rodents. Asian Pac. J. Trop. Med. 2018, 11, 202–208. [Google Scholar] [CrossRef]
- Yotsuya, Y.; Hasegawa, Y. Nacre extract from pearl oyster attenuates amyloid beta-induced memory impairment. J. Nat. Med. 2022, 76, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Omachi, T.; Matsuyama, N.; Hasegawa, Y. Nacre extract from pearl oyster suppresses LPS-induced depression and anxiety. J. Funct. Foods 2023, 100, 105373. [Google Scholar] [CrossRef]
- Omachi, T.; Hasegawa, Y. Effect of nacre extract from pearl oyster shells against behavioral and psychological symptoms of dementia in senescence-accelerated mouse P8 (SAMP8). J. Funct. Foods 2024, 116, 106280. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Oura, K.; Hasegawa, Y. Nacre extract from pearl oyster shell prevents D-galactose-induced brain and skin aging. Mar. Biotechnol. 2023, 25, 503–518. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Hasegawa, Y. Oral administration of nacre extract from pearl oyster shells has anti-aging effects on skin and muscle, and extends the lifespan in SAMP8 mice. Pharmaceuticals 2024, 17, 713. [Google Scholar] [CrossRef]
- Yamagami, H.; Fuji, T.; Wako, M.; Hasegawa, Y. Sulfated polysaccharide isolated from the nacre of pearl oyster improves scopolamine-induced memory impairment. Antioxidants 2021, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Andersson, E.; Janelidze, S.; Ossenkoppele, R.; Insel, P.; Strandberg, O.; Zetterberg, H.; Rosen, H.J.; Rabinovici, G.; Chai, X.; et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci. Adv. 2020, 6, eaaz2387. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and Tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef]
- Lee, H.K.; Kumar, P.; Fu, Q.; Rosen, K.M.; Querfurth, H.W. The insulin/Akt signaling pathway is targeted by intracellular β-amyloid. Mol. Biol. Cell 2009, 20, 1533–1544. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, F.; Wang, Y.; Yu, G.; Jia, B.L. Silencing of SAA1 inhibits palmitate- or high-fat diet induced insulin resistance through suppression of the NF-κB pathway. Mol. Med. 2019, 25, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Sawyer, T.W.; Tse, Y.C.; Fan, C.; Hennes, G.; Barnes, J.; Josey, T.; Weiss, T.; Nelson, P.; Wong, T.P. Primary blast-induced changes in Akt and GSK3β phosphorylation in rat hippocampus. Front. Neurol. 2017, 8, 413. [Google Scholar] [CrossRef]
- Das, T.K.; Chakrabarti, S.K.; Zulkipli, I.N.; Abdul Hamid, M.R.W. Curcumin ameliorates the impaired insulin signaling involved in the pathogenesis of Alzheimer’s disease in rats. J. Alzheimers Dis. Rep. 2019, 3, 59–70. [Google Scholar] [CrossRef]
- Alves, S.S.; Servilha-Menezes, G.; Rossi, L.; da Silva Junior, R.M.P.; Garcia-Cairasco, N. Evidence of disturbed insulin signaling in animal models of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2023, 152, 105326. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Lakic, I.; Ruzicic, A.; Jasnic, N.; Djurasevic, S.; Djordjevic, J.; Vujovic, P. The expression of insulin in the central nervous system: What have we learned so far? Int. J. Mol. Sci. 2023, 24, 6586. [Google Scholar] [CrossRef]
- Nemoto, T.; Toyoshima-Aoyama, F.; Yanagita, T.; Maruta, T.; Fujita, H.; Koshida, T.; Yonaha, T.; Wada, A.; Sawaguchi, A.; Murakami, M. New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: Amyloid-β1–42-Induced reduction of proinsulin level via glycogen synthase Kinase-3β. Cell. Signal. 2014, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shi, X.; Huang, J.; Zhang, S.; Yan, Y.; Ma, D.; Xu, W.; Xu, W.; Dong, K.; Tao, J.; et al. Exendin-4 improves cognitive function of diabetic mice via increasing brain insulin synthesis. Curr. Alzheimer Res. 2021, 18, 546–557. [Google Scholar] [CrossRef]
- Inotsuka, R.; Udono, M.; Yamatsu, A.; Kim, M.; Katakura, Y. Exosome-mediated activation of neuronal cells triggered by γ-aminobutyric acid (GABA). Nutrients 2021, 13, 2544. [Google Scholar] [CrossRef]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Yang, W.; Zhong, M.; Lin, Z.X.; Gray, N.E.; Xian, Y.F. Animal models of Alzheimer’s disease: Preclinical insights and challenges. Acta Mater. Med. 2023, 2, 192–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).