A Study of the Influence of Synthesis Parameters on the Preparation of High Performance SSZ-13 Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Seed Crystal
2.2. Seeding Procedure
2.3. Hydrothermal Synthesis of SSZ-13 Zeolite Membrane
2.4. Gas-Tightness Measurement
2.5. Template Removal of SSZ-13 Membranes
2.6. Single and Mixed Gas Permeation Measurement
3. Results and Discussion
3.1. Impact of Pore Size in Intermediate Layers
3.2. Impact of Seeding Concentration
3.3. Impact of Sonication and Suspension Liquid on Deagglomeration of Seeds
3.4. Impact of Sources of Si and Al
3.5. Impact of Si/Al Ratio
3.6. Impact of Alkalinity (OH/Si)
3.7. Impact of H2O/Si
3.8. Impact of TMAdaOH/Si
3.9. Impact of Ageing Time
3.10. Impact of Filling Factor of the Autoclave
3.11. Impact of Synthesis Time
3.12. Impact of Synthesis Temperature
3.13. Impact of Calcination Temperature
3.14. Mixed Gas Permeation Measurement
3.15. Comparison with Related Work
4. Conclusions
- The membrane was synthesized on an alumina support with a pore size of 200 nm
- A seeding procedure was employed with 1 wt% concentration of the SSZ-13 crystals in EtOH followed by 30 min of sonication
- The gel composition had the ratio of 1 SiO2:0.1 TMAdaOH:0.2 NaOH:0.05 Al(OH)3:80 H2O after 24 h of ageing
- Crystallizing at 160 °C for 48 h and calcining at 450 °C yielded the highest CO2/CH4 separation performance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adewole, J.K.; Ahmad, A.L.; Ismail, S.; Leo, C.P. Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Taherizadeh, A.; Simon, A.; Richter, H.; Stelter, M.; Voigt, I. Exploring the separation properties of high-Si CHA membranes for the CO2 capturing technology: Impact of the selective layer thickness and growth mechanism. J. Membr. Sci. 2024, 697, 122565. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Lee, D.-H. Gas Membranes for CO2/CH4 (Biogas) Separation: A Review. Environ. Eng. Sci. 2015, 32, 71–85. [Google Scholar] [CrossRef]
- Smart, S.; Lin, C.X.C.; Ding, L.; Thambimuthu, K.; Da Diniz Costa, J.C. Ceramic membranes for gas processing in coal gasification. Energy Environ. Sci. 2010, 3, 268. [Google Scholar] [CrossRef]
- Aydani, A.; Brunetti, A.; Maghsoudi, H.; Barbieri, G. CO2 separation from binary mixtures of CH4, N2, and H2 by using SSZ-13 zeolite membrane. Sep. Purif. Technol. 2021, 256, 117796. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Nasef, M.M.; Abdullah, T.A.T.; Ahmad, A.; Ting, T.M. CO2 adsorption and CO2/CH4 separation using fibrous amine-containing adsorbents: Isothermal, kinetic, and thermodynamic behaviours. Environ. Sci. Pollut. Res. 2023, 30, 116906–116920. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Taherizadeh, A.; Simon, A.; Richter, H.; Stelter, M.; Voigt, I. Development and investigation of a multilayer PDMS/zeolite composite membrane for CO2 separation applications. Sep. Purif. Technol. 2024, 346, 127344. [Google Scholar] [CrossRef]
- Hassan, T.N.A.T.; Shariff, A.M.; Pauzi, M.M.M.; Khidzir, M.S.; Surmi, A. Insights on Cryogenic Distillation Technology for Simultaneous CO2 and H2S Removal for Sour Gas Fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Rufford, T.E.; Smart, S.; Watson, G.; Graham, B.F.; Boxall, J.; Da Diniz Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2 -Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Taherizadeh, A.; Simon, A.; Richter, H.; Stelter, M.; Voigt, I. Characterization and synthesis of high permeance SSZ-13 membranes to separate CO2 from CH4 for biogas upgrading. J. Membr. Sci. 2024, 703, 122845. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chew, T.L.; Zhu, P.W.; Mohamed, A.R.; Chai, S.-P. Synthesis and performance of microporous inorganic membranes for CO2 separation: A review. J. Porous Mater. 2013, 20, 1457–1475. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.S.; Hedlund, J. Industrially relevant CHA membranes for CO2/CH4 separation. J. Membr. Sci. 2022, 641, 119888. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Wang, S.; Hong, Z.; Wang, X.; Gu, X. SSZ-13 zeolite membranes on four-channel α-Al2O3 hollow fibers for CO2 separation. Sep. Purif. Technol. 2021, 267, 118611. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Meng, D.; Kong, X.; Kong, L.; Qiu, H.; Xu, N.; Guo, W.; Yang, S.; Zhang, Y. Efficient synthesis of thin SSZ-13 membranes by gel-less method. J. Membr. Sci. 2021, 620, 118920. [Google Scholar] [CrossRef]
- Palomino, M.; Corma, A.; Rey, F.; Valencia, S. New insights on CO2-methane separation using LTA zeolites with different Si/Al ratios and a first comparison with MOFs. Langmuir 2010, 26, 1910–1917. [Google Scholar] [CrossRef]
- Kim, E.; Lee, M.; Baik, H.; Han, D.-Y.; Ha, J.-M.; Choi, J. On the synthesis and characterization of all-silica CHA zeolite particles. Microporous Mesoporous Mater. 2014, 184, 47–54. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, N.; Wang, H.; Bu, N.; Zhang, F.; Zhou, R. Preparation of steam-stable high-silica CHA (SSZ-13) membranes for CO2/CH4 and C2H4/C2H6 separation. J. Membr. Sci. 2015, 475, 303–310. [Google Scholar] [CrossRef]
- Xu, L.; Du, A.; Wei, Y.; Wang, Y.; Yu, Z.; He, Y.; Zhang, X.; Liu, Z. Synthesis of SAPO-34 with only Si(4Al) species: Effect of Si contents on Si incorporation mechanism and Si coordination environment of SAPO-34. Microporous Mesoporous Mater. 2008, 115, 332–337. [Google Scholar] [CrossRef]
- Ji, Y.; Deimund, M.A.; Bhawe, Y.; Davis, M.E. Organic-Free Synthesis of CHA-Type Zeolite Catalysts for the Methanol-to-Olefins Reaction. ACS Catal. 2015, 5, 4456–4465. [Google Scholar] [CrossRef]
- Taherizadeh, A.; Harpf, A.; Simon, A.; Choi, J.; Richter, H.; Voigt, I.; Stelter, M. Thermochemical study of the structural stability of low-silicate CHA zeolite crystals. Results Chem. 2022, 4, 100466. [Google Scholar] [CrossRef]
- Aydani, A.; Maghsoudi, H.; Brunetti, A.; Barbieri, G. Silica sol gel assisted defect patching of SSZ-13 zeolite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 277, 119518. [Google Scholar] [CrossRef]
- Karakiliç, P.; Wang, X.; Kapteijn, F.; Nijmeijer, A.; Winnubst, L. Defect-free high-silica CHA zeolite membranes with high selectivity for light gas separation. J. Membr. Sci. 2019, 586, 34–43. [Google Scholar] [CrossRef]
- Wu, T.; Diaz, M.C.; Zheng, Y.; Zhou, R.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Influence of propane on CO2/CH4 and N2/CH4 separations in CHA zeolite membranes. J. Membr. Sci. 2015, 473, 201–209. [Google Scholar] [CrossRef]
- Mehio, N.; Dai, S.; Jiang, D. Quantum mechanical basis for kinetic diameters of small gaseous molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef]
- Simon, A.; Seyring, M.; Kämnitz, S.; Richter, H.; Voigt, I.; Rettenmayr, M.; Ritter, U. Carbon nanotubes and carbon nanofibers fabricated on tubular porous Al2O3 substrates. Carbon 2015, 90, 25–33. [Google Scholar] [CrossRef]
- Ding, W.; Xiang, S.; Ye, F.; Gui, T.; Li, Y.; Zhang, F.; Hu, N.; Zhu, M.; Chen, X. Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes. Membranes 2020, 10, 41. [Google Scholar] [CrossRef]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Guo, M.; Liu, B.; Zhou, R.; Lai, Z. High-performance 7-channel monolith supported SSZ-13 membranes for high-pressure CO2/CH4 separations. J. Membr. Sci. 2021, 629, 119277. [Google Scholar] [CrossRef]
- Kong, X.; Qiu, H.; Meng, D.; Tang, X.; Yang, S.; Guo, W.; Zhang, Y.; Kong, L.; Zhang, Y.; Zhang, Z. Reproducible synthesis of all-silica CHA zeolite membranes in a homogeneous mother liquor. Sep. Purif. Technol. 2021, 274, 119104. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Borghuis, G.J.; Sripathi, V.G.; Hensen, E.J. Influence of the Si/Al ratio on the separation properties of SSZ-13 zeolite membranes. J. Membr. Sci. 2015, 484, 140–145. [Google Scholar] [CrossRef]
- Mei, W.; Du, Y.; Wu, T.; Gao, F.; Wang, B.; Duan, J.; Zhou, J.; Zhou, R. High-flux CHA zeolite membranes for H2 separations. J. Membr. Sci. 2018, 565, 358–369. [Google Scholar] [CrossRef]
- Song, S.; Gao, F.; Zhang, Y.; Li, X.; Zhou, M.; Wang, B.; Zhou, R. Preparation of SSZ-13 membranes with enhanced fluxes using asymmetric alumina supports for N2/CH4 and CO2/CH4 separations. Sep. Purif. Technol. 2019, 209, 946–954. [Google Scholar] [CrossRef]
- Hamilton, K.E.; Coker, E.N.; Sacco, A.; Dixon, A.G.; Thompson, R.W. The effects of the silica source on the crystallization of zeolite NaX. Zeolites 1993, 13, 645–653. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, L.; Wang, X.; Qiu, H.; Ji, M.; Gu, X. Effect of Si/Al ratio in the framework on the pervaporation properties of hollow fiber CHA zeolite membranes. Microporous Mesoporous Mater. 2019, 273, 196–202. [Google Scholar] [CrossRef]
- Araki, S.; Yamashita, R.; Li, K.; Yamamoto, H. Preparation and gas permeation properties of all-silica CHA zeolite hollow fiber membranes prepared on amorphous-silica hollow fibers. J. Membr. Sci. 2021, 634, 119338. [Google Scholar] [CrossRef]
- Wu, T.; Shu, C.; Liu, S.; Xu, B.; Zhong, S.; Zhou, R. Separation Performance of Si-CHA Zeolite Membrane for a Binary H2/CH4 Mixture and Ternary and Quaternary Mixtures Containing Impurities. Energy Fuels 2020, 34, 11650–11659. [Google Scholar] [CrossRef]
- Nazir, L.S.M.; Yeong, Y.F.; Chew, T.L. Methods and synthesis parameters affecting the formation of FAU type zeolite membrane and its separation performance: A review. J. Asian Ceram. Soc. 2020, 8, 553–571. [Google Scholar] [CrossRef]
- Debost, M.; Klar, P.B.; Barrier, N.; Clatworthy, E.B.; Grand, J.; Laine, F.; Brázda, P.; Palatinus, L.; Nesterenko, N.; Boullay, P.; et al. Synthesis of Discrete CHA Zeolite Nanocrystals without Organic Templates for Selective CO2 Capture. Angew. Chem. Int. Ed. 2020, 59, 23491–23495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, T.; Sun, Z.; Liu, Y.; Chen, X.; Zhu, M.; Zhang, F.; Hu, N.; Li, Y.; Gui, T.; et al. Influence of sodium ion on high-silica SSZ-13 membranes for efficient CO2/CH4 and N2/CH4 separations. J. Membr. Sci. 2022, 661, 120918. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, M.; Chen, L.; Zhong, C.; Yang, Y.; Wu, T.; Wang, H.; Kumakiri, I.; Chen, X.; Kita, H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes 2018, 8, 43. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Li, X.; Zhong, S.; Zhou, R. Highly CO2-selective and moisture-resistant bilayer silicalite-1/SSZ-13 membranes with gradient pores for wet CO2/CH4 and CO2/N2 separations. J. Membr. Sci. 2021, 636, 119565. [Google Scholar] [CrossRef]
- Tang, H.; Bai, L.; Wang, M.; Zhang, Y.; Li, M.; Wang, M.; Kong, L.; Xu, N.; Zhang, Y.; Rao, P. Fast synthesis of thin high silica SSZ-13 zeolite membrane using oil-bath heating. Int. J. Hydrogen Energy 2019, 44, 23107–23119. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Gücüyener, C.; Szyja, B.M.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. High flux high-silica SSZ-13 membrane for CO2 separation. J. Mater. Chem. A 2014, 2, 13083–13092. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhu, M.-H.; Wu, T.; Jiang, Q.-L.; Zhang, F.; Wu, Y.-F.; Chen, X.-S. Template Removal and Surface Modification of an SSZ-13 Membrane with Heated Sodium Chloride for CO2/CH4 Gas Separation. ACS Omega 2022, 7, 6721–6727. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhang, Y.; Kong, L.; Kong, X.; Tang, X.; Meng, D.; Xu, N.; Wang, M.; Zhang, Y. High performance SSZ-13 membranes prepared at low temperature. J. Membr. Sci. 2020, 603, 118023. [Google Scholar] [CrossRef]
- Li, X.; Yu, K.; He, Z.; Liu, B.; Zhou, R.; Xing, W. Improved SSZ-13 thin membranes fabricated by seeded-gel approach for efficient CO2 capture. Chin. J. Chem. Eng. 2023, 56, 273–280. [Google Scholar] [CrossRef]
- Kim, J.; Jang, E.; Hong, S.; Kim, D.; Kim, E.; Ricther, H.; Simon, A.; Choi, N.; Korelskiy, D.; Fouladvand, S.; et al. Microstructural control of a SSZ-13 zeolite film via rapid thermal processing. J. Membr. Sci. 2019, 591, 117342. [Google Scholar] [CrossRef]
- Gui, T.; Chen, X.; Zhu, M.; An, X.; Wang, H.; Wu, T.; Zhang, F.; Chen, X.; Kita, H. Gas Separation Performance of SSZ-13 Zeolite Membranes on Different Supports. Energy Fuels 2021, 35, 14852–14859. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Meng, D.; Kong, X.; Yang, S.; Guo, W.; Qiu, H.; Kong, L.; Zhang, Y.; Zhang, Z. Fast synthesis of thin SSZ-13 membranes by a hot-dipping method. J. Membr. Sci. 2021, 629, 119297. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Natsui, M.; Ikeda, A. Gas Permeation Properties of High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 249. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Preparation and gas permeation properties on pure silica CHA-type zeolite membranes. J. Membr. Sci. 2017, 522, 363–370. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wu, T.; Song, S.; Wang, B.; Zhong, S.; Zhou, R. High-performance SSZ-13 membranes prepared using ball-milled nanosized seeds for carbon dioxide and nitrogen separations from methane. Chin. J. Chem. Eng. 2020, 28, 1285–1292. [Google Scholar] [CrossRef]
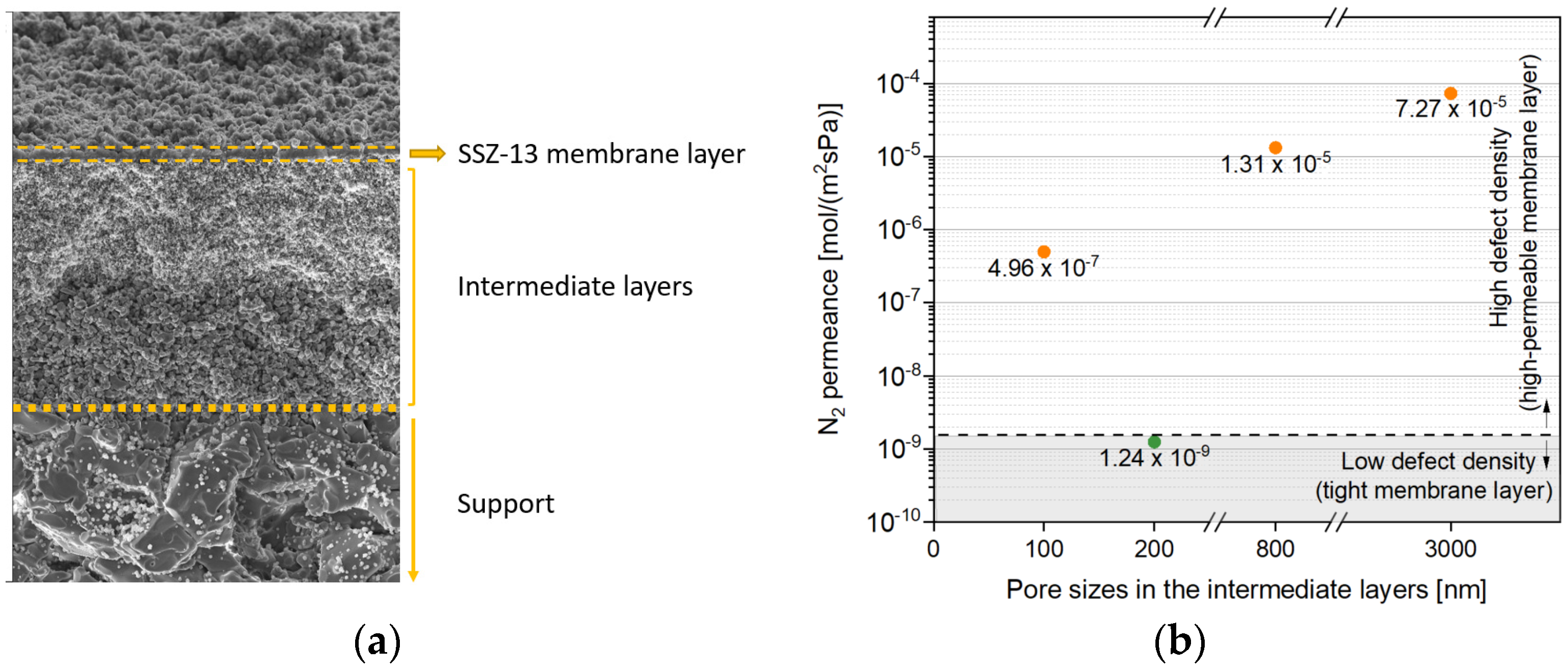

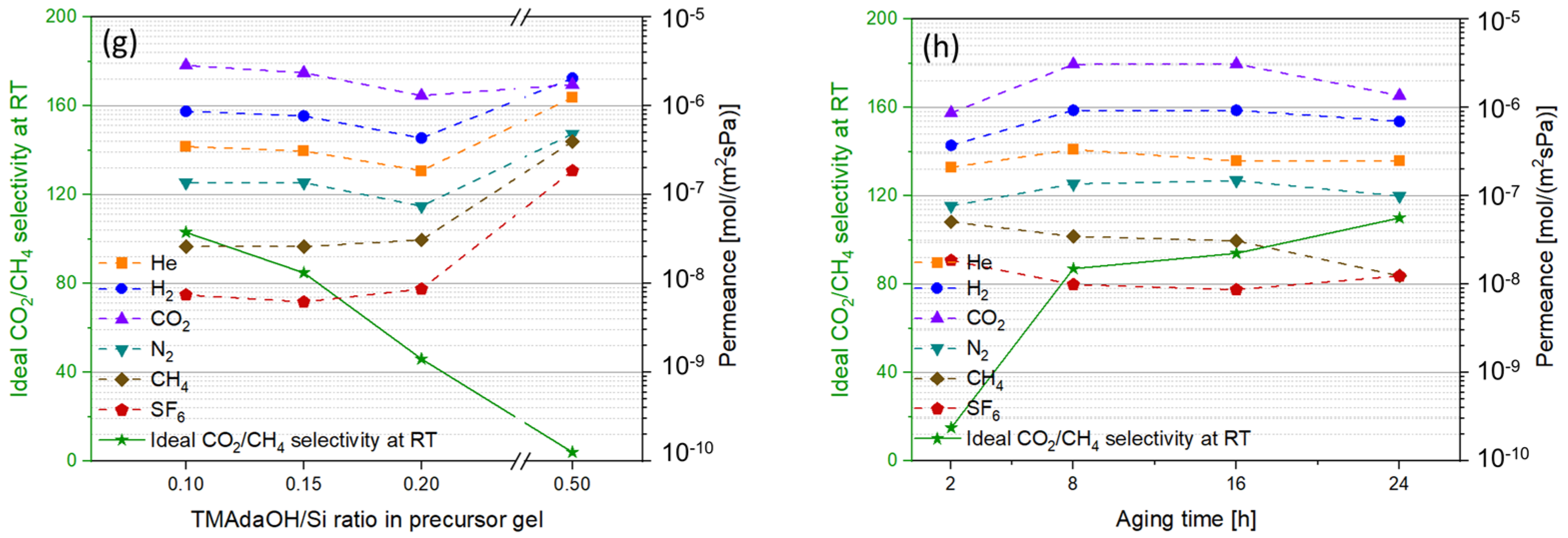
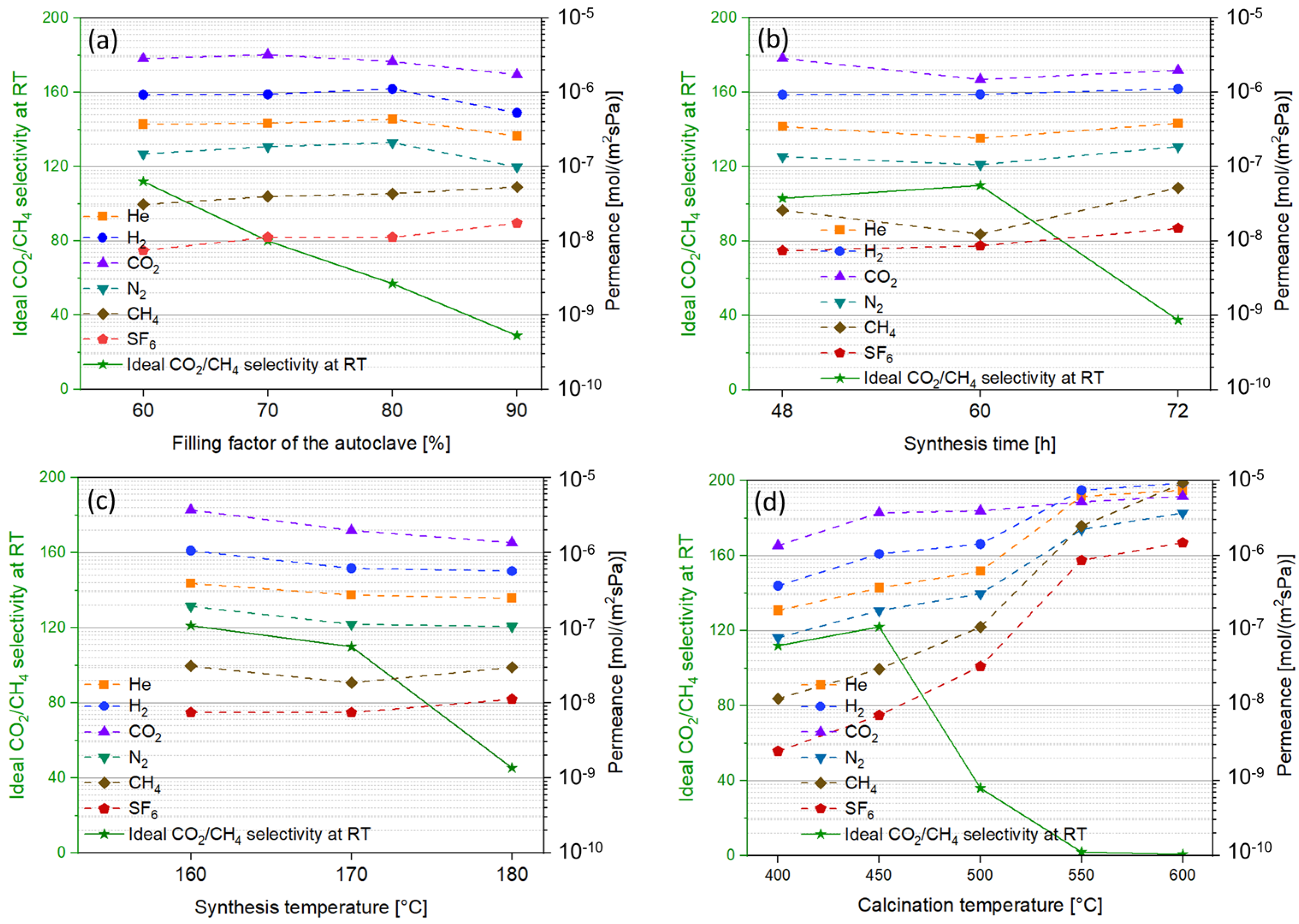

| Support Type and Pore Size | Synthesis Condition | Pressure Drop | Membrane Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness | Single Gas | Mixed Gas | ||||||||
| Gel Precursor Molar Composition | Time | Temperature | CO2 Permeance | Ideal CO2/CH4 Selectivity | CO2 Permeance | CO2/CH4 Selectivity | Ref. | |||
| [nm] | SiO2:Al2O3:Na2O:TMAdaOH:H2O | [h] | [K] | [MPa] | [μm] | 10−7 × [mol/(m2sPa)] | 10−7 × [mol/(m2sPa)] | |||
| Al2O3, 300 | 1.05:005:0.1:0.2:44 | 144 | 433 | 0.6 | 4 to 6 | 3 | 20 | 2.8 | 36 | [52] |
| Mullite, 130 | 1:0.005:0.2:0.1:80 | - | - | 0.4 | - | 3.2 | 34 | - | - | [53] |
| Al2O3, 200 | 1:0.05:0.1:0.5:80 | 48 | 433 | 0.2 | 6 | 8.1 | 75 | 5.6 | 57 | [39] |
| Al2O3, 200 | 1:0.1:0.1:0.6:44 | 240 | 373 | 0.14 | 0.67 | 43 | 123 | 39 | 162 | [54] |
| Al2O3, 200 | 1:0.01:0.1:0.6:44 | 48 | 433 | 0.14 | 1.7 | - | - | 14 | 116 | [21] |
| Al2O3, 100 | 1:0.005:0.1:0.4:44 | 24 | 453 | 0.2 | 1 | 1.1 | 91 | 13.2 | 125 | [55] |
| Al2O3 | 1:0.025:0.2:0.2:80 | 72 | 433 | 0.2 | 3 | - | - | 2 | 22 | [56] |
| Al2O3 | 1:0.005:1:1:80 | 48 | 433 | 0.2 | 3 to 4 | - | - | 2.9 | 56 | [57] |
| Al2O3, 200 | 1:0.01:0.1:0.6:44 | 48 | 453 | 0.14 | 3.27 | - | - | 3.8 | 135 | [58] |
| Al2O3, 300 | 1:0.025:0.1:0.07:100 | 20 | 433 | 0.2 | 3 | 5.1 | - | 5.3 | 240 | [59] |
| Al2O3, 100 | 1:0.025:0.1:0.4:44 | 96 | 433 | 0.3 | 6 | 15 | 45 | - | - | [60] |
| Al2O3, 200 | 1:0.01:0.1:0.6:44 | 2 | 473 | 0.2 | 1 | 11 | 138 | 5.5 | 112 | [49] |
| Al2O3, 150 | 1.05:0.004:0.2:0.2:44 | 72 | 433 | 0.2 | 5.3 | 4 | 130 | 2.5 | 119 | [20] |
| Al2O3, 80 | 1:0.005:0.09:0.19:42.75 | 18 | 433 | 0.2 | 2 | 3.56 | 31 | 3.4 | 30 | [29] |
| Al2O3 | 1:0.025:0.2:0.1:80 | 48 | 433 | 0.2 | 3 | 6.5 | 37 | 0.87 | 100 | [28] |
| Al2O3, 200 | 1:0.05:0.05:0.2:80 | 72 | 453 | 0.2 | 3.5 | 0.23 | 46 | 9 | 34 | [35] |
| Al2O3, 200 | 1:0.005:0.1:0.2:40 | 96 | 453 | 0.2 | 2 | 10.1 | 108 | 12.1 | 183 | [61] |
| Al2O3, 200 | 1:0.025:0.1:0.1:80 | 48 | 433 | 0.15 | 2 | 37.2 | 122 | 8.5 | 111 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taherizadeh, A.; Simon, A.; Richter, H.; Stelter, M.; Voigt, I. A Study of the Influence of Synthesis Parameters on the Preparation of High Performance SSZ-13 Membranes. Appl. Sci. 2024, 14, 7836. https://doi.org/10.3390/app14177836
Taherizadeh A, Simon A, Richter H, Stelter M, Voigt I. A Study of the Influence of Synthesis Parameters on the Preparation of High Performance SSZ-13 Membranes. Applied Sciences. 2024; 14(17):7836. https://doi.org/10.3390/app14177836
Chicago/Turabian StyleTaherizadeh, Alireza, Adrian Simon, Hannes Richter, Michael Stelter, and Ingolf Voigt. 2024. "A Study of the Influence of Synthesis Parameters on the Preparation of High Performance SSZ-13 Membranes" Applied Sciences 14, no. 17: 7836. https://doi.org/10.3390/app14177836






