Sulfated Polysaccharides Isolated from Nacre Extract Suppress Chronic Scopolamine Administration-Induced Amyloid-Beta Deposition
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of the Nacre Extract and Sulfated Polysaccharides
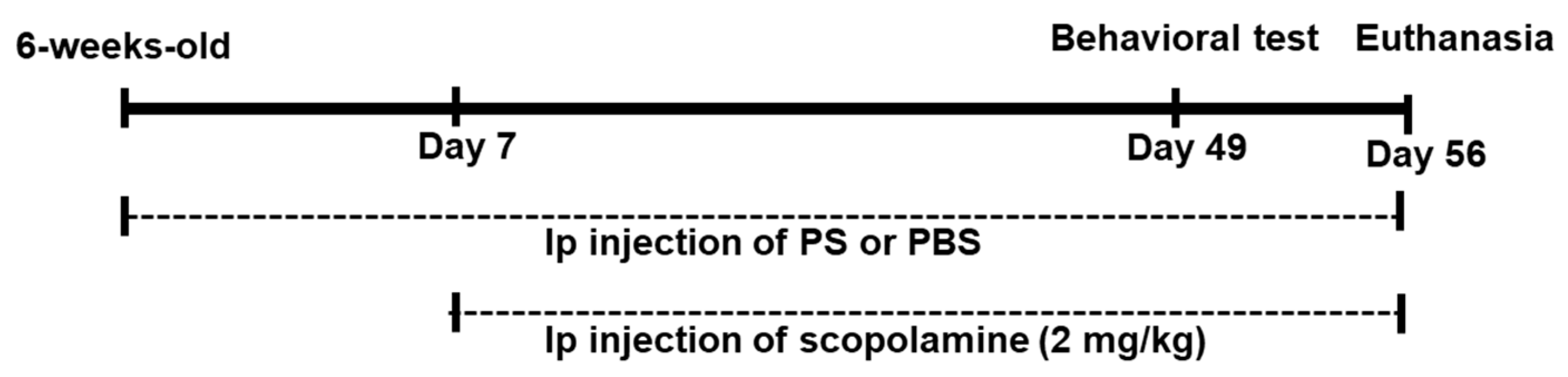
2.3. Animals
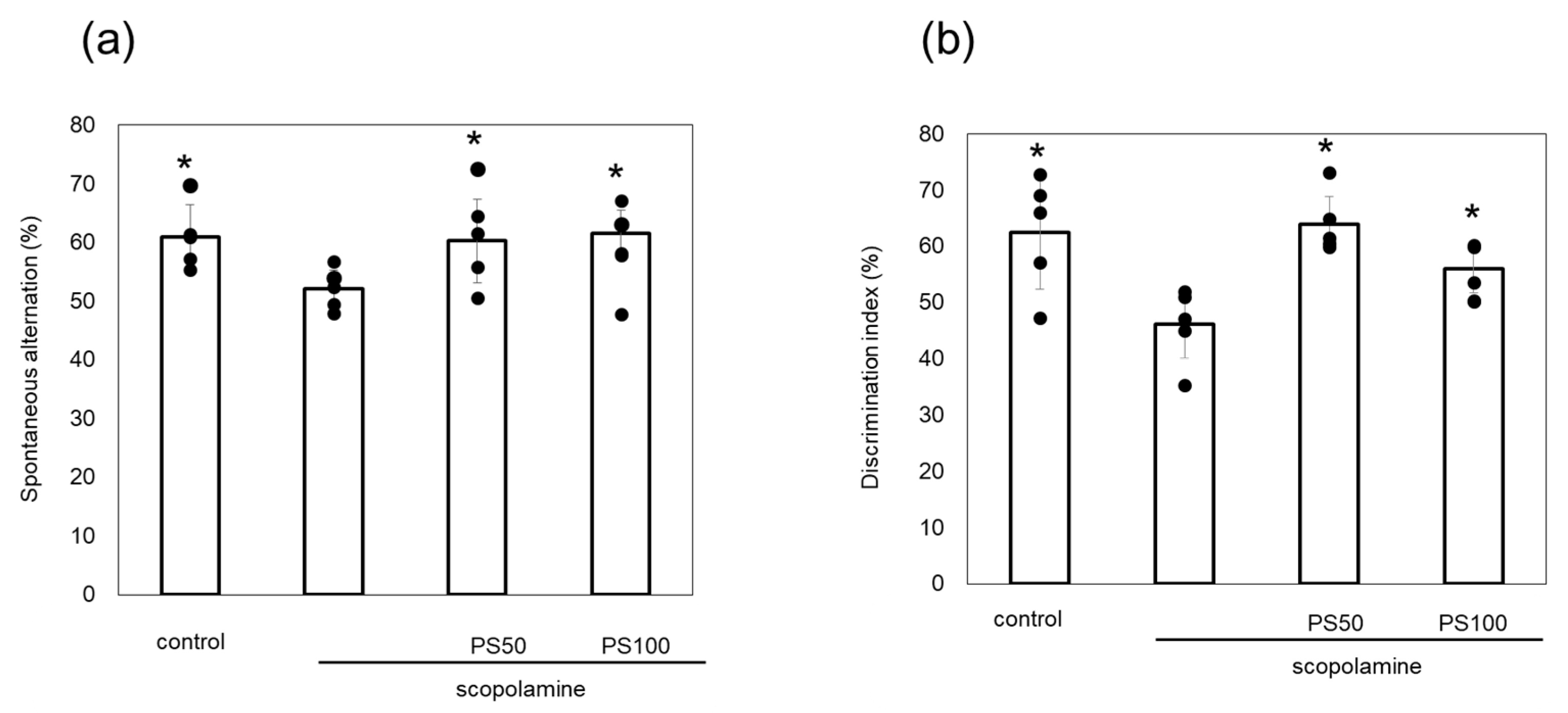
2.4. Novel Object Recognition Test
2.5. Y-Maze Test
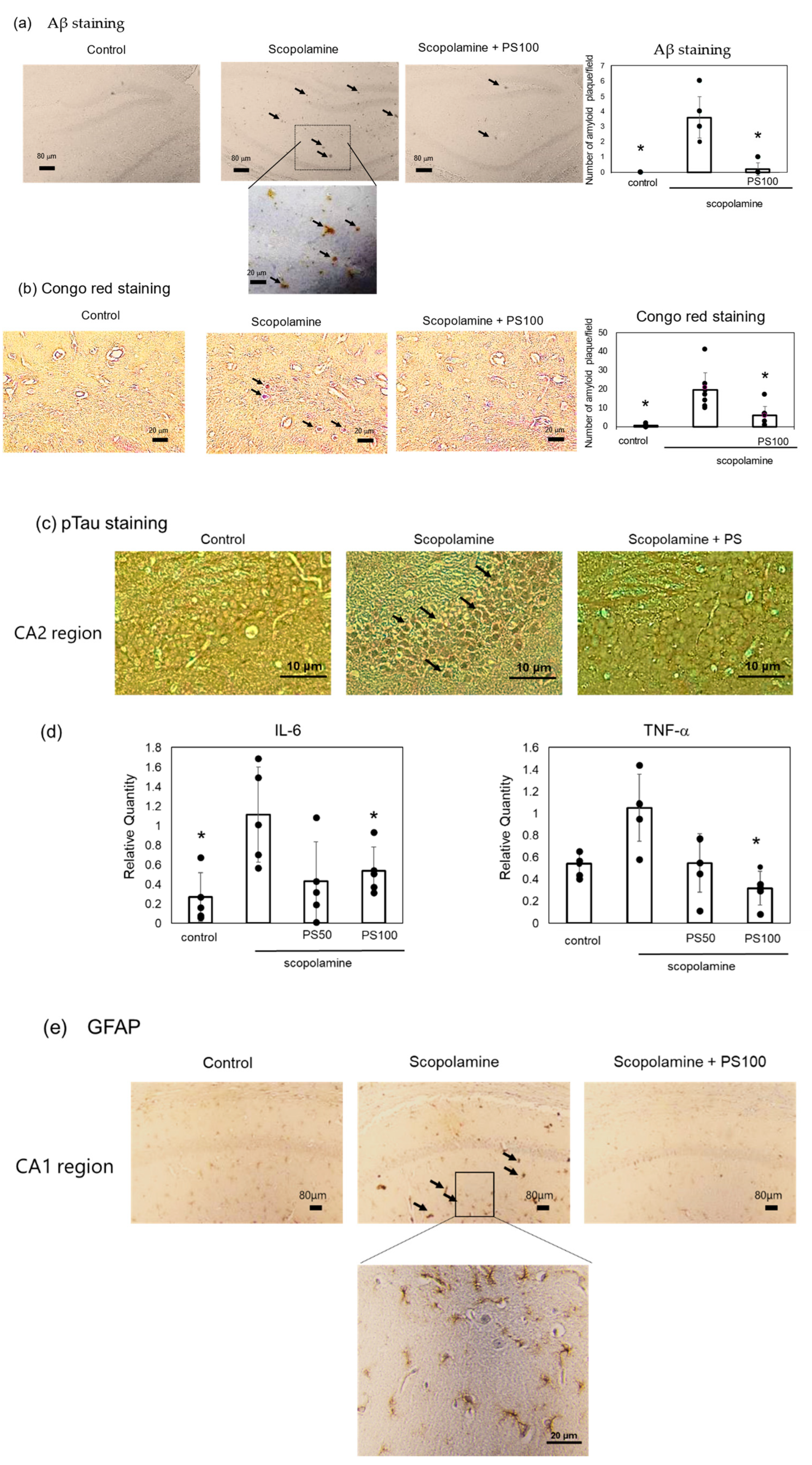
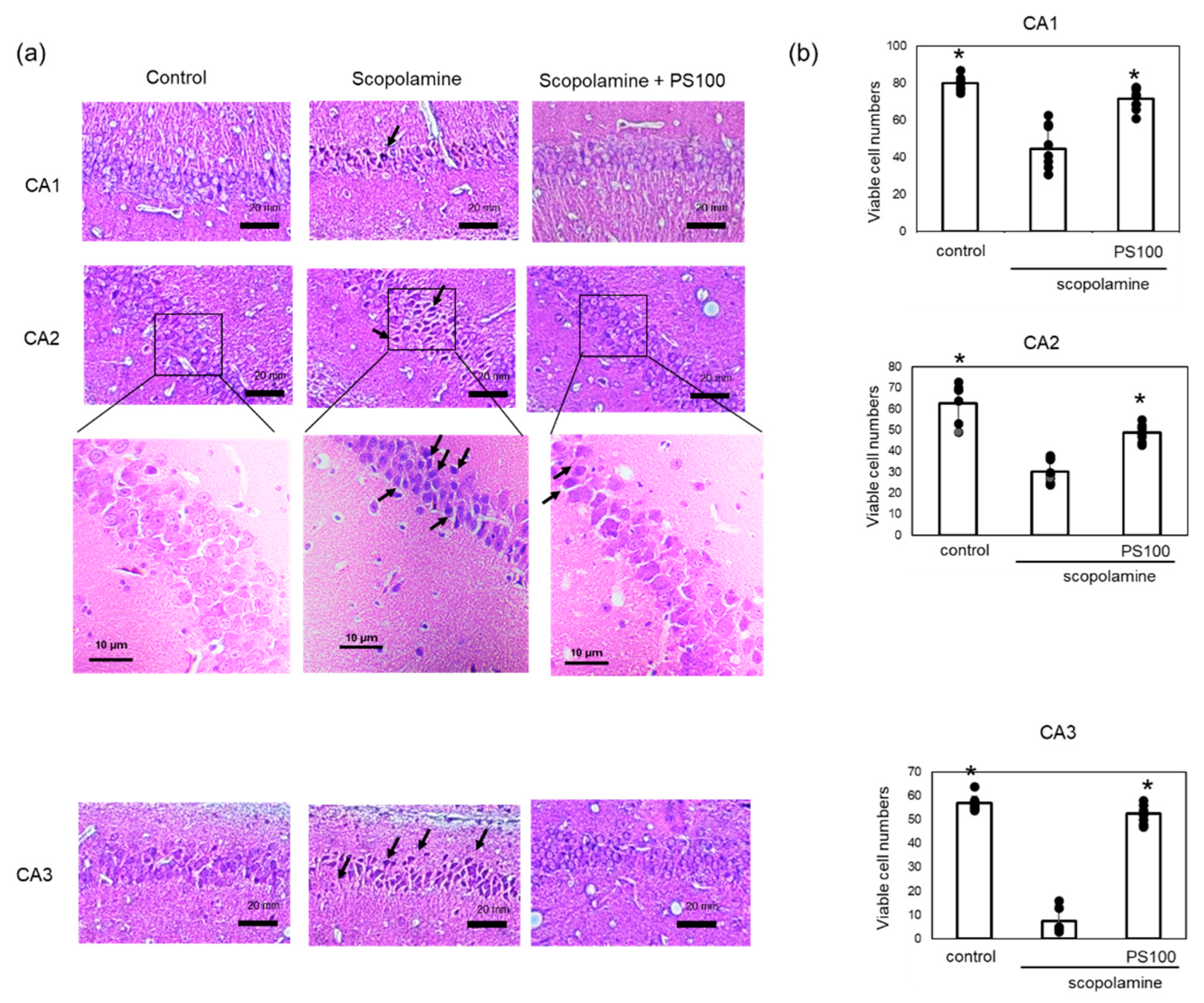
2.6. Histochemistry
2.7. RT-qPCR
2.8. Western Blot Analyses
2.9. Statistical Analyses
3. Results
3.1. Effect of Sulfated Polysaccharides on Chronic Scopolamine-Induced Memory Impairment
3.2. Effect of Sulfated Polysaccharides on Aβ Deposition
3.3. Effects of Sulfated Polysaccharides on Hippocampal Neurons
3.4. Effects of Sulfated Polysaccharide on the Aβ Metabolism-Associated Enzyme Levels
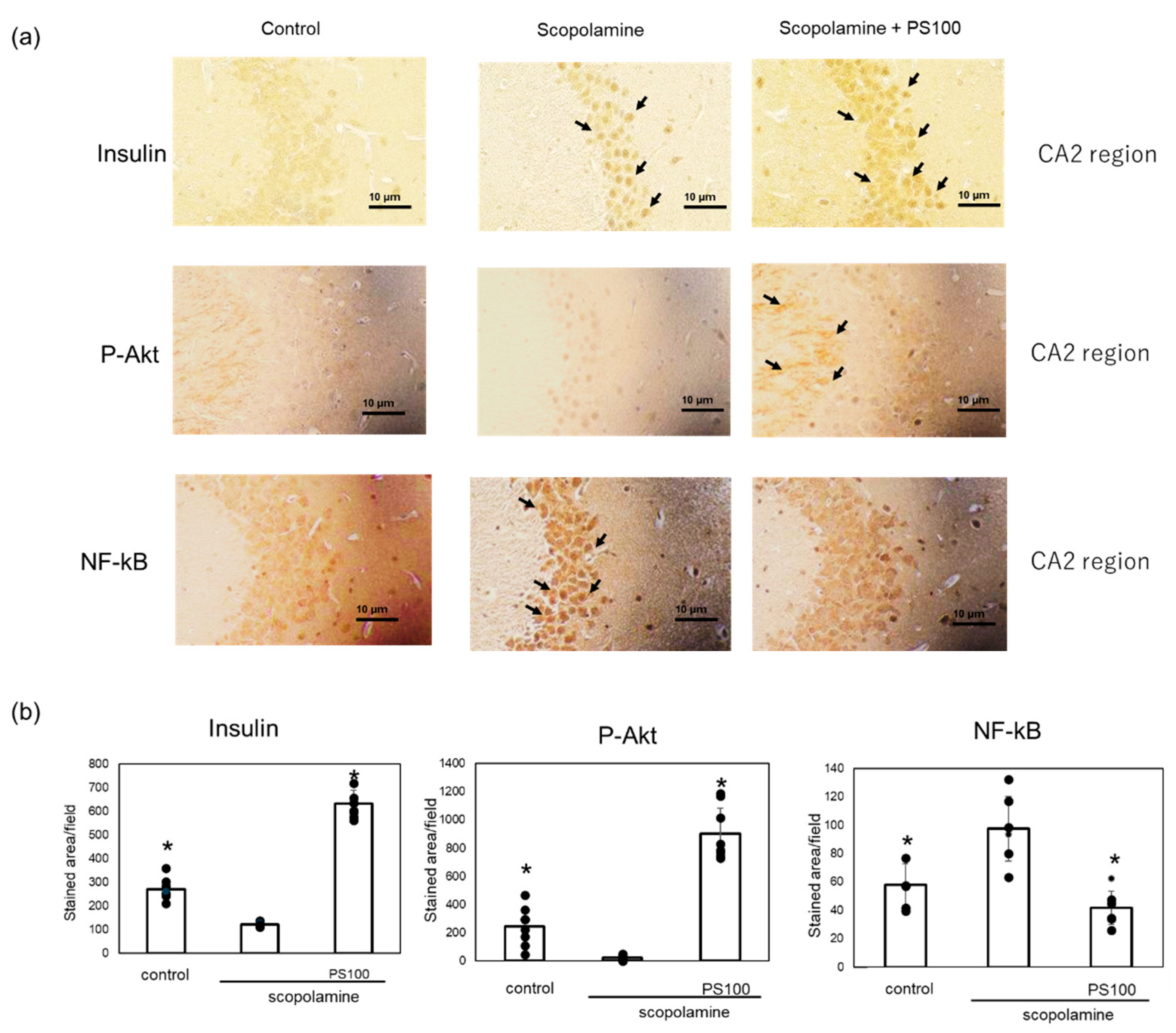
3.5. Effect of Sulfated Polysaccharides on Insulin Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. New pathways identify novel drug targets for the prevention and treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 5383. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. Oligomerization and conformational change turn monomeric β-amyloid and tau proteins toxic: Their role in Alzheimer’s pathogenesis. Molecules 2020, 25, 1659. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Experimental approaches for altering the expression of Abeta-degrading enzymes. J. Neurochem. 2023, 164, 725–763. [Google Scholar] [CrossRef]
- Sikanyika, N.L.; Parkington, H.C.; Smith, A.I.; Kuruppu, S. Powering amyloid beta degrading enzymes: A possible therapy for Alzheimer’s disease. Neurochem. Res. 2019, 44, 1289–1296. [Google Scholar] [CrossRef]
- Żukowska, J.; Moss, S.J.; Subramanian, V.; Acharya, K.R. Molecular basis of selective amyloid-b degrading enzymes in Alzheimer’s disease. FEBS J. 2023, 291, 2999–3029. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Beckett, C.; Belyaev, N.D.; Turner, A.J. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J. Neurochem. 2012, 120 (Suppl. S1), 167–185. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, G.; Zhang, M. Insulin-degrading enzyme: Roles and pathways in ameliorating cognitive impairment associated with Alzheimer’s disease and diabetes. Ageing Res. Rev. 2023, 90, 101999. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Leissring, M.A.; Eckman, E.A.; Bertram, L.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid β-protein. Am. J. Pathol. 2004, 164, 1425–1434. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of Cerebral Aβ in Alzheimer’s disease: Reassessing the Role of Microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.-H.; Tan, L.; Cao, X.; Yu, J.-T.; Tan, L. Clearance of amyloid beta and tau in Alzheimer’s disease: From mechanisms to therapy. Neurotox. Res. 2018, 34, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Leissring, M.A.; Farris, W.; Chang, A.Y.; Walsh, D.M.; Wu, X.; Sun, X.; Frosch, M.P.; Selkoe, D.J. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003, 40, 1087–1093. [Google Scholar] [CrossRef]
- Ohyagi, Y.; Takei, S.I. Insulin signaling as a therapeutic target in Alzheimer’s disease: Efficacy of apomorphine. Neurol. Clin. Neurosci. 2020, 8, 146–154. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ishikuro, R.; Tanida, M.; Suzuki, K.; Ikeda-Matsuo, Y.; Sobue, K. Insulin-signaling Pathway Regulates the Degradation of Amyloid β-protein via Astrocytes. Neurosci 2018, 385, 227–236. [Google Scholar] [CrossRef]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: Implications for Alzheimer’s disease intervention. J. Neurosci. 2004, 24, 11120–11126. [Google Scholar] [CrossRef]
- Inoue, Y.; Masuda, T.; Misumi, Y.; Ando, Y.; Ueda, M. Metformin attenuates vascular pathology by increasing expression of insulin-degrading enzyme in a mixed model of cerebral amyloid angiopathy and type 2 diabetes mellitus. Neurosci. Lett. 2021, 762, 136136. [Google Scholar] [CrossRef]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Chronic administration of scopolamine increased GSK3βP9, beta secretase, amyloid beta, and oxidative stress in the hippocampus of Wistar rats. Mol. Neurobiol. 2020, 57, 3979–3988. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Fuji, T.; Inoue, T.; Hasegawa, Y. Nacre extract prevents scopolamine-induced memory deficits in rodents. Asian Pac. J. Trop. Med. 2018, 11, 202–208. [Google Scholar] [CrossRef]
- Yotsuya, Y.; Hasegawa, Y. Nacre extract from pearl oyster attenuates amyloid beta-induced memory impairment. J. Nat. Med. 2022, 76, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Omachi, T.; Matsuyama, N.; Hasegawa, Y. Nacre extract from pearl oyster suppresses LPS-induced depression and anxiety. J. Funct. Foods 2023, 100, 105373. [Google Scholar] [CrossRef]
- Omachi, T.; Hasegawa, Y. Effect of nacre extract from pearl oyster shells against behavioral and psychological symptoms of dementia in senescence-accelerated mouse P8 (SAMP8). J. Funct. Foods 2024, 116, 106280. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Oura, K.; Hasegawa, Y. Nacre extract from pearl oyster shell prevents D-galactose-induced brain and skin aging. Mar. Biotechnol. 2023, 25, 503–518. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Hasegawa, Y. Oral administration of nacre extract from pearl oyster shells has anti-aging effects on skin and muscle, and extends the lifespan in SAMP8 mice. Pharmaceuticals 2024, 17, 713. [Google Scholar] [CrossRef]
- Yamagami, H.; Fuji, T.; Wako, M.; Hasegawa, Y. Sulfated polysaccharide isolated from the nacre of pearl oyster improves scopolamine-induced memory impairment. Antioxidants 2021, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Andersson, E.; Janelidze, S.; Ossenkoppele, R.; Insel, P.; Strandberg, O.; Zetterberg, H.; Rosen, H.J.; Rabinovici, G.; Chai, X.; et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci. Adv. 2020, 6, eaaz2387. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and Tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef]
- Lee, H.K.; Kumar, P.; Fu, Q.; Rosen, K.M.; Querfurth, H.W. The insulin/Akt signaling pathway is targeted by intracellular β-amyloid. Mol. Biol. Cell 2009, 20, 1533–1544. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, F.; Wang, Y.; Yu, G.; Jia, B.L. Silencing of SAA1 inhibits palmitate- or high-fat diet induced insulin resistance through suppression of the NF-κB pathway. Mol. Med. 2019, 25, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Sawyer, T.W.; Tse, Y.C.; Fan, C.; Hennes, G.; Barnes, J.; Josey, T.; Weiss, T.; Nelson, P.; Wong, T.P. Primary blast-induced changes in Akt and GSK3β phosphorylation in rat hippocampus. Front. Neurol. 2017, 8, 413. [Google Scholar] [CrossRef]
- Das, T.K.; Chakrabarti, S.K.; Zulkipli, I.N.; Abdul Hamid, M.R.W. Curcumin ameliorates the impaired insulin signaling involved in the pathogenesis of Alzheimer’s disease in rats. J. Alzheimers Dis. Rep. 2019, 3, 59–70. [Google Scholar] [CrossRef]
- Alves, S.S.; Servilha-Menezes, G.; Rossi, L.; da Silva Junior, R.M.P.; Garcia-Cairasco, N. Evidence of disturbed insulin signaling in animal models of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2023, 152, 105326. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Lakic, I.; Ruzicic, A.; Jasnic, N.; Djurasevic, S.; Djordjevic, J.; Vujovic, P. The expression of insulin in the central nervous system: What have we learned so far? Int. J. Mol. Sci. 2023, 24, 6586. [Google Scholar] [CrossRef]
- Nemoto, T.; Toyoshima-Aoyama, F.; Yanagita, T.; Maruta, T.; Fujita, H.; Koshida, T.; Yonaha, T.; Wada, A.; Sawaguchi, A.; Murakami, M. New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: Amyloid-β1–42-Induced reduction of proinsulin level via glycogen synthase Kinase-3β. Cell. Signal. 2014, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shi, X.; Huang, J.; Zhang, S.; Yan, Y.; Ma, D.; Xu, W.; Xu, W.; Dong, K.; Tao, J.; et al. Exendin-4 improves cognitive function of diabetic mice via increasing brain insulin synthesis. Curr. Alzheimer Res. 2021, 18, 546–557. [Google Scholar] [CrossRef]
- Inotsuka, R.; Udono, M.; Yamatsu, A.; Kim, M.; Katakura, Y. Exosome-mediated activation of neuronal cells triggered by γ-aminobutyric acid (GABA). Nutrients 2021, 13, 2544. [Google Scholar] [CrossRef]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Yang, W.; Zhong, M.; Lin, Z.X.; Gray, N.E.; Xian, Y.F. Animal models of Alzheimer’s disease: Preclinical insights and challenges. Acta Mater. Med. 2023, 2, 192–215. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wako, M.; Ohara, K.; Hasegawa, Y. Sulfated Polysaccharides Isolated from Nacre Extract Suppress Chronic Scopolamine Administration-Induced Amyloid-Beta Deposition. Appl. Sci. 2024, 14, 7830. https://doi.org/10.3390/app14177830
Wako M, Ohara K, Hasegawa Y. Sulfated Polysaccharides Isolated from Nacre Extract Suppress Chronic Scopolamine Administration-Induced Amyloid-Beta Deposition. Applied Sciences. 2024; 14(17):7830. https://doi.org/10.3390/app14177830
Chicago/Turabian StyleWako, Mayumi, Kanae Ohara, and Yasushi Hasegawa. 2024. "Sulfated Polysaccharides Isolated from Nacre Extract Suppress Chronic Scopolamine Administration-Induced Amyloid-Beta Deposition" Applied Sciences 14, no. 17: 7830. https://doi.org/10.3390/app14177830
APA StyleWako, M., Ohara, K., & Hasegawa, Y. (2024). Sulfated Polysaccharides Isolated from Nacre Extract Suppress Chronic Scopolamine Administration-Induced Amyloid-Beta Deposition. Applied Sciences, 14(17), 7830. https://doi.org/10.3390/app14177830









