Comparative Analysis of Concrete Cracking and Reinforcement Corrosion in Concrete and Ultra-High-Performance Concrete Short Columns after Accelerated Corrosion
Abstract
1. Introduction
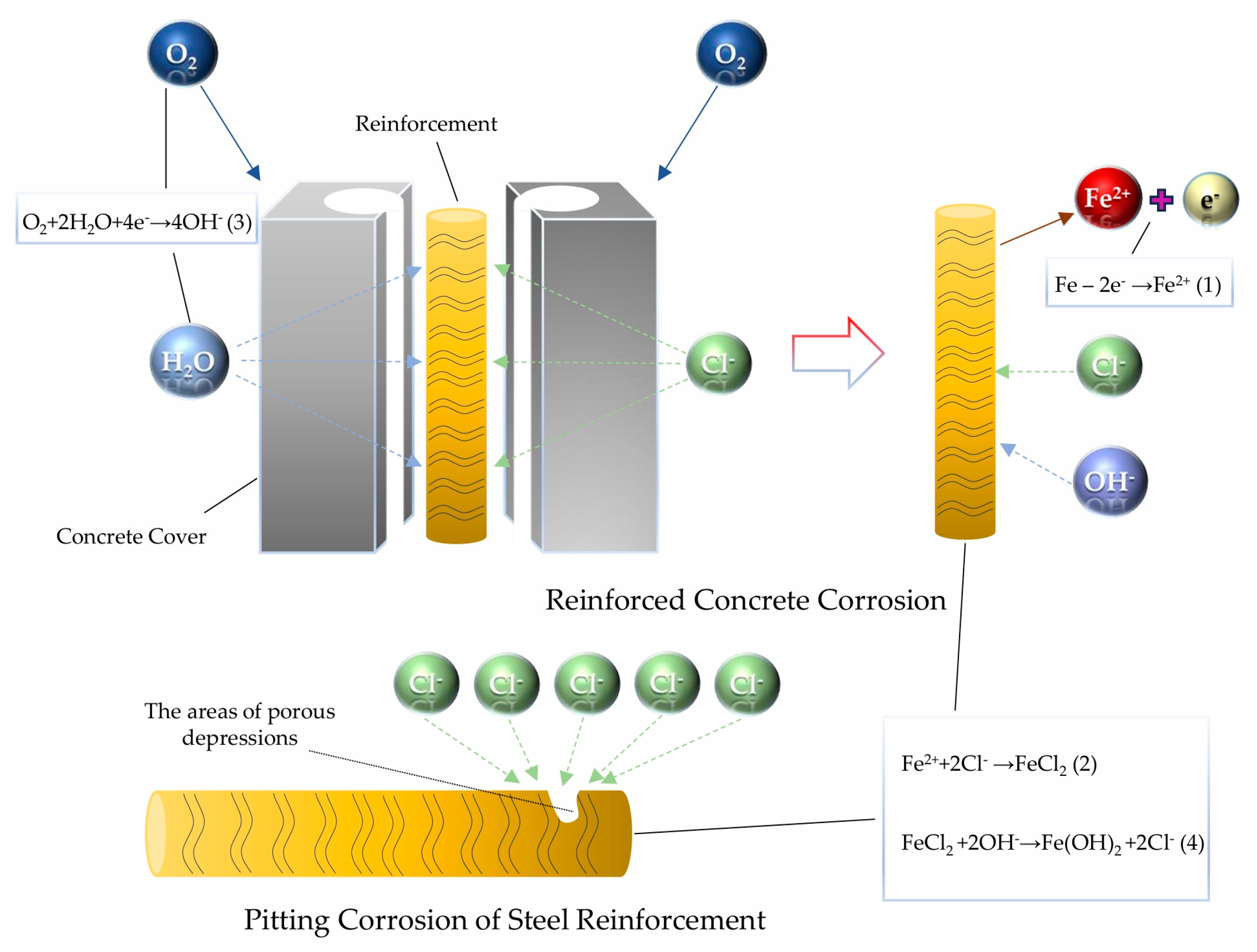
2. The Corrosion Mechanism of Reinforced Concrete
3. Design of Reinforced Concrete Corrosion Testing
3.1. Corrosion Method
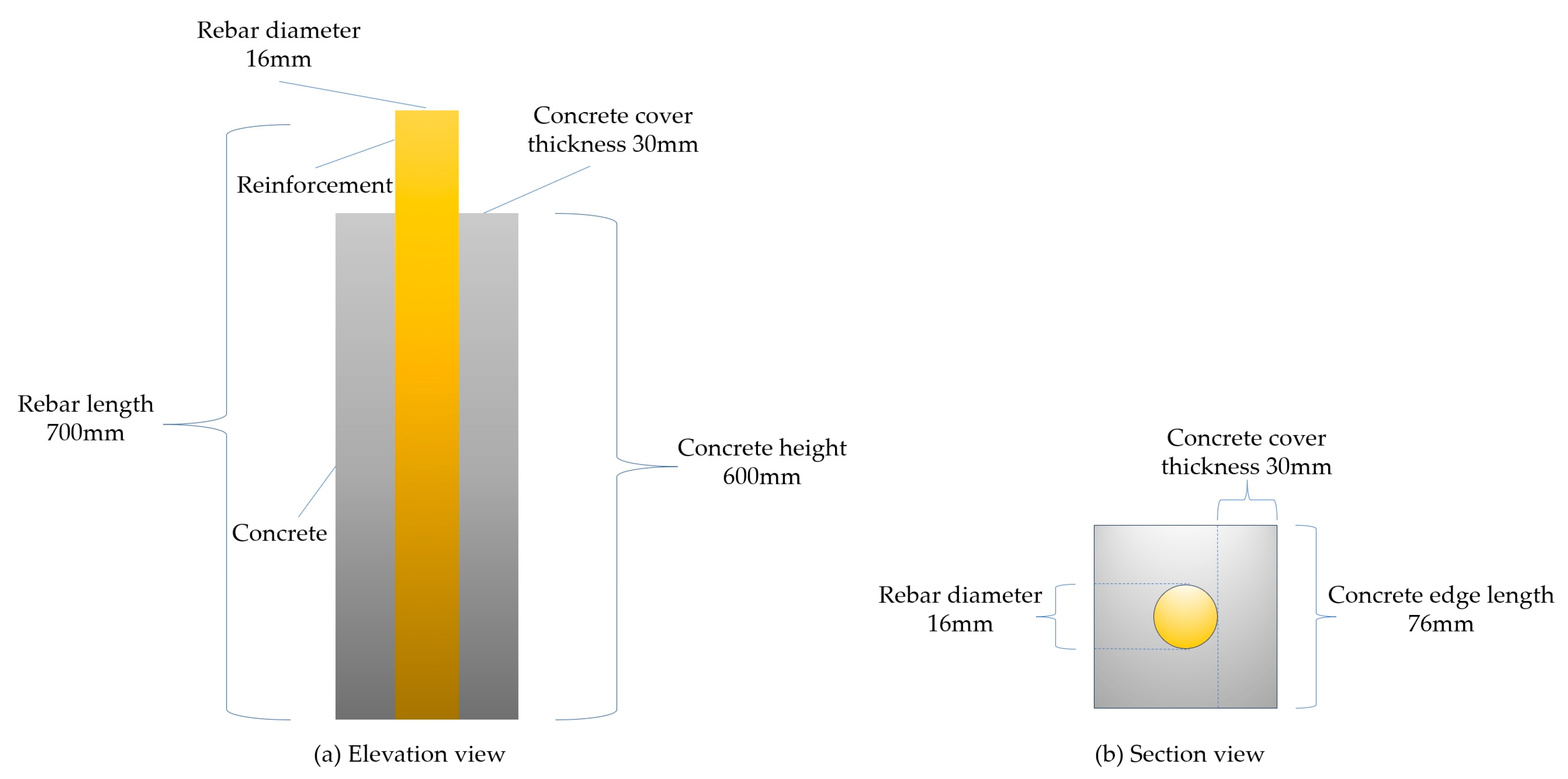
3.2. Preparation of Concrete and UHPC Short Column Specimens
3.3. Determination of Accelerated Corrosion Parameters
3.3.1. Current Density
3.3.2. Corrosion Height
3.3.3. Theoretical Corrosion Rate
3.3.4. The Actual Corrosion Rate
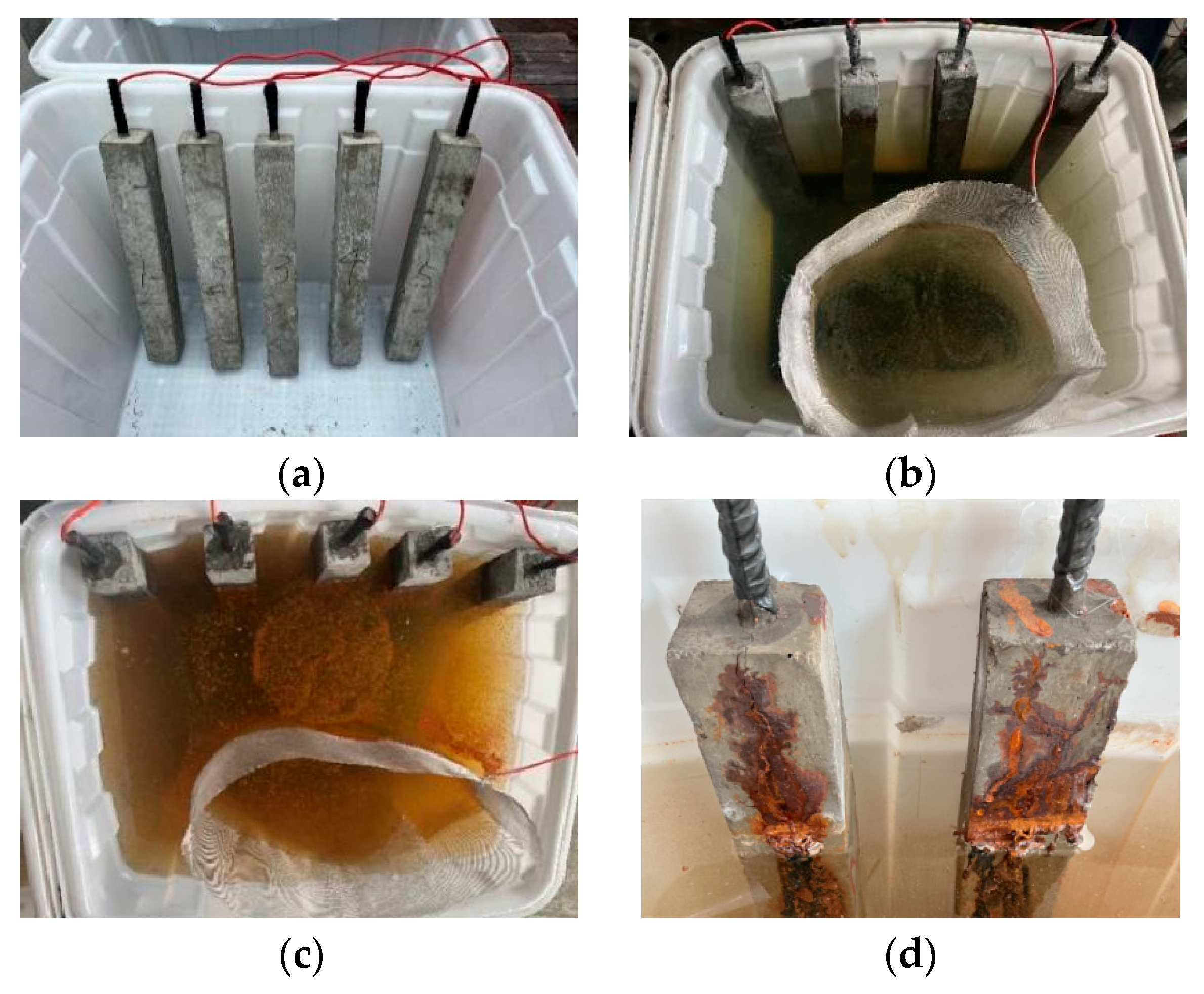
3.4. Accelerated Corrosion Testing Process
4. Analysis of Accelerated Corrosion Results for Short Columns
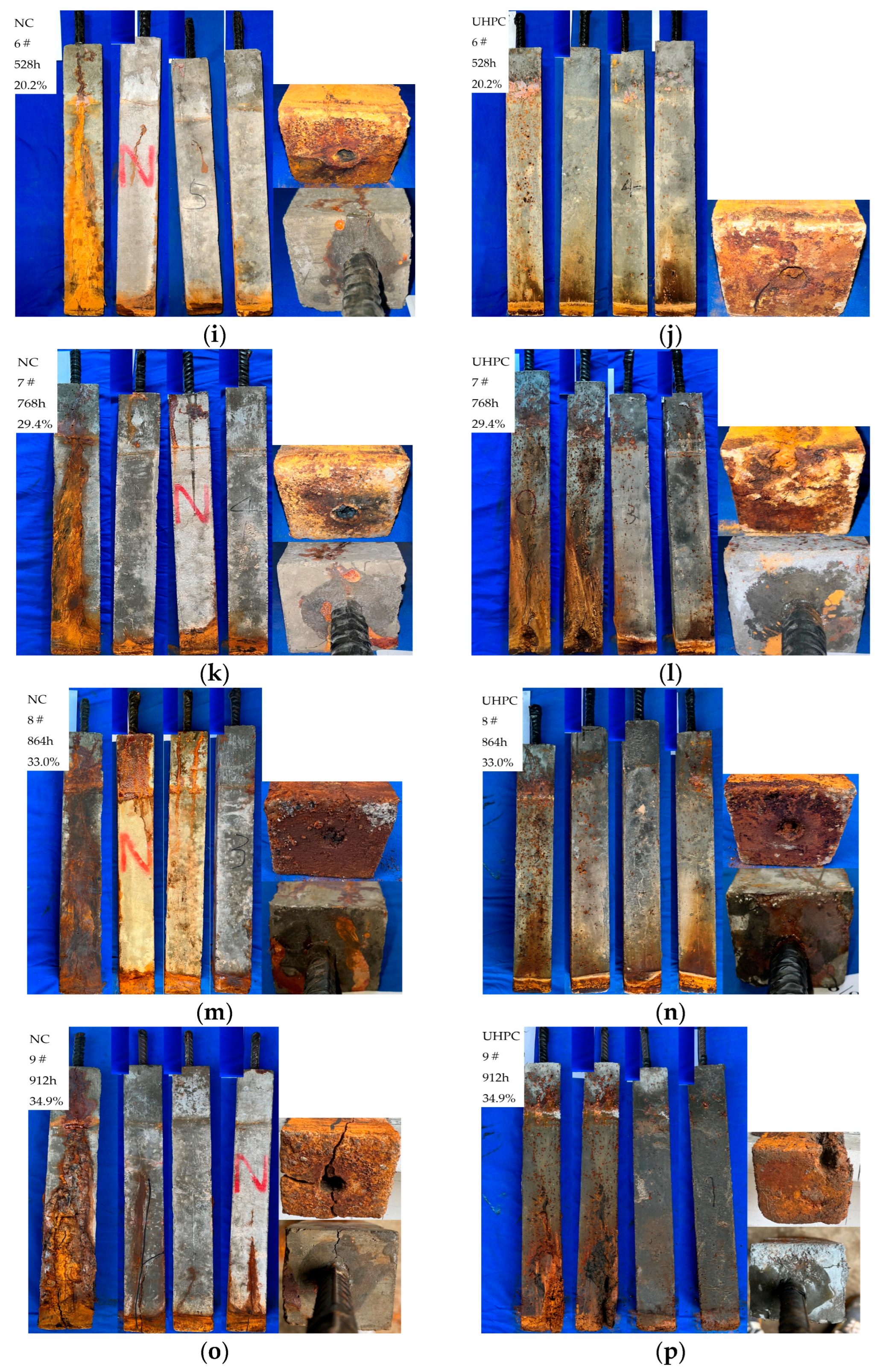
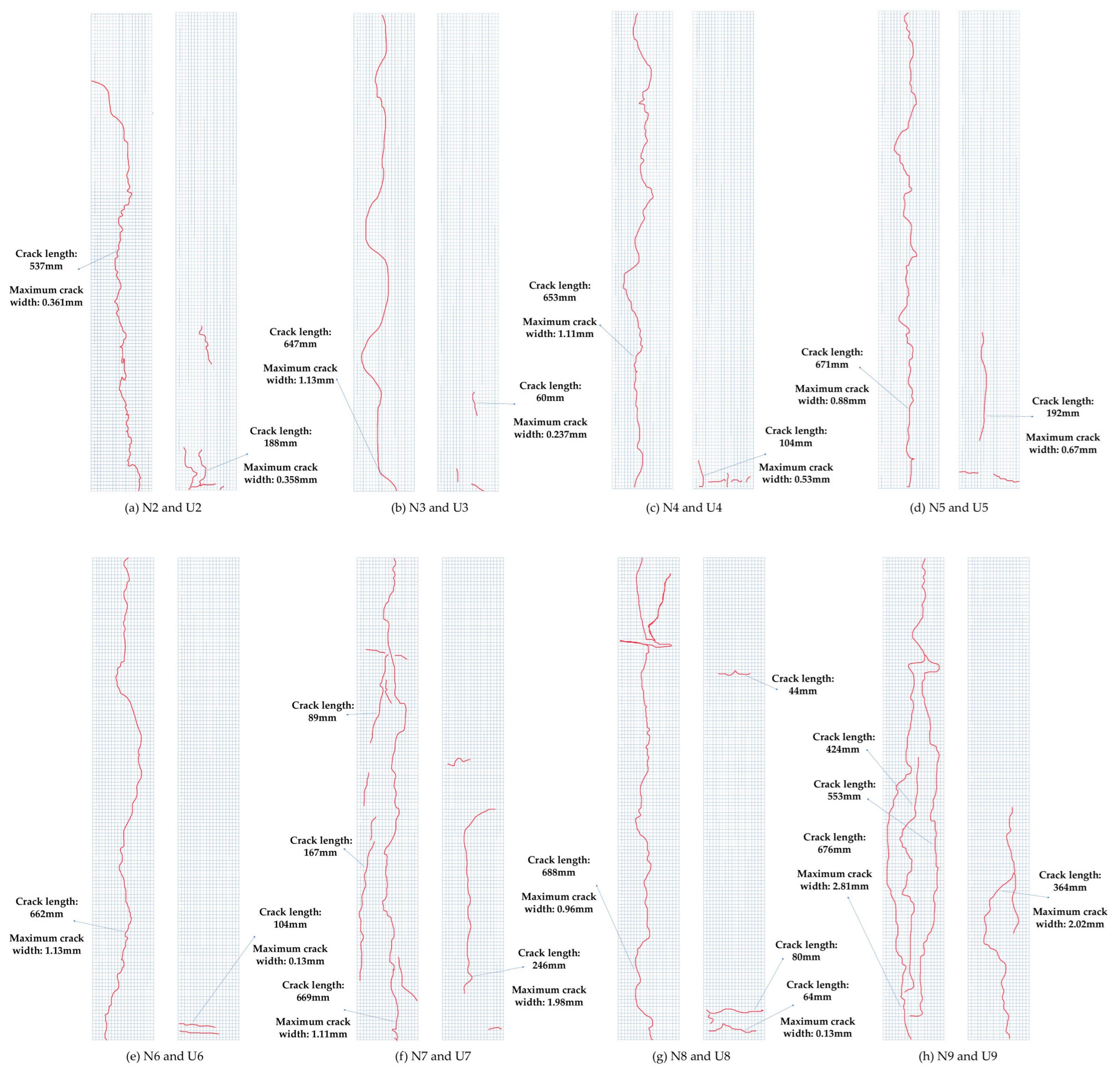
4.1. Analysis of Concrete Cracking Phenomena
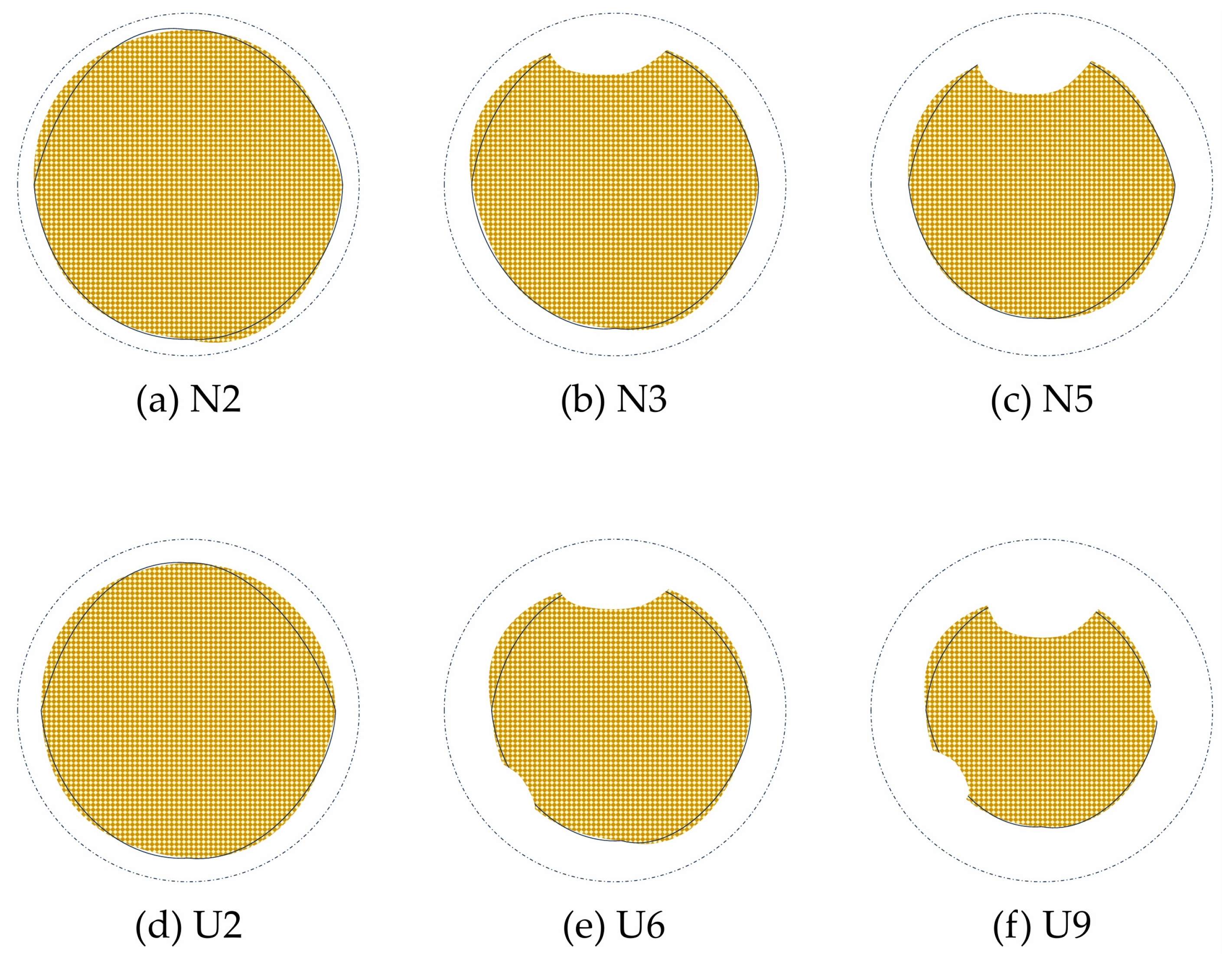
4.2. Analysis of Reinforcement Corrosion Phenomena
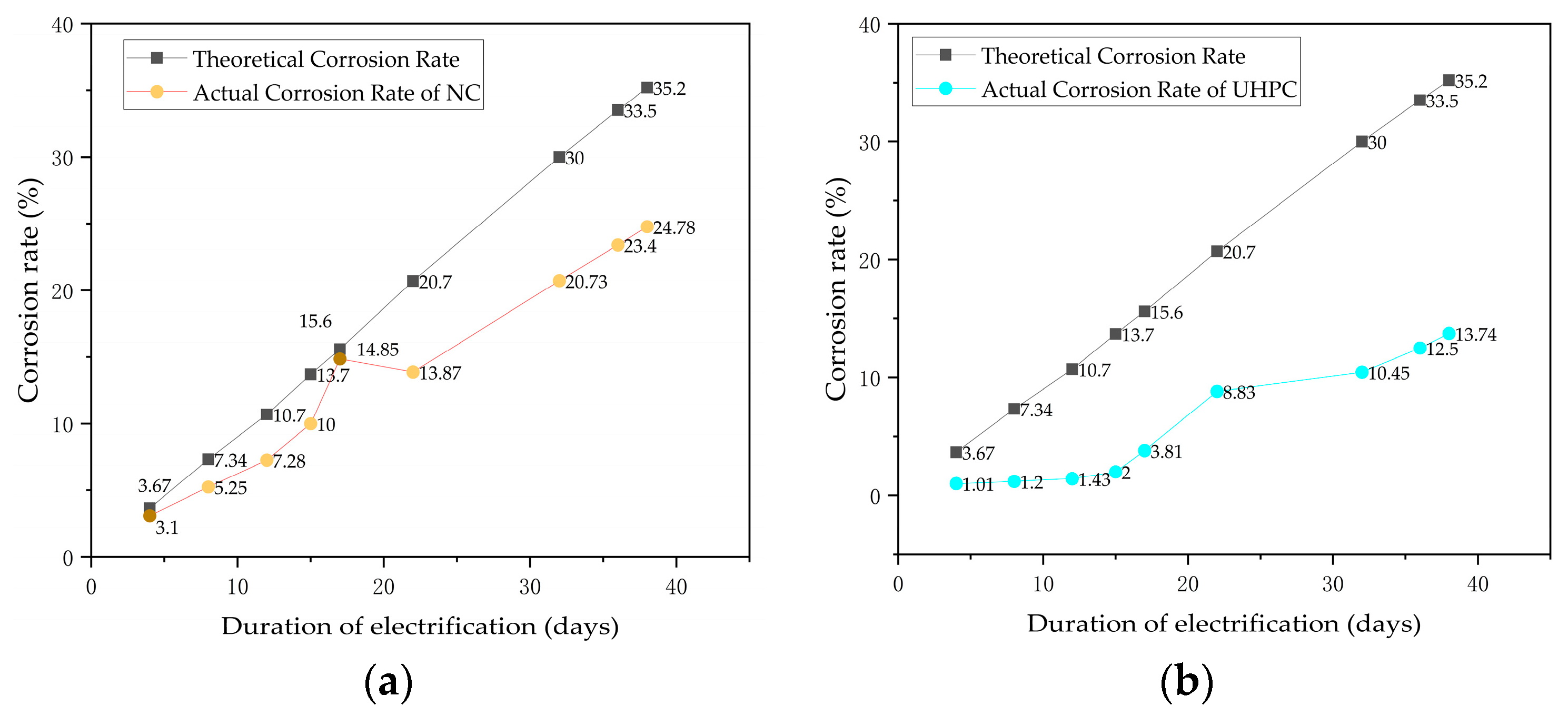
4.3. Analysis of the Corrosion Rate of Steel Reinforcements in Concrete and UHPC Short Columns
5. Conclusions
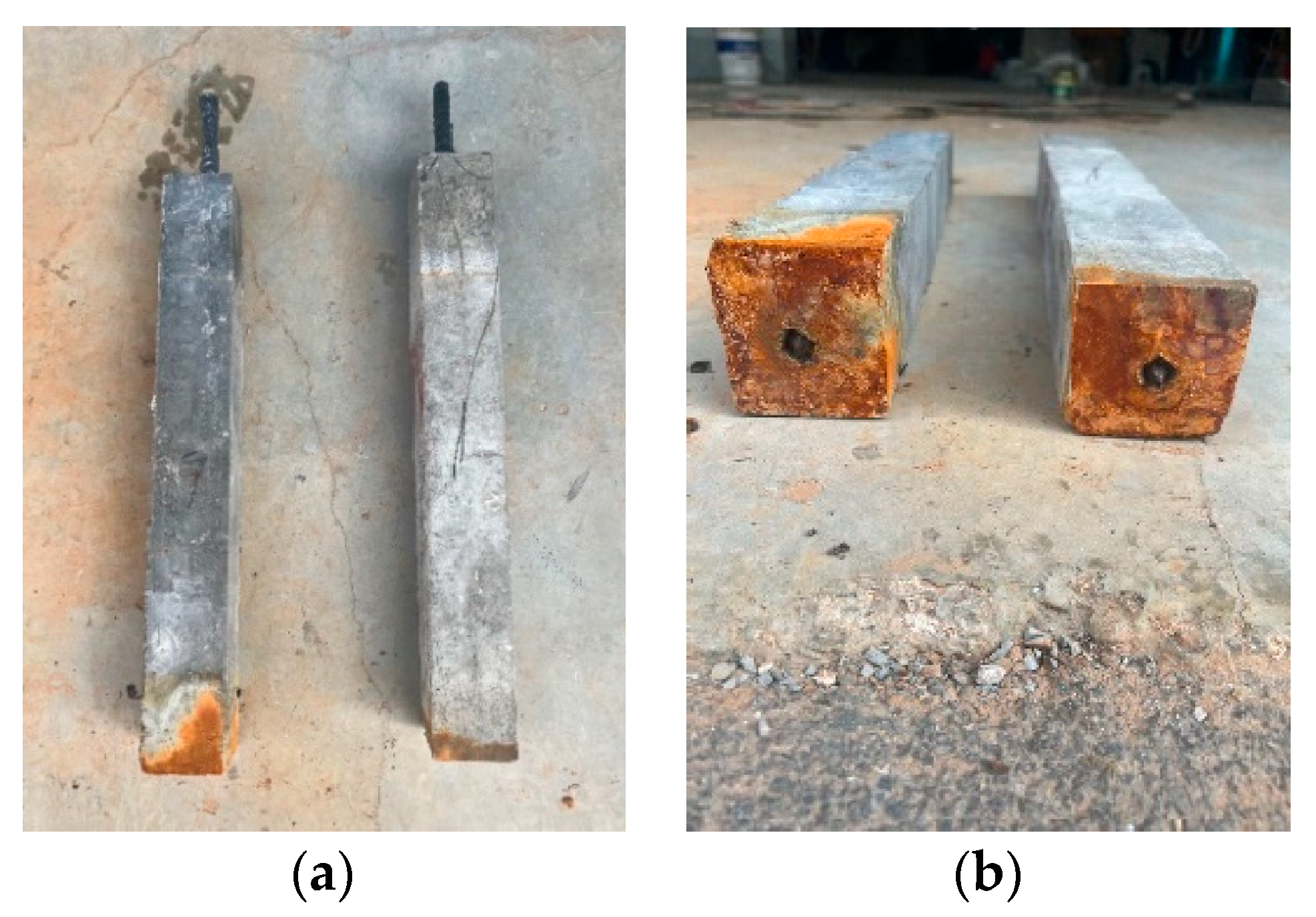
- For the concrete specimens, as the corrosion rate increased, corrosion cracks in the concrete began at the bottom and gradually developed upwards. Specimen N3 exhibited longitudinal cracks through the material, with corrosion products continuously precipitating on the surface, extending from the bottom to the top along the cracks. In contrast, the corrosion phenomena in UHPC were less pronounced. In the early stages of corrosion, only a few point-like corrosion products appeared on one side of the UHPC specimens. Starting with specimen U4, corrosion cracks and spalling began on the side with more corrosion products, with an increase in the corrosion products. As corrosion progressed, spalling eventually occurred at the bottom of the UHPC specimens.
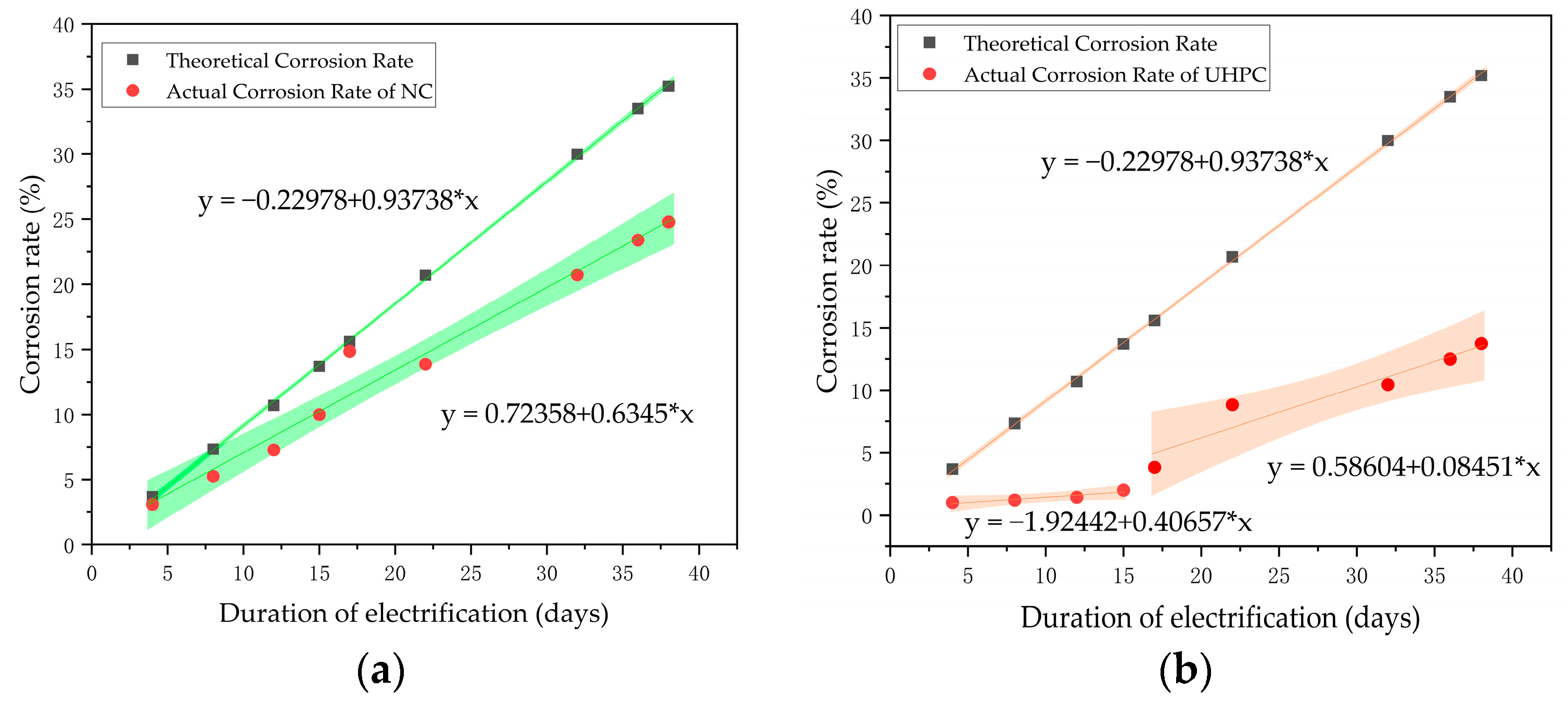
- Starting from N3, the concrete short columns showed noticeable uneven corrosion of the reinforcing steel, whereas the UHPC short columns exhibited uniform corrosion throughout the entire process. Due to the earlier cracking of the concrete specimens, the corrosion rate of their reinforcing steel remained relatively stable, around 70% of the theoretical value. In contrast, the UHPC specimens performed better throughout the corrosion process, with the corrosion rate displaying two distinct phases. The overall actual corrosion rate of the UHPC reinforcing steel was around 40% of the theoretical value.
- From the experimental observations, it is evident that the corrosion rates of both concrete and UHPC short columns after accelerated corrosion are significantly lower than the theoretical corrosion rates. This discrepancy has also been noted in previous research. The theoretical corrosion rates calculated based on Faraday’s law do not adequately account for factors such as chloride ion concentration and cover thickness. Therefore, it is suggested that future studies focus on this area to develop a more realistic corrosion rate calculation equation that better reflects actual engineering conditions.
- This study compared the corrosion phenomena and patterns of ordinary concrete and UHPC specimens. This research allowed us to investigate the corrosion behavior of different material components in actual structures on a smaller scale and at a lower cost. Future research can build on this study to further explore the bond strength of internal steel reinforcements after corrosion and the seismic performance of both materials post corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cairns, J.; Plizzari, G.; Du, Y.; Law, D.; Franzoni, C. Mechanical properties of corrosion-damaged reinforcement. ACI Mater. J. 2005, 102, 256–264. [Google Scholar]
- Chen, J.; Jia, J.; Zhu, M. Development of admixtures on seawater sea sand concrete: A critical review on Concrete hardening, chloride ion penetration and steel corrosion. Constr. Build. Mater. 2024, 411, 134219. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride diffusivity, chloride threshold, and corrosion initiation in reinforced alkali-activated mortars: Role of calcium, alkali, and silicate content. Cem. Concr. Res. 2018, 111, 56–71. [Google Scholar] [CrossRef]
- Ji, H.; Tian, Y.; Fu, C.; Ye, H. Transfer learning enables prediction of steel corrosion in concrete under natural environments. Cem. Concr. Compos. 2024, 148, 105488. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-Term Durability of Marine Reinforced Concrete Structures. J. Mar. Sci. Eng. 2020, 8, 290. [Google Scholar] [CrossRef]
- Kijjanon, A.; Sumranwanich, T.; Tangtermsirikul, S. Steel corrosion and critical chloride content in concrete with calcined kaolinite clay, fly ash, and limestone powder under natural and accelerated marine environments. Structures 2024, 62, 106211. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Wang, J. Corrosion of steel rebar in concrete induced by chloride ions under natural environments. Constr. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- De Domenico, D.; Messina, D.; Recupero, A. Seismic vulnerability assessment of reinforced concrete bridge piers with corroded bars. Struct. Concr. 2023, 24, 56–83. [Google Scholar] [CrossRef]
- Zucca, M.; Reccia, E.; Longarini, N.; Eremeyev, V.; Crespi, P. On the structural behaviour of existing RC bridges subjected to corrosion effects: Numerical insight. Eng. Fail. Anal. 2023, 152, 107500. [Google Scholar] [CrossRef]
- Duan, M.; Miao, J.; Wu, J.; Dong, F. Reliability Assessment Approach for Fire Resistance Performance of Prestressed Steel–Concrete Box Girder Bridges. Fire 2023, 6, 472. [Google Scholar] [CrossRef]
- Duan, M.; Xue, J.; Wang, X.; Wu, Y.; Tao, H. Experimental study on shear performance of perfobond steel plate in ultra-high performance concrete (UHPC)-normal concrete (NC) connection. Structures 2024, 66, 106783. [Google Scholar] [CrossRef]
- Wu, H.; Xu, F.; Li, B.; Gao, Q. Study on Expansion Rate of Steel Slag Cement-Stabilized Macadam Based on BP Neural Network. Materials 2024, 17, 3558. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Guo, A.; Li, H. Equivalent elastic modulus of reinforcement to consider bond-slip effects of coastal bridge piers with non-uniform corrosion. Eng. Struct. 2020, 210, 110382. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Li, Y.; Zhu, W.; Zheng, H.; Zheng, S. Exploring the shear performance and predictive shear capacity of corroded RC columns utilizing the modified compression-field theory: An investigative study. Eng. Struct. 2024, 302, 117390. [Google Scholar] [CrossRef]
- Miao, Z.; Yuan, H.; Liu, Y.; Geng, X. Seismic performance analysis of coastal reinforced concrete frames considering the pitting corrosion in the longitudinal reinforcement bars. J. Build. Eng. 2024, 90, 109478. [Google Scholar] [CrossRef]
- Khaksefidi, S.; Ghalehnovi, M.; Rahdar, H. Bond-slip relationship between deformed rebar and ultra-high-performance concrete with corrosion effect. Case Stud. Constr. Mater. 2024, 21, e03585. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A. Bond strength between corroded steel reinforcement and recycled aggregate concrete. Structures 2019, 19, 369–385. [Google Scholar] [CrossRef]
- Lin, Y.; Zhong, S.; Yu, G.; Sun, L. Effect of corrosion on bond slipping between steel and concrete in SRC structures. J. Constr. Steel Res. 2024, 220, 108857. [Google Scholar] [CrossRef]
- Papakonstantinou, C.G.; Balaguru, P.N.; Auyeung, Y. Influence of FRP confinement on bond behavior of corroded steel reinforcement. Cem. Concr. Compos. 2011, 33, 611–621. [Google Scholar] [CrossRef]
- Tung Pham, T.; Tan Nguyen, N.; Thao Nguyen, T.-T.; Linh Nguyen, N. Numerical analysis of the shear behavior for steel fiber reinforced concrete beams with corroded reinforcing bars. Structures 2023, 57, 105081. [Google Scholar] [CrossRef]
- Haskett, M.; Oehlers, D.J.; Ali, M.S.M.; Wu, C. Yield Penetration Hinge Rotation in Reinforced Concrete Beams. J. Struct. Eng. 2009, 135, 130–138. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, T.; Zhao, Y. Effects of hydrostatic pressure on chloride ion penetration into concrete. Mag. Concr. Res. 2016, 68, 877–886. [Google Scholar] [CrossRef]
- Jensen, M.M.; De Weerdt, K.; Johannesson, B.; Geiker, M.R. Use of a multi-species reactive transport model to simulate chloride ingress in mortar exposed to NaCl solution or sea-water. Comput. Mater. Sci. 2015, 105, 75–82. [Google Scholar] [CrossRef]
- Tahri, W.; Hu, X.; Shi, C.; Zhang, Z. Review on corrosion of steel reinforcement in alkali-activated concretes in chloride-containing environments. Constr. Build. Mater. 2021, 293, 123484. [Google Scholar] [CrossRef]
- Marcotte, T.; Hansson, C.M. Corrosion products that form on steel within cement paste. Mater. Struct. 2006, 40, 325–340. [Google Scholar] [CrossRef]
- Neville, A. Chloride attack of reinforced concrete: An overview. Mater. Struct. 1995, 28, 63–70. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Okada, K.; KobayashiI, K.; Miyagawa, T. Influence of Longitudinal Cracking Due to Reinforcement Corrosion on Characteristics of Reinforced Concrete Members. Aci Struct. J. 1988, 85, 134–140. [Google Scholar]
- Fuhaid, A.F.A.; Niaz, A. Carbonation and Corrosion Problems in Reinforced Concrete Structures. Buildings 2022, 12, 586. [Google Scholar] [CrossRef]
- Naidu Gopu, G.; Joseph, S.A. Corrosion Behavior of Fiber-Reinforced Concrete—A Review. Fibers 2022, 10, 38. [Google Scholar] [CrossRef]
- Ye, H.; Jin, N.; Fu, C.; Jin, X. Rust distribution and corrosion-induced cracking patterns of corner-located rebar in concrete cover. Constr. Build. Mater. 2017, 156, 684–691. [Google Scholar] [CrossRef]
- Almusallam, A.A. Effect of degree of corrosion on the properties of reinforcing steel bars. Constr. Build. Mater. 2001, 15, 361–368. [Google Scholar] [CrossRef]
- GB/T50152-2012; Standard for Test Method of Mechanical Properties of Normal Concrete. Ministry of Housing and Urban-Rural Development of China: Beijing, China, 2012. (In Chinese)
- SIA 2052:2016 Bauwesen; Ultra-Hochleistungs-Faserbeton (UHFB)—Baustoffe, Bemessung und Ausführung. SIA Zurich: Zürich, Switzerland, 2016. (In German)
- Vu, N.S.; Yu, B.; Li, B. Stress-strain model for confined concrete with corroded transverse reinforcement. Eng. Struct. 2017, 151, 472–487. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Shang, Z.; Chen, J.; Wang, D. Experimental study on the seismic behavior of ECE corroded reinforced concrete short pier columns. Constr. Build. Mater. 2022, 348, 128681. [Google Scholar] [CrossRef]
- Chen, L.; Mindess, S.; Morgan, D.R.; Shah, S.P.; Johnston, C.D.; Pigeon, M. Comparative Toughness Testing of Fiber Reinforced Concrete. In SP-155: Testing of Fiber Reinforced Concrete; American Concrete Institute: Indianapolis, IN, USA, 1995. [Google Scholar]
- Liu, Y.; Hao, H.; Hao, Y.; Cui, J. Experimental study of dynamic bond behaviour between corroded steel reinforcement and concrete. Constr. Build. Mater. 2022, 356, 129272. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Zhang, L.; Yang, X. Bond properties between corroded steel bars and recycled aggregate concrete after high-temperature exposure. J. Build. Eng. 2024, 93, 109835. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Lu, Y. Stochastic modeling for bond deterioration between rebar and concrete due to reinforcement corrosion. Structures 2024, 66, 106904. [Google Scholar] [CrossRef]
- JTG/T 2231-02—2021; Specifications for Seismic Performance Evaluation of Highway Bridges. Ministry of Transport of China: Beijing, China, 2021. (In Chinese)
- Maaddawy, T.A.E.; Soudki, K.A. Effectiveness of Impressed Current Technique to Simulate Corrosion of Steel Reinforcement in Concrete. J. Mater. Civ. Eng. 2003, 15, 41–47. [Google Scholar] [CrossRef]
- Vu, K.A.T.; Stewart, M.G. Structural reliability of concrete bridges including improved chloride-induced corrosion models. Struct. Saf. 2000, 22, 313–333. [Google Scholar] [CrossRef]
- GB/T50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of PRC; General Administration of Quality Supervision, Inspection and Quarantine of PRC: Beijing, China, 2009.
- Zheng, Y.; Zheng, S.-S.; Yang, L.; Dong, L.-G.; Zhang, Y.-B. Experimental study and analytical model of the bond behavior of corroded reinforcing steel bars in concrete. Constr. Build. Mater. 2022, 327, 126991. [Google Scholar] [CrossRef]
- Yalciner, H.; Kumbasaroglu, A.; Ertuc, İ.; Turan, A.İ. Confinement Effect of Geo-grid and Conventional Shear Reinforcement Bars Subjected to Corrosion. Structures 2018, 13, 139–152. [Google Scholar] [CrossRef]
- Yalciner, H.; Kumbasaroglu, A.; Turan, A.İ. Torsional behavior of reinforced concrete beams with corroded reinforcement. Structures 2019, 20, 476–488. [Google Scholar] [CrossRef]
- Yalciner, H.; Eren, O.; Sensoy, S. An experimental study on the bond strength between reinforcement bars and concrete as a function of concrete cover, strength and corrosion level. Cem. Concr. Res. 2012, 42, 643–655. [Google Scholar] [CrossRef]



| fc,n (MPa) | fc,u (MPa) | fck,n (MPa) | fck,u (MPa) | ft,u (MPa) |
|---|---|---|---|---|
| 55.7 | 185.9 | 36.3 | 121.6 | 9.8 |
| Sample Number | Electrification Duration (Days) | Current Density (μA/cm2) | Theoretical Corrosion Rate (%) |
|---|---|---|---|
| N1 | 4 | 900 | 3.67 |
| U1 | |||
| N2 | 8 | 900 | 7.34 |
| U2 | |||
| N3 | 12 | 900 | 11.00 |
| U3 | |||
| N4 | 15 | 900 | 13.8 |
| U4 | |||
| N5 | 17 | 900 | 15.6 |
| U5 | |||
| N6 | 22 | 900 | 20.2 |
| U6 | |||
| N7 | 32 | 900 | 29.4 |
| U7 | |||
| N8 | 36 | 900 | 33.5 |
| U8 | |||
| N9 | 38 | 900 | 35.2 |
| U9 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Concrete Short Column | 50.0 | 49.3 | 50.0 | 50.0 | 49.8 | 49.6 | 48.4 | 49.4 | 48.0 |
| UHPC Short Column | 50.0 | 48.7 | 48.8 | 48.4 | 48.0 | 49.5 | 46.2 | 49.3 | 43.0 |
| Difference | 0.0 | 0.6 | 1.2 | 1.6 | 1.8 | −0.1 | 2.2 | 0.1 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Tong, T.; Wang, X.; Huang, J. Comparative Analysis of Concrete Cracking and Reinforcement Corrosion in Concrete and Ultra-High-Performance Concrete Short Columns after Accelerated Corrosion. Appl. Sci. 2024, 14, 7969. https://doi.org/10.3390/app14177969
Wu H, Tong T, Wang X, Huang J. Comparative Analysis of Concrete Cracking and Reinforcement Corrosion in Concrete and Ultra-High-Performance Concrete Short Columns after Accelerated Corrosion. Applied Sciences. 2024; 14(17):7969. https://doi.org/10.3390/app14177969
Chicago/Turabian StyleWu, Hengyu, Teng Tong, Xu Wang, and Jingzhi Huang. 2024. "Comparative Analysis of Concrete Cracking and Reinforcement Corrosion in Concrete and Ultra-High-Performance Concrete Short Columns after Accelerated Corrosion" Applied Sciences 14, no. 17: 7969. https://doi.org/10.3390/app14177969
APA StyleWu, H., Tong, T., Wang, X., & Huang, J. (2024). Comparative Analysis of Concrete Cracking and Reinforcement Corrosion in Concrete and Ultra-High-Performance Concrete Short Columns after Accelerated Corrosion. Applied Sciences, 14(17), 7969. https://doi.org/10.3390/app14177969







