Does the Transcranial Direct Current Stimulation Selectively Modulate Prefrontal Cortex Hemodynamics? An Immediate Effect-Controlled Trial on People with and without Depression
Abstract
:1. Introduction
2. Materials and Methods
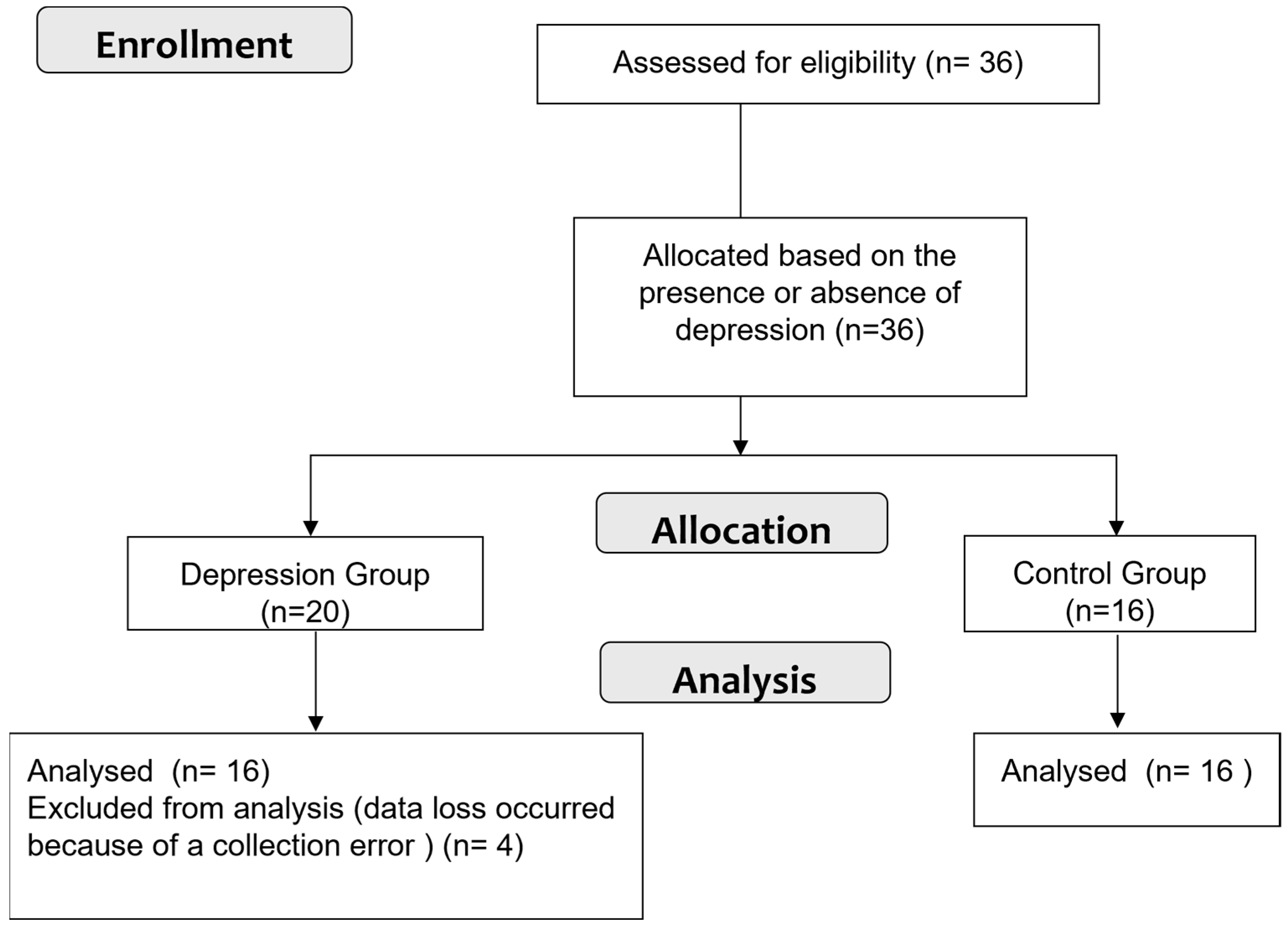
2.1. Participants
2.2. Transcranial Direct Current Stimulation (tDCS)
2.3. fNIRS Recording
2.4. Experimental Testing
2.5. Statistical Analysis
3. Results
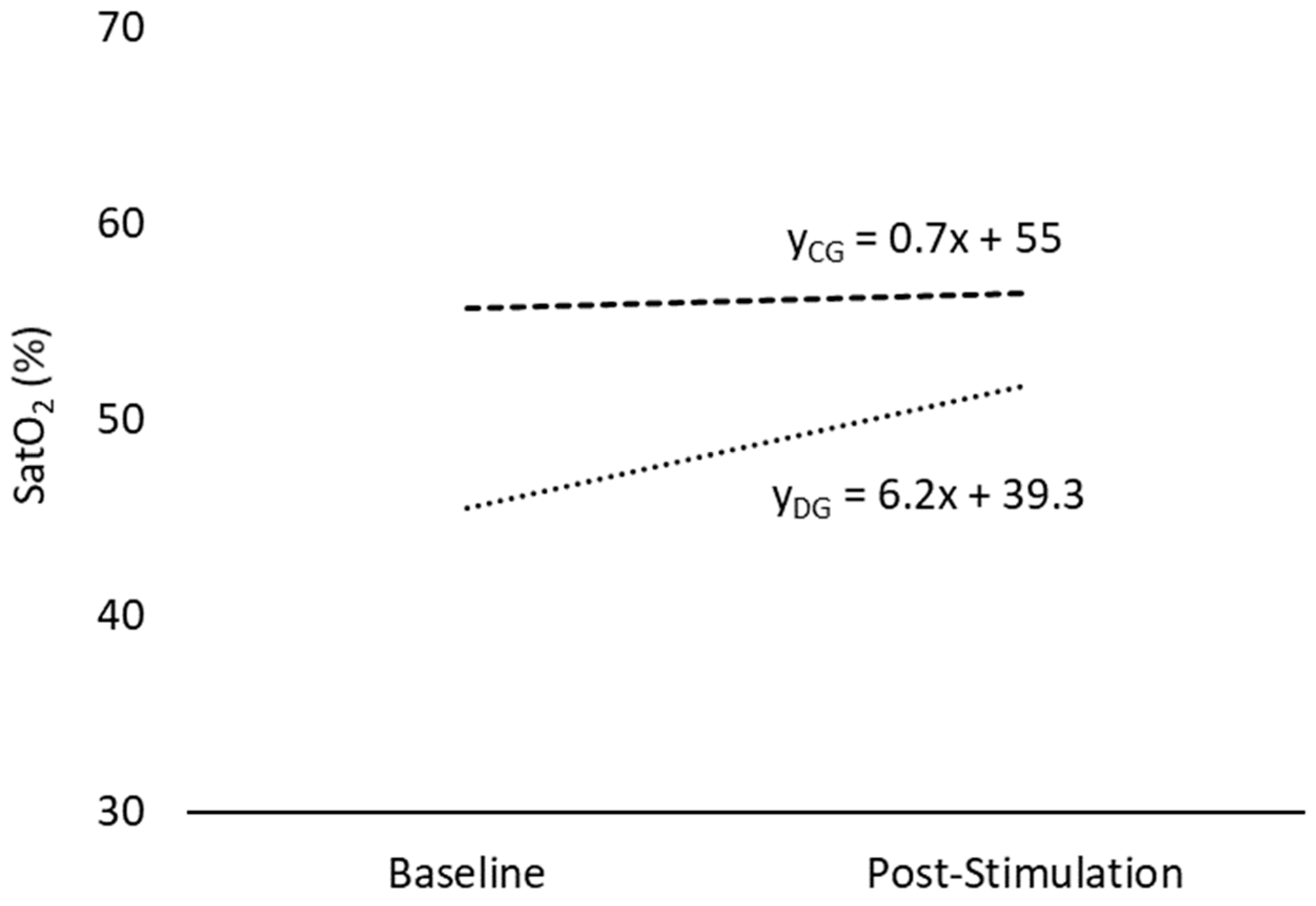
3.1. Oxygen Saturation (SatO2)
3.2. Oxyhemoglobin (HbO)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-W.; Chou, P.-H.; Brunoni, A.R.; Hung, K.-C.; Tseng, P.-T.; Liang, C.-S.; Carvalho, A.F.; Vieta, E.; Tu, Y.-K.; Lin, P.-Y.; et al. Comparing Different Non-Invasive Brain Stimulation Interventions for Bipolar Depression Treatment: A Network Meta-Analysis of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2024, 156, 105483. [Google Scholar] [CrossRef]
- Borrione, L.; Cavendish, B.A.; Aparicio, L.V.M.; Luethi, M.S.; Goerigk, S.; Carneiro, A.M.; Valiengo, L.; Moura, D.O.; de Souza, J.P.; Baptista, M.; et al. Home-Use Transcranial Direct Current Stimulation for the Treatment of a Major Depressive Episode. JAMA Psychiatry 2024, 81, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Kim, H.; Roh, D.; Kim, D.H. Home-Based, Remotely Supervised, 6-Week TDCS in Patients With Both MCI and Depression: A Randomized Double-Blind Placebo-Controlled Trial. Clin. EEG Neurosci. 2023, 55, 15500594231215847. [Google Scholar] [CrossRef]
- Meron, D.; Hedger, N.; Garner, M.; Baldwin, D.S. Transcranial Direct Current Stimulation (TDCS) in the Treatment of Depression: Systematic Review and Meta-Analysis of Efficacy and Tolerability. Neurosci. Biobehav. Rev. 2015, 57, 46–62. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. TDCS for the Treatment of Depression: A Comprehensive Review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef]
- Khadka, N.; Bikson, M. Neurocapillary-Modulation. Neuromodulation 2022, 25, 1299–1311. [Google Scholar] [CrossRef]
- Bikson, M. Transcranial Direct Current Stimulation and Neurovascular Modulation. Eur. J. Neurol. 2023, 30, 802–803. [Google Scholar] [CrossRef]
- Gellner, A.; Frase, S.; Reis, J.; Fritsch, B. Direct Current Stimulation Increases Blood Flow and Permeability of Cortical Microvasculature in Vivo. Eur. J. Neurol. 2023, 30, 362–371. [Google Scholar] [CrossRef]
- Yang, D.; Shin, Y.-I.; Hong, K.-S. Systemic Review on Transcranial Electrical Stimulation Parameters and EEG/FNIRS Features for Brain Diseases. Front. Neurosci. 2021, 15, 629323. [Google Scholar] [CrossRef]
- Chao, J.; Zheng, S.; Wu, H.; Wang, D.; Zhang, X.; Peng, H.; Hu, B. FNIRS Evidence for Distinguishing Patients With Major Depression and Healthy Controls. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2211–2221. [Google Scholar] [CrossRef]
- Kang, L.; Wang, W.; Nie, Z.; Gong, Q.; Yao, L.; Xiang, D.; Zhang, N.; Tu, N.; Feng, H.; Zong, X.; et al. Dysregulated Cerebral Blood Flow, Rather than Gray Matter Volume, Exhibits Stronger Correlations with Blood Inflammatory and Lipid Markers in Depression. Neuroimage Clin. 2024, 41, 103581. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, Y.; Qiu, H.; Zhang, Z.; Tan, X.; Huang, P.; Zhang, M.; Miao, D.; Zhu, X. A New Perspective for Evaluating the Efficacy of tACS and tDCS in Improving Executive Functions: A Combined tES and fNIRS Study. Hum. Brain Mapp. 2024, 45, e26559. [Google Scholar] [CrossRef] [PubMed]
- Wachter, D.; Wrede, A.; Schulz-Schaeffer, W.; Taghizadeh-Waghefi, A.; Nitsche, M.A.; Kutschenko, A.; Rohde, V.; Liebetanz, D. Transcranial Direct Current Stimulation Induces Polarity-Specific Changes of Cortical Blood Perfusion in the Rat. Exp. Neurol. 2011, 227, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yahata, N.; Funane, T.; Takizawa, R.; Katura, T.; Atsumori, H.; Nishimura, Y.; Kinoshita, A.; Kiguchi, M.; Koizumi, H.; et al. A NIRS–FMRI Investigation of Prefrontal Cortex Activity during a Working Memory Task. Neuroimage 2013, 83, 158–173. [Google Scholar] [CrossRef]
- Poorzargar, K.; Pham, C.; Ariaratnam, J.; Lee, K.; Parotto, M.; Englesakis, M.; Chung, F.; Nagappa, M. Accuracy of Pulse Oximeters in Measuring Oxygen Saturation in Patients with Poor Peripheral Perfusion: A Systematic Review. J. Clin. Monit. Comput. 2022, 36, 961–973. [Google Scholar] [CrossRef]
- Tsukuda, J.; Fujitani, S.; Rahman, M.; Morisawa, K.; Kawaguchi, T.; Taira, Y. Monitoring Tissue Oxygenation Index Using Near-infrared Spectroscopy during Pre-hospital Resuscitation among Out-of-hospital Cardiac Arrest Patients: A Pilot Study. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 42. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal Cortex and Depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Kuo, P.H.; Su, M.I.; Huang, W.L. The Efficacy of Non-Invasive, Non-Convulsive Electrical Neuromodulation on Depression, Anxiety and Sleep Disturbance: A Systematic Review and Meta-Analysis. Psychol. Med. 2022, 52, 801–812. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Hamaoka, T.; McCully, K.K. Review of Early Development of Near-Infrared Spectroscopy and Recent Advancement of Studies on Muscle Oxygenation and Oxidative Metabolism. J. Physiol. Sci. 2019, 69, 799–811. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current Intensity- and Polarity-specific Online and Aftereffects of Transcranial Direct Current Stimulation: An FMRI Study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef]
- Hu, S.; Zheng, T.; Dong, Y.; Du, J.; Liu, L. Effect of Anodal Direct-Current Stimulation on Cortical Hemodynamic Responses With Laser-Speckle Contrast Imaging. Front. Neurosci. 2018, 12, 503. [Google Scholar] [CrossRef]
- Figeys, M.; Zeeman, M.; Kim, E.S. Effects of Transcranial Direct Current Stimulation (TDCS) on Cognitive Performance and Cerebral Oxygen Hemodynamics: A Systematic Review. Front. Hum. Neurosci. 2021, 15, 623315. [Google Scholar] [CrossRef]
- Trofimov, A.O.; Agarkova, D.I.; Sergeev, D.; Dubrovin, A.; Trofimova, K.A.; Novosadova, O.; Martynov, D.; Lidji-Goryaev, K.; Bragin, D.E. NIRS-Based Study of Local Cerebral Oxygenation During Transcranial Direct Current Stimulation in Patients with Mild Traumatic Brain Injury. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switherland, 2022; Volume 1395, pp. 59–63. ISBN 9783031141904. [Google Scholar]
- Liu, M.L.; Karabanov, A.N.; Piek, M.; Petersen, E.T.; Thielscher, A.; Siebner, H.R. Short Periods of Bipolar Anodal TDCS Induce No Instantaneous Dose-Dependent Increase in Cerebral Blood Flow in the Targeted Human Motor Cortex. Sci. Rep. 2022, 12, 9580. [Google Scholar] [CrossRef]
- Zanardi, R.; Poletti, S.; Prestifilippo, D.; Attanasio, F.; Barbini, B.; Colombo, C. Transcranial Direct Current Stimulation: A Novel Approach in the Treatment of Vascular Depression. Brain Stimul. 2020, 13, 1559–1565. [Google Scholar] [CrossRef]
- Merzagora, A.C.; Foffani, G.; Panyavin, I.; Mordillo-Mateos, L.; Aguilar, J.; Onaral, B.; Oliviero, A. Prefrontal Hemodynamic Changes Produced by Anodal Direct Current Stimulation. Neuroimage 2010, 49, 2304–2310. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z. Aberrant Pattern of Cerebral Blood Flow in Patients with Major Depressive Disorder: A Meta-Analysis of Arterial Spin Labelling Studies. Psychiatry Res. Neuroimaging 2022, 321, 111458. [Google Scholar] [CrossRef]
- Willeumier, K.; Taylor, D.V.; Amen, D.G. Decreased Cerebral Blood Flow in the Limbic and Prefrontal Cortex Using SPECT Imaging in a Cohort of Completed Suicides. Transl. Psychiatry 2011, 1, e28. [Google Scholar] [CrossRef]
- Barbosa, A. Does tDCS Selectively Modulate the Pre-Frontal Cortex Hemo-Dynamics? An Immediate Effect-Controlled Trial on Depressed and Healthy People. Mendeley Data, V1. 2024. Available online: https://data.mendeley.com/datasets/vgpm3sx7pz/1 (accessed on 1 August 2024).
| Characteristics | Depression Group | Control Group | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | n (%) | Mean ± SD | n (%) | ||
| Sex | |||||
| Male | 4 (25) | 3 (18.75) | ns | ||
| Female | 12 (75) | 13 (81.25) | |||
| Age (years) | 36.4 ± 18.9 | 24.1 ± 2.29 | <0.001 * | ||
| Weight (kg) | 69.4 ± 16.8 | 71.1 ± 14.1 | ns | ||
| Height (m) | 1.65 ± 0.07 | 1.67 ± 0.04 | ns | ||
| (BMI) | 25.5 ± 5.5 | 25.3 ± 4.10 | ns | ||
| Outcome (%) | Depression Group (n = 16) Mean ± SD | Within-Group Differences | Control Group (n = 16) Mean ± SD | Within-Group Differences | Between-Group Differences | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Stimulation | Baseline | Post-Stimulation | Baseline | Post-Stimulation | |||
| Mean | 51.1 ± 7.35 | 53.7 ± 10.0 | ns | 57.3 ± 5.46 | 57.8 ± 5.52 | ns | ns | ns |
| Minimum | 45.5 ± 14.3 | 51.7 ± 10.8 | 0.006 * | 55.7 ± 5.29 | 56.4 ± 6.31 | ns | 0.012 * | ns |
| Maximum | 54.5 ± 7.18 | 55.6 ± 9.87 | ns | 58.6 ± 5.63 | 58.5 ± 5.56 | ns | ns | ns |
| Outcome (g/dL) | Depression Group (n = 16) Mean ± SD | Within-Group Differences | Control Group (n = 16) Mean ± SD | Within-Group Differences | Between-Group Differences | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Stimulation | Baseline | Post-Stimulation | Baseline | Post-Stimulation | |||
| Mean | 5.94 ± 0.97 | 6.29 ± 1.35 | ns | 6.76 ± 0.75 | 6.84 ± 0.76 | ns | ns | ns |
| Minimum | 5.25 ± 1.74 | 6.04 ± 1.43 | 0.002 * | 6.54 ± 0.72 | 6.64 ± 0.86 | ns | 0.04 * | ns |
| Maximum | 6.39 ± 0.96 | 6.55 ± 1.35 | ns | 6.94 ± 0.78 | 6.93 ± 0.77 | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, L.O.; Macedo, M.d.C.G.S.; Monerat, V.K.V.; Ferreira, K.R.d.R.; Santos, M.E.C.d.; Esquirio, A.F.; Alves, A.L.G.; Gama, G.L.; Barbosa, M.A.; Barbosa, A.C. Does the Transcranial Direct Current Stimulation Selectively Modulate Prefrontal Cortex Hemodynamics? An Immediate Effect-Controlled Trial on People with and without Depression. Appl. Sci. 2024, 14, 7901. https://doi.org/10.3390/app14177901
Campos LO, Macedo MdCGS, Monerat VKV, Ferreira KRdR, Santos MECd, Esquirio AF, Alves ALG, Gama GL, Barbosa MA, Barbosa AC. Does the Transcranial Direct Current Stimulation Selectively Modulate Prefrontal Cortex Hemodynamics? An Immediate Effect-Controlled Trial on People with and without Depression. Applied Sciences. 2024; 14(17):7901. https://doi.org/10.3390/app14177901
Chicago/Turabian StyleCampos, Laura Oliveira, Maria de Cassia Gomes Souza Macedo, Vheyda Katheleen Vespasiano Monerat, Kariny Realino do Rosário Ferreira, Mayra Evelise Cunha dos Santos, Arthur Ferreira Esquirio, Ana Luiza Guimarães Alves, Gabriela Lopes Gama, Michelle Almeida Barbosa, and Alexandre Carvalho Barbosa. 2024. "Does the Transcranial Direct Current Stimulation Selectively Modulate Prefrontal Cortex Hemodynamics? An Immediate Effect-Controlled Trial on People with and without Depression" Applied Sciences 14, no. 17: 7901. https://doi.org/10.3390/app14177901





