Quantitative Analysis of Sterols and Oxysterols in Foods by Gas Chromatography Coupled with Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fat Extraction from Food Products
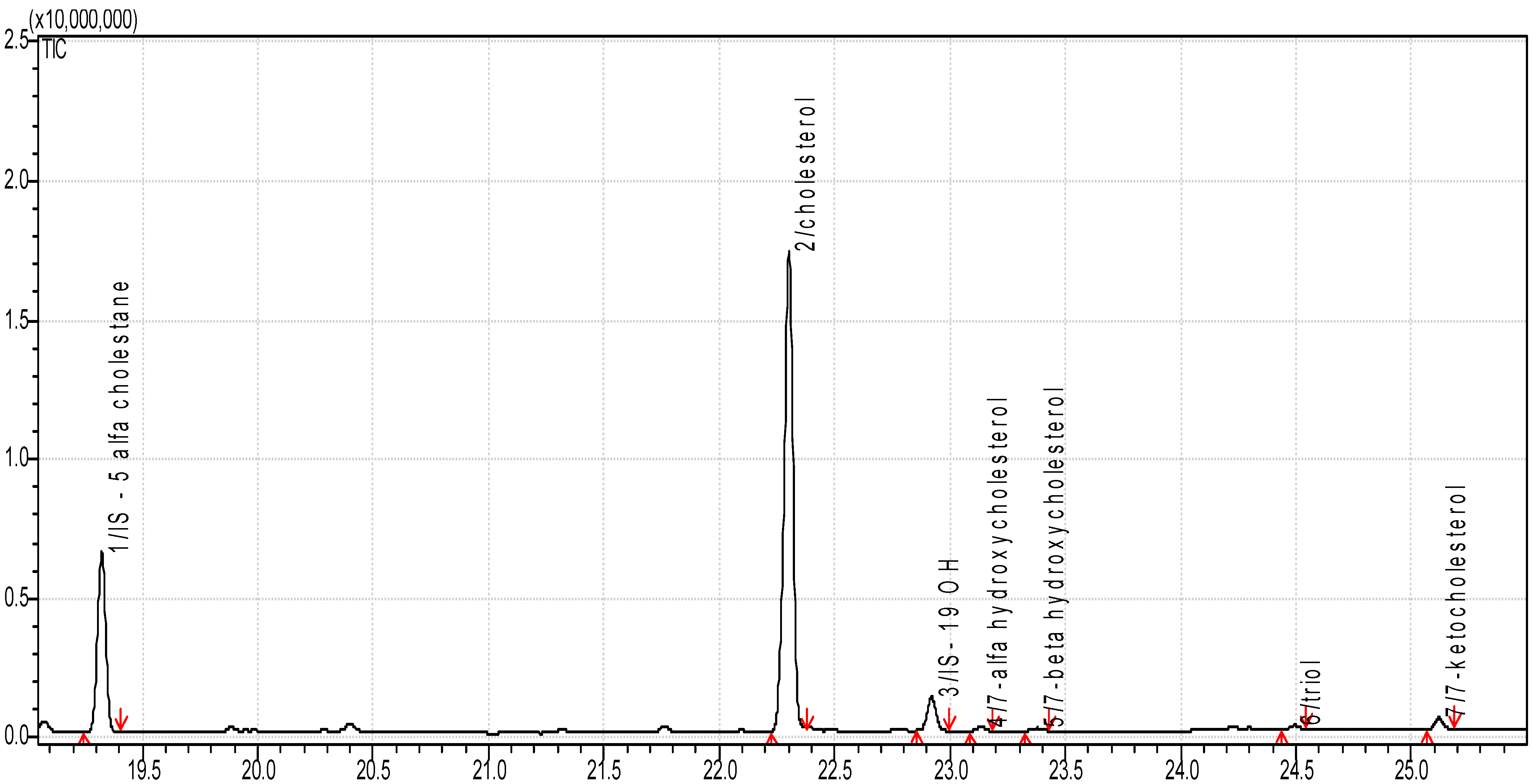
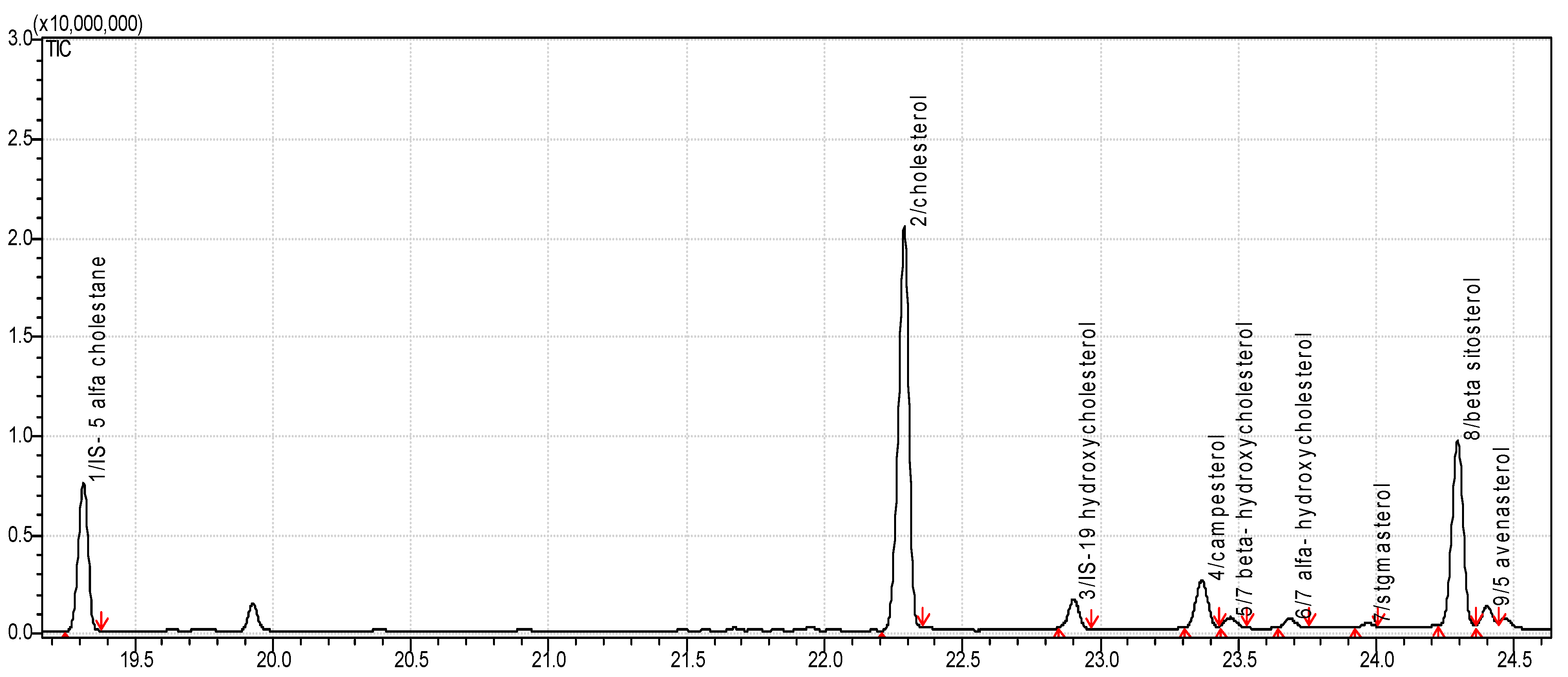
2.3. Determination of Cholesterol and Oxysterols
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sterol Content in Food Products
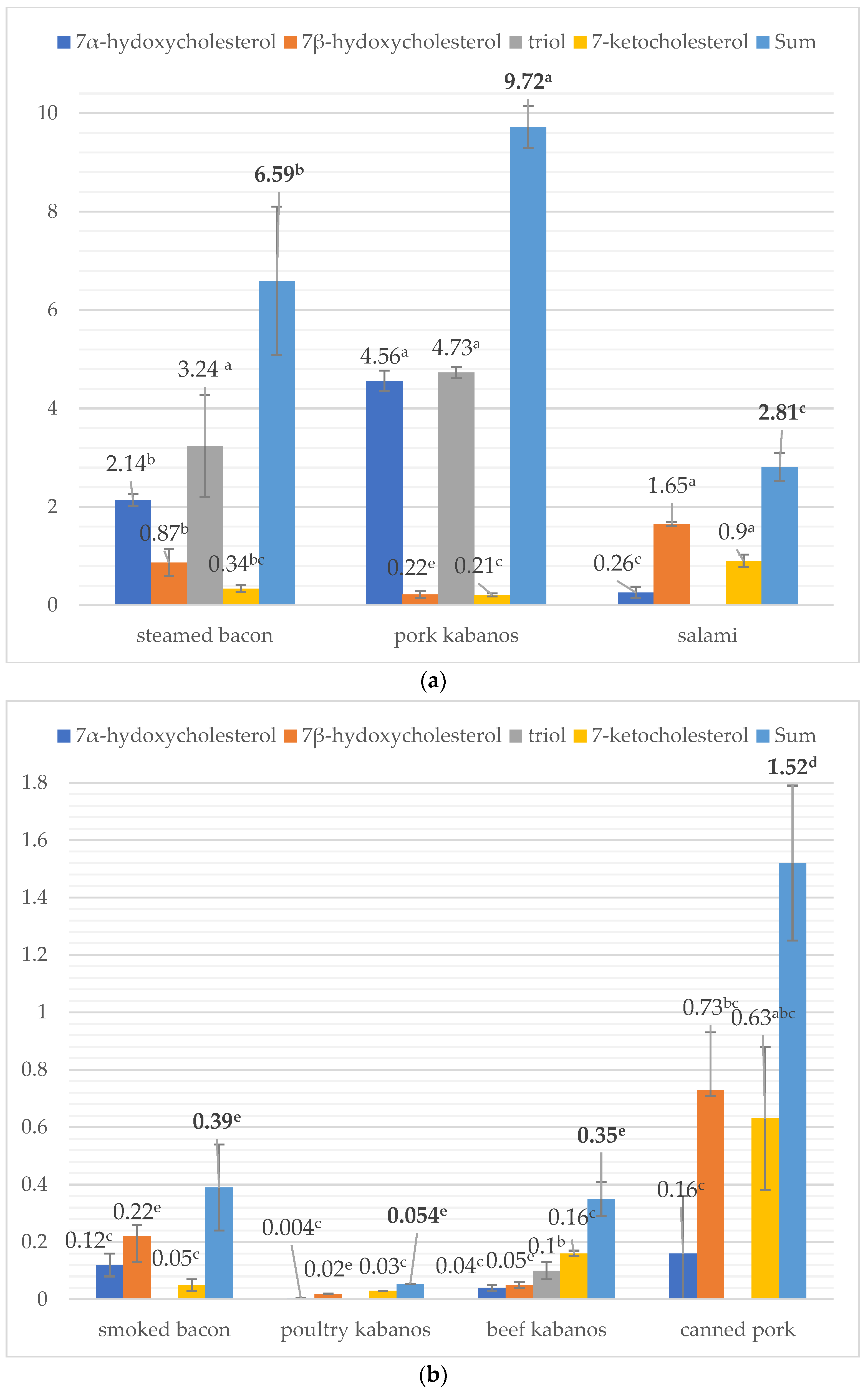
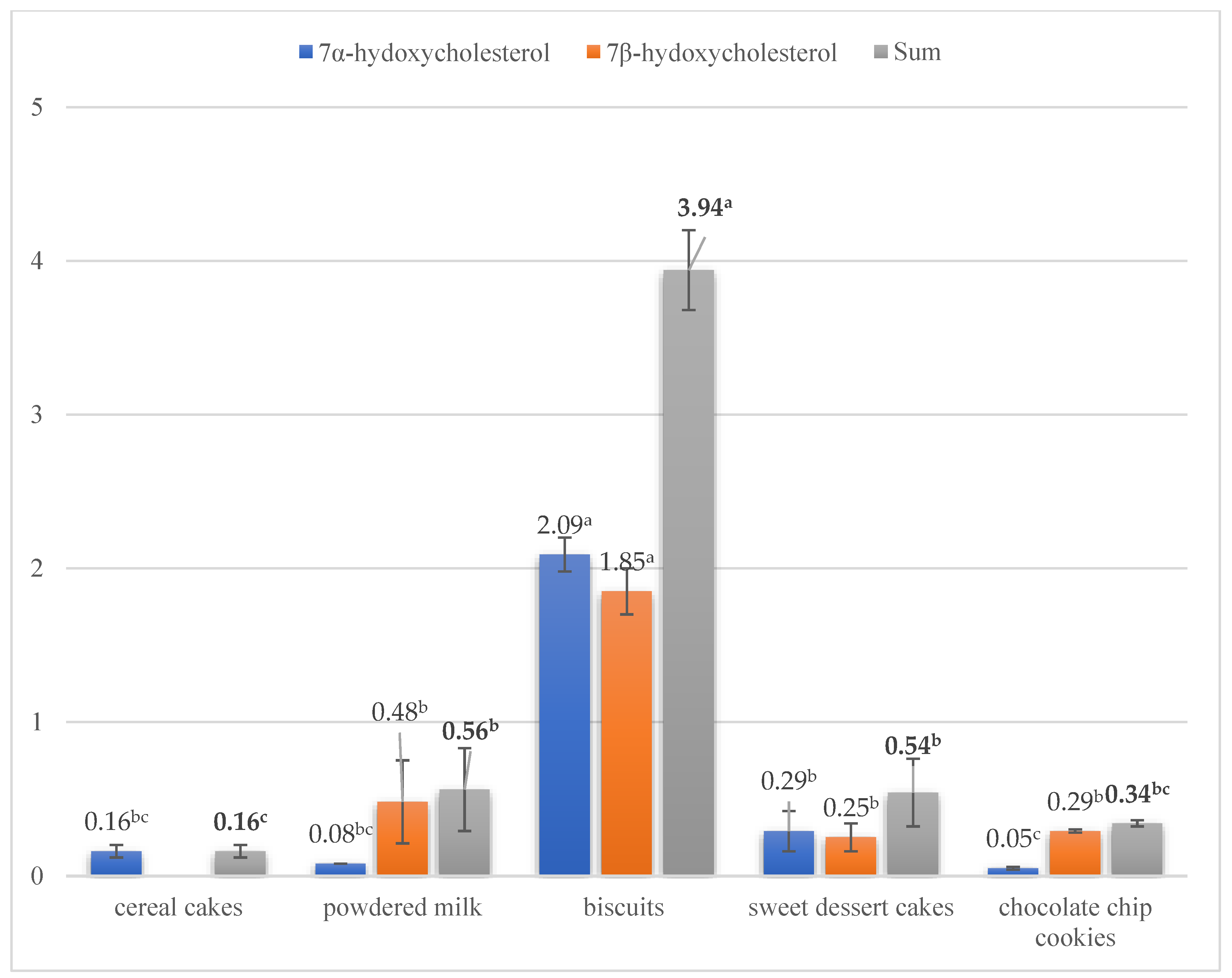
3.2. Concentration of Sterol Oxidation Products in Food Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnus, Z. Oznaczanie wolnych sterol roślinnych i zwierzęcych w estrach metylowych kwasów tłuszczowych z wykorzystaniem techniki chromatografii gazowej. Nafta-Gaz 2013, LXIX, 632–645. [Google Scholar]
- Kropiwiec, K.; Babicz, M.; Całyniku, Z. Zawartość cholesterolu w surowcach i produktach pochodzenia zwierzęcego. In Przegląd Wybranych Zagadnień z Zakresu Przemysłu Spożywczego; Szala, M., Kropiwiec, K., Eds.; Wydawnictwa Naukowe Tygiel: Lublin, Poland, 2016; pp. 75–83. [Google Scholar]
- Li, L.-H.; Dutkiewicz, E.P.; Huang, Y.-C.; Zhou, H.-B.; Hsu, C.-C. Analytical methods for cholesterol quantification. J. Food Drug Anal. 2019, 27, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek-Chełmińska, Z. Nowoczesna Dietetyczna Książka Kucharska; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2011; pp. 15–18. [Google Scholar]
- Drozdowski, B. Lipidy. In Chemia Żywności t. 2, Sacharydy, Lipidy i Białka; Sikorski, Z.E., Ed.; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 2012; pp. 73–83. [Google Scholar]
- Kołodziejczyk, A. Steroidy. In Naturalne Związki Organiczne; Polskie Wydawnictwo Naukowe PWN: Warsaw, Poland, 2015; pp. 466–476. [Google Scholar]
- Czarkowska, J. Zdrowy Styl Życia; Wydawnictwa Podlaski Ośrodek Doradztwa Rolniczego: Szepietów, Poland, 2018; pp. 1–20. [Google Scholar]
- Kołakowska, A. Lipidy. In Chemia Żywności 1. Główne Składniki Żywności; Polskie Wydawnictwo Naukowe: Warsaw, Poland, 2017; pp. 153–214. [Google Scholar]
- Zdrojewski, T.; Wyrzykowski, B.; Szczęch, R.; Wierucki, Ł.; Naruszewicz, M.; Narkiewicz, K.; Zarzeczna-Baran, M. Epidemiology and prevention of arterial hypertension in Poland. Blood Press. 2005, 14 (Suppl. 2), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, W.; Czabak-Garbacz, R.; Szkutnik, J. Fitosterole w profilaktyce i terapii. Lek Polsce 2013, 23, 36–41. [Google Scholar]
- Szymańska, R.; Kruk, J. Fitosterole-występowanie i znaczenie dla człowieka. Kosm. Probl. Nauk. Biol. 2007, 56, 107–114. [Google Scholar]
- Wełnicki, M.; Szeligowska, J.; Mamcarz, A. Zastosowanie steroli roślinnych w prewencji chorób układu sercowo-naczyniowego. Mit czy realna możliwość potencjalizacji efektów klasycznych leków hipolipemizujących? Chor. Serca Naczyń 2014, 11, 225–229. [Google Scholar]
- Otaegui-Arrazola, M.; Menendez-Carreno, M.; Ansorena, D.; Astiasaran, I. Oxysterol: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current Knowledge about Oxysterols: A Review. J. Food Sci. 2016, 81, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Derewiaka, D. Occurrence and Formation of Sterol Oxidation Products in Selected Food Products. Ph.D. Thesis, Warsaw University of Life Sciences, Warsaw, Poland, 2008. [Google Scholar]
- Hafid, N. Cholesterol Oxidation Products (COPs) in Ruminant Meat: A Biological and Pathological Approach: A review. Asian J. Dairy Food Res. 2019, 38, 191–203. [Google Scholar]
- Karwowska, M.; Libera, J.; Wójciak, K.M. Znaczenie żywieniowe utlenionych pochodnych cholesterolu w mięsie i produktach mięsnych. In Trendy w Żywieniu Człowieka; Karwowska, M., Gustawa, W., Eds.; Wydawnictwo Polskie Towarzystwo Technologów Żywności: Kraków, Poland, 2015; pp. 91–97. [Google Scholar]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Gray Jerome, W., III; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2019, 29, 101380. [Google Scholar] [CrossRef] [PubMed]
- Derewiaka, D. Food Lipids: Sources, Health Implications, and Future Trends; Lorenzo, J., Munekata, P., Pateiro, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 173–205. ISBN 978-0-12-823371-9. [Google Scholar] [CrossRef]
- Czerwonka, M.; Gielecińska, A.; Białek, A.; Białek, M.; Bobrowska-Korczak, B. Cholesterol and Its Oxidation Derivatives Content in Market Dairy Products. Nutrients 2024, 16, 1371. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, M.; Białek, A.; Bobrowska-Korczak, B. A Novel Method for the Determination of Squalene, Cholesterol and Their Oxidation Products in Food of Animal Origin by GC-TOF/MS. Int. J. Mol. Sci. 2024, 25, 2807. [Google Scholar] [CrossRef] [PubMed]
- Chudy, S.; Teichert, J. Oxysterols in stored powders as potential health hazards. Sci. Rep. 2021, 11, 21192. [Google Scholar] [CrossRef] [PubMed]
- Derewiaka, D. The influence of the food matrix on the content of cholesterol and oxysterols during simulated process of digestion. Eur. J. Lipid Sci. Technol. 2024, 126, 2200175. [Google Scholar] [CrossRef]
- Kołodziej-Skalska, A.; Matysiak, B.; Grudziński, M. Mięso wieprzowe a zdrowie człowieka. Kosmos. Probl. Nauk. Biol. 2016, 65, 535–542. [Google Scholar]
- Lercker, G.; Rodriguez-Estrada, M.T. Cholesterol Oxidation: Presence of 7-ketocholesterol in Different Food Products. J. Food Compos. Anal. 2000, 13, 625–631. [Google Scholar] [CrossRef]
- Pieszka, M.; Pompa-Roborzyński, M.; Baran, J. Surowce i produkty drobiarskiej a zdrowie konsumenta. Wiadomości Zootech. 2017, 4, 118–123. [Google Scholar]
- Bonczar, G.; Chrzanowska, K.; Maciejowski, K.; Walczycka, M. Zawartość cholesterolu i jego pochodnych w mleku i produktach mleczarskich—Uwarunkowania surowcowe i technologiczne. Żywność Nauka Technol. Jakość 2011, 1, 15–27. [Google Scholar]
- Kopeć, A.; Nowacka, E.; Piątkowska, E.; Leszczyńska, T. Charakterystyka i prozdrowotne właściwości steroli roślinnych. Żywność Nauka Technol. Jakość 2011, 3, 5–14. [Google Scholar]
- Rudzińska, M.; Kazuś, T.; Wąsowicz, E. Sterole i ich utlenione pochodne w olejach roślinnych rafinowanych i tłoczonych na zimno. Rośliny Oleiste 2001, 22, 477–497. [Google Scholar]

| Steamed Bacon | Smoked Bacon | Pork Kabanos | Poultry Kabanos | Beef Kabanos | Salami | Canned Pork | |
|---|---|---|---|---|---|---|---|
| Cholesterol | 214.72 ± 56.44 bc | 101.68 ± 5.08 c | 276.55 ± 77.72 b | 356.62 ± 67.57 ab | 98.1 ± 19.06 c | 237.19 ± 49.67 bc | 275.02 ± 43.36 b |
| Campasterol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.65 ± 0.63 |
| Stigmasterol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.22 ± 0.45 |
| β-sitosterol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.01 ± 0.95 |
| Sum of phytosterols | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.88 ± 2.03 |
| Cereal Cakes | Powdered Milk | Biscuits | Sweet Dessert Cakes | Chocolate Chip Cookies | |
|---|---|---|---|---|---|
| Cholesterol | 8.72 ± 1.42 c | 266.96 ± 82.55 b | 2007.11 ± 167.57 a | 95.78 ± 2.06 bc | 46.26 ± 2.19 c |
| Brassicasterol | 42.88 ± 13.93 | n.d. | n.d. | n.d. | n.d. |
| Campesterol | 103.00 ± 1.86 a | n.d. | 23.68 ± 1.65 b | 12.03 ± 0.83 c | 7.52 ± 0.46 d |
| Stigmasterol | 7.74 ± 0.34 a | n.d. | n.d. | 2.08 ± 0.17 c | 6.82 ± 0.29 b |
| β -sitosterol | 227.18 ± 6.11 a | n.d. | 60.15 ± 4.13 b | 43.01 ± 3.4 c | 29.81 ± 1.19 d |
| Avenasterol | n.d. | n.d. | n.d. | 3.65 ± 0.28 a | 1.38 ± 0.55 b |
| Sum of phytosterols | 380.8 ± 22.24 a | n.d. | 83.83 ± 5.78 b | 60.76 ± 4.68 bc | 45.53 ± 2.49 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derewiaka, D.; Pydyn, M. Quantitative Analysis of Sterols and Oxysterols in Foods by Gas Chromatography Coupled with Mass Spectrometry. Appl. Sci. 2024, 14, 7934. https://doi.org/10.3390/app14177934
Derewiaka D, Pydyn M. Quantitative Analysis of Sterols and Oxysterols in Foods by Gas Chromatography Coupled with Mass Spectrometry. Applied Sciences. 2024; 14(17):7934. https://doi.org/10.3390/app14177934
Chicago/Turabian StyleDerewiaka, Dorota, and Mateusz Pydyn. 2024. "Quantitative Analysis of Sterols and Oxysterols in Foods by Gas Chromatography Coupled with Mass Spectrometry" Applied Sciences 14, no. 17: 7934. https://doi.org/10.3390/app14177934
APA StyleDerewiaka, D., & Pydyn, M. (2024). Quantitative Analysis of Sterols and Oxysterols in Foods by Gas Chromatography Coupled with Mass Spectrometry. Applied Sciences, 14(17), 7934. https://doi.org/10.3390/app14177934







