Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Culture
2.2. Extraction of Plant Material
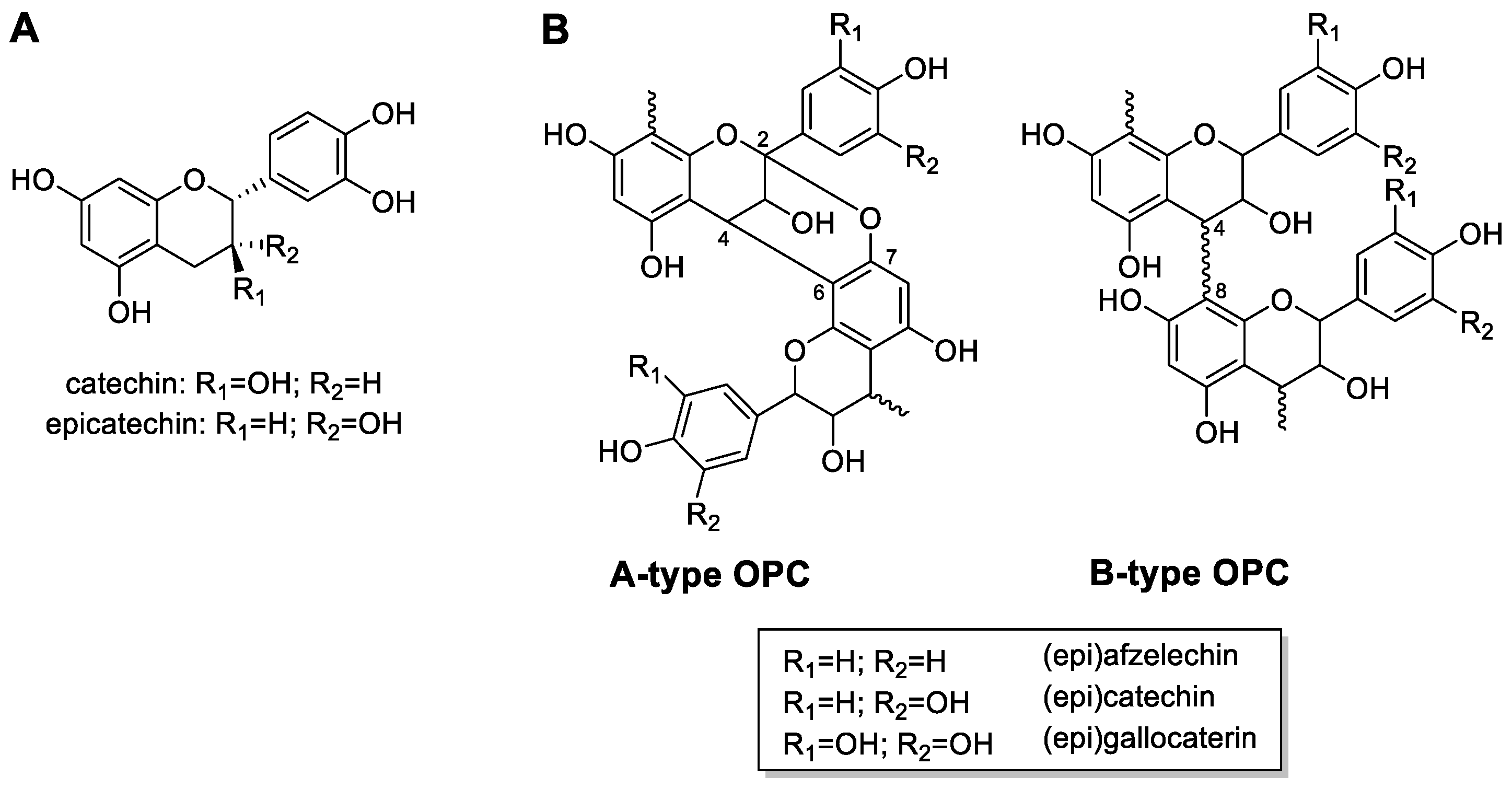
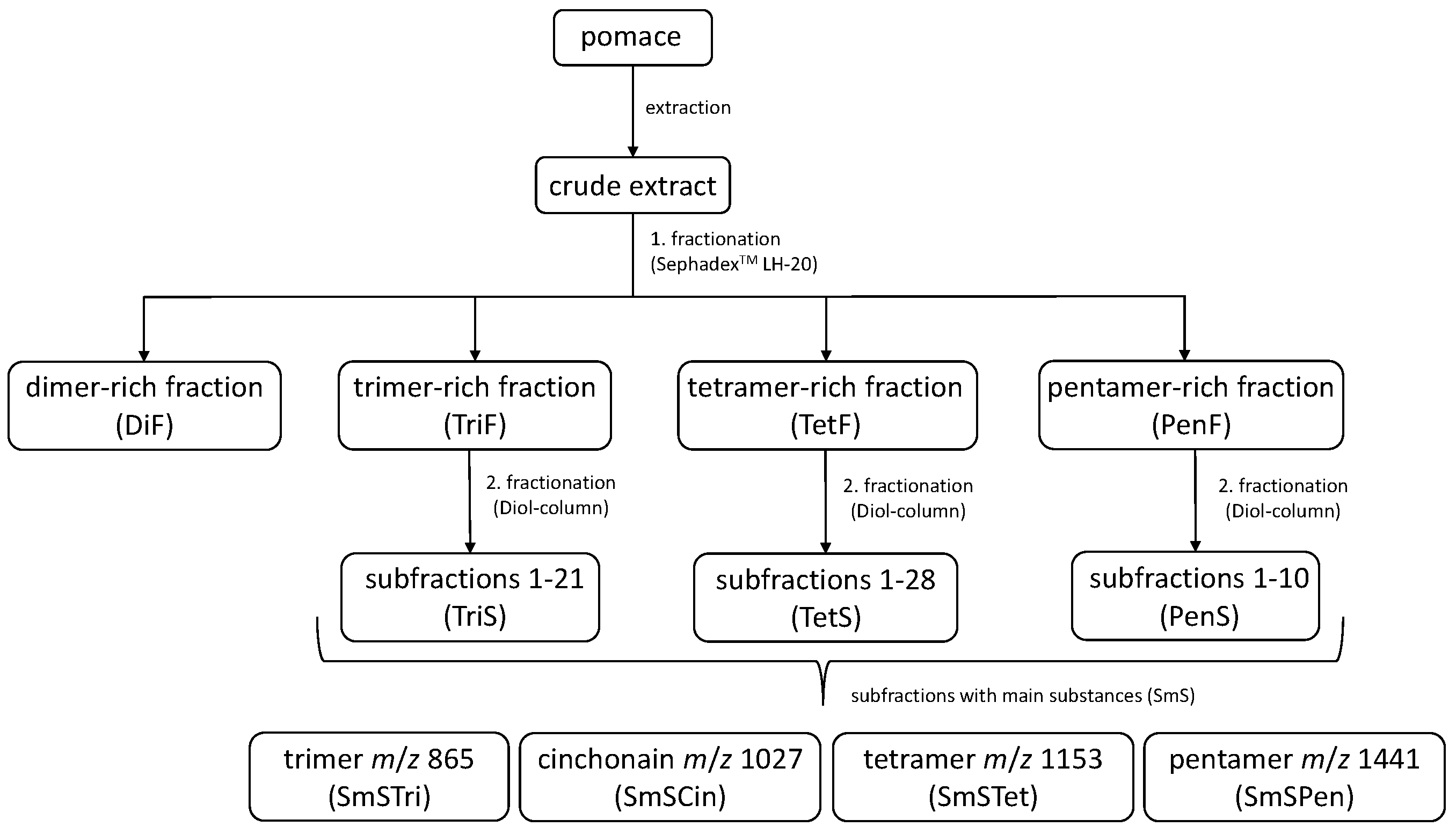
2.3. Isolation and Characterization of OPCs
2.4. Evaluation of Antioxidant Capacity Using the Trolox Equivalent Antioxidant Capaciy (TEAC) Assay
2.5. Cell Culture
2.6. Assessment of the Antioxidant Potential Using a Modified Dichlorofluoresceine Diacetate (DCFH2-DA) Assay
2.7. Software and Statistics
3. Results
3.1. Analysis of the Subfractions of Chokeberry Crude Extract Using LC-HRMS
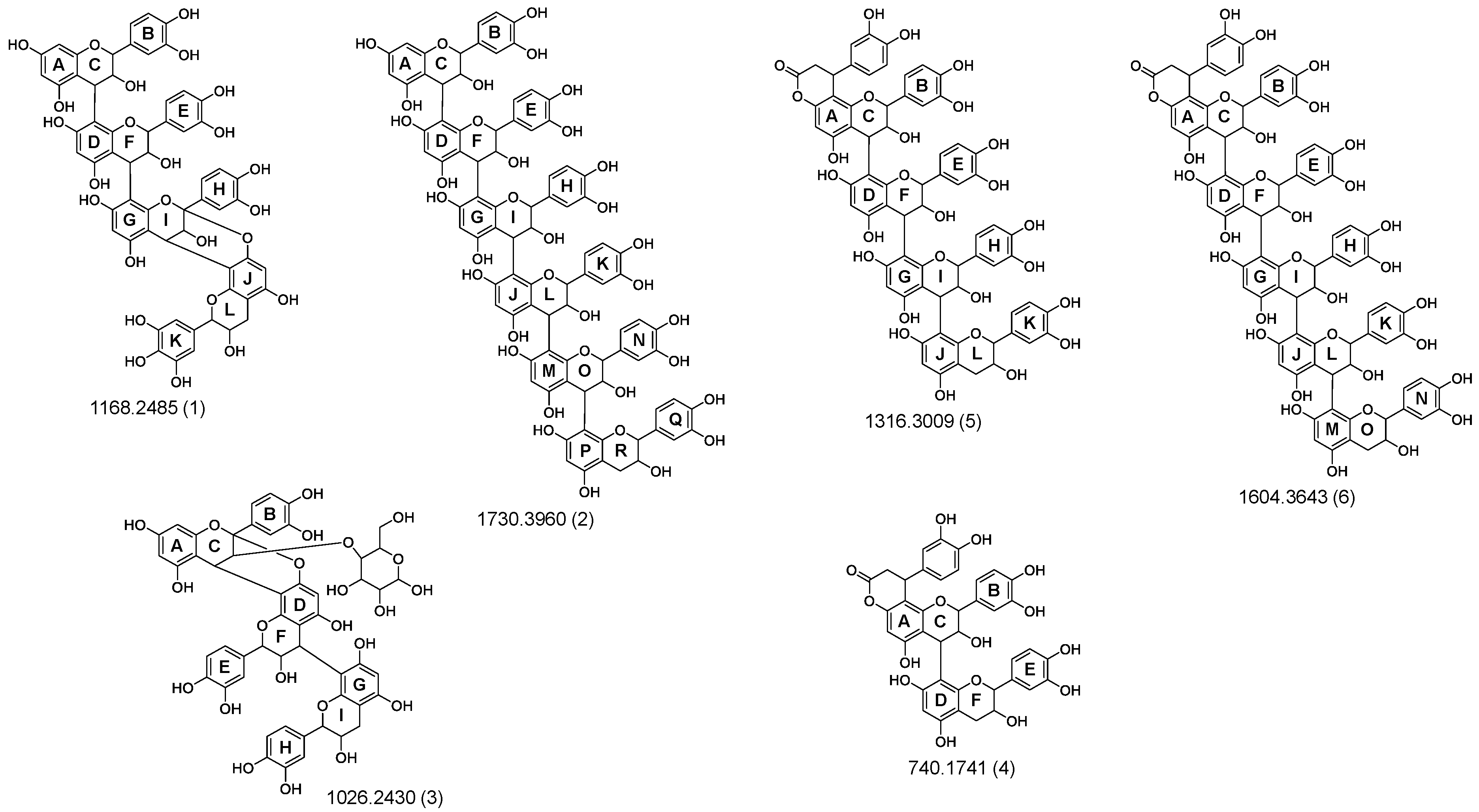
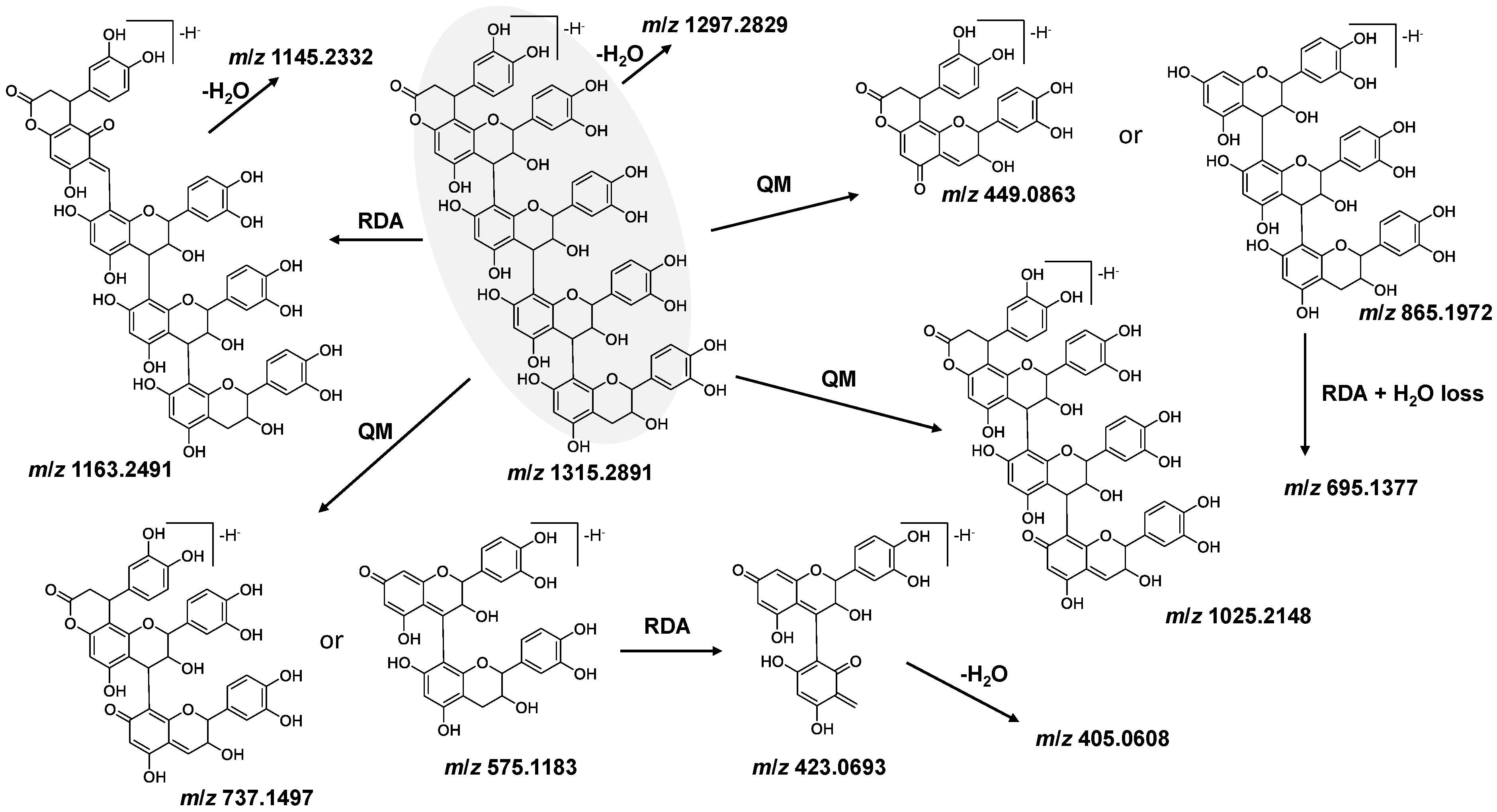
3.2. Identification and Characterization of OPCs in Purified Subfractions via Fragment Spectra
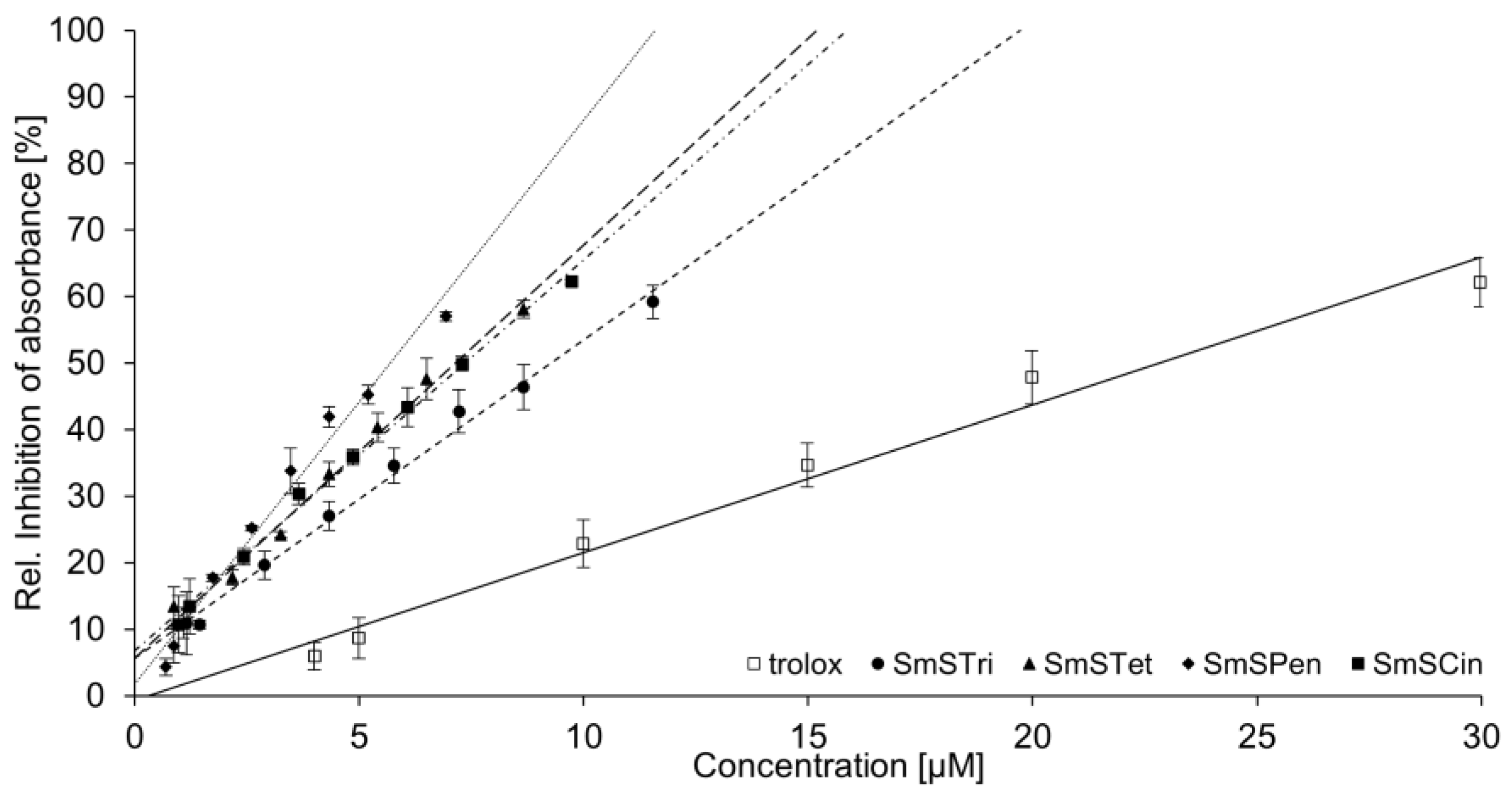
3.3. Trolox Equivalent Antioxidant Capacity of Crude Extract and Selected Enriched OPC Subfractions
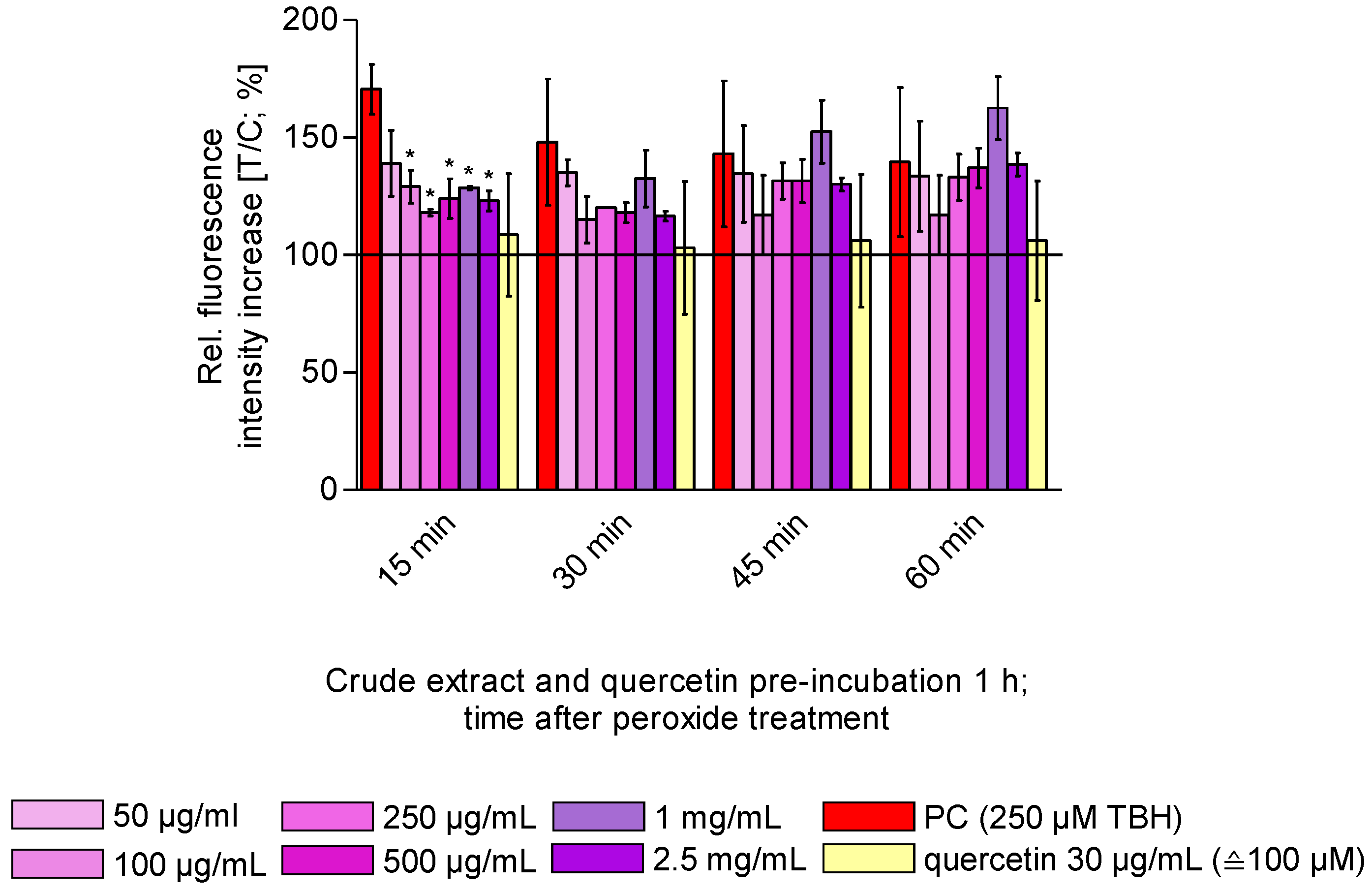
3.4. Antioxidant Capacity of Selected Enriched OPC Subfractions and Crude Extract In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.-V.; Flachowsky, H. Obstzüchtung und Wissenschaftliche Grundlagen; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54084-8. [Google Scholar]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: A meta analysis of controlled clinical trials. J. Diet. Suppl. 2021, 18, 517–530. [Google Scholar] [CrossRef]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Müller, L.; Weever, F.; Hübner, F.; Humpf, H.-U.; Esselen, M. Characterization of Oligomeric Proanthocyanidin-Enriched Fractions from Aronia melanocarpa (Michx.) Elliott via High-Resolution Mass Spectrometry and Investigations on Their Inhibitory Potential on Human Topoisomerases. J. Agric. Food Chem. 2021, 69, 11053–11064. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant Enzymes and Human Health. In Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0789-7. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Fogh, J.; Trempe, G. New Human Tumor Cell Lines. In Human Tumor Cells In Vitro; Fogh, J., Ed.; Springer: Boston, MA, USA, 1975; pp. 115–159. ISBN 978-1-4757-1649-8. [Google Scholar]
- Li, H.-J.; Deinzer, M.L. The mass spectral analysis of isolated hops A-type proanthocyanidins by electrospray ionization tandem mass spectrometry. J. Mass. Spectrom. 2008, 43, 1353–1363. [Google Scholar] [CrossRef]
- Rzeppa, S.; von Bargen, C.; Bittner, K.; Humpf, H.-U. Analysis of Flavan-3-ols and procyanidins in food samples by reversed phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry (RP-HPLC-ESI-MS/MS). J. Agric. Food Chem. 2011, 59, 10594–10603. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Alfke, J.; Kampermann, U.; Kalinina, S.; Esselen, M. Isolation and structural elucidation of dimeric epigallocatechin-3-gallate autoxidation products and their antioxidant capacity. Eur. Food Res. Technol. 2021, 247, 2961–2975. [Google Scholar] [CrossRef]
- Alfke, J.; Esselen, M. Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants 2022, 11, 1746. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Kolter, T. Cinchonaine; Thieme Gruppe: Stuttgart, Germany, 2017. [Google Scholar]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Sendker, J.; Petereit, F.; Lautenschläger, M.; Hellenbrand, N.; Hensel, A. Phenylpropanoid-substituted procyanidins and tentatively identified procyanidin glycosides from hawthorn (Crataegus spp.). Planta Med. 2013, 79, 45–51. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Seminotti, B.; Grings, M.; Tucci, P.; Leipnitz, G.; Saso, L. Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell. Neurosci. 2021, 15, 785057. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The Glutathione System: A Journey from Cyanobacteria to Higher Eukaryotes. Antioxidants 2023, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Comp. Rev. Food Sci. Food Safe 2012, 11, 471–489. [Google Scholar] [CrossRef]

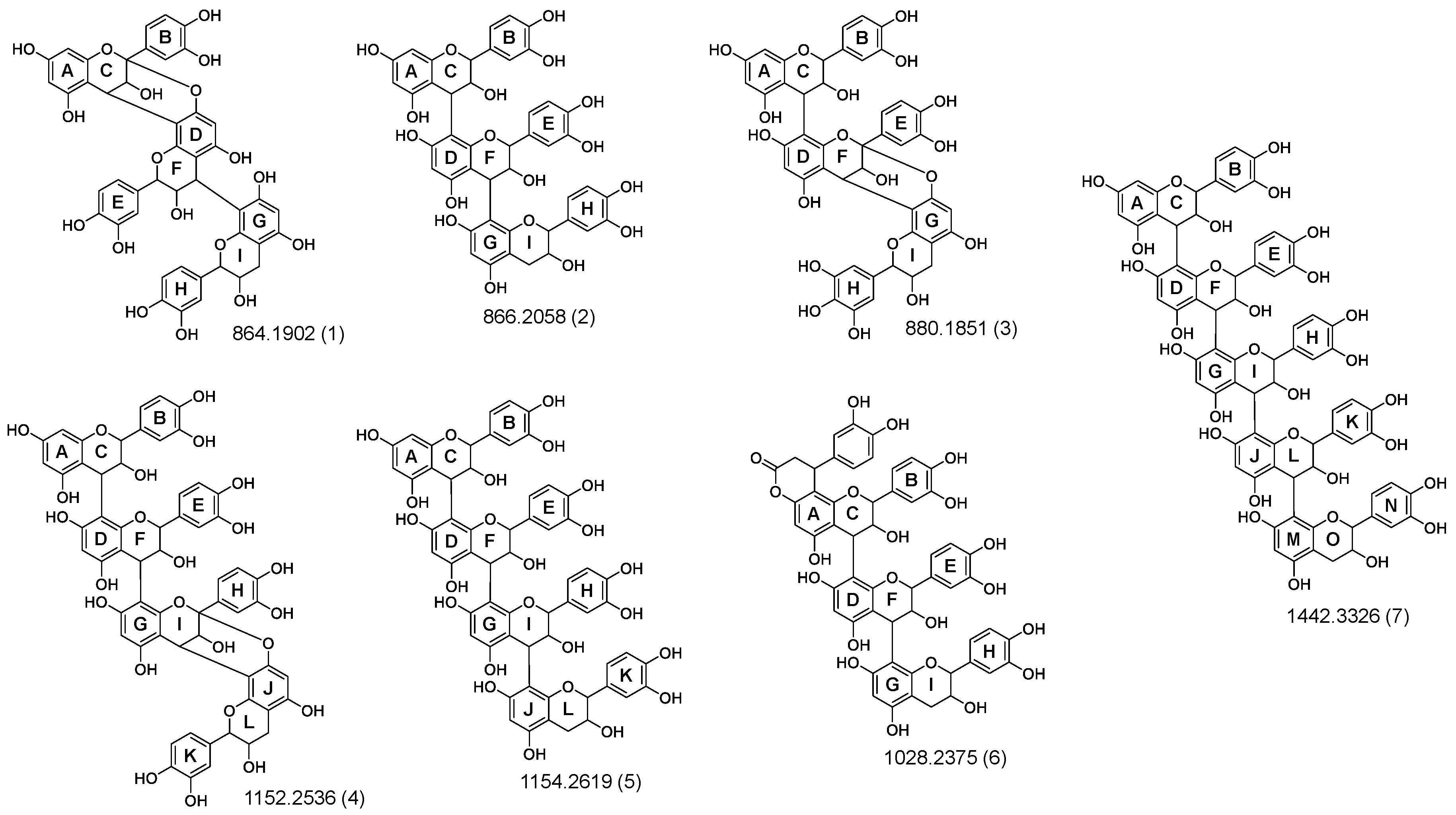


| DP | Postulated Linkage | tR (min) | m/z [M-H]− * m/z [M-2H]2− | Ion Formula | m/z MS2 Ions |
|---|---|---|---|---|---|
| Dimer | B-type | 7.07 | 739.1650 | C39H31O15− | 587, 435, 339, 449, 451, 569, 289, 629, 721 |
| 7.46 | 739.1654 | C39H31O15− | |||
| 8.09 | 739.1647 | C39H31O15− | |||
| 8.67 | 739.1660 | C39H31O15− | |||
| 9.44 | 739.1653 | C39H31O15− | |||
| Trimer | A-type | 6.36 | 863.1807 | C45H35O18− | 711, 411, 451, 693, 559, 573, 289, 290 |
| 6.64 | 863.1803 | C45H35O18− | |||
| 7.95 | 863.1802 | C45H35O18− | |||
| A-type | 6.85 | 879.1747 | C45H35O19− | 591, 709, 573, 465, 753, 303, 861, 727, 691, 287 | |
| B-type | 5.71 | 865.1969 | C45H37O18− | 695, 407, 577, 739, 713, 451, 587, 425, 543, 287, 847 | |
| 5.84 | 865.1974 | C45H37O18− | |||
| 6.13 | 865.1965 | C45H37O18− | |||
| 6.89 | 865.1971 | C45H37O18− | |||
| 7.33 | 865.1962 | C45H37O18− | |||
| 7.88 | 865.1968 | C45H37O18− | |||
| 8.22 | 865.1981 | C45H37O18− | |||
| A-type | 7.52 | 1025.2311 | C51H45O23− | 735, 573, 873, 609, 447, 855, 703, 721 | |
| 8.37 | 1025.2336 | C51H45O23− | |||
| 8.65 | 1025.2343 | C51H45O23− | |||
| B-type | 7.38 | 1027.2283 | C54H43O21− | 737, 575, 875, 857, 449, 585, 577, 705, 695, 339, 451, 1009 | |
| 8.45 | 1027.2268 | C54H43O21− | |||
| 8.90 | 1027.2290 | C54H43O21− | |||
| Tetramer | A-type | 7.02 | 1151.2450 | C60H47O24− | 981, 861, 407, 739, 577, 999, 573, 709 |
| 7.12 | 1151.2448 | C60H47O24− | |||
| 7.64 | 1151.2446 | C60H47O24− | |||
| 8.32 | 1151.2455 | C60H47O24− | |||
| 8.84 | 1151.2452 | C60H47O24− | |||
| B-type | 6.07 | 1153.2607 | C60H49O24− | 983, 865, 575, 1027, 577, 739, 701, 1135, 407, 1001, 695, 701, 449, 847, 451 | |
| 6.33 | 1153.2605 | C60H49O24− | |||
| 6.54 | 1153.2588 | C60H49O24− | |||
| 6.83 | 1153.2603 | C60H49O24− | |||
| 7.19 | 1153.2568 | C60H49O24− | |||
| 7.58 | 1153.2599 | C60H49O24− | |||
| 8.39 | 1153.2605 | C60H49O24− | |||
| A-type | 5.47 | 1167.2379 | C60H47O25− | 591, 997, 879, 863, 573, 1149, 861, 1015, 753, 465, 1041 | |
| 5.76 | 1167.2378 | C60H47O25− | |||
| 6.26 | 1167.2373 | C60H47O25− | |||
| B-type | 7.90 | 1315.2897 | C69H55O27− | 737, 575, 1145, 1025, 865, 695, 1297, 1163, 449, 405, 423 | |
| 8.37 | 1315.2891 | C69H55O27− | |||
| 8.94 | 1315.2905 | C69H55O27− | |||
| Pentamer | B-type | 6.33 | 720.1581 * | C75H61O30− | 863, 577, 289, 575, 1151, 1315, 451, 865, 407, 1027, 701, 898, 739 |
| 6.68 | 720.1585 * | C75H61O30− | |||
| 6.88 | 720.1588 * | C75H61O30− | |||
| 7.35 | 720.1586 * | C75H61O30− | |||
| 7.51 | 720.1585 * | C75H61O30− | |||
| 7.81 | 720.1586 * | C75H61O30− | |||
| B-type | 7.94 | 801.1775 * | C84H67O33− | 575, 737, 1025, 577, 449, 865, 1027, 1313 | |
| 8.54 | 801.1757 * | C84H67O33− | |||
| 8.70 | 801.1757 * | C84H67O33− | |||
| 8.91 | 801.1757 * | C84H67O33− | |||
| 9.24 | 801.1754 * | C84H67O33− | |||
| Hexamer | B-type | 6.29 | 864.1909 * | C90H73O36− | 1151, 575, 577, 287, 449, 407, 1153, 989, 1315, 739, 1439 |
| 6.65 | 864.1910 * | C90H73O36− | |||
| 7.30 | 864.1903 * | C90H73O36− | |||
| 7.38 | 864.1902 * | C90H73O36− | |||
| 7.72 | 864.1904 * | C90H73O36− | |||
| 7.90 | 864.1903 * | C90H73O36− |
| Compound | TEAC Value |
|---|---|
| SmSTri | 2.15 |
| SmSTet | 2.80 |
| SmSPen | 3.82 |
| SmSCin | 2.64 |
| Crude extract | 0.05 |
| Quercetin | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiners, A.; Hübner, F.; Esselen, M. Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential. Appl. Sci. 2024, 14, 7839. https://doi.org/10.3390/app14177839
Meiners A, Hübner F, Esselen M. Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential. Applied Sciences. 2024; 14(17):7839. https://doi.org/10.3390/app14177839
Chicago/Turabian StyleMeiners, Amelie, Florian Hübner, and Melanie Esselen. 2024. "Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential" Applied Sciences 14, no. 17: 7839. https://doi.org/10.3390/app14177839
APA StyleMeiners, A., Hübner, F., & Esselen, M. (2024). Isolation and Characterization of Novel Oligomeric Proanthocyanidins in Chokeberries Using High-Resolution Mass Spectrometry and Investigation of Their Antioxidant Potential. Applied Sciences, 14(17), 7839. https://doi.org/10.3390/app14177839






